Abstract
Background
While progress has been made in reducing HIV incidence rates among cisgender women, it continues to fall short of reaching the goal of ending the HIV epidemic with no new cases.
Objective
This study aims to use innovative electronic methods (e.g., social media with community-informed advertisements) to recruit and retain a large (N = 1,800), diverse national sample of women at higher risk for HIV seroconversion who are 14 years of age and older to better understand the predictors of HIV-related sexual risk and HIV incidence within the context of a theoretically-grounded social-ecological framework.
Methods
A US-based national longitudinal cohort study was launched among cisgender women with greater likelihood of HIV seroconversion Participants complete a survey with items related to demographics, substance use, mental health symptoms, interpersonal violence and other social factors. Biospecimens include self-collected vaginal and rectal swabs, and blood in microtainers to test for HIV, syphilis, chlamydia, gonorrhea, and trichomoniasis every 6 months for 2 years.
Results
Participant recruitment began in June 2023 and baseline enrollment is scheduled to finish in July 2025.
Discussion
Innovative and culturally sensitive strategies to improve access to HIV prevention and treatment services for cisgender women are vital to curb the burden of the HIV epidemic for this key population. Findings from this study will inform future research, intervention strategies, and public policies.
Keywords: HIV, STI, Self-sampling, Cisgender women, Cohort
Introduction
An estimated 7,190 new HIV infections occurred among cisgender women (hereafter referred to as women) in 2022 [1–3]. Several factors have been associated with HIV infection among women in the US including socioeconomic conditions (e.g., poverty, unemployment), substance use (e.g., alcohol, cannabis, injecting or using other substances), and sexual risk behaviors [2, 4–7], such as transactional sex, condomless intercourse, sex with partners with unknown HIV status, and multiple concurrent sex partners [8–10]. However, cohort studies in the US have failed to link individual level behaviors with HIV risk [11, 12]. Factors associated with increased risk for HIV included sex partner and community characteristics and socioeconomic factors that may lead women to enter sex work or engage in transactional sex to survive environments characterized by poverty [13–15]. Additionally, women with partners with a history of incarceration have shown to be at higher risk for HIV acquisition due to low rates of condom use, lack of HIV testing, and lack of information about prison-related risks for HIV acquisition [16]. Disparities in incarceration among men of color may exacerbate HIV incidence among women of color through gender imbalances and increased risk behaviors of their previously incarcerated partners [16–19].
Furthermore, substance use prior to sexual encounters has been associated with greater HIV risk among women [6, 7]. Women who use alcohol, cannabis or other substances before or during sexual encounters are more likely to have condomless intercourse and multiple sex partners [7, 20–22]. Studies indicate that women who use or inject drugs and share needles have higher odds of acquiring HIV than men who engage in similar risk-taking behaviors [7, 23, 24]. Women who use or inject drugs face a variety of gender-specific risk environments such as poverty, gender-based or intimate partner violence as well as legal and social risk environments [7, 14, 23].
While many individual level behaviors increase the odds of HIV exposure, social and structural barriers such as racism, sexism, poverty, discrimination, violence, and HIV stigma have a major impact on access to health care, HIV prevention opportunities, and ultimately HIV transmission. These barriers fuel the racial/ethnic inequities in HIV acquisition among women. Due to the unequal distribution of HIV incidence and prevalence among women of color, the National HIV Strategic Plan (2021–2025) lists Black women as a priority population. Risk-based screening and contextualizing a woman’s risk for HIV solely based on known behavioral risks greatly reduces the ability to offer resources and prevention opportunities to those who do not disclose stigmatized activities or may be unable to identify risk factors but are at risk for contextual or structural reasons [25–27].
Network structure and the characteristics of a network have significant implications for disease transmission, prevention information dissemination, and opportunities to promote behavior change. Elements of social network structure, including network size, density (connectedness between network members), duration (length of relationship to network members), and quality of relationships have been shown to influence HIV risk behaviors, with supportive networks associated with lower likelihood of HIV acquisition and condomless sex, and higher likelihood of HIV testing [28]. The impact of social network dynamics on HIV risk [29], has been clearly demonstrated in young men who have sex with men (YMSM), transgender women, and people who inject drugs (PWID) [29–31], but has yet to be established in large cohorts of women. Some studies highlight that vulnerability to HIV among women largely depends on their partners’ behaviors or network group [32–37]. Women who frequently have sex with men from multiple sexual networks that have high prevalence rates of HIV and low awareness of status or risk factors have a higher risk of acquiring HIV [38]. Thus, understanding the structural dynamics and characteristics of social and sexual networks appears to be important in understanding HIV transmission among women [33]. The American Women: Assessing Risk Epidemiologically (AWARE) Cohort Study aims to combine epidemiologic methods, digital technology, and data science approaches to better understand HIV prevention and transmission for women living in the US.
Methods
Study design
The AWARE study will utilize a prospective cohort study design that employs innovative electronic methods to recruit a large racially diverse sample of women at high risk of HIV acquisition and examine geospatial factors and micro epidemic areas (“hot spots” for HIV) to understand differences in HIV risk behavior and incidence across geographic areas in the US and its territories. AWARE aims to build a knowledgebase of integrated data, including data from an epidemiologic cohort of women, disease surveillance, social determinants of health, and network data. It will use innovative electronic methods (e.g., social media with community-informed advertisements) to recruit and retain a large (N = 1,800), diverse national cohort of high-risk women 14 years of age and older to better understand the correlates of HIV-related sexual risk and HIV incidence within the context of a social-ecological framework.
