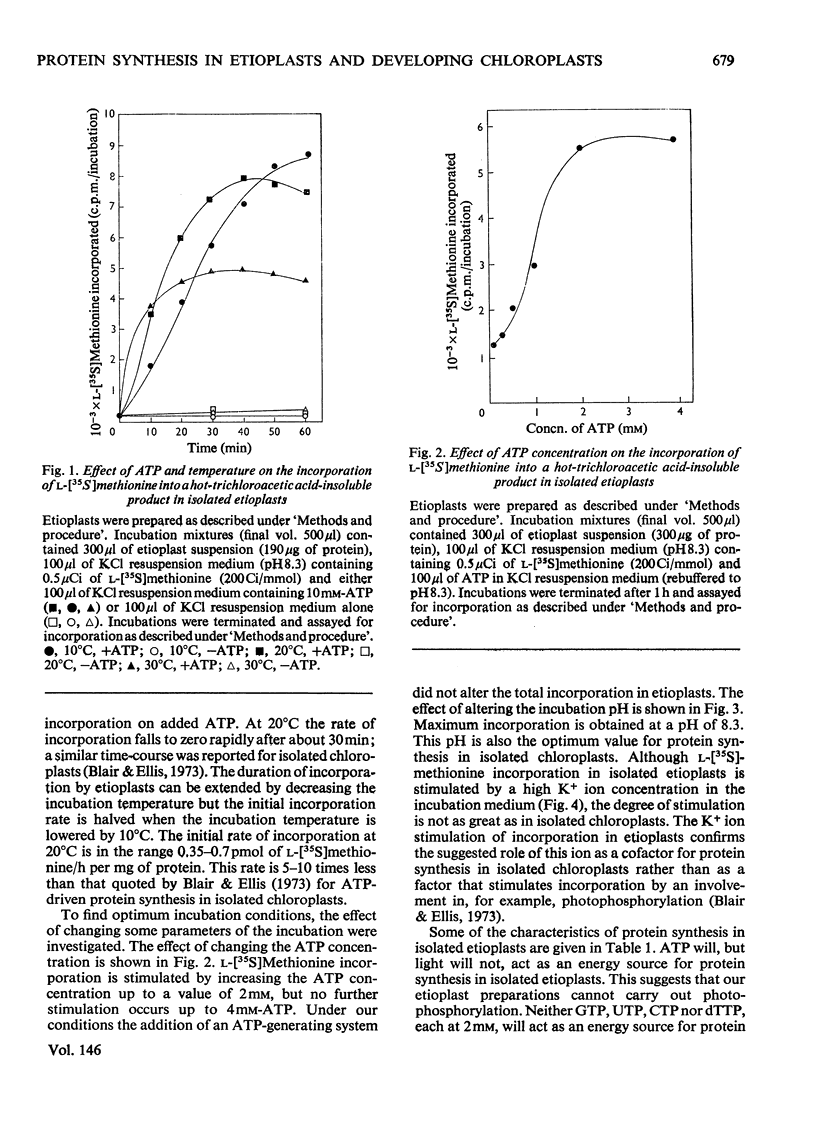
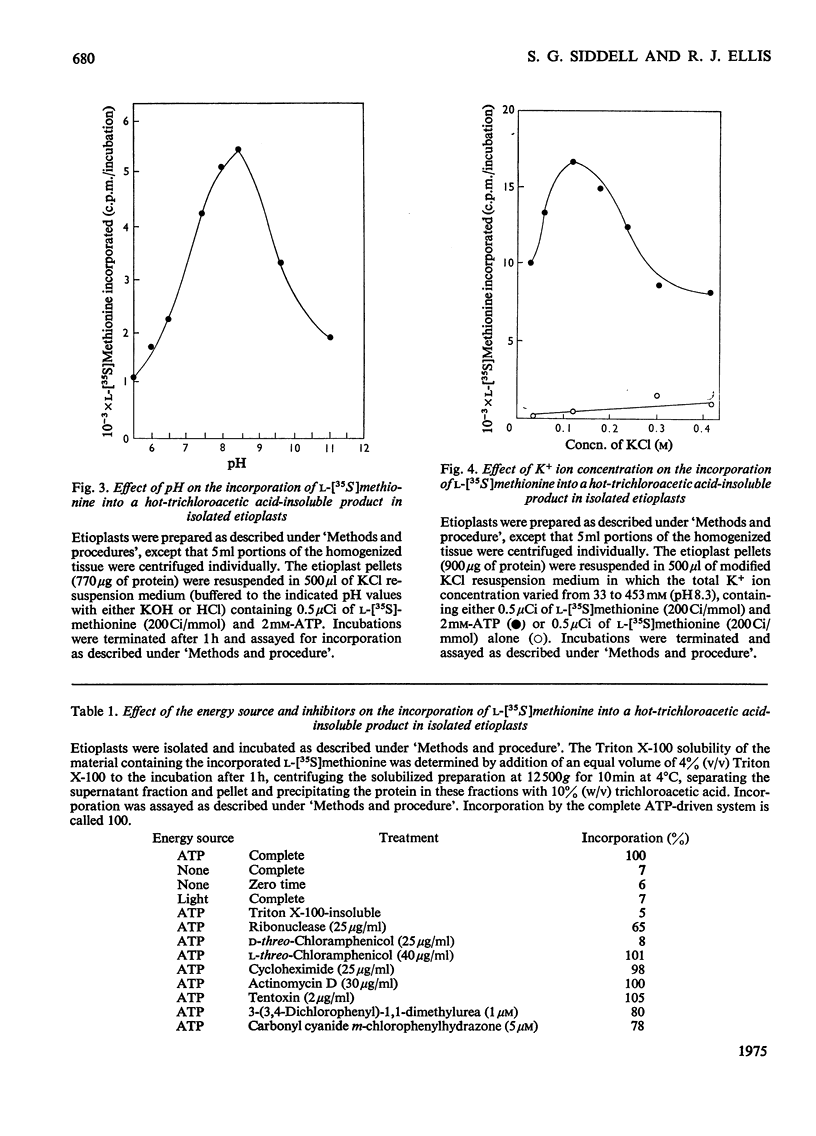
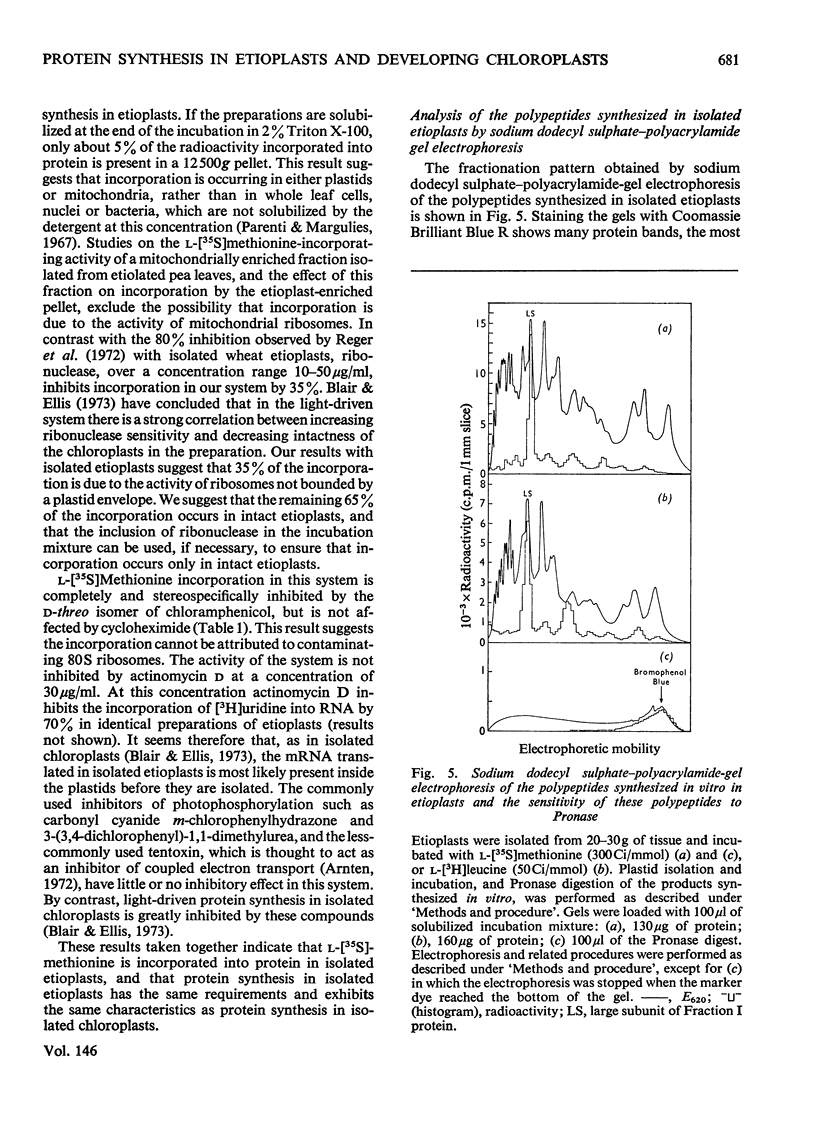
Abstract
The function of plastid ribosomes in pea (Pisum sativum L.) was investigated by characterizing the products of protein synthesis in vitro in plastids isolated at different stages during the transition from etioplast to chloroplast. Etioplasts and plastids isolated after 24, 48 and 96h of greening in continuous white light, use added ATP to incorporate labelled amino acids into protein. Plastids isolated from greening leaves can also use light as the source of energy for protein synthesis. The labelled polypeptides synthesized in isolated plastids were analysed by electrophoresis in sodium dodecyl sulphate-ureapolyacrylamide gels. Six polypeptides are synthesized in etioplasts with ATP as energy source. Only one of these polypeptides is present in a 150 