Abstract
Inflammatory mediators orchestrate the host immune and metabolic response to acute bacterial infections and mediate the events leading to septic shock. Tumor necrosis factor (TNF) has long been identified as one of the proximal mediators of endotoxin action. Recent studies have implicated peroxisome proliferator-activated receptor alpha (PPARα) as a potential target to modulate regulation of the immune response. Since PPARα activators, which are hypolipidemic drugs, are being prescribed for a significant population of older patients, it is important to determine the impact of these drugs on the host response to acute inflammation. Therefore, we examined the role of PPARα activators on the regulation of TNF expression in a mouse model of endotoxemia. CD-1 mice treated with dietary fenofibrate or Wy-14,643 had fivefold-higher lipopolysaccharide (LPS)-induced TNF plasma levels than LPS-treated control-fed animals. Higher LPS-induced TNF levels in drug-fed animals were reflected physiologically in significantly lower glucose levels in plasma and a significantly lower 50% lethal dose than those in LPS-treated control-fed animals. Utilizing PPARα wild-type (WT) and knockout (KO) mice, we showed that the effect of fenofibrate on LPS-induced TNF expression was indeed mediated by PPARα. PPARα WT mice fed fenofibrate also had a fivefold increase in LPS-induced TNF levels in plasma compared to control-fed animals. However, LPS-induced TNF levels were significantly decreased and glucose levels in plasma were significantly increased in PPARα KO mice fed fenofibrate compared to those in control-fed animals. Data from peritoneal macrophage studies indicate that Wy-14,643 modestly decreased TNF expression in vitro. Similarly, overexpression of PPARα in 293T cells decreased activity of a human TNF promoter-luciferase construct. The results from these studies suggest that any anti-inflammatory activity of PPARα in vivo can be masked by other systemic effects of PPARα activators.
Septic or endotoxic shock is a complex syndrome characterized by hypotension, poor organ perfusion, and severe systemic metabolic derangements. Although the pathogenesis of septic shock is complex, it is well documented that microorganisms elicit the release of large amounts of inflammatory cytokines from activated macrophages. The inflammatory mediators are released in a cascade and include tumor necrosis factor (TNF), interleukin 6 (IL-6), and IL-1, among others (1). The inflammatory cytokines induce the acute-phase response as well as a number of deleterious effects leading to the shock syndrome. Despite the cascade of inflammatory mediators induced by bacteria or their toxic products, a single endogenous factor, TNF, can mimic the lethal systemic responses elicited by endotoxin (37). Therefore, attempts have been made to develop reagents that block and/or attenuate the release of TNF during sepsis.
The discovery of a new family of nuclear hormone receptors, the peroxisome proliferator-activated receptors (PPARs), has opened the possibility of elucidating additional mechanisms for the regulation of cytokine production (32). PPARs are members of the class II family of nuclear steroid receptors which heterodimerize with the 9-cis retinoic acid receptor (RXR) (12). PPAR-RXR heterodimers act as transcription factors which bind to 6-bp direct repeats separated by a single base pair, termed a PPAR response element (PPRE). PPREs have been identified in a number of genes involved in lipid metabolism, such as acyl coenzyme A (CoA) oxidase (9), peroxisomal β-oxidation bifunctional enzyme (40), cytochrome P450 IVA6 enzyme (23), phosphoenolpyruvate carboxykinase (PEPCK) (36), and lipoprotein lipase (LPL) (33). PPARα mediates the pleiotropic response to peroxisome proliferators and the hypolipidemic effect of fibrates (reviewed in reference 32). This was demonstrated when PPARα knockout mice were shown to be refractory to the effects of the classical peroxisome proliferators, clofibrate and Wy-14,643 (19).
The interest in PPARα and its role in modulating the immune response has emerged due to a number of diverse in vivo and in vitro studies. In mice lacking the PPARα gene, the response to topical inflammatory mediators was prolonged compared to the response in wild-type mice (7). Also, numerous animal studies have shown that dietary administration of (n-3) polyunsaturated fatty acids (PUFA), which are PPARα activators, resulted in increased survival of the animals when they were challenged with bacteria or endotoxin (reviewed in references 3 and 38). In addition, a number of human studies have been conducted examining the effect of dietary (n-3) fatty acids, which are potential PPAR ligands, on cytokine production in endotoxin-stimulated peripheral blood monocytes. The consensus of these studies is that circulating levels of proinflammatory cytokines (TNF, IL-6, IL-1) are decreased following administration of fish oil supplements (3).
Despite the fact that PPARα activators, such as the fibrates, have long been prescribed for a significant population of older patients, no one to our knowledge has examined the effect of these hypolipidemic drugs on the host response to acute inflammation, such as endotoxemia. Therefore, since TNF is one of the proximal mediators of endotoxic shock, we chose to examine the effect of PPARα activators on TNF expression in well-characterized in vivo and in vitro models of endotoxemia.
