Abstract
Background
This study aimed to summarize the pathogenic spectrum of infective endocarditis (IE) and analyze the risk factors for poor prognosis in surgical patients in a tertiary hospital in China.
Methods
We retrospectively included patients diagnosed with IE between January 2013 and January 2022. The pathogenic spectrum was summarized; the risk factors for early postoperative mortality and embolic events were analyzed using multivariate logistic regression.
Results
Among 630 patients who underwent blood cultures, the positivity rate was 56.83%. The most prevalent pathogens were viridans streptococci, Staphylococcus aureus, enterococci, and coagulase-negative staphylococci (CoNS). The prevalence of viridans streptococci significantly increased in the surgically treated group, compared to the medically treated group (50.80% vs. 27.78%, P < 0.001), while that of CoNS decreased (5.60% vs. 12.04%, P = 0.034). There has been a declining trend in the blood culture positivity in recent years compared to earlier years (2018–2022 vs. 2013–2017 = 60.95% vs. 47.30%, P = 0.037), with an increasing trend in viridans streptococci and a decreasing trend in CoNS. Multivariate logistic regression analysis identified male gender, coronary artery disease, platelet count < 100 × 109/L, albumin < 35 g/L, elevated creatinine, and prosthetic valve as independent risk factors for early postoperative mortality. Risk factors for embolic events included recent cerebral infarction within 3 months, history of peripheral vasculopathy, and hemoglobin (Hb) < 90 g/L.
Conclusions
Viridans streptococci predominates as the most common IE pathogen, with its incidence rising recently, especially among surgical patients. Blood culture positivity is decreasing. Understanding risk factors for early postoperative mortality and embolic events is crucial for optimizing patient management and prognosis.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10350-y.
Keywords: Pathogenic spectrum, Infective endocarditis, Risk factors, Early postoperative mortality, Embolic events
Background
Despite recent improvements in diagnostic and therapeutic strategies, the age-standardized morbidity and mortality rates of infective endocarditis (IE) have continued to rise over the past three decades [1]. As one of the most lethal cardiac diseases, IE presents significant public health challenges, with a 1-year mortality rate approaching 30% [2]. This alarming statistic underscores the necessity for improved understanding and management of this condition.
Identifying potentially pathogenic microorganisms in IE is critical for initiating effective anti-infective therapy and determining the appropriate duration of treatment. Current guidelines recommend empirical antimicrobial therapy using a combination of at least three antibiotics while awaiting pathogen identification [3]. However, the reliance on broad-spectrum antibiotics, particularly against multidrug-resistant organisms, raises concerns about adverse events and the potential for antibiotic overuse. Understanding local epidemiology is essential not only for optimizing treatment strategies but also for addressing these concerns. Surgical intervention has emerged as a cornerstone in IE treatment, with over half of all patients undergoing surgical intervention. However, the complexity of IE increases surgical risks, with mortality rates ranging from 8 to 30% within 30 postoperative days [4, 5]. Furthermore, embolic events are notably common complications of IE, occurring in 13–49% of cases, with cerebral embolism posing a particularly challenging treatment scenario and increasing mortality risk [6].
This study uniquely contributes to the existing literature by providing a comprehensive analysis of the pathogenic spectrum of bacteria associated with IE and identifying risk factors for poor postoperative outcomes specifically in surgical patients treated at our center over the past 9 years. Unlike previous studies that often focus on isolated aspects of IE, our research consolidates a wide range of clinical experiences and outcomes, offering valuable insights that could enhance therapeutic efficacy and inform future clinical practices. Additionally, our findings may serve as a foundation for future multicenter studies, which are essential for validating and generalizing these insights across diverse populations and clinical settings.
Methods
Study participants and data collection
This study received approval from the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University (2018 [100]). Clinical trial number: not applicable. This retrospective study included patients hospitalized between January 2013 and January 2022 and diagnosed with IE based on the modified Duke criteria, including native and prosthetic valve endocarditis [3]. Exclusion criteria comprised absence of blood cultures, age < 18 years, lack of essential primary data, pregnancy, and malignancy. The data from the electronic case system included patient demographics, medical histories, clinical manifestations, laboratory and echocardiographic findings, procedural details, hospital discharge summaries, embolic events, and recent (30-day postoperative) follow-up outcomes. Two independent reviewers conducted data extraction and cross-validation, with discrepancies resolved in consultation with a third reviewer. All examinations and laboratory results were collected at the time of hospital admission. Missing data were minimal (2% of variables) and were addressed using mean imputation for continuous variables and mode imputation for categorical variables. This approach ensured that the dataset remained robust for statistical analysis while minimizing bias.
Pathogenic bacterial profiles were established through blood cultures, summarizing flora profiles across medically and surgically treated patients. Surgical interventions were indicated based on guidelines for select patients who eventually underwent cardiac surgery [3]. Finally, 685 cases were included, 438 of whom had undergone cardiac surgery. Risk factors for early postoperative mortality and perioperative embolic events were analyzed among surgical patients.
Definitions
Primary outcomes included early postoperative cardiac mortality and preoperative embolic events. Early postoperative mortality was defined as all-cause mortality during hospitalization or within the first postoperative month. Embolic events were diagnosed based on clinical and imaging data, confirmed by specialized experts.
Statistical analysis
All statistical analyses were conducted using SPSS software version 26.0 (SPSS Inc., Chicago, IL). Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range) based on normality and compared using the unpaired Student t-test or Mann–Whitney U test, as appropriate. Categorical variables were assessed using either the chi-squared or Fisher’s exact test. Risk factors were identified through both univariate and multivariate logistic regression analyses. Multivariate logistic regression models were used to adjust for potential confounders, incorporating variables with p < 0.1 in univariate analysis. Sensitivity analyses, including alternative model specifications and stratified analyses by gender, were conducted to confirm the robustness of the findings. To address multicollinearity in our logistic regression, we calculated the Variance Inflation Factor (VIF) for each variable. Variables with VIFs over 5 were assessed, and the least relevant ones were removed until all remaining variables had VIFs below the threshold. To test our regression model for overfitting issues, we employ L2 regularization via Ridge regression as a way to mitigate this risk (See Supplementary Material). All tests were two-tailed, and statistical significance was set at P < 0.05.