000g supernatant fraction. This polypeptide has been identified as the large subunit of Fraction I protein (3-phospho-D-glycerate carboxylyase EC 4.1.1.39) by comparing the tryptic 'map' of its L-(35S)methionine-labelled peptides with the tryptic 'map' of large subunit peptides from Fraction I labelled with L-(35S)methionine in vivo. The same gel pattern of six polypeptides is seen when plastids isolated from greening leaves are incubated with either added ATP or light as the energy source. However, the rates of synthesis of particular polypeptides are different in plastids isolated at different stages of the etioplast to chloroplast transition. The results support the idea that plastid ribosomes synthesize only a small number of proteins, and that the number and molecular weight of these proteins does not alter during the formation of chloroplasts from etioplasts.
Full text
PDF



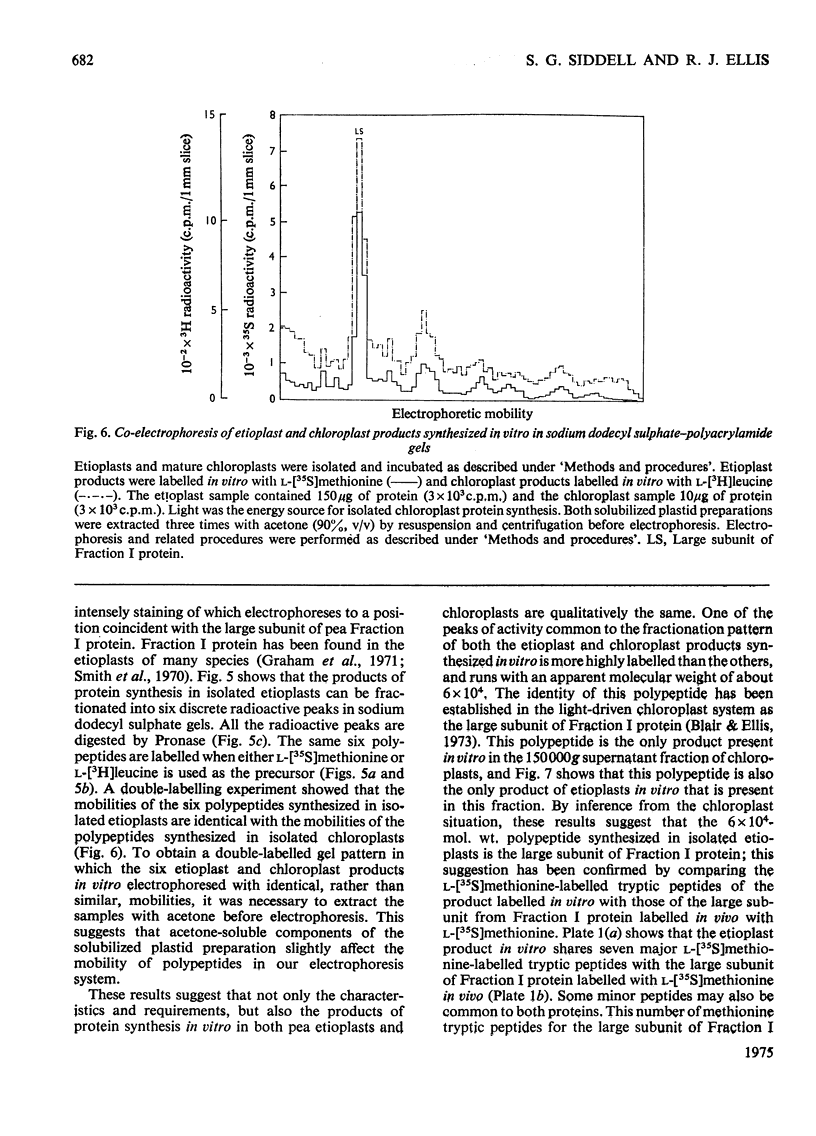
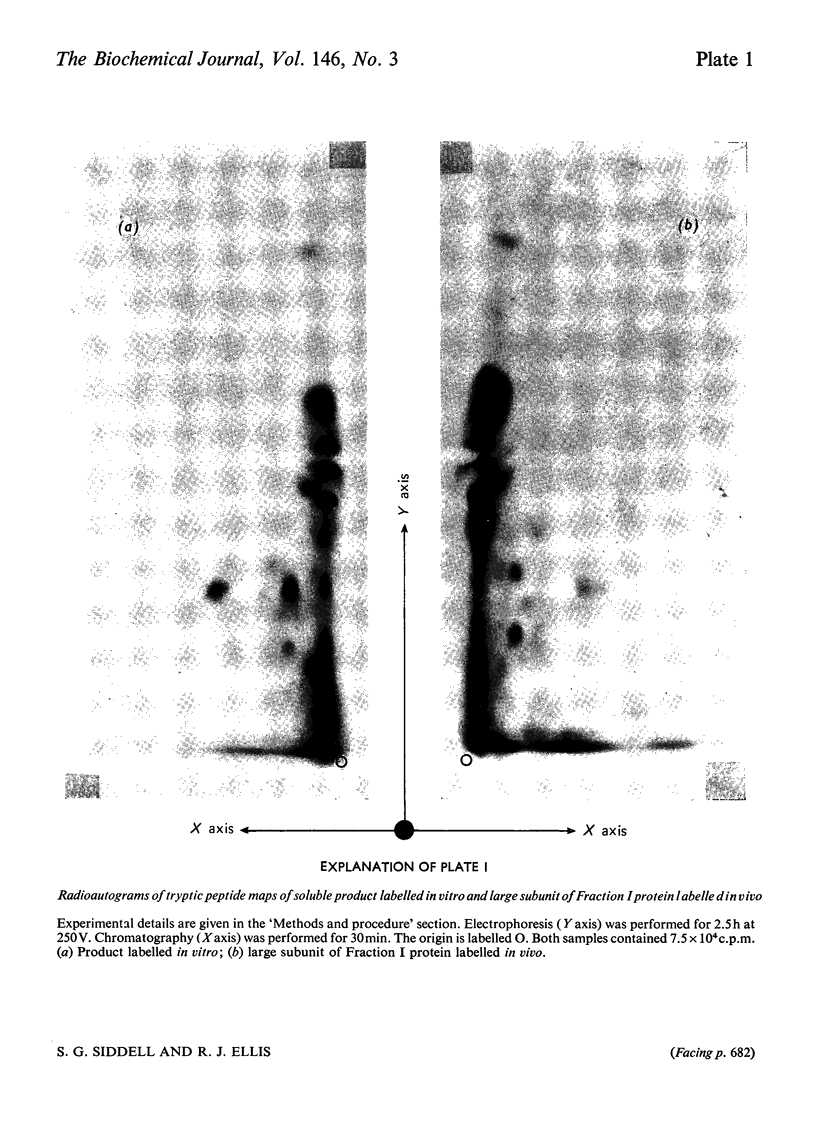
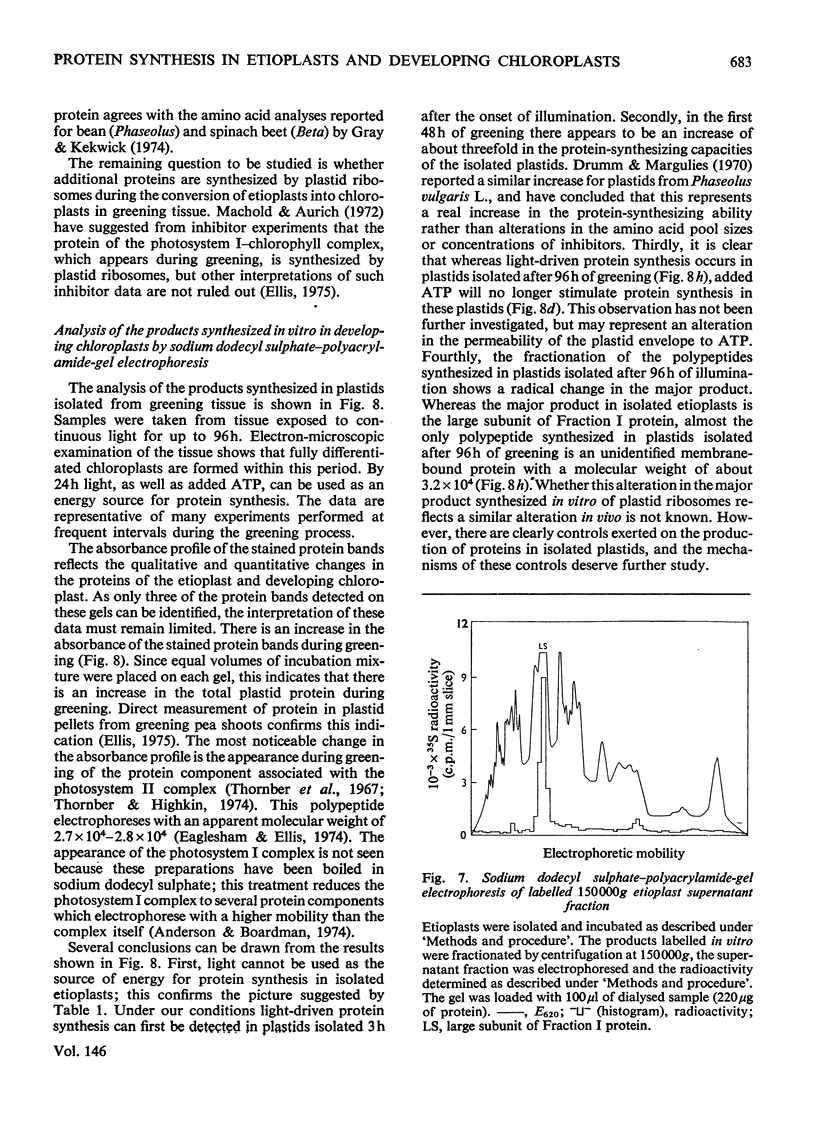
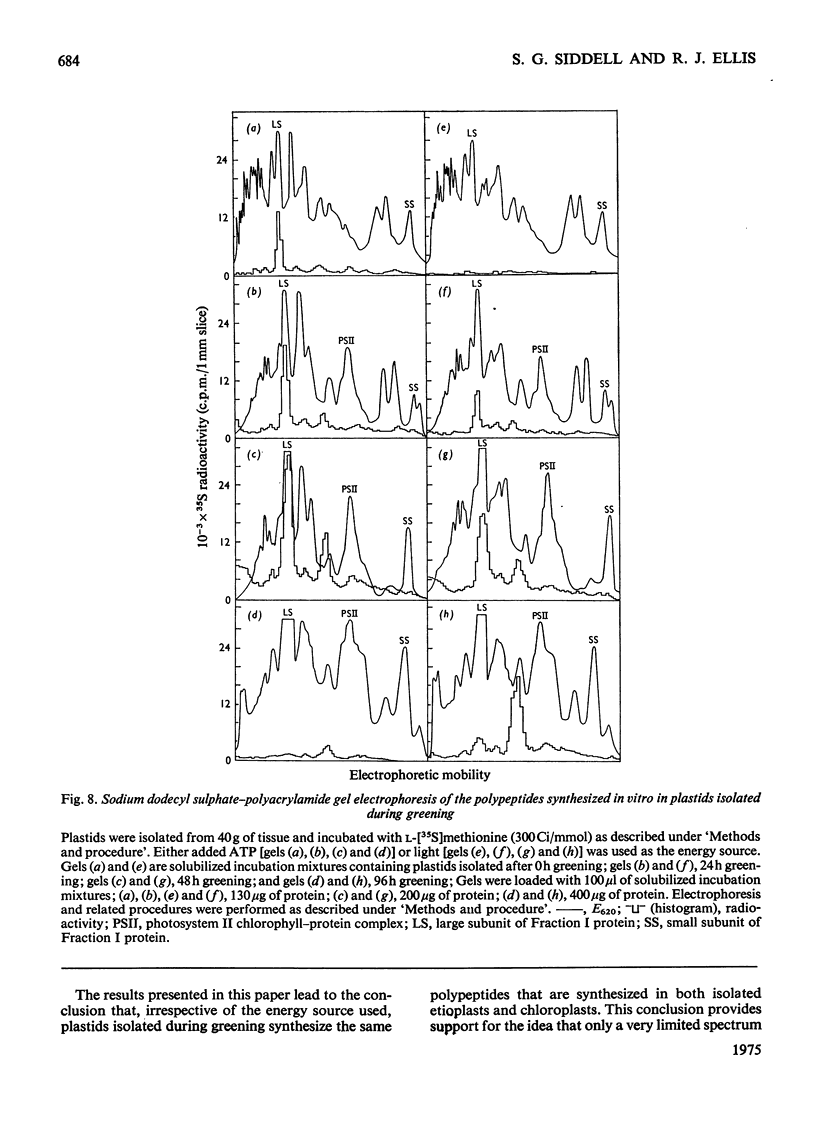

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Arntzen C. J. Inhibition of photophosphorylation by tentoxin, a cyclic tetrapeptide. Biochim Biophys Acta. 1972 Dec 14;283(3):539–542. doi: 10.1016/0005-2728(72)90273-3. [DOI] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bray D., Brownlee S. M. Peptide mapping of proteins from acrylamide gels. Anal Biochem. 1973 Sep;55(1):213–221. doi: 10.1016/0003-2697(73)90306-0. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Wildman S. G. Chloroplast DNA codes for the primary structure of the large subunit of fraction I protein. Biochim Biophys Acta. 1972 Sep 14;277(3):677–680. doi: 10.1016/0005-2787(72)90115-3. [DOI] [PubMed] [Google Scholar]