MATERIALS AND METHODS
Chemicals.
All chemicals were purchased from Sigma (St. Louis, Mo.) unless otherwise noted. [4-Chloro-6-(2,3-xylidine)-pyrimidinylthio]acetic acid (Wyeth [Wy]-14,643) was purchased from ChemSyn Science Laboratories (Lenexa, Kans.). Pelleted Purina rodent chow 5001 was prepared with control (no drug), 0.1% Wy-14,643, or 0.5% fenofibrate (Bioserv, Frenchtown, N.J.).
Animals.
All animals were housed and cared for according to the National Institutes of Health guidelines for the care and use of laboratory animals. All experiments involving animals were approved by the Oklahoma University Health Sciences Center Institutional Animal Care and Use Committee. Male CD-1 mice (20 to 25 g) were purchased from Charles River Laboratory (Wilmington, Mass.). All experiments were conducted after the animals had acclimated for 1 week. The CD-1 mice were used in feeding experiments and fed a diet containing no drug, fenofibrate (0.5%), or Wy-14,643 (0.1%) ad libitum for 14 days. Experiments were also conducted with age- and sex-matched C57BL/6N × Sv/129 homozygous wild-type (WT) or knockout (KO) mice for PPARα (19). In all experiments (except the lethality study), the animals were injected intraperitoneally (i.p.) with either 0.5 ml of sterile saline or a sublethal dose of Escherichia coli O111:B4 lipopolysaccharide (LPS) (12 mg/kg of body weight) (15). At the appropriate time interval, the animals were anesthetized with metaphane and decapitated to remove trunk blood. Livers were removed and weighed. The plasma was separated and stored at −70°C. In the lethality study, CD-1 mice were fed as described earlier and challenged i.p. with LPS in doses ranging from 6 to 60 mg/kg (four mice/dose). The 50% lethal dose (LD50) dose was calculated by using the Reed–Muench method (27).
Macrophage studies.
Peritoneal macrophages were elicited with thioglycollate and harvested from WT and KO mice as previously described (14). Following attachment, the cells (105 per well) were treated with medium (Dulbecco’s modified Eagle medium [DMEM], 10% fetal bovine serum, 1% sodium pyruvate, 100 U of penicillin/ml, and 100 μg of streptomycin/ml) plus vehicle (ethanol) or medium plus Wy-14,643 at a final concentration of 50 μM. After 3 days of incubation, the cells were treated with LPS at a final concentration of 10 ng/ml for 2, 4, 6, or 24 h. Cell culture supernatant fractions were harvested and stored at −70°C for TNF measurements.
TNF ELISA.
Plasma samples were assayed for TNF by using a sandwich enzyme-linked immunosorbent assay (ELISA). The capture (rat anti-mouse TNF) and detecting (biotinylated rat anti-mouse TNF) antibodies were purchased from Pharmingen (San Diego, Calif.). Concentrations of TNF in the plasma samples or culture supernatant fractions were calculated from a standard curve determined by using recombinant mouse TNF (Genzyme, Cambridge, Mass.). The assay was linear between 50 and 3,200 pg/ml.
Lipid analysis.
Plasma levels of total triglyceride (TG) and total cholesterol (TC) were measured by the Lipid Analysis Laboratory at Oklahoma Medical Research Foundation (Oklahoma City, Okla.).
Glucose assay.
Glucose levels in plasma were determined by using a colorimetric glucose oxidase assay kit from Sigma Chemical Co.
Plasmids.
A human TNF promoter fragment (−615 to +90) was cloned into the SmaI site of p19Luc (8). The mammalian expression vector, pEF-BOS was utilized to prepare the PPAR and RXRα expression vectors (22). The murine (m) PPARα, mPPARγ2, mPPARδ (20), and mRXRα (kindly provided by Ron Evans, Salk Institute) cDNAs were excised from their original vectors, ligated to BstXI linkers, and subcloned into the BstXI site of pEF-BOS (29).
Transfections.
Transfections were carried out in 293T human renal epithelial cells by using the calcium phosphate method. The cells were plated at a density of 5 × 104 cells per 35-mm2 dish 1 day prior to transfection. The calcium phosphate-DNA coprecipitate was prepared by using 2 μg of each expression vector and 1 μg of TNF-luc per well. PPAR and RXR expression vectors have been shown by antibody staining to express protein following transfection in 293T cells (11). Total DNA transfected in each group was equalized with the empty vector, pEF-BOS. Following an overnight incubation, the cells were fed with fresh medium, incubated an additional 24 h, and harvested in a 100-μl volume of 25 mM glycylglycine, 15 mM MgSO4, 1 mM dithiothreitol, and 1% Triton X-100. Luciferase assays were performed over a 20-s period by using a 25-μl aliquot of cell lysate and 100 μl of reaction buffer (0.5 mM d-luciferin, 2.5 mM ATP, 7.5 mM MgSO4, 100 mM KH2PO4) in a Monolight 2010 Luminometer (Analytical Luminescence Laboratory, San Diego, Calif.). Luciferase values were normalized relative to cell lysate protein and are reported as means ± standard errors. Protein was quantitated by using the bicinchoninic acid (BCA) protein assay kit from Pierce Chemical Co. (Rockford, Ill.).
