Abstract
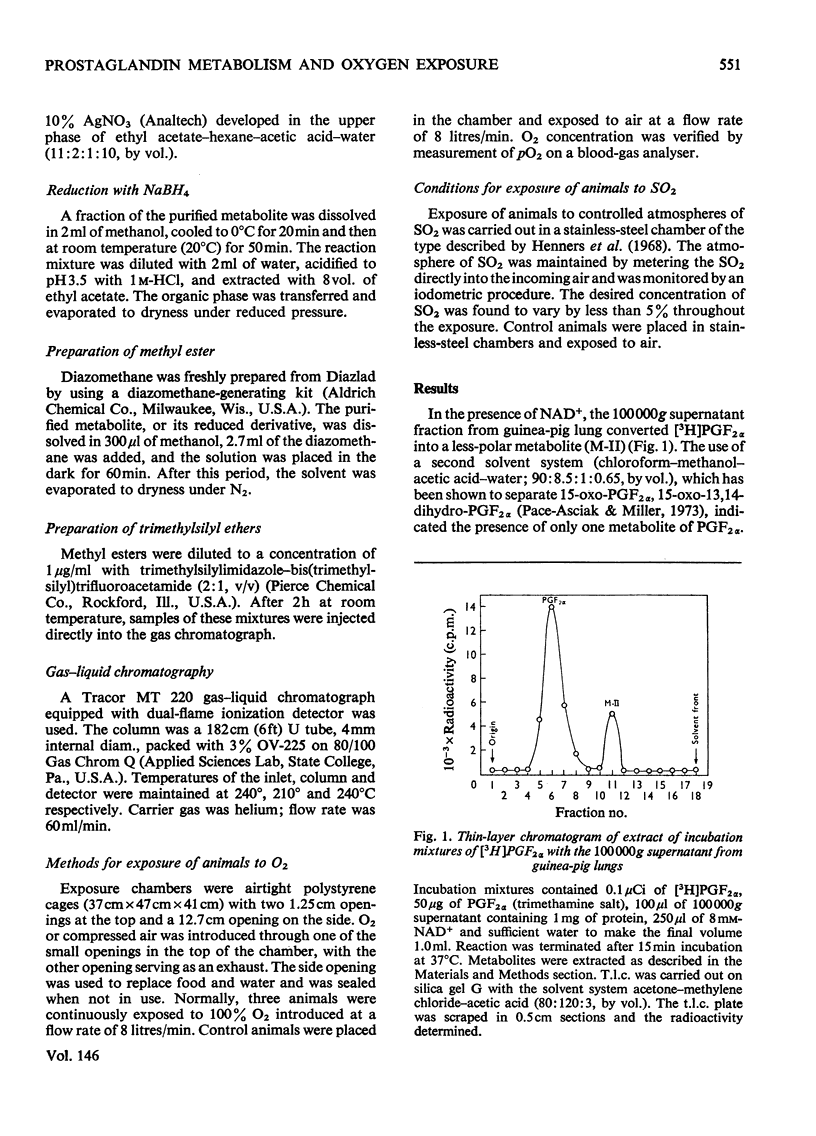
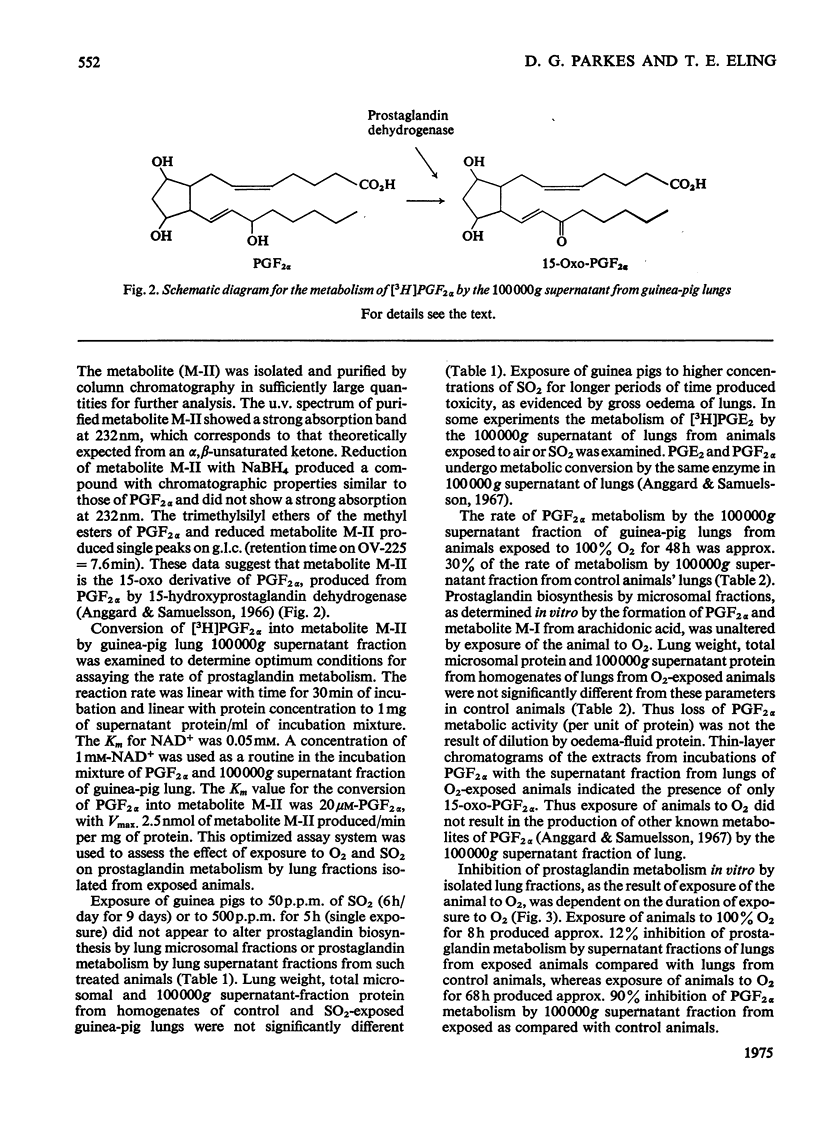
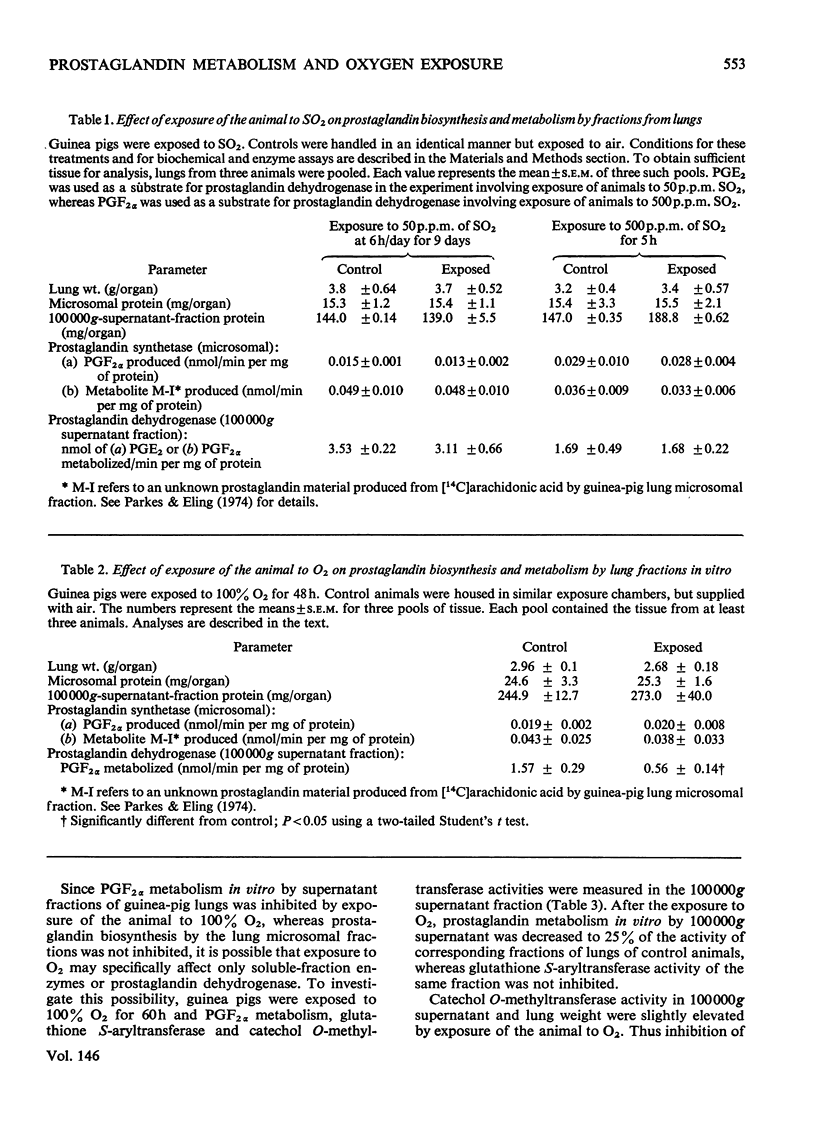
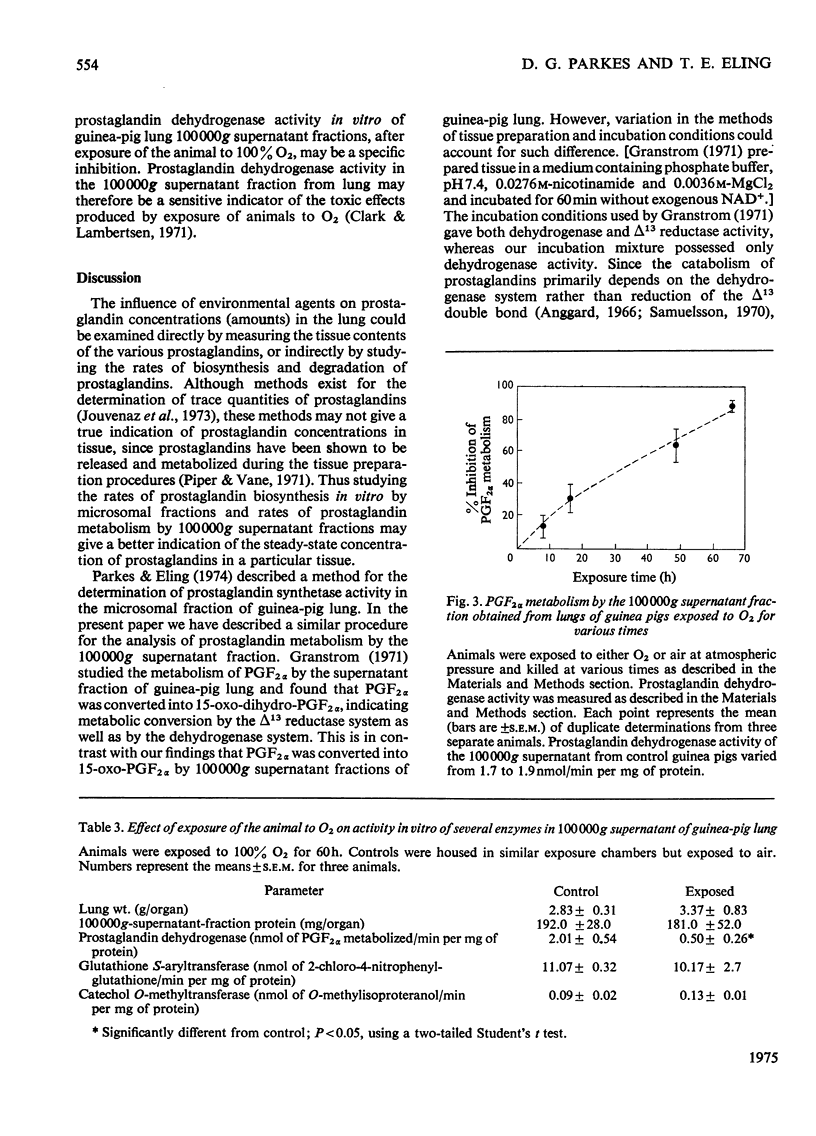
Enzymes in the 100 000g supernatant fraction of guinea-pig lungs, in the presence of NAD-+, converted PGF-2 alpha (prostaglanding F-2 alpha) into a less-polar compound. The u.v. spectrum of this metabolite showed a strong absorption band at 230 nm, which is characteristic of a carbonyl group in conjugation with a double bond. Reduction of this metabolite with NaBH4 resulted in a compound that behaved like PGF2 ALPHA on t.l.c. and g.l.c. From this evidence we concluded that PGF2alpha is metabolized in vitro to 15-oxo-PGF2 alpha by the NAD-+-dependent prostaglandin dehydrogenase system of guinea-pig lung. The effect of exposure of the animal to SO-2 and O2 on the rate of prostaglanding biosynthesis and catabolism by lung fractions in vitro was studied. Exposure of guinea pigs to 500 p.m. of SO2 for 5h or to 50p.p.m for 9 days (6h/day) did not alter the production or degradation of prostaglandings by lung fractions in vitro. In contrast, exposure of guinea pigs to 100% O2 for 48 h inhibited the rate of prostaglanding metabolism in vitro by 60-70% without significantly altering the rate of biosynthesis by