- Drumm H. E., Margulies M. M. In vitro protein synthesis by plastids of Phaseolus vulgaris. IV. Amino acid incorporation by etioplasts and effect of illumination of leaves on incorporation by plastids. Plant Physiol. 1970 Apr;45(4):435–442. doi: 10.1104/pp.45.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Blair G. E., Hartley M. R. The nature and function of chloroplast protein synthesis. Biochem Soc Symp. 1973;(38):137–162. [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- HENDLER R. W. PROCEDURE FOR SIMULTANEOUS ASSAY OF TWO BETA-EMITTING ISOTOPES WITH THE LIQUID SCINTILLATION COUNTING TECHNIQUE. Anal Biochem. 1964 Jan;7:110–120. doi: 10.1016/0003-2697(64)90125-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Machold O., Aurich O. Sites of synthesis of chloroplast lamellar proteins in Vicia faba. Biochim Biophys Acta. 1972 Sep 29;281(1):103–112. doi: 10.1016/0005-2787(72)90192-x. [DOI] [PubMed] [Google Scholar]
- Moore N. F., Burke D. C. Characterization of the structural proteins of different strains of Newcastle disease virus. J Gen Virol. 1974 Nov;25(2):275–289. doi: 10.1099/0022-1317-25-2-275. [DOI] [PubMed] [Google Scholar]
- Parenti F., Margulies M. M. In Vitro Protein Synthesis by Plastids of Phaseolus vulgaris. I. Localization of Activity in the Chloroplasts of a Chloroplast Containing Fraction from Developing Leaves. Plant Physiol. 1967 Sep;42(9):1179–1186. doi: 10.1104/pp.42.9.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J. M., Campo F. F., Arnon D. I. Photosynthetic phosphorylation as energy source for protein synthesis and carbon dioxide assimilation by chloroplasts. Proc Natl Acad Sci U S A. 1968 Feb;59(2):606–612. doi: 10.1073/pnas.59.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger B. J., Smillie R. M., Fuller R. C. Protein synthesis by isolated etioplasts and chloroplasts from pea and wheat and the effects of chloramphenicol and cycloheximide. Plant Physiol. 1972 Jul;50(1):19–23. doi: 10.1104/pp.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent J. R., Vadlamudi B. P. Electrophoresis of peptides on thin layers of silica gel. Anal Biochem. 1968 Oct 24;25(1):583–587. doi: 10.1016/0003-2697(68)90137-1. [DOI] [PubMed] [Google Scholar]
- Talbot D. N., Yphantis D. A. Fluorescent monitoring of SDS gel electrophoresis. Anal Biochem. 1971 Nov;44(1):246–253. doi: 10.1016/0003-2697(71)90367-8. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Gregory R. P., Smith C. A., Bailey J. L. Studies on the nature of the chloroplast lamella. I. Preparation and some properties of two chlorophyll-protein complexes. Biochemistry. 1967 Feb;6(2):391–396. doi: 10.1021/bi00854a004. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]