Abstract
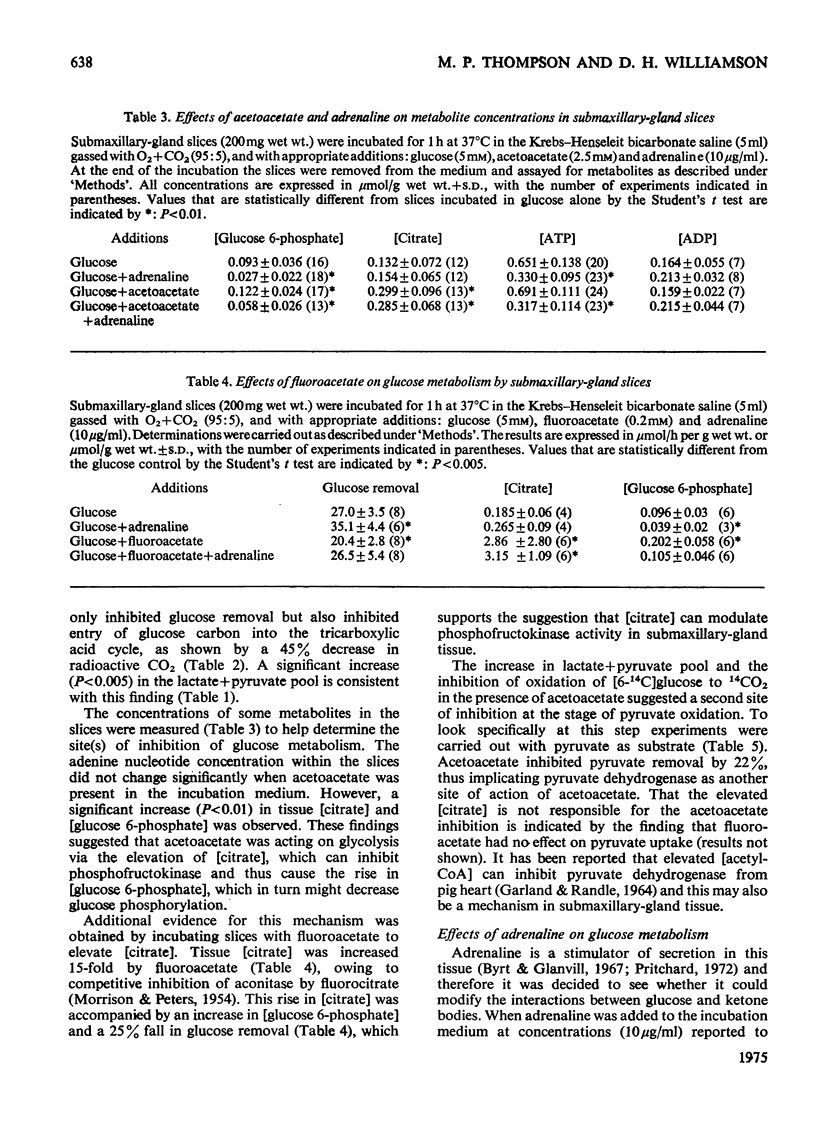
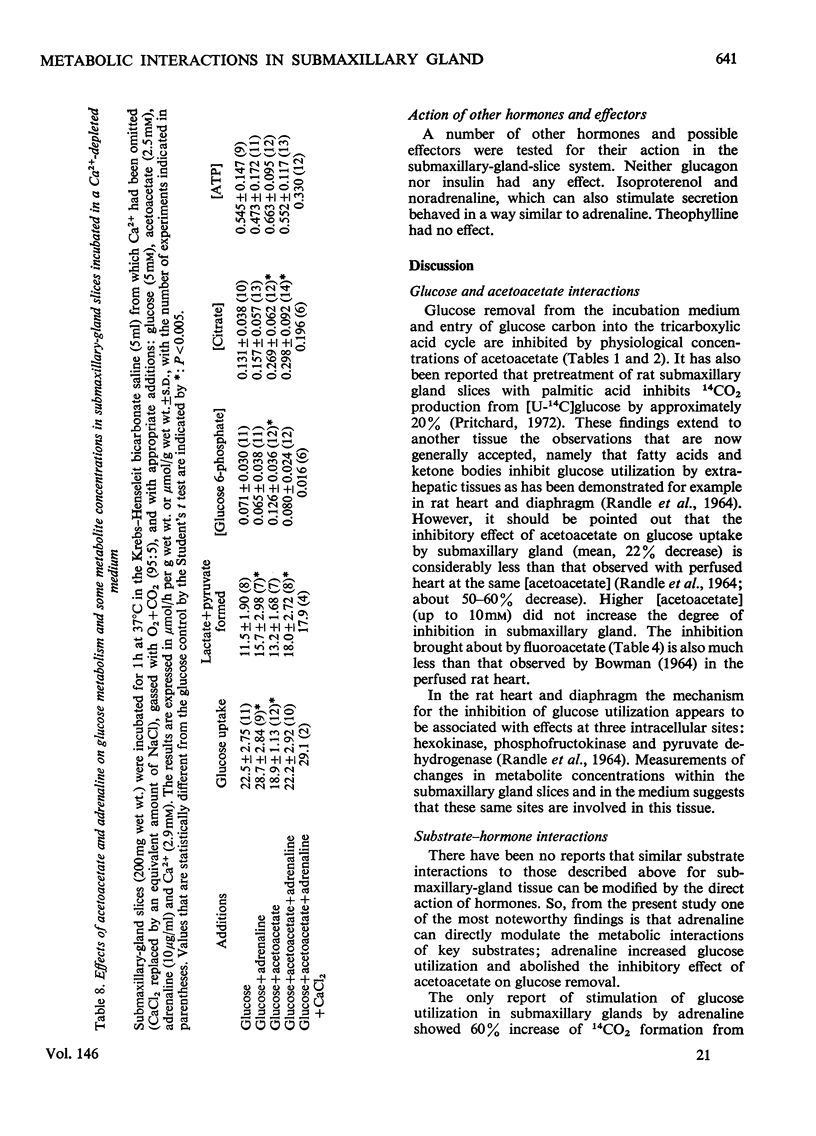
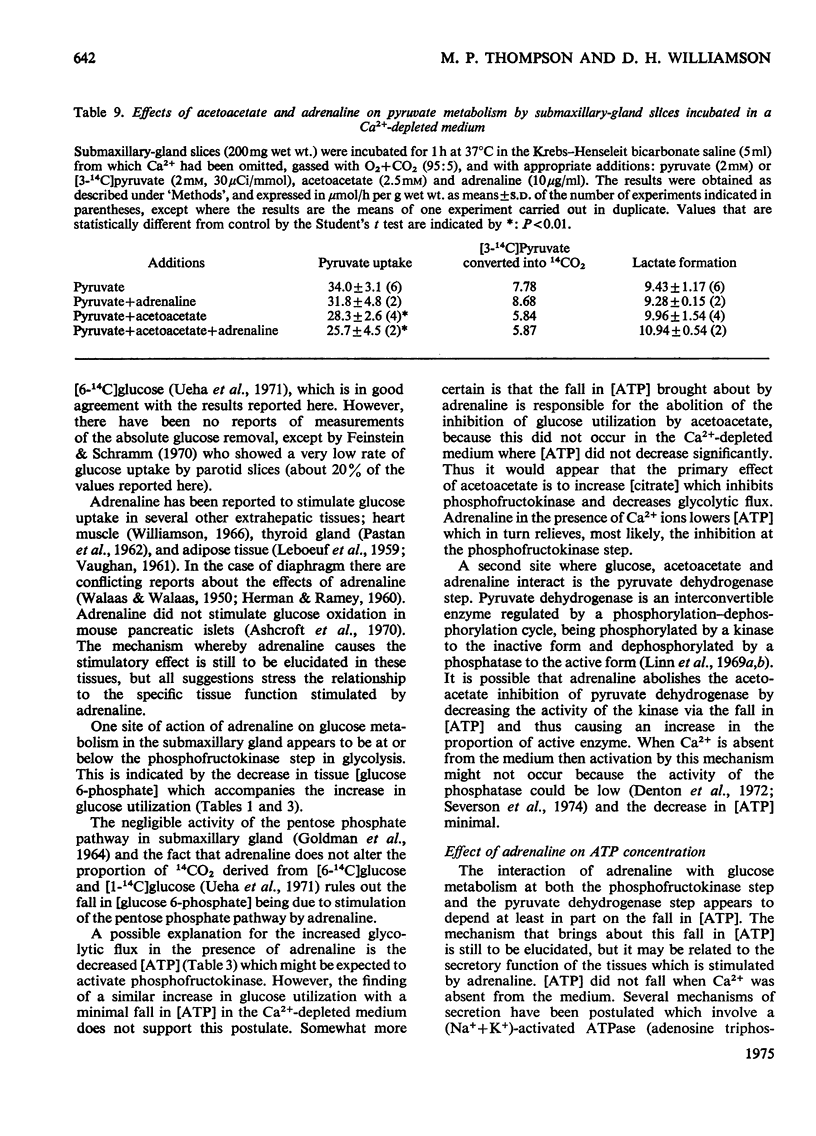
1. The metabolic interactions between glucose, acetoacetate and adrenaline were studied in submaxillary-gland slices. 2. Acetoacetate (2.5 mM) inhibited glucose removal by 22% and entry of glucose carbon into the tricarboxylic acid cycle by 54%. 3. Acetoacetate caused an increase in (glucose 6-phosphate) together with an increase in (citrate), a finding that suggests that the phosphofructokinase step might be inhibited by the elevated (citrate). Support for this suggestion was obtained in experiments in which fluoracetate was used to elevate (citrate). 4. A further site of action of acetoacetate at the pyruvate dehydrogenase step was suggested by an increase in the lactate+pyruvate pool, and the finding that pyruvate removal and (3-14C)pyruvate oxidation were inhibited by acetoacetate. 5. Adrenaline, a stimulator of secretion by this tissue, increased glucose removal by 25%. Adrenaline increased glucose removal to the same extent when acetoacetate was also present in the incubation medium. In both cases the increase was accompanied by a fall in (glucose 6-phosphate). 6. Adrenaline also overcame the inhibition of pyruvate removal caused by acetoacetate. 7. The tissue (ATP) decreased by about 50% on addition of adrenaline, and a similar fall was observed in vivo after adrenergic stimulation by isoproterenol. 8. Omission of Ca-2+ from the medium prevented the fall in (glucose 6-phosphate) and (ATP) caused by adrenaline, although adrenaline was still able to stimulate glucose removal. The inhibitory effect of acetoacetate on gluocse removal was reversed by adrenaline, but there was no stimulation above the control rates. Inhibition of pyruvate removal by acetoacetate was not overcome by adrenaline in the absence of Ca-2+. 