Results
Pathogen spectrum of patients with IE
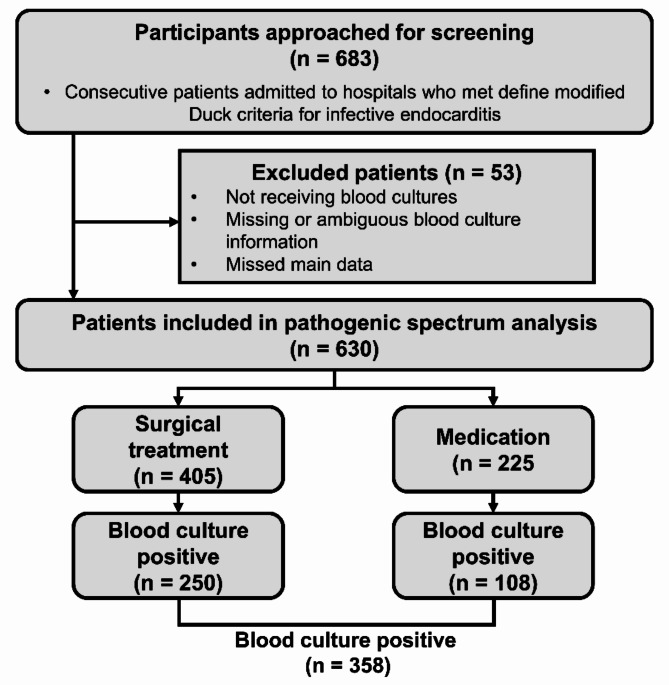
The pathogen spectrum analysis included 630 patients diagnosed with IE who underwent a blood culture. Among them, 405 patients were treated surgically, and 225 received medical treatment. The overall blood culture positivity rate was 56.83% (n = 358), with rates of 61.73% (n = 250) in the surgical group and 48% (n = 108) in the medical group (Fig. 1).
Fig. 1.
Flow chart of patients with infective endocarditis who underwent blood culture testing
The distribution of bacterial pathogens is detailed in Table 1. Viridans streptococci were predominant, accounting for 43.85% of cases, followed by Staphylococcus aureus, enterococci, and coagulase-negative staphylococci (13.69%, 7.82%, and 7.54%, respectively). Streptococci collectively constituted the majority (56.70%) and included β-hemolytic streptococci (3.35%), Streptococcus bovis (1.68%), Streptococcus pneumoniae (0.84%), Granulicatella species, and Abiotrophia species (5.31%) and other streptococci (1.68%). HACEK organisms and fungi were each detected in 1.68% of cases, while multiple bacterial infections were present in 1.4% of patients.
Table 1.
Pathogenic spectrum of blood culture-positive infective endocarditis
| Pathogen | Total (n = 358) | Surgical treatment (n = 250) |
Medical treatment (n = 108) |
P-value |
|---|---|---|---|---|
| Staphylococcus | 76 (21.23) | 45 (18.00) | 31 (28.70) | 0.023 |
| Staphylococcus aureus | 49 (13.69) | 31 (12.40) | 18 (16.67) | 0.281 |
| Coagulase-negative staphylococci | 27 (7.54) | 14 (5.60) | 13 (12.04) | 0.034 |
| Streptococcus | 203 (56.70) | 161 (64.40) | 42 (38.89) | < 0.001 |
| Viridans Streptococci | 157 (43.85) | 127 (50.80) | 30 (27.78) | < 0.001 |
| β-hemolytic streptococci | 12 (3.35) | 11 (4.40) | 1 (0.93) | 0.094 |
| Streptococcus bovis | 6 (1.68) | 4 (1.60) | 2 (1.85) | 0.865 |
| Streptococcus pneumoniae | 3 (0.84) | 3 (1.20) | 0 (0.00) | 0.253 |
| Granulicatella sp. and Abiotrophia sp. | 19 (5.31) | 13 (5.20) | 6 (5.56) | 0.890 |
| Other streptococcus | 6 (1.68) | 3 (1.20) | 3 (2.78) | 0.286 |
| Enterococcus | 28 (7.82) | 21 (8.40) | 7 (6.48) | 0.535 |
| Other G + bacteria | 11 (3.07) | 6 (2.40) | 5 (4.63) | 0.262 |
| Escherichia coli | 3 (0.84) | 0 (0.00) | 3 (2.78) | 0.008 |
| HACEK* | 6 (1.68) | 4 (1.60) | 2 (1.85) | 0.865 |
| Other G- bacteria | 20 (5.59) | 10 (4.00) | 10 (9.26) | 0.047 |
| Fungi | 6 (1.68) | 1 (0.40) | 5 (4.63) | 0.004 |
| Multiple bacteria | 5 (1.40) | 2 (0.80) | 3 (2.78) | 0.143 |
*HACEK: Hemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; G+: Gram-positive; G-: Gram-negative
Further analysis revealed significantly higher proportions of streptococci (64.40% vs. 38.89%, P < 0.001), particularly viridans streptococci (50.80% vs. 27.78%, P < 0.001), in the surgically treated group compared to the medically treated group. Conversely, staphylococci (18.00% vs. 28.70%, P = 0.023), especially coagulase-negative staphylococci (5.60% vs. 12.04%, P = 0.034), were significantly lower in the surgically treated cohort. Moreover, the surgically treated group exhibited lower percentages of Escherichia coli, fungi, and other Gram-negative bacteria than the medically treated group (all P < 0.05).