Statistical analysis.
Statistical analysis of the data was performed by using Student’s t test for significant differences (P < 0.05), with Minitab statistical software.
RESULTS
Effect of PPARα activators on LPS-induced TNF levels in CD-1 mice.
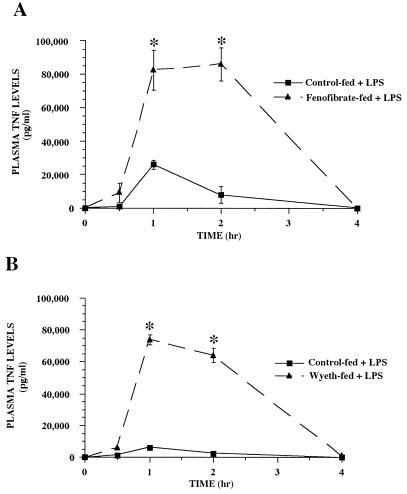
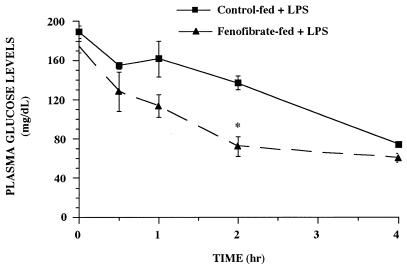
TNF has long been identified as one of the proximal mediators of septic or endotoxic shock. Since recent reports suggest that the PPARs may be important in immune regulation, we tested the effect of PPARα activators on TNF expression during endotoxemia. CD-1 mice were fed for 2 weeks with rodent chow containing no drug, fenofibrate (0.5%), or Wy-14,643 (0.1%) and challenged with E. coli O111:B4 LPS (12 mg/kg). TNF levels in plasma were assessed over time in control-fed versus drug-fed mice after LPS challenge (Fig. 1). TNF was not detectable in the saline-treated groups from either control-fed or fenofibrate-fed animals. In LPS-treated control-fed animals, TNF in plasma was detectable by 0.5 h and peaked 1 h after challenge. In LPS-treated fenofibrate-fed mice, TNF levels were significantly elevated at the 1 and 2 h time points compared to the LPS-treated control-fed group (P < 0.05). TNF was cleared from the bloodstream by 4 h in both LPS-treated groups. Similar results were obtained in animals fed Wy-14,643 (Fig. 1B). We also measured glucose levels in plasma in the fenofibrate-fed animals, since hypoglycemia is a marker of acute endotoxemia and TNF, like LPS and IL-1, induces hypoglycemia (17). As shown previously in this model (15, 16), plasma glucose levels decreased over time, with hypoglycemia observed 4 h after LPS treatment (Fig. 2). Plasma glucose levels were also significantly lower (P < 0.05) at the 2 h time point in LPS-treated fenofibrate-fed versus LPS-treated control-fed animals.
FIG. 1.
Time course of fenofibrate (A) and Wy 14,643 (B) on TNF levels in plasma from mice during endotoxemia. CD-1 mice were fed rodent chow containing no drug, fenofibrate (0.5%), or Wy-14,643 (0.1%) for 2 weeks. The animals were then challenged with 0.5 ml of saline or LPS (12 mg/kg), and plasma samples were collected at the indicated times. The data are taken from a representative experiment (n = 4 animals per group), and the values are expressed as the means ± standard errors of the means. Asterisks denote values that are significantly different (P < 0.05), as determined by Student’s t test, from those of LPS-treated control-fed animals. The values for saline-treated animals were not plotted, since no TNF was detected in the plasma samples.
FIG. 2.
Time course of fenofibrate on glucose levels in plasma from mice during endotoxemia. CD-1 mice were fed rodent chow containing no drug or fenofibrate (0.5%) for 2 weeks. The animals were then challenged with 0.5 ml of saline or LPS (12 mg/kg), and plasma samples were collected at the indicated times. The data are taken from a representative experiment (n = four animals per group), and the values are expressed as means ± standard errors of the means. Asterisks denote values that are significantly different (P < 0.05), as determined by Student’s t test, from LPS-treated control-fed animals.