9. Dibutyryl cyclic AMP had no effect on glucose removal or on (ATP). 10. Possible mechanisms by which adrenaline can bring about its metabolic effects are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Ohad I., Schramm M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969 Jun;41(3):753–773. doi: 10.1083/jcb.41.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A., Desai K., Halperin M. L. Free fatty acid and ATP levels in adipocytes during lipolysis. Metabolism. 1971 Jan;20(1):87–99. doi: 10.1016/0026-0495(71)90062-x. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BDOLAH A., SCHRAMM M. THE FUNCTION OF 3'5' CYCLIC AMP IN ENZYME SECRETION. Biochem Biophys Res Commun. 1965 Feb 3;18:452–454. doi: 10.1016/0006-291x(65)90730-8. [DOI] [PubMed] [Google Scholar]
- Babad H., Ben-Zvi R., Bdolah A., Schramm M. The mechanism of enzyme secretion by the cell. 4. Effects of inducers, substrates and inhibitors on amylase secretion by rat parotid slices. Eur J Biochem. 1967 Mar;1(1):96–101. doi: 10.1111/j.1432-1033.1967.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Batzri S., Selinger Z. Enzyme secretion mediated by the epinephrine -receptor in rat parotid slices. Factors governing efficiency of the process. J Biol Chem. 1973 Jan 10;248(1):356–360. [PubMed] [Google Scholar]
- Bihler I., Jeanrenaud B. ATP content of isolated fat cells. Effects of insulin, ouabain, and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):496–506. doi: 10.1016/0005-2760(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Byrt P. N., Glanvill S. Effect of isoprenaline on the secretion of sialoproteins from rat salivary glands. Biochim Biophys Acta. 1967 Oct 9;148(1):215–221. doi: 10.1016/0304-4165(67)90296-6. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch W., Raper H. S. Respiration and functional activity. J Physiol. 