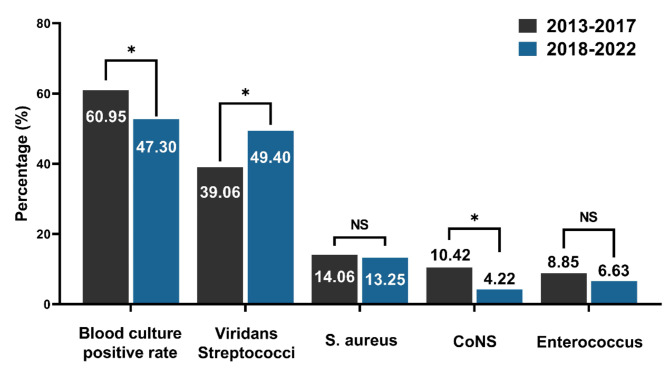
The flora percentages were compared between 2013 and 2017 and 2018–2022 (Fig. 2). The results indicate a decrease in the positivity rates of blood cultures in recent years compared to the past (2018–2022 vs. 2013–2017 = 60.95% vs. 47.30%, P = 0.037). The proportion of viridans streptococci showed an increasing trend, while coagulase-negative staphylococci showed a decreasing trend (all P < 0.05). Differences in the remaining flora were insignificant.
Fig. 2.
Comparison of blood culture positivity rates and major pathogens in patients with endocarditis across two periods, 2013–2017 and 2018–2022
Clinical characteristics of patients with IE who underwent surgical treatment
Surgical treatment has become the mainstay for IE; among the 683 patients with IE, 438 received surgical treatment, accounting for 64.13%. The clinical characteristics of the surgically treated patients with IE are summarized in Table 2. The mean age of the patients was 44.52 ± 15.74 years, and 70.09% were men. There were 419 (95.66%) patients with native valve endocarditis and 19 (4.34%) with prosthetic valve endocarditis. Moreover, 11.43% of the patients had cerebral infarction within 3 months, while 2.28% had a history of peripheral vasculopathy. Seventy-two patients had moderate to severe anemia (Hb < 90 g/L), and 203 had hypoproteinemia (albumin < 35 g/L). Class III (47.72%) and Class IV (12.33%) heart failure accounted for 60.05% of the total population.
Table 2.
Clinical characteristics of surgically treated patients with infective endocarditis
| Variables | Total (n = 438) |
|---|---|
| Early mortality | 35 (7.99) |
| Demographic characteristics | |
| Age (years) | 44.52 ± 15.74 |
| Male gender (%) | 307 (70.09) |
| Current smoker (%) | 79 (18.04) |
| Medical history | |
| Hypertension (%) | 63 (14.38) |
| Coronary heart disease (%) | 26 (5.94) |
| Diabetes (%) | 26 (5.94) |
| COPD (%) | 5 (1.14) |
| Recurrence or previous IE (%) | 9 (2.05) |
| Previous cardiac surgery (%) | 30 (6.85) |
| Prosthetic valve endocarditis (%) | 19 (4.34) |
| Cerebral infarction within 3 months (%) | 50 (11.42) |
| History of peripheral vasculopathy | 10 (2.28) |
| Preoperative embolization event (%) | 112 (25.57) |
| Moderate or severe anemia* (%) | 72 (16.44) |
| Renal insufficiency (%) | 29 (6.62) |
| Hypoalbuminemia* (%) | 203 (46.35) |
| Symptoms and signs | |
| Atrial fibrillation or atrial flutter (%) | 30 (6.85) |
| Cardiac conduction block (%) | 17 (3.88) |
| NYHA | |
| I (%) | 18 (4.11) |
| II (%) | 157 (35.84) |
| III (%) | 209 (47.72) |
| IV (%) | 54 (12.33) |
| Laboratory finding | |
| White blood cell counts, ×109/L | 9.24 ± 3.98 |
| Platelet count, ×109/L | 247.8 ± 95.27 |
| Hemoglobin, g/L | 111.4 ± 23.09 |
| NT-proBNP, pg/mL | 805.35 (203.38-2405.25) |
| Serum creatinine, µmol/L | 77.00 (64.00–93.00) |
| Blood urea nitrogen, mmol/L | 5.20 (4.10–7.30) |
| Serum albumin, g/L | 35.43 ± 5.87 |
| Total bilirubin, µmol/L | 12.70 (9.30–17.10) |
| Aspartate transaminase, U/L | 23.00 (18.00–32.00) |
| Echocardiographic characteristic | |
| Left heart (%) | 401 (91.55) |
| Right heart (%) | 26 (5.94) |
| Bilateral heart* (%) | 11 (2.51) |
| Aortic valve alone (%) | 144 (32.88) |
| Mitral valve alone (%) | 185 (42.24) |
| Pulmonary valve alone (%) | 9 (2.05) |
| Tricuspid valve alone (%) | 17 (3.88) |
| Aortic and mitral valve (%) | 54 (12.33) |
| Other multiple valve lesions (%) | 11 (2.51) |
| Infection of other sites (%) | 18 (4.11) |
| Vegetation formation (%) | 401 (91.55) |
| Size of vegetation*, mm | 11.74 ± 6.75 |
| > 10 mm (%) | 256 (58.45) |
| > 15 mm (%) | 139 (31.74) |
| > 20 mm (%) | 55 (12.56) |
| Left ventricular ejection fraction, % | 67.08 ± 8.16 |
| Paravalvular abscess (%) | 42 (9.59) |
| Left atrial thrombus (%) | 4 (0.91) |
| Perforated valves (%) | 144 (32.88) |
| Ruptured tendon cords (%) | 65 (14.84) |
| Surgery-related information | |
| Valve replacement (%) | 406 (92.69) |
| Biological valve replacement (%) | 104 (23.74) |
| Mechanical valve replacement (%) | 302 (68.95) |
| Isolated valvuloplasty (%) | 32 (7.31) |
| Combined CABG (%) | 7 (1.60) |
| Combined aortic-related surgery (%) | 16 (3.65) |
| Combined congenital heart disease surgery (%) | 67 (15.30) |
*Moderate or severe anemia was defined as hemoglobin < 90 g/L; Hypoalbuminemia was defined as albumin < 35 g/L; Entire heart surgery refers to cases where patients underwent both left- and right-side cardiac surgery; Size of vegetation refers to the largest diameter of vegetation measured by echocardiography
COPD, chronic obstructive pulmonary disease; IE, infective endocarditis; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; CABG, coronary artery bypass graft
Ultrasound findings showed that 91.55% of cases involved left-sided endocarditis, 5.94% involved right-sided endocarditis, and 2.51% involved both sides (total heart endocarditis). The most commonly involved valve was the mitral valve (42.24%). Nearly all patients had valvular vegetations (91.55%), with a mean size of 11.74 ± 6.75 mm. Perivalvular abscess was present in 42 cases (9.59%), valve perforation in 144 (32.88%), and ruptured tendon cords in 65 (14.84%).