The efficacy of the feeding regimen was assessed by measuring body weight, liver weight, plasma TG, and plasma TC levels of control and drug-fed animals (Table 1). Animals that were fed fenofibrate or Wy-14,643 had significantly larger livers (P < 0.05) than control-fed animals, due to the peroxisome and cell proliferative effect of the compounds (24). Body weight was also significantly decreased (P < 0.05) in animals fed Wy-14,643 compared to the body weight of control-fed animals. As expected, TG and TC levels were significantly decreased (P < 0.05) by approximately 50% in fenofibrate-fed mice compared to control-fed mice (34).
TABLE 1.
Effect of PPAR activators on body weight, liver weight, and lipid levels in CD-1 mice
| Measure | Results for indicated expt and group (mean ± SEM)
|
|||
|---|---|---|---|---|
| Expt 1
|
Expt 2
|
|||
| Control | Fenofibrate | Normal | Wy-14,643 | |
| Body weighta | 33.3 ± 3.4 | 28.0 ± 3.2 | 31.1 ± 2.5 | 22.1 ± 1.8b |
| Liver weighta | 2.1 ± 0.3 | 4.5 ± 0.8b | 2.0 ± 0.2 | 3.4 ± 0.4b |
| TGc | 110.2 ± 8.5 | 56.6 ± 14.6b | NDd | ND |
| TCc | 116.0 ± 8.8 | 65.9 ± 6.0b | ND | ND |
n = 32.
P < 0.05 compared to level for control-fed animals, as determined by Student’s t test.
n = 9.
ND, not determined.
Since LPS-induced TNF levels were significantly higher in fenofibrate-fed mice, we conducted a lethality study to determine if fenofibrate treatment altered mortality in response to LPS. CD-1 mice were fed control chow or fenofibrate chow for 2 weeks as described earlier and challenged with LPS (6 to 60 mg/kg). Mortality was monitored for 7 days, although all deaths occurred between 24 and 48 h. The LD50 for control-fed CD-1 mice was 530 μg/mouse (18.6 mg/kg when adjusted for body weight) versus 350 μg/mouse (14 mg/kg when adjusted for body weight) for fenofibrate-fed CD-1 mice. Thus, fenofibrate treatment decreased the LD50 for LPS by 25% compared to that for control-fed mice.
Effect of LPS on plasma TNF and glucose levels in PPARα WT versus KO mice.
The results described earlier suggest that activation of PPARα by fenofibrate or Wy-14,643 may be involved in the regulation of TNF during endotoxemia. To test this hypothesis, we utilized age- (3 to 6 months old) and sex-matched PPARα WT and KO mice to assess TNF expression during endotoxemia. WT and KO mice were fed either control chow or chow containing fenofibrate (0.5%) ad libitum for 2 weeks as described earlier. While the WT mice fed fenofibrate exhibited a significant increase in liver weight, the KO mice did not (data not shown), consistent with the original observations of Lee et al. (19). The animals were challenged i.p. with E. coli LPS (12 mg/kg), and plasma was collected 2 h later. Data from a representative experiment (one of two experiments) are shown in Table 2. Consistent with the results in CD-1 mice, LPS-induced plasma TNF levels were fivefold higher (P < 0.05) in fenofibrate-fed WT mice than in control-fed WT mice. However, LPS-induced plasma TNF levels were fivefold lower (P < 0.05) in fenofibrate-fed KO mice than in control-fed KO mice. There was no significant difference in LPS-induced TNF levels in control-fed WT mice versus control-fed KO mice. Glucose levels in plasma were also measured, and the results are shown in Table 3. LPS treatment significantly decreased glucose levels in plasma in control-fed or fenofibrate-fed WT mice compared to those in mice challenged with saline. Consistent with the observation in CD-1 mice described earlier, glucose levels in the plasma of LPS-challenged mice were significantly lower in fenofibrate-fed WT mice than in control-fed WT mice. However, in KO mice, LPS treatment significantly lowered glucose levels in the plasma of control-fed mice but not in that of fenofibrate-fed mice. These data indicate that PPARα is necessary to mediate the LPS-inducible, TNF-enhancing effects of fenofibrate. Moreover, the data suggest that in the absence of PPARα, fenofibrate treatment decreased the magnitude of TNF expression and subsequent hypoglycemia during endotoxemia.
TABLE 2.
TNF levels in plasma in fenofibrate-fed PPARα WT and KO mice 2 h after endotoxin treatment
| Type of chow | Mean ± SEM level of TNF in plasma from indicated mice treated with saline or LPS (pg/ml)a
|
|||
|---|---|---|---|---|
| WT
|
KO
|
|||
| Saline | LPS | Saline | LPS | |
| Control | 0 ± 0 | 36,789 ± 14,568 | 0 ± 0 | 30,597 ± 9,466b |
| Fenofibrate | 0 ± 0 | 203,769 ± 43,690bc | 0 ± 0 | 5,354 ± 2,904bc |
n = 4 to 5 animals per group.
P < 0.05 compared to level for animals receiving saline.