lung fractions. Inhibition of prostaglandin dehydrogenase activity in vitro by lung fractions after exposure of the animal to O2 was dependent on the duration of exposure. Gluthathione S-aryltransferase and catechol O-methyltransferase activites of guinea-pig lung 100 000g supernatant were unaltered by exposure of the animal to O2. Thus it appears that inhibition of pulmonary prostaglandin dehydrogenase by exposure of the animal to O2 is not the result of a general toxic response. It was postulated that the inhibition of prostaglanding dehydrogenase may occur after exposure of the animal to other oxidant gases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anggård E., Larsson C., Samuelsson B. The distribution of 15-hydroxy prostaglandin dehydrogenase and prostaglandin-delta 13-reductase in tissues of the swine. Acta Physiol Scand. 1971 Mar;81(3):396–404. doi: 10.1111/j.1748-1716.1971.tb04914.x. [DOI] [PubMed] [Google Scholar]
- Anggård E., Samuelsson B. Biosynthesis of prostaglandins from arachidonic acid in guinea pig lung. Prostaglandins and related factors. 38. J Biol Chem. 1965 Sep;240(9):3518–3521. [PubMed] [Google Scholar]
- Anggård E. The biological activities of three metabolites of prostaglandin E 1. Acta Physiol Scand. 1966 Apr;66(4):509–510. doi: 10.1111/j.1748-1716.1966.tb03231.x. [DOI] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Fanburg B. L. Prostaglandins and the lung. Am Rev Respir Dis. 1973 Sep;108(3):482–489. doi: 10.1164/arrd.1973.108.3.482. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Granström E. Metabolism of prostaglandin F2-alpha in guinea pig lung. Eur J Biochem. 1971 Jun 29;20(4):451–458. doi: 10.1111/j.1432-1033.1971.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Haugaard N. Cellular mechanisms of oxygen toxicity. Physiol Rev. 1968 Apr;48(2):311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- Hinman J. W. Prostaglandins. Annu Rev Biochem. 1972;41:161–178. doi: 10.1146/annurev.bi.41.070172.001113. [DOI] [PubMed] [Google Scholar]
- Hinners R. G., Burkart J. K., Punte C. L. Animal inhalation exposure chambers. Arch Environ Health. 1968 Feb;16(2):194–206. doi: 10.1080/00039896.1968.10665043. [DOI] [PubMed] [Google Scholar]
- Horton E. W. Hypotheses on physiological roles of prostaglandins. Physiol Rev. 1969 Jan;49(1):122–161. doi: 10.1152/physrev.1969.49.1.122. [DOI] [PubMed] [Google Scholar]
- Jouvenaz G. H., Nugteren D. H., van Dorp D. A. Gas chromatographic determination of nanogram amounts of prostaglandins E and F. Prostaglandins. 