Valve replacement was performed in 92.69% of cases, with 23.74% having undergone bioprosthetic valve replacement and 68.95% having undergone mechanical valve replacement. Valvuloplasty alone accounted for 7.31% of the procedures. Furthermore, there were seven cases (1.60%) of combined CABG, 16 (3.65%) of combined aortic-related surgery, and 67 (15.30%) of combined congenital heart surgery.
Analysis of risk factors for prognosis after surgical treatment
Early postoperative mortality occurred in 35 patients (7.99%). Multivariate logistic regression analysis identified male gender, coronary artery disease, platelet count < 100 × 109/L, albumin < 35 g/L, elevated creatinine, and prosthetic valve as independent risk factors for early postoperative mortality (Table 3).
Table 3.
Multivariable analyses of risk factors for early mortality in patients with surgically treated infective endocarditis
| Variable | OR | 95% PI | P Value | |
|---|---|---|---|---|
| Male gender | 3.722 | (1.175–11.795) | 0.026* | |
| Coronary artery disease | 4.207 | (1.329–13.323) | 0.015* | |
| Platelet count < 100 × 109/L | 25.035 | (7.407–84.612) | < 0.001* | |
| Albumin < 35 g/L | 4.202 | (1.622–10.881) | 0.003* | |
| Creatinine, µmol/L | 1.004 | (1.001–1.007) | 0.005* | |
| Prosthetic valve | 25.697 | (5.028–131.334) | < 0.001* | |
| Pulmonary valve replacement | 8.496 | (0.868–83.122) | 0.066 |
OR, odds ratio; PI, prediction interval
A total of 112 patients (25.57%) experienced new embolic events: cerebral embolism in 59 cases, splenic embolism in 24, peripheral arterial embolism in nine, abdominal vascular embolism in two, pulmonary embolism in two, multiple organ embolism in 14 cases, and other types in two. Cerebral embolism was the most common (52.68%) and was most frequently seen in cases of multiple organ embolism (71.43%). Multivariate logistic regression analysis showed that cerebral infarction within 3 months, history of peripheral vasculopathy, and hemoglobin < 90 g/L were independent risk factors for embolic events (Table 4).
Table 4.
Multivariable analyses of risk factors for embolic events in patients with surgically treated infective endocarditis
| Variable | OR | 95% PI | P Value |
|---|---|---|---|
| Cerebral infarction within 3 months | 8.229 | (4.307–15.724) | < 0.001* |
| History of peripheral vasculopathy | 15.365 | (3.120-75.664) | 0.001* |
| HB < 90 g/L | 2.102 | (1.178–3.750) | 0.012* |
OR, odds ratio; PI, prediction interval
Discussion
This study summarized the total population of patients with IE at our center over the past 9 years, comparing the pathogenic spectrum of the medically and surgically treated cohorts, and identifying the trends in the pathogenic spectrum over time. Since operation is emerging as an essential and effective therapeutic strategy for treating IE, a risk factor analysis was performed on patients undergoing surgical procedures. Male gender, coronary artery disease, platelet count < 100 × 109/L, albumin < 35 g/L, elevated creatinine, and prosthetic valve were independent risk factors for early postoperative mortality. Moreover, cerebral infarction within 3 months, history of peripheral vasculopathy, and Hb < 90 g/L were independent risk factors for embolic events.
The overall blood culture positivity rate in our study was 56.83%, consistent across both medical and surgical treatment groups, and showing a decreasing trend in recent years. This rate is notably lower than reported in some other regions, such as Europe (95.1%) [7], the United States (74.7%) [8], and Latin America (76.1%) [9], but aligns with other studies from China, which report positivity rates of 35–65% [10–14]. The discrepancy in blood culture positivity rates between regions may be attributed to variations in healthcare infrastructure, diagnostic capabilities, and initial patient management practices. In Chinese high-level medical centers, where most IE studies are conducted, many patients receive empirical antibiotic therapy at local clinics prior to referral. This pre-admission antibiotic exposure is a well-documented factor leading to culture-negative results, as it can reduce bacterial load and impact pathogen detection. This challenge is further compounded by infections with fastidious or slow-growing organisms, such as Coxiella burnetii, Bartonella spp., and certain fungi, which may require specialized culture conditions or media beyond standard blood culture capabilities. Additionally, the increase in healthcare-associated endocarditis, including infections related to intravascular devices, may also contribute to a higher proportion of culture-negative cases, as these infections often involve biofilm-forming organisms that are challenging to culture [15]. The decline in blood culture positivity underscores the importance of advancing diagnostic approaches. Molecular techniques, including metagenomic next-generation sequencing (mNGS) and PCR-based diagnostics, offer promising solutions by identifying pathogens in culture-negative cases, including atypical and previously undetectable organisms. As mNGS becomes more widely implemented, it may improve pathogen detection rates, enhance understanding of IE’s microbiologic spectrum, and ultimately support more targeted therapeutic strategies, especially in cases where initial empirical antibiotic therapy may obscure pathogen identification. For centers without immediate access to advanced diagnostic techniques like mNGS, enhancing conventional methods (e.g., blood culture optimization, PCR-based diagnostics) and adopting cost-effective syndromic panels are practical interim solutions. Additionally, regional collaboration to develop microbial surveillance networks can support evidence-based empirical therapy. Incremental capacity-building efforts for diagnostic upgrades remain a long-term goal to improve pathogen identification in resource-limited settings.