P < 0.05 compared to level for control-fed animals receiving LPS.
TABLE 3.
Glucose levels in plasma from fenofibrate-fed PPARα WT and KO mice 2 h after endotoxin treatment
| Type of chow | Mean ± SEM level of glucose in plasma from indicated mice treated with saline or LPS (mg/dl)a
|
|||
|---|---|---|---|---|
| Wild type
|
KO
|
|||
| Saline | LPS | Saline | LPS | |
| Control | 195.8 ± 6.1 | 114.7 ± 3.9b | 161.4 ± 3.9 | 115.7 ± 10.5b |
| Fenofibrate | 164.3 ± 4.3 | 79.0 ± 3.6bc | 157.6 ± 5.3 | 152.5 ± 24.0 |
n = 4 to 5 animals per group.
P < 0.05 compared to level for animals receiving saline.
P < 0.05 compared to level for control-fed animals receiving LPS.
Effect of Wy-14,643 on LPS-induced TNF production in peritoneal macrophages from WT versus KO mice.
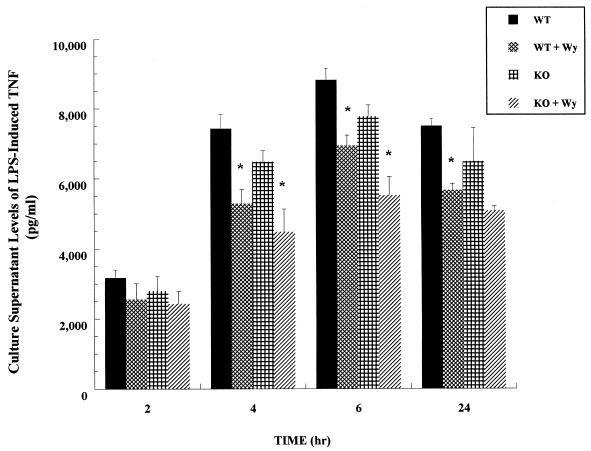
Since TNF is a principal cytokine induced in vivo in response to LPS and is known to be produced by LPS-stimulated macrophages in vitro, we examined the effect of LPS treatment over time on TNF production in primary macrophages from WT versus KO mice in the presence or absence of Wy-14,643. We have shown previously that the concentration of LPS (10 ng/ml) used in these studies is nonlethal, as evidenced by cell viability (>95%, as judged by trypan blue exclusion) over the course of the experiment (14). The results of a representative experiment are shown in Fig. 3. No TNF was detectable in the culture supernatant fractions from saline-treated macrophages, and therefore, the saline-treated groups are not represented in the figure. Consistent with the results observed in intact WT and KO animals, we observed no significant differences in LPS-induced TNF production in macrophages from WT versus KO mice. However, short-term (72-h) in vitro treatment with Wy-14,643 significantly reduced (P < 0.05) LPS-induced TNF production in peritoneal macrophages from both WT and KO mice at the 4 and 6 h time points. These results suggest that, in cultured primary macrophages, the effects of Wy-14,643 on TNF expression are not mediated through PPARα.
FIG. 3.
Time course of Wy-14,643 on LPS-induced TNF expression in primary macrophages from PPARα wild-type (WT) versus knockout (KO) mice. Culture supernatant fractions were collected from LPS-treated (10 ng/ml) primary peritoneal macrophages at the times indicated. The data presented are from a representative experiment (n = 4 wells per group). Asterisks denote values that are significantly different (P < 0.05), as determined by Student’s t test, from LPS-treated cells in the absence of Wy-14,643.
Effect of overexpression of PPARs on TNF promoter activity.
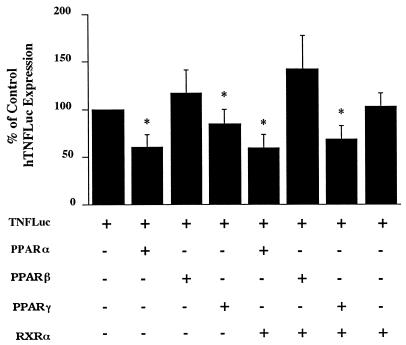
To test further whether PPARs are regulators of TNF expression, we tested mPPAR expression vectors alone and in combination with an mRXRα expression vector for changes in reporter activity when cotransfected with a human TNF promoter-luciferase reporter construct (hTNF-luc). PPARs can directly regulate target genes by heterodimerizing with RXRα and binding to promoter elements termed PPREs. PPARs may also indirectly regulate target genes by influencing the activity of other transcription factors, such as NFκB.