1936 Aug 19;87(3):275–286. doi: 10.1113/jphysiol.1936.sp003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch W., Raper H. S. The respiration and metabolism of submaxillary gland tissue of the cat. J Physiol. 1938 May 14;92(4):439–458. doi: 10.1113/jphysiol.1938.sp003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. The influence of calcium on the secretory response of the submaxillary gland to acetylcholine or to noradrenaline. J Physiol. 1963 Mar;165(3):528–541. doi: 10.1113/jphysiol.1963.sp007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach R. H., Gerlach E. Adenosine phosphate of rat salivary and lacrimal glands in vitro. Proc Soc Exp Biol Med. 1967 Oct;126(1):281–282. doi: 10.3181/00379727-126-32424. [DOI] [PubMed] [Google Scholar]
- Feinstein H., Schramm M. Energy production in rat parotid gland. Relation tonzyme secretion and effects of caium. Eur J Biochem. 1970 Mar 1;13(1):158–163. doi: 10.1111/j.1432-1033.1970.tb00912.x. [DOI] [PubMed] [Google Scholar]
- GOLDMAN J., ROSALES F., VILLAVICENCIO M., GUERRA R. PATHWAYS OF GLUCOSE METABOLISM IN RAT SUBMAXILLARY GLAND. Biochim Biophys Acta. 1964 Feb 10;82:303–312. doi: 10.1016/0304-4165(64)90301-0. [DOI] [PubMed] [Google Scholar]
- HERMAN M. S., RAMEY E. R. Epinephrine action on glucose uptake by rat diaphragm; effect of ionic composition. Am J Physiol. 1960 Aug;199:226–228. doi: 10.1152/ajplegacy.1960.199.2.226. [DOI] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- LEBOEUF B., FLINN R. B., CAHILL G. F., Jr Effect of epinephrine on glucose uptake and glycerol release by adipose tissue in vitro. Proc Soc Exp Biol Med. 1959 Oct-Dec;102:527–529. doi: 10.3181/00379727-102-25306. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON J. F., PETERS R. A. Biochemistry of fluoroacetate poisoning: the effect of fluorocitrate on purified aconitase. Biochem J. 1954 Nov;58(3):473–479. doi: 10.1042/bj0580473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Metabolism of the heart in health and disease. I. Am Heart J. 1968 Nov;76(5):685–698. doi: 10.1016/0002-8703(68)90168-3. [DOI] [PubMed] [Google Scholar]
- PASTAN I., HERRING B., JOHNSON P., FIELD J. B. Studies on the mechanism by which epinephrine stimulates glucose oxidation in the thyroid. J Biol Chem. 1962 Feb;237:287–290. [PubMed] [Google Scholar]
- Pritchard E. T. Palmitic acid oxidation by secreting submandibular gland tissue in vitro. FEBS Lett. 1972 Jul 1;23(3):314–316. doi: 10.1016/0014-5793(72)80304-1. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDHU R. S., GESSART C. F., MCINTYRE A. R. STIMULATION BY ACETYLCHOLINE AND NOREPINEPHRINE OF GLUCOSE OXIDATION IN RAT SUBMAXILLARY GLAND SLICES, AS INFLUENCED BY CALCIUM. Biochem Pharmacol. 1964 Jul;13:1100–1103. doi: 10.1016/0006-2952(64)90108-x. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Naim E., Lasser M. ATP-dependent calcium uptake by microsomal preparations from rat parotid and submaxillary glands. Biochim Biophys Acta. 1970 Apr 21;203(2):326–334. doi: 10.1016/0005-2736(70)90147-1. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueha T., Catanzaro O., Hanson R., Lindsay R. H. Metabolic alterations accompanying alpha-amylase secretion by rat parotid tissue in vitro. Am J Physiol. 1971 Feb;220(2):312–318. doi: 10.1152/ajplegacy.1971.220.2.312. [DOI] [PubMed] [Google Scholar]
- VAUGHAN M. Effect of hormones on glucose metabolism in adipose tissue. J Biol Chem. 1961 Aug;236:2196–2199. [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R. Metabolic effects of epinephrine in the perfused rat heart. II. Control steps of glucose and glycogen metabolism. Mol Pharmacol. 1966 May;2(3):206–220. [PubMed] [Google Scholar]