Our study indicates that viridans streptococci remain the dominant group in IE in our cohort, accounting for 43.85% of the blood culture-positive cases. This prevalence is consistent with reports from several regions in China, where rates range from approximately 37.5–52.9% [10, 12, 13, 16]. However, this figure is significantly higher than those observed in Europe (approximately 17.4%) [7], the United States (approximately 26.6%) [8], and Latin America (approximately 17.8%) [9], while being somewhat comparable to the overall prevalence in Africa (approximately 34%) [17]. Despite the increasing rates of intracardiac implants and invasive procedures in China, viridans streptococci-mediated IE remains predominant in many centers, exhibiting an upward trend at our institution [10–13]. This pattern may be attributed to a higher incidence of community-acquired IE, particularly involving native valves. Notably, studies have reported an incidence of viridans streptococci as high as 39.4% in cases of native valve endocarditis [18]. Additionally, the association between viridans streptococci and poor oral health underscores a significant public health issue in our population, where inadequate oral hygiene contributes to bacteremia that can precipitate IE [19]. Daily activities such as brushing, flossing, and chewing can lead to severe bacteremia, resulting in IE, especially in individuals with poor oral hygiene [20]. Thus, it is vital to advocate for regular dental checkups every 6 months and promote oral hygiene education among high-risk groups for IE in China [21]. Historically, viridans streptococci were the most common pathogens in Europe, the United States, and Latin America; however, their prevalence has declined in these regions over the past two decades. In contrast, patients with viridans streptococci-associated IE tend to be older and often have underlying cardiac conditions, including rheumatic heart disease in resource-limited settings and valvular diseases such as aortic stenosis and mitral valve prolapse in more affluent countries. In our study, Staphylococcus aureus ranked second at 13.69%, significantly lower than reported rates in Europe (31.4%) [22], the United States (40%) [23], Latin America (18.6%) [9], and Africa (41.3%) [17]. The increasing incidence of Staphylococcus aureus IE has raised concerns, particularly as it has become the leading pathogen in several countries, partly attributed to the opioid crisis and the associated rise in injection drug use. Staphylococcus aureus accounts for a majority of IE cases in people who inject drugs [24]. The expanding indications for cardiovascular implantable electronic devices and the rising number of valve replacement surgeries have also contributed to an increased incidence of device-related infections, predominantly caused by staphylococci [23]. Recent large-scale epidemiological studies have suggested a temporal association between various invasive medical and surgical procedures-such as gastrointestinal endoscopy and bronchoscopy-and the development of IE, particularly in high-risk populations [21].
Regarding treatment, there is no need to adjust the guideline-recommended first-line treatment, as the viridans streptococci cultured in this study did not show resistance to penicillin. Since five cases of methicillin-resistant S Aureus (MRSA) flora were cultured in this cohort, the use of MRSA-active drugs is necessary when indicated. These results reinforce the continued relevance of guideline-recommended empiric antibiotic regimens. Enterococcus-mediated IE ranked third, accounting for approximately 7.82% of cases. It is a classic community-acquired pathogen and a cause of IE in older male patients with underlying gastrointestinal or genitourinary tract abnormalities [24]. Considering the difficulty in treating enterococci, guidelines recommend combination antibiotic therapy [25]. Coagulase-negative staphylococci ranked fourth and are typically opportunistic pathogens associated with retained or implanted foreign bodies, leading to IE [26]. Since coagulase-negative staphylococci are part of the skin flora, contamination during blood culture collection and tissue processing must be avoided to prevent its misidentification as a pathogen.
Importantly, our analysis found that early postoperative mortality occurred in 35 patients (7.99%). We identified several independent risk factors for early mortality through multivariate logistic regression. Male gender was identified as a significant risk factor for early postoperative mortality (OR 3.722). However, the female gender was earlier demonstrated to be a risk factor for postoperative death in IE, suggesting that although the prevalence is lower in women, the symptoms are more severe [27–30]. This conclusion is controversial, as some studies show that female gender is not a risk factor for postoperative death [31, 32], and others indicate that the age-adjusted mortality rate is higher in men compared to women [33]. Recent gender-specific studies have found that women exhibit more severe IE manifestations and significantly higher 30-day and 1-year mortality rates. However, multivariate analysis revealed that it is not the female gender but the underlying comorbidities that determine clinical outcomes [31]. These differences in findings may be attributable to geographical variations in populations.