The cotransfection results are shown in Fig. 4. The control value is the luciferase activity in cells transfected with the reporter gene (hTNF-luc) plus the empty pEF-BOS expression vector and is expressed as 100%. The luciferase activity in 293T cells transfected with hTNF-luc was relatively high (1 × 106 to 3 × 106 RLU/mg of protein), even in the absence of any exogenously added TNF inducers or activators. PPARα decreased hTNF-luc activity to 60% of control levels in the presence or absence of RXRα. PPARβ had no significant effect on hTNF-luc activity in the presence or absence of RXRα. PPARγ decreased TNF-luc activity to approximately 80% of control levels and to approximately 65% of control levels in the presence of RXRα. RXRα alone had no effect on hTNF-luc activity. Although the cotransfection analyses we performed in this study show that PPARs influence TNF expression, the results do not allow us to conclude if the effect of PPARs on TNF expression was direct or indirect.
FIG. 4.
Effect of overexpression of PPARs and RXRα on hTNF-luciferase activity. 293T cells were cotransfected, by using the calcium phosphate method, with a TNF promoter (−615 to +90)-luciferase construct plus PPARα, PPARβ, or PPARγ with or without RXRα. The medium was changed 24 h after transfection, and cell extracts were harvested 24 h later. Luciferase activity and protein concentration were determined, and the results are expressed as the percentage of activity of hTNF-luciferase alone. The results represent four independent experiments (n = 3 wells/group/experiment).
DISCUSSION
The data presented here show that PPARα activators markedly up-regulated TNF expression, decreased plasma glucose levels, and increased mortality in mice during endotoxemia. Conversely, PPARα activators modestly down-regulated TNF expression in vitro. Although the mechanism of action of fenofibrate or Wy-14,643 on TNF expression is unclear, we propose several possible explanations.
Firstly, PPARα could bind directly to the TNF promoter. If PPARα is a positive transcription factor for TNF, fenofibrate or Wy-14,643 could increase TNF transcription by activating PPARα to form heterodimers with its partner, RXRα, and interact with a PPRE in the promoter of the TNF gene. This could result in a larger pool of nascent TNF mRNA present in macrophages that is translated and secreted when exposed to LPS. Alternatively, PPARα activators could increase expression of transcription factors or activation of another transcription factor(s) that regulates TNF expression. In previous reports, Wy-14,643 increased hepatic TNF mRNA transcripts by twofold, and antibodies to TNF blocked the proliferative effect of Wy-14,643 (4, 30). Subsequently, Rusyn et al. (31) showed that Wy-14,643 increased NF-κB binding in electrophoretic mobility shift assays with nuclear extracts from primary Kupffer cells. NF-κB is well documented to be an important transcription factor that increases TNF transcription (2). However, our data from in vitro studies do not support this idea. Short-term (72-h) treatment with Wy-14,643 decreased LPS-induced TNF expression in primary macrophages from either WT or KO mice, suggesting that the effect of Wy-14,643 was not mediated through PPARα. In fact, there have been contradictory reports about the expression of PPARα in macrophages. Ricote et al. (28) reported that activated macrophages express only PPARβ and PPARγ, but Chinetti et al. (6) have reported evidence for the presence of PPARα in human monocyte-derived macrophages. This issue remains to be resolved. Although believed to be primarily a PPARα ligand, it is possible that Wy-14,643 decreased TNF expression in primary macrophages through activation of PPARγ. It has been shown that treatment of macrophages with ligands that activate PPARγ, such as prostaglandin J2, decreased cytokine expression in vitro (5, 28). Also, the results of transfection studies suggest that overexpression of PPARα and PPARγ have inhibitory rather than stimulatory effects on TNF expression in 293T cells.
A second mechanism of action for PPARα ligands on TNF expression in vivo might be explained by the hypolipidemic effect of these drugs. A number of studies have demonstrated that hypolipidemic animals are more susceptible to the deleterious effects of endotoxin, such as increased TNF production and mortality. Circulating lipoproteins provide a detoxifying mechanism by binding and removing foreign lipids from the circulation, thus providing a biological explanation for the hypertriglyceridemia observed in endotoxic animals. Fenofibrate has been shown to promote the clearance of chylomicrons (13), and our data indicate that plasma lipid levels (cholesterol and triglycerides) were reduced almost by half in fenofibrate-fed animals. Feingold et al. have shown that hypolipidemic animals have higher LPS-induced TNF levels and higher mortality (10). In addition, they showed that exogenous administration of chylomicrons improved survival in endotoxin-treated animals, presumably due to the ability of triglyceride-rich particles to bind endotoxin and prevent it from stimulating macrophages (10, 26). If fenofibrate-fed animals have lower levels of chylomicrons, macrophages will be exposed to a higher concentration of endotoxin than that in control-fed animals with normal levels of lipids, thus accounting for higher TNF levels. Further supporting this idea was the fact that LPS-induced levels of TNF were dramatically higher in fenofibrate-fed WT mice but dramatically lower in fenofibrate-fed KO mice. Peters et al. (25) reported that the basal lipid profile in KO mice is different than that in WT mice, as evidenced by higher levels of total serum cholesterol, high-density lipoprotein cholesterol, hepatic apolipoprotein A-I (apoA-I) mRNA, and serum apoA-I. Although treatment with Wy-14,643 lowered apoA-I and triglyceride serum levels in WT mice, no change was observed in KO mice. These results suggest that in the absence of hypolipidemia, PPARα activators lower TNF expression, perhaps through activation of PPARγ. Although Wy-14,643 is primarily a PPARα ligand, it has weak stimulatory activity for PPARγ as well (18, 39).
