Abstract
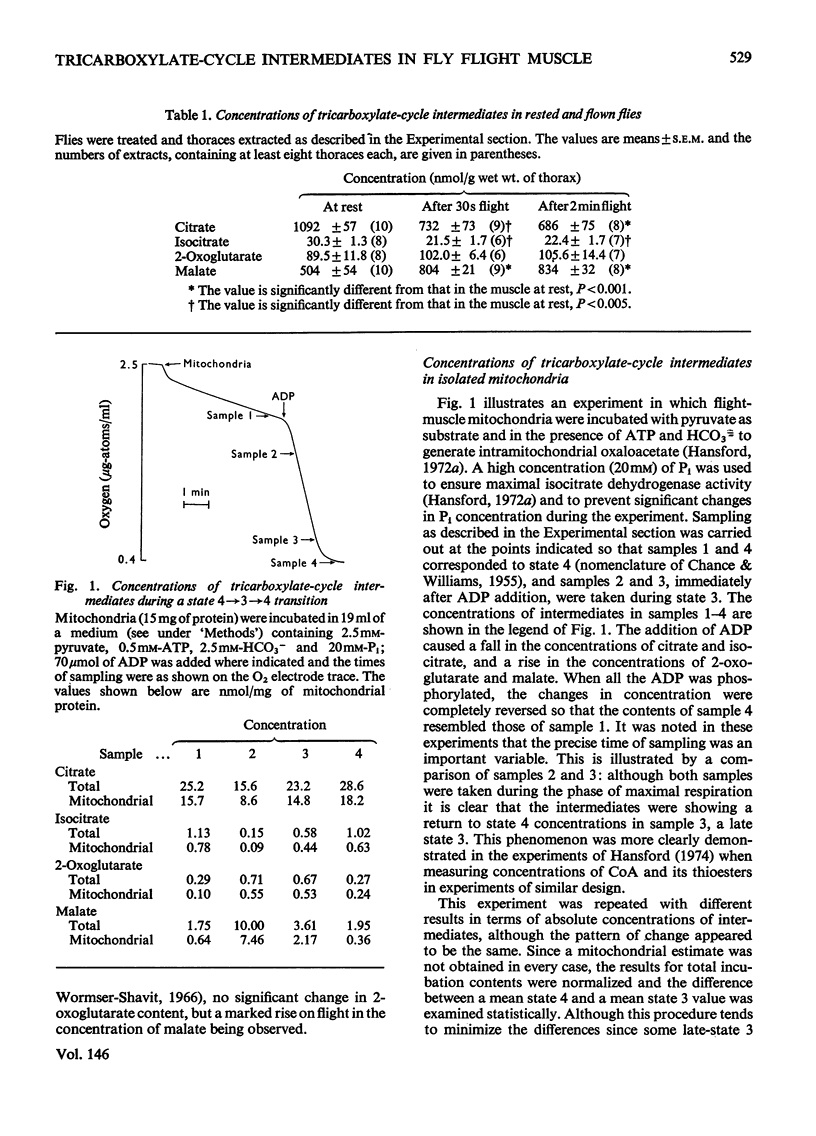
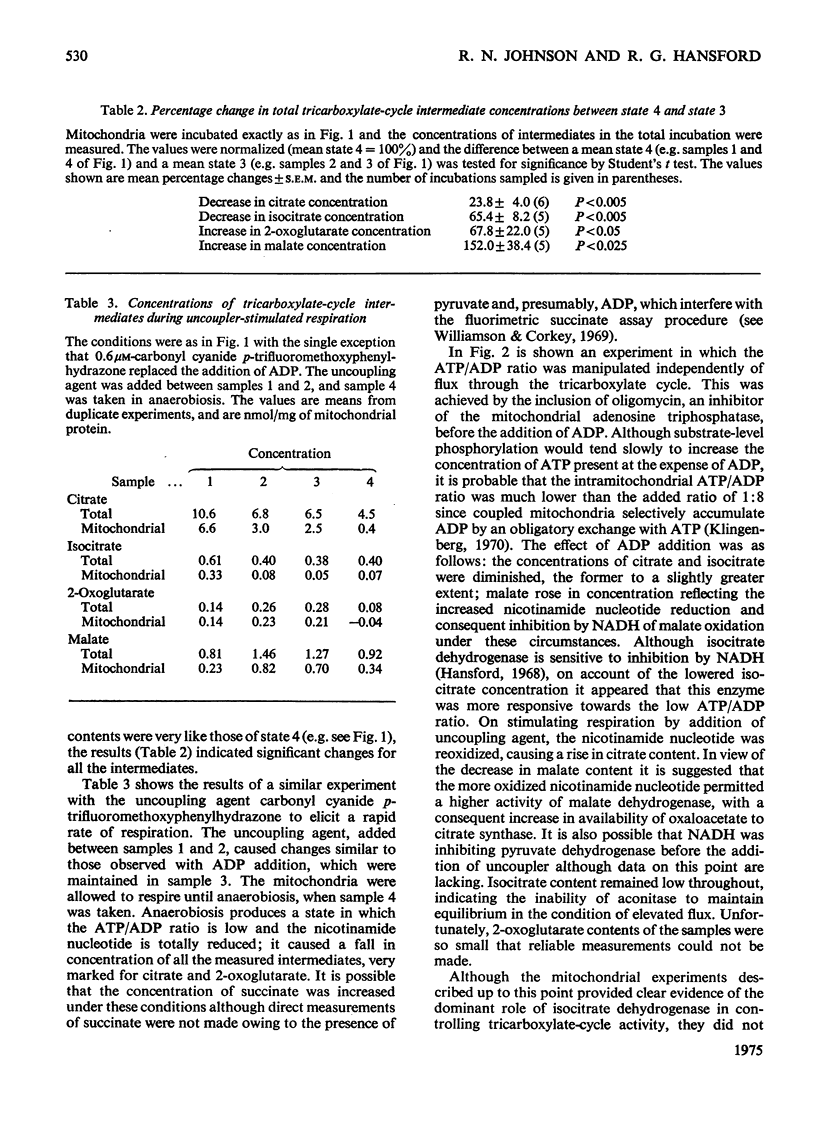
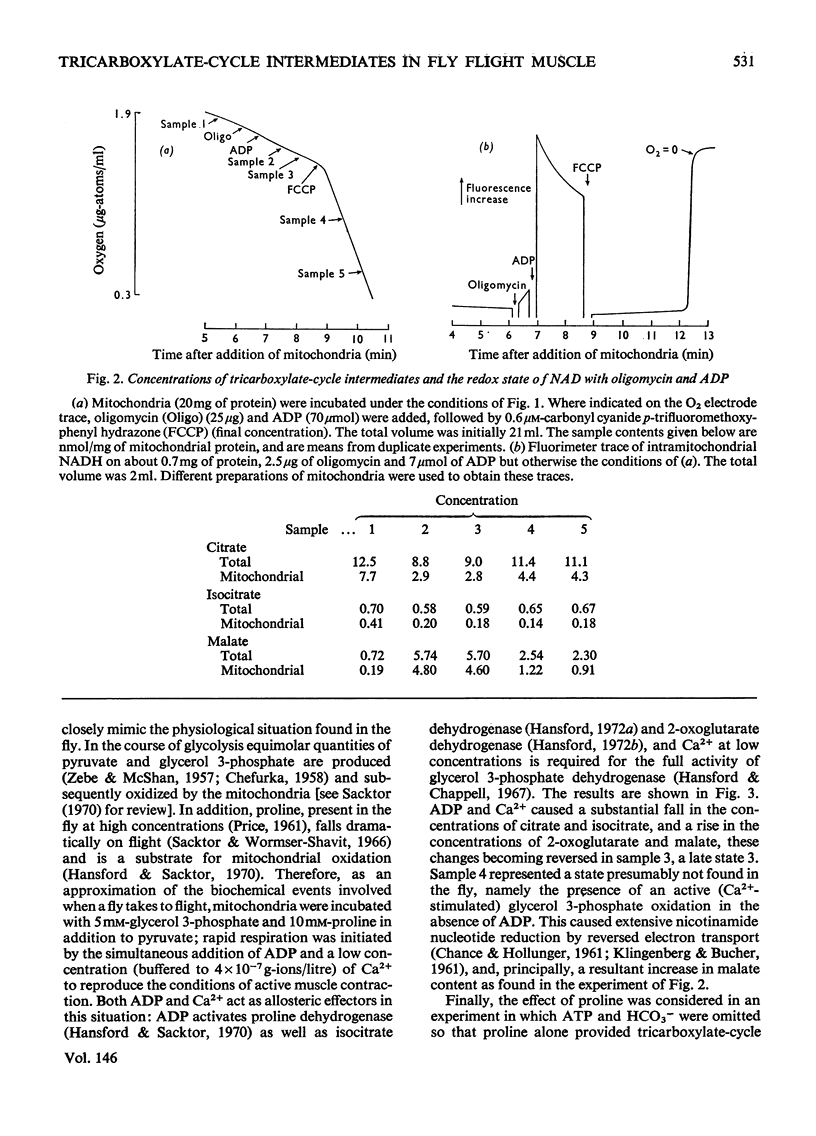
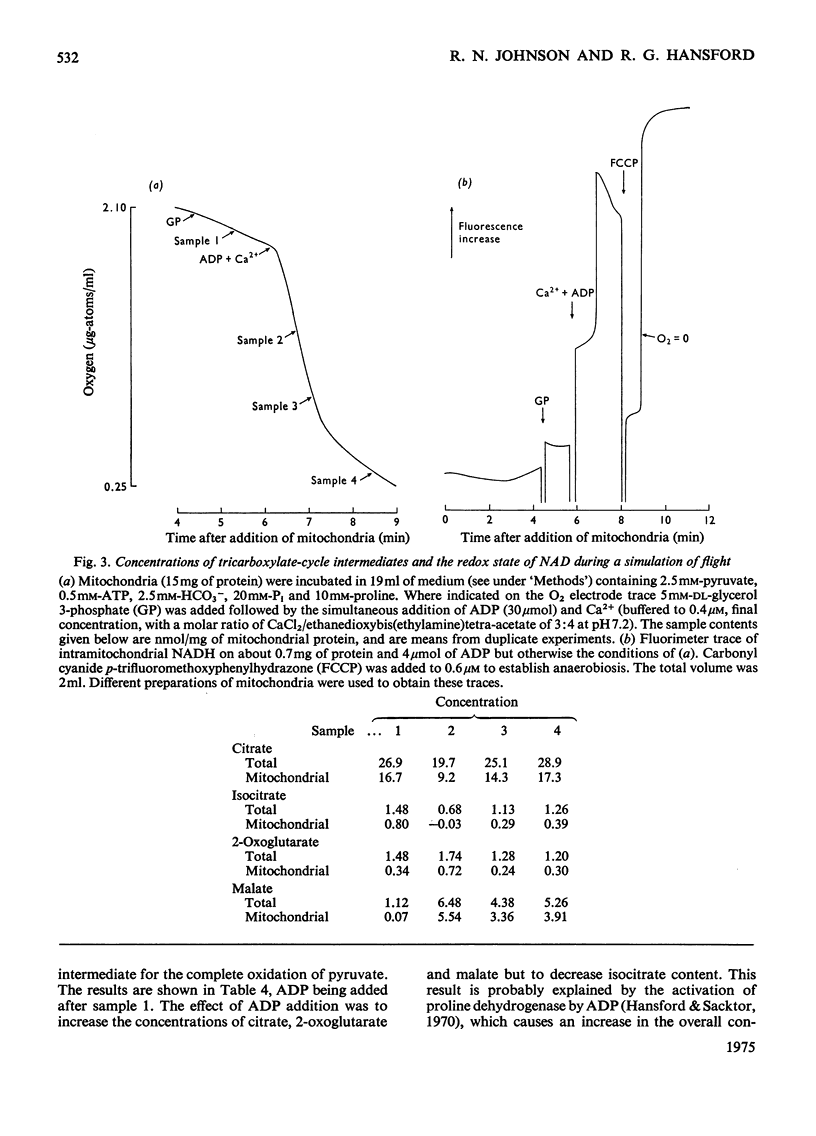
1. Blowfly (Phormia regina) flight-muscle mitochondria were allowed to oxidize pyruvate under a variety of experimental conditions, and determinations of the citrate, isocitrate, 2-oxoglutarate and malate contents of both the mitochondria and the incubation medium were made. For each intermediate a substantial portion of the total was present within the mitochondria. 2. Activation of respiration by either ADP or uncoupling agent resulted in a decreased content of citrate and isocitrate and an increased content of 2-oxoglutarate and malate when the substrate was pyruvate, APT and HCO3 minus. Such a decrease in citrate content was obscured when the substrate was pyruvate and proline owing to a large rise in the total content of tricarboxylate-cycle intermediates in the presence of proline and ADP. 3. An experiment involving oligomycin and uncoupling agent demonstrated that the ATP/ADP ratio is the main determinant of flux through the tricarboxylate cycle, with the redox state of nicotinamide nucleotide being of lesser importance. 4. Addition of ADP and Ca-2+ to activate the oxidation of both glycerol 3-phosphate and pyruvate, simulating conditions on initiation of flight, gave a decrease in citrate and isocitrate and an increase in 2-oxoglutarate and malate content. 