Hypoproteinemia was found to be a significant risk factor for early postoperative mortality (OR 4.202). Low serum albumin levels (< 35 g/L) often indicate malnutrition or chronic disease, leading to a compromised immune response and poorer wound healing, which can increase the risk of complications after surgery. A machine learning-based prediction model for early postoperative death after IE revealed that serum albumin levels were the most significant factor in explaining patient risk [34]. Hypoproteinemia has a high incidence of IE and predicts poor postoperative prognosis [35]. Thrombocytopenia, with a platelet count of less than 100 × 10⁹/L, was associated with an alarming odds ratio of 25.035, indicating a profound risk for mortality. Thrombocytopenia can stem from various causes, including sepsis, bone marrow suppression, or increased destruction of platelets, complicating the surgical recovery process, particularly in patients with concurrent cardiac issues. Thrombocytopenia is an important risk factor, especially significant in patients with heart failure [36]. Coronary artery disease (CAD) was found to significantly increase mortality risk (OR 4.207). Patients with CAD often present with reduced myocardial perfusion and can experience postoperative complications related to cardiac function, particularly during the stress of surgery [37]. The presence of a prosthetic valve was associated with a very high odds ratio of 25.697, which is aligns with previous studies [27, 29, 30, 38]. Patients with prosthetic valve infections face increased risks of both perioperative complications and mortality due to the complexities of managing infection and the inherent risks associated with prior surgical interventions. Elevated creatinine levels (OR 1.004 per 1 µmol/L) serve as a marker for renal dysfunction, which is particularly critical in the context of IE. Impaired kidney function can complicate the management of fluid and electrolytes post-surgery, thereby increasing the risk of adverse outcomes. This is consistent with previous research [29, 30, 37, 38]. The identification of these factors emphasizes the importance of comprehensive preoperative assessments and tailored postoperative care strategies. By addressing these risk factors, particularly in high-risk populations, we can enhance clinical outcomes and potentially reduce early postoperative mortality in patients with IE.
This study further analyzed the risk factors for embolic events in IE and found that cerebral infarction within the past 3 months, a history of peripheral vasculopathy, and Hb levels < 90 g/L were independent risk factors. The presence of pre-existing cerebrovascular disease and peripheral vasculopathy indicate more severe vascular conditions, making small emboli more likely to cause embolic events, consistent with previous studies [6, 39]. These findings suggest that patients with these vascular conditions may benefit from intensified monitoring for embolic complications or consideration of early surgical intervention to reduce embolic risk. Moreover, while studies have shown a strong association between anemia and cerebrovascular events, this study uniquely identifies anemia (Hb < 90 g/L) as an independent risk factor for embolism in IE. Anemia induces a hyperkinetic circulatory state, potentially promoting thrombus formation by influencing endothelial adhesion molecule expression [40]. Recognizing anemia as a risk factor supports the consideration of preoperative anemia management, including targeted nutritional or pharmacologic interventions, which may reduce embolic risk in susceptible patients. By incorporating these findings, clinicians may improve risk stratification for embolic events in IE, allowing for tailored management strategies that prioritize early intervention and address modifiable risk factors like anemia. Future studies should aim to refine these strategies in multicenter settings to establish more broadly applicable guidelines.
Limitations
Several limitations are associated with this study. Firstly, the relatively low blood culture positivity rate may have resulted in some data loss; however, the ongoing implementation of metagenomic analysis at our center will enhance pathogen detection in further studies. Secondly, the observational and retrospective study design introduces inherent biases. Although we have accounted for this in our analysis, a prospective study design would provide more definitive evidence regarding risk factors for mortality and embolic events in IE. Thirdly, the results of this single-center study could reflect local practices and should be cautiously generalized. Future multicenter or international studies could help to validate and broaden the applicability of these findings across different clinical settings. Lastly, the high rate of surgical interventions in our study population may be influenced by selection bias from tertiary cardiac centers. Patients referred to tertiary centers typically exhibit fewer comorbidities and are better candidates for operation, potentially contributing to the observed lower mortality rate. Conversely, patients with more severe diseases who are unsuitable for operation and at higher risk of mortality may not have been represented in our study cohort. Our study predominantly reflects the risk factors in surgically managed IE cases, which may not fully represent the medically treated cohort. Patients managed without surgery often differ in disease severity and comorbidities. Future studies incorporating both surgical and medically managed cohorts across diverse healthcare settings are needed to provide a more comprehensive understanding of IE. Due to the small number of resistant isolates identified in this cohort, particularly methicillin-resistant Staphylococcus aureus (MRSA), it was not feasible to accurately analyze resistance trends over the study period. Larger multicenter studies are necessary to provide a more comprehensive understanding of resistance patterns to better guide empirical therapy. In addition, residual confounding due to unmeasured variables, such as socioeconomic factors or preoperative functional status, may exist. Prospective multicenter studies with standardized data collection are necessary to validate our findings and explore the influence of unmeasured confounders.
Conclusion
In this study, we identified viridans streptococci as the most common pathogen causing IE in our surgical cohort, with an increasing incidence in recent years. Blood culture positivity rates were observed to be low and declining, highlighting the need for advanced diagnostic methods to enhance pathogen identification, such as metagenomic sequencing, which may improve early targeted therapy. Our exploration of risk factors for early postoperative mortality and embolic events suggests that intensified preoperative risk assessment, including stratification for embolic risks and individualized surgical planning, could enhance patient management. Given the high prevalence of viridans streptococci, tailoring empirical antibiotic protocols to reflect local epidemiology might optimize initial treatment and reduce the risk of adverse outcomes. Future studies could focus on multicenter collaborations to validate these findings and refine risk prediction models, ultimately enhancing the management and prognosis of IE patients across different clinical settings.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our gratitude to Bullet Edits Limited for the language editing and proofreading of the manuscript.
Author contributions
WZK, HSQ and HJ contributed to the conception or design of the study. HSQ, CJT, CTX, LZY, LL, LQ and FKN contributed to the acquisition, analysis, or interpretation ofdata. HSQ, CJT and CTX drafted the manuscript. WZK and HJ critically revised the manuscript. All the authors gave final approval and agreed to be accountable for all aspects of work for ensuring integrity and accuracy. All authors read and approved the final manuscript.
Funding
This study was supported by the National Key R&D Program of China (2023YFC2706200) and National Science Foundation of China (82370271, 82070297).