Although these studies focused on the effect of PPARα activators on TNF expression during endotoxemia, the results raise a number of questions about the regulation of PPARs following LPS treatment. Previous studies have shown that glucocorticoids increase and insulin decreases PPARα expression in rat liver or rat hepatocytes (21, 35). Our laboratory has also shown that PPARα and PPARγ are decreased in white adipose and brown adipose tissues during endotoxemia in CD-1 mice (16). However, to our knowledge, no one has examined whether or not PPAR activators prevent decreased expression of PPARs or alter glucocorticoid or insulin levels during endotoxemia.
In summary, the effect of PPARα ligands on endotoxemia is complex. The agents may exert an anti-inflammatory effect through a repression of TNF transcription. This action of PPARα on the TNF promoter may be direct or indirect. However, the systemic hypolipidemic effect of PPARα ligands in vivo may be proinflammatory. The absence of serum lipids to sequester circulating endotoxin may increase macrophage activation. Further studies are under way to define the mechanism of PPARα actions on inflammation.
ACKNOWLEDGMENTS
We thank Ron Evans for the RXR expression vector. We also thank Karen Reynolds, Damaris Brisco, and Jenny Gipson for expert technical assistance.
This work was supported by the Oklahoma Center for Advancement of Science and Technology H97-008 (M.R.H.) and NIH CA 50898 (J.M.G.).
REFERENCES
- 1.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Blok W L, Katan M B, van der Meer J. Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J Nutr. 1996;126:1515–1533. doi: 10.1093/jn/126.6.1515. [DOI] [PubMed] [Google Scholar]
- 4.Bojes H K, Germolec D R, Simeonova P, Bruccoleri A, Schoonhoven R, Luster M I, Thurman R G. Antibodies to tumor necrosis factor alpha prevent increases in cell replication in liver due to the potent peroxisome proliferator, Wy-14,643. Carcinogenesis. 1997;18:669–674. doi: 10.1093/carcin/18.4.669. [DOI] [PubMed] [Google Scholar]
- 5.Chengyu J, Ting A T, Seed B. PPAR gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 6.Chinetti G, Griglio S, Antonucci M, Torra I P, Delerive P, Majd Z, Fruchart J-C, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis in human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 7.Devchand P, Keller H, Peters J, Vazquez M, Gonzalez F, Wahli W. The PPAR alpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 8.DeWet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 10.Feingold K, Funk J, Moser A, Shigenaga J, Rapp J, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect Immun. 1995;63:2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimble J M, Robinson C E, Wu X, Kelly K A, Rodriguez B R, Kliewer S A, Lehmann J M, Morris D C. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- 12.Green S. PPAR: a mediator of peroxisome proliferator action. Mutat Res. 1995;333:101–109. doi: 10.1016/0027-5107(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 13.Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem. 1995;270:13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- 14.Hill M, Kelly K, Wu X, Wanker F, Bass H, Morgan C, Wang C-S, Gimble J. Lipopolysaccharide regulation of lipoprotein lipase expression in murine macrophages. Infect Immun. 1995;63:858–864. doi: 10.1128/iai.63.3.858-864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill M, McCallum R. Identification of tumor necrosis factor as a transcriptional regulator of the phosphoenolpyruvate carboxykinase gene following endotoxin treatment of mice. Infect Immun. 1992;60:4040–4050. doi: 10.1128/iai.60.10.4040-4050.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill M R, Young M D, McCurdy C M, Gimble J M. Decreased expression of murine PPARγ in adipose tissue during endotoxemia. Endocrinology. 1997;138:3073–3076. doi: 10.1210/endo.138.7.5379. [DOI] [PubMed] [Google Scholar]
- 17.Hill M R, Stith R D, McCallum R E. Interleukin-1: a regulatory role in glucocorticoid-regulated hepatic metabolism. J Immunol. 1986;137:858–862. [PubMed] [Google Scholar]