1973 Feb;3(2):175–187. doi: 10.1016/0090-6980(73)90085-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mathé A. A., Hedqvist P., Holmgren A., Svanborg N. Bronchial hyperreactivity to prostaglandin F 2 and histamine in patients with asthma. Br Med J. 1973 Jan 27;1(5847):193–196. doi: 10.1136/bmj.1.5847.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Mizuno K., Miyamoto T., Suzuki T., Oshima Y. The effects of ozone, nitrogen dioxide, and sulfur dioxide on experimentally induced allergic respiratory disorder in guinea pigs. IV. Effects on respiratory sensitivity to inhaled acetylcholine. Am Rev Respir Dis. 1972 Feb;105(2):262–267. doi: 10.1164/arrd.1972.105.2.262. [DOI] [PubMed] [Google Scholar]
- Matsumura Y. The effects of ozone, nitrogen dioxide, and sulfur dioxide on the experimentally induced allergic respiratory disorder in guinea pigs. I. The effect on sensitization with albumin through the airway. Am Rev Respir Dis. 1970 Sep;102(3):430–437. doi: 10.1164/arrd.1970.102.3.430. [DOI] [PubMed] [Google Scholar]
- Oesterling T. O., Morozowich W., Roseman T. J. Prostaglandins. J Pharm Sci. 1972 Dec;61(12):1861–1895. doi: 10.1002/jps.2600611202. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C., Miller D. Prostaglandins during development. I. Age-dependent activity profiles of prostaglandin 15-hydroxydehydrogenase and 13,14-reductase in lung tissue from late prenatal, early postnatal and adult rats. Prostaglandins. 1973 Sep;4(3):351–362. doi: 10.1016/0090-6980(73)90022-1. [DOI] [PubMed] [Google Scholar]
- Parkes D. G., Eling T. E. Characterization of prostaglandin synthetase in guinea pig lung. Isolation of a new prostaglandin derivative from arachidonic acid. Biochemistry. 1974 Jun 4;13(12):2598–2604. doi: 10.1021/bi00709a020. [DOI] [PubMed] [Google Scholar]
- Pike J. E. Prostaglandins. Sci Am. 1971 Nov;225(5):84–93. doi: 10.1038/scientificamerican1171-84. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Vane J. R. Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature. 1969 Jul 5;223(5201):29–35. doi: 10.1038/223029a0. [DOI] [PubMed] [Google Scholar]
- Piper P., Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971 Apr 30;180:363–385. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Dao N. Release of vasoactive substances from guinea-pig lungs by slow-reacting substance c and arachidonic acid. Its blockade by nonsteroid anti-inflammatory agents. Pharmacology. 1971;6(2):99–108. doi: 10.1159/000136231. [DOI] [PubMed] [Google Scholar]
- Weeks J. R. Prostaglandins. Annu Rev Pharmacol. 1972;12:317–336. doi: 10.1146/annurev.pa.12.040172.001533. [DOI] [PubMed] [Google Scholar]