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study received approval from the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University (2018 [100]). Prior approval by the institution’s human research committee indicates that the study protocol conforms with the ethical criteria outlined in the 1975 Declaration of Helsinki. Prior informed consent has been sought for all the participating subjects in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Suiqing Huang, Jiantao Chen and Tongxin Chu contributed equally to this work.
Contributor Information
Zeyu Li, Email: lizy95@mail3.sysu.edu.cn.
Jian Hou, Email: chnhouj@163.com.
Zhongkai Wu, Email: wuzhk@mail.sysu.edu.cn.
References
- 1.Yang X, Chen H, Zhang D, Shen L, An G, Zhao S. Global magnitude and temporal trend of infective endocarditis, 1990–2019: results from the Global Burden of Disease Study. Eur J Prev Cardiol. 2022;29(8):1277–86. [DOI] [PubMed] [Google Scholar]
- 2.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882–93. [DOI] [PubMed] [Google Scholar]
- 3.Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, Caselli S, Doenst T, Ederhy S, Erba PA, et al. : 2023 ESC Guidelines for the management of endocarditis. Eur Heart J. 2023;44(39):3948–4042. [DOI] [PubMed] [Google Scholar]
- 4.Said SM, Abdelsattar ZM, Schaff HV, Greason KL, Daly RC, Pochettino A, Joyce LD, Dearani JA. Outcomes of surgery for infective endocarditis: a single-centre experience of 801 patients. Eur J Cardiothorac Surg. 2018;53(2):435–9. [DOI] [PubMed] [Google Scholar]
- 5.Sevilla T, Lopez J, Gomez I, Vilacosta I, Sarria C, Garcia-Granja PE, Olmos C, Di Stefano S, Maroto L, San Roman JA. Evolution of Prognosis in Left-Sided Infective Endocarditis: A Propensity Score Analysis of 2 Decades. J Am Coll Cardiol. 2017;69(1):111–2. [DOI] [PubMed] [Google Scholar]
- 6.Sambola A, Lozano-Torres J, Boersma E, Olmos C, Ternacle J, Calvo F, Tribouilloy C, Reskovic-Luksic V, Separovic-Hanzevacki J, Park SW, et al. Predictors of embolism and death in left-sided infective endocarditis: the European Society of Cardiology EURObservational Research Programme European Infective Endocarditis registry. Eur Heart J. 2023;44(43):4566–75. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosioni J, Hernandez-Meneses M, Durante-Mangoni E, Tattevin P, Olaison L, Freiberger T, Hurley J, Hannan MM, Chu V, Hoen B, et al. Epidemiological Changes and Improvement in Outcomes of Infective Endocarditis in Europe in the Twenty-First Century: An International Collaboration on Endocarditis (ICE) Prospective Cohort Study (2000–2012). Infect Dis Ther. 2023;12(4):1083–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA. 2017;317(16):1652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urina-Jassir M, Jaimes-Reyes MA, Martinez-Vernaza S, Quiroga-Vergara C, Urina-Triana M. Clinical, Microbiological, and Imaging Characteristics of Infective Endocarditis in Latin America: A Systematic Review. Int J Infect Dis. 2022;117:312–21. [DOI] [PubMed] [Google Scholar]
- 10.Ren Z, Mo X, Chen H, Peng J. A changing profile of infective endocarditis at a tertiary hospital in China: a retrospective study from 2001 to 2018. BMC Infect Dis. 2019;19(1):945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Chen Y, Xiao T, Niu T, Shi Q, Xiao Y. Epidemiology and risk factors of infective endocarditis in a tertiary hospital in China from 2007 to 2016. BMC Infect Dis. 2020;20(1):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JB, Lu CC, Wen ZK, Yang JR, Li JJ. Surgical treatment of left-sided infective endocarditis with symptomatic neurological complications before surgery in China. Front Cardiovasc Med. 2023;10:1217148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Jin F, Lu Y, Ni F, Xu Y, Xia W. Clinical Characteristics and Risk Factors for in-Hospital Mortality in 240 Cases of Infective Endocarditis in a Tertiary Hospital in China: A Retrospective Study. Infect Drug Resist. 2022;15:3179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HL, Tromp J, Teramoto K, Tse YK, Yu SY, Lam LY, Li KY, Wu MZ, Ren QW, Wong PF, et al. Temporal trends and patterns of infective endocarditis in a Chinese population: A territory-wide study in Hong Kong (2002–2019). Lancet Reg Health West Pac. 2022;22:100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsouli A, Massad MG. Current issues in the diagnosis and management of blood culture-negative infective and non-infective endocarditis. Ann Thorac Surg. 2013;95(4):1467–74. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Ge Y, Ma H, Zhu B, Miao Q. Infective endocarditis at a tertiary-care hospital in China. J Cardiothorac Surg. 2020;15(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noubiap JJ, Nkeck JR, Kwondom BS, Nyaga UF. Epidemiology of infective endocarditis in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2022;10(1):e77–86. [DOI] [PubMed] [Google Scholar]
- 18.Angsutararux T, Angkasekwinai N. Cumulative incidence and mortality of infective endocarditis in Siriraj hospital-Thailand: a 10-year retrospective study. BMC Infect Dis. 2019;19(1):1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, Petersen PE, Wang HY, Bian JY, Zhang BX. Oral health knowledge, attitudes and behaviour of adults in China. Int Dent J. 2005;55(4):231–41. [DOI] [PubMed] [Google Scholar]