- 18.Kliewer S, Lenhard J M, Willson T M, Patel I, Morris D C, Lehmann J M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee SS-T, Pineau T, Drago J, Lee E, Owens J, Kroetz D, Fernadez-Salguero P, Westphal A H, Gonzalez F J. Targeted disruption of the α isoform of the peroxisome proliferator-actiated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann J, Moore L, Smith-Oliver T, Wilkison W, Willson S K T. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR-gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 21.Lemberger T, Saladin R, Vazquez M, Assimacopoulos F, Staels B, Desvergne B, Wahli W, Auwerx J. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J Biol Chem. 1996;271:1764–1769. doi: 10.1074/jbc.271.3.1764. [DOI] [PubMed] [Google Scholar]
- 22.Mangelsdorf D J, Borgmeyer U, Heyman R A, Zhou J Y, Ong E S, Oro A E, Kakizuka A, Evans R M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 23.Muerhoff A S, Griffen K J, Johnson E F. The peroxisome proliferator-activated receptor mediates the induction of CYP4A6, a cytochrome P450 fatty acid ω-hydroxylase, by clofibric acid. J Biol Chem. 1992;267:19051–19053. [PubMed] [Google Scholar]
- 24.Peters J M, Cattley R C, Gonzalez F J. Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 1997;18:2029–2033. doi: 10.1093/carcin/18.11.2029. [DOI] [PubMed] [Google Scholar]
- 25.Peters J M, Hennuyer N, Staels B, Fruchart J, Fievet C, Gonzalez F, Auwerx J. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor α-deficient mice. J Biol Chem. 1997;272:27307–27312. doi: 10.1074/jbc.272.43.27307. [DOI] [PubMed] [Google Scholar]
- 26.Read T, Grunfeld C, Kumwenda Z, Calhoun M, Kane J, Feingold K, Rapp J. Triglyceride-rich lipoproteins prevent septic death in rats. J Exp Med. 1995;182:267–272. doi: 10.1084/jem.182.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed L J, Muench H A. A simple method for estimating 50% end points. Am J Hyg. 1938;27:493–499. [Google Scholar]
- 28.Ricote M, Li A C, Willson T M, Kelly C J, Glass C K. The peroxisome proliferator-activated receptor gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 29.Robinson C E, Wu X, Morris D C, Gimble J M. DNA bending is induced by binding of the peroxisome proliferator-activated receptor gamma2 heterodimer to its response element in the murine lipoprotein lipase promoter. Biochem Biophys Res Commun. 1998;244:671–677. doi: 10.1006/bbrc.1998.8305. [DOI] [PubMed] [Google Scholar]
- 30.Rose M L, Germolec D R, Schoonhoven R, Thurman R G. Kupffer cells are causally responsible for the mitogenic effect of peroxisome proliferators. Carcinogenesis. 1997;18:1453–1456. doi: 10.1093/carcin/18.8.1453. [DOI] [PubMed] [Google Scholar]
- 31.Rusyn I, Tsukamoto H, Thurman R G. Wy-14 643 rapidly activates nuclear factor kappaB in Kupffer cells before hepatocytes. Carcinogenesis. 1998;19:1217–1222. doi: 10.1093/carcin/19.7.1217. [DOI] [PubMed] [Google Scholar]
- 32.Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 33.Schoonjans K, Peinado-Onsurbe J, Heyman R, Briggs M, Caayet D, Deeb S, Staels B, Auwerx J. PPAR alpha and PPAR gamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 34.Staels B, Tol A V, Fruchart J-C, Auwerx J. Effects of hypolipidemic drugs on the expression of genes involved in high density lipoprotein metabolism in the rat. Isr J Med Sci. 1996;32:490–498. [PubMed] [Google Scholar]
- 35.Steineger H, Sorensen H, Tugwood J, Skrede S, Spydevold O, Gautvik K. Dexamethasone and insulin demonstrate marked and opposite regulation of the steady-state mRNA level of the peroxisomal proliferator-activated receptor (PPAR) in hepatic cells. Eur J Biochem. 1994;225:967–974. doi: 10.1111/j.1432-1033.1994.0967b.x. [DOI] [PubMed] [Google Scholar]
- 36.Tontonoz P, Hu E, Devine J, Beale E G, Spiegelman B M. PPARγ2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tracey K, Beutler B, Lowry S, Merryweather J, Wolpe S, Milsark I, Hariri R, Fahey III T, Zentella A, Albert J, Shires T, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 38.Whelan J. Antagonistic effects of dietary arachidonic acid and n-3 polyunsaturated fatty acids. J Nutr. 1996;126:1086S–1091S. doi: 10.1093/jn/126.suppl_4.1086S. [DOI] [PubMed] [Google Scholar]
- 39.Yu K, Bayona W, Kallen C B, Harding H P, Ravera C P, McMahon G, Brown M, Lazar M A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Marcus L L, Sajadi F G, Alvares K, Reddy J K, Subramani S, Rachubinski R A, Capone J P. Identification of a peroxisome proliferator-responsive element upstream of the gene encoding rat peroxisomal enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase. Proc Natl Acad Sci USA. 1992;89:7541–7545. doi: 10.1073/pnas.89.16.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]