5. There was a good correlation between these results with isolated flight-muscle mitochondria and the changes found in fly thoraces after 30s and 2 mihorax. 6. It is concluded that NAD-isocitrate dehydrogenase (EC 1.1.1.41) controls the rate of pyruvate oxidation in both resting fly flight muscle in vivo and isolated mitochondria in state 4 (nomenclature of Change & Williams, 1955).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLAMY D. The endogenous citric acid-cycle intermediates and amino acids of mitochondria. Biochem J. 1962 Jan;82:218–224. doi: 10.1042/bj0820218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J Biol Chem. 1961 May;236:1534–1543. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- CHEFURKA W. On the importance of alpha-glycerophosphate dehydrogenase in glycolysing insect muscle. Biochim Biophys Acta. 1958 Jun;28(3):660–661. doi: 10.1016/0006-3002(58)90545-6. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Chappell J. B. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967 Jun 23;27(6):686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Sacktor B. The control of the oxidation of proline by isolated flight muscle mitochondria. J Biol Chem. 1970 Mar 10;245(5):991–994. [PubMed] [Google Scholar]
- Hansford R. G. Some properties of pyruvate and 2-oxoglutarate oxidation by blowfly flight-muscle mitochondria. Biochem J. 1972 Mar;127(1):271–283. doi: 10.1042/bj1270271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. The control of tricarboxylate-cycle of oxidations in blowfly flight muscle. The steady-state concentrations of coenzyme A, acetyl-coenzyme A and succinyl-coenzyme A in flight muscle and isolated mitochondria. Biochem J. 1974 Sep;142(3):509–519. doi: 10.1042/bj1420509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. The control of tricarboxylate-cycle oxidations in blowfly flight muscle. The oxidized and reduced nicotinamide-adenine dinucleotide content of flight muscle and isolated mitochondria, the adenosine triphosphate and adenosine diphosphate content of mitochondria, and the energy status of the mitochondria during controlled respiration. Biochem J. 1975 Mar;146(3):537–547. doi: 10.1042/bj1460537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. The effect of adenine nucleotides upon the 2-oxoglutarate dehydrogenase of blowfly flight muscle. FEBS Lett. 1972 Mar 15;21(2):139–141. doi: 10.1016/0014-5793(72)80122-4. [DOI] [PubMed] [Google Scholar]
- KLINGENBERG M., BUECHER T. [Glycerin-1-phosphate and flight muscle mitochondria]. Biochem Z. 1961;334:1–17. [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- LaNoue K. F., Bryla J., Williamson J. R. Feedback interactions in the control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1972 Feb 10;247(3):667–679. [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- Price G. M. Some aspects of amino acid metabolism in the adult housefly Musca