- 20.Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, Bahrani-Mougeot FK, Sasser HC. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140(10):1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayer MJ, Quintero-Martinez JA, Thornhill MH, Chambers JB, Pettersson GB, Baddour LM. Recent Insights Into Native Valve Infective Endocarditis: JACC Focus Seminar 4/4. J Am Coll Cardiol. 2024;83(15):1431–43. [DOI] [PubMed] [Google Scholar]
- 22.Bourget M, Pasquie M, Charbonneau H, Bonnet E. Comparable clinical course between coagulase-negative staphylococcal and Staphylococcus aureus endocarditis. Infection. 2022;50(2):483–90. [DOI] [PubMed] [Google Scholar]
- 23.Wang A, Gaca JG, Chu VH. Management Considerations in Infective Endocarditis: A Review. JAMA. 2018;320(1):72–83. [DOI] [PubMed] [Google Scholar]
- 24.Talha KM, DeSimone DC, Sohail MR, Baddour LM. Pathogen influence on epidemiology, diagnostic evaluation and management of infective endocarditis. Heart. 2020;106(24):1878–82. [DOI] [PubMed] [Google Scholar]
- 25.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. [DOI] [PubMed] [Google Scholar]
- 26.Noshak MA, Rezaee MA, Hasani A, Mirzaii M. The Role of the Coagulase-negative Staphylococci (CoNS) in Infective Endocarditis; A Narrative Review from 2000 to 2020. Curr Pharm Biotechnol. 2020;21(12):1140–53. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Selles M, Munoz P, Arnaiz A, Moreno M, Galvez J, Rodriguez-Roda J, de Alarcon A, Garcia Cabrera E, Farinas MC, Miro JM, et al. Valve surgery in active infective endocarditis: a simple score to predict in-hospital prognosis. Int J Cardiol. 2014;175(1):133–7. [DOI] [PubMed] [Google Scholar]
- 28.Di Mauro M, Dato GMA, Barili F, Gelsomino S, Sante P, Corte AD, Carrozza A, Ratta ED, Cugola D, Galletti L, et al. A predictive model for early mortality after surgical treatment of heart valve or prosthesis infective endocarditis. The EndoSCORE. Int J Cardiol. 2017;241:97–102. [DOI] [PubMed] [Google Scholar]
- 29.Varela Barca L, Fernandez-Felix BM, Navas Elorza E, Mestres CA, Munoz P, Cuerpo-Caballero G, Rodriguez-Abella H, Montejo-Baranda M, Rodriguez-Alvarez R, Gutierrez Diez F, et al. Prognostic assessment of valvular surgery in active infective endocarditis: multicentric nationwide validation of a new score developed from a meta-analysis. Eur J Cardiothorac Surg. 2020;57(4):724–31. [DOI] [PubMed] [Google Scholar]
- 30.Varela Barca L, Navas Elorza E, Fernandez-Hidalgo N, Moya Mur JL, Muriel Garcia A, Fernandez-Felix BM, Miguelena Hycka J, Rodriguez-Roda J, Lopez-Menendez J. Prognostic factors of mortality after surgery in infective endocarditis: systematic review and meta-analysis. Infection. 2019;47(6):879–95. [DOI] [PubMed] [Google Scholar]
- 31.Weber C, Gassa A, Rokohl A, Sabashnikov A, Deppe AC, Eghbalzadeh K, Merkle J, Hamacher S, Liakopoulos OJ, Wahlers T. Severity of Presentation, Not Sex, Increases Risk of Surgery for Infective Endocarditis. Ann Thorac Surg. 2019;107(4):1111–7. [DOI] [PubMed] [Google Scholar]
- 32.Leterrier J, Iung B, de Tymoski C, Deconinck L, Para M, Duval X, Provenchere S, Mesnier J, Delhomme C, Haviari S et al. Sex differences and outcomes in surgical infective endocarditis. Eur J Cardiothorac Surg 2024, 65(4). [DOI] [PubMed]
- 33.Agha A, Nazir S, Minhas AMK, Kayani W, Issa R, Moukarbel GV, DeAnda A, Cram P, Jneid H. Demographic and Regional Trends of Infective Endocarditis-Related Mortality in the United States, 1999 to 2019. Curr Probl Cardiol. 2023;48(1):101397. [DOI] [PubMed] [Google Scholar]
- 34.Luo L, Huang SQ, Liu C, Liu Q, Dong S, Yue Y, Liu KZ, Huang L, Wang SJ, Li HY, et al. Machine Learning-Based Risk Model for Predicting Early Mortality After Surgery for Infective Endocarditis. J Am Heart Assoc. 2022;11(11):e025433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang S, Zhou Z, Luo L, Yue Y, Liu Q, Feng K, Hou J, Wang K, Chen J, Li H, et al. Preoperative serum albumin: a promising indicator of early mortality after surgery for infective endocarditis. Ann Transl Med. 2021;9(18):1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Huang S, Hou J, Feng K, Wu H, Liu Q, Zhou Z, Li H, Luo L, Shang L et al. Impact of heart failure and preoperative platelet count on the postoperative short-term outcome in infective endocarditis patients. Clin Cardiol 2024, 47(1):e24171 [DOI] [PMC free article] [PubMed]
- 37.Caes F, Bove T, Van Belleghem Y, Vandenplas G, Van Nooten G, Francois K. Reappraisal of a single-centre policy on the contemporary surgical management of active infective endocarditis. Interact Cardiovasc Thorac Surg. 2014;18(2):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olmos C, Vilacosta I, Habib G, Maroto L, Fernandez C, Lopez J, Sarria C, Salaun E, Di Stefano S, Carnero M, et al. Risk score for cardiac surgery in active left-sided infective endocarditis. Heart. 2017;103(18):1435–42. [DOI] [PubMed] [Google Scholar]
- 39.Papadimitriou-Olivgeris M, Guery B, Ianculescu N, Auberson D, Tozzi P, Kirsch M, Monney P. Risk of embolic events before and after antibiotic treatment initiation among patients with left-side infective endocarditis. Infection. 2024;52(1):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiafa G, Savopoulos C, Kanellos I, Mylonas KS, Tsikalakis G, Tegos T, Kakaletsis N, Hatzitolios AI. Anemia and stroke: Where do we stand? Acta Neurol Scand. 2017;135(6):596–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.