domestica. Biochem J. 1961 Aug;80(2):420–428. doi: 10.1042/bj0800420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor B., Hurlbut E. C. Regulation of metabolism in working muscle in vivo. II. Concentrations of adenine nucleotides, arginine phosphate, and inorganic phosphate in insect flight muscle during flight. J Biol Chem. 1966 Feb 10;241(3):632–634. [PubMed] [Google Scholar]
- Sacktor B., Wormser-Shavit E. Regulation of metabolism in working muscle in vivo. I. Concentrations of some glycolytic, tricarboxylic acid cycle, and amino acid intermediates in insect flight muscle during flight. J Biol Chem. 1966 Feb 10;241(3):624–631. [PubMed] [Google Scholar]
- Shepherd D., Garland P. B. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J. 1969 Sep;114(3):597–610. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Colleen M., Williamson John R. Inhibition of citrate synthase by succinyl-CoA and other metabolites. FEBS Lett. 1971 Oct 15;18(1):35–38. doi: 10.1016/0014-5793(71)80400-3. [DOI] [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]
- ZEBE E. C., MCSHAN W. H. Lactic and alpha-glycerophosphate dehydrogenases in insects. J Gen Physiol. 1957 May 20;40(5):779–790. doi: 10.1085/jgp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den BERGH S., SLATER E. C. The respiratory activity and permeability of housefly sarcosomes. Biochem J. 1962 Feb;82:362–371. doi: 10.1042/bj0820362. [DOI] [PMC free article] [PubMed] [Google Scholar]