Inclusion criteria
To participate in the study, participants must: 1) be between 14 and 64 years of age (14 years is the youngest age that an individual can take an HIV home test without parental consent in all U.S. states and jurisdictions); 2) be assigned female sex at birth; 3) identify as female; 4) understand and read English or Spanish; 5) live within the U.S. and its territories; 6) be HIV-negative; and 7) self-report condomless vaginal or anal sex with a male in the past 6 months. Potential participants must also meet one or more of the following criteria in the past 6 months to be eligible for participation: injection or non-injection drug use (i.e., heroin, cocaine, crack cocaine, methamphetamine, or prescription drugs apart from those prescribed by a licensed provider); alcohol dependency or binge drinking; self-reported history of sexually transmitted infections (e.g., gonorrhea, chlamydia, or syphilis); exchange of sex for commodities, such as drugs, money, or shelter; male sexual partner with reported history of either injection or non-injection drug use, alcohol dependency or binge drinking, history of sexually transmitted infections, HIV diagnosis; history of intimate partner violence (IPV) or sexual assault, or incarceration of partner or self (jail or prison ≥ 24 h) within the past 5 years.
Exclusion criteria
We exclude women who staff determine participation may be detrimental to the participant or to the study (e.g., severe cognitive deficit) and persons unable or unwilling to provide consent for study participation. We carefully considered whether to include transgender women or individuals who identify along the transfeminine spectrum but opted to exclude them because mechanisms of HIV risk are considerably different for transgender women compared to cisgender women.
Recruitment
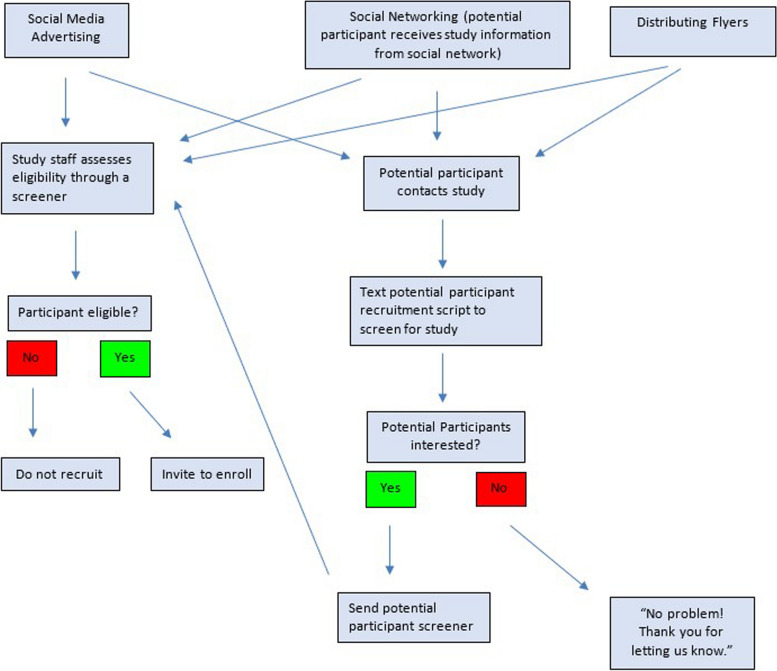
We are recruiting participants using community-informed advertisements posted on platforms like Instagram or Facebook. Figure 1 illustrates sample advertisements for this study. In addition, recruitment materials are distributed at physical locations such as Community-Based Organizations (CBOs). Physical recruitment materials contain QR codes that will automatically direct potential participants to the study screener, or to the Study Team for more information regarding the study before screening. See Fig. 2 for more details about the recruitment process.
Fig. 1.
Sample study advertisement
Fig. 2.
AWARE recruitment protocol
Screening procedures
Clicking on an advertisement prompts potential participants to complete a brief consent form and be screened for eligibility. Once they provide consent, participants will be electronically screened through REDCap for eligibility using the full screening instrument. Participants sign an e-consent form indicating their willingness to receive a study package and provide their address so the study team can send them the package.
Study packages
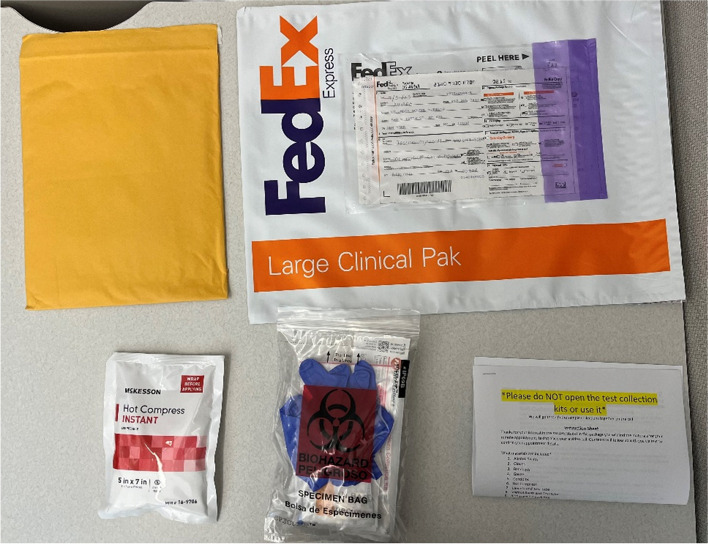
Prior to the study visit, participants receive a study package via FedEx. The study package includes: One cushioned envelope, a FedEx Envelope or Clinical Pak and Air bill pre-addressed to the lab, package inserts, sample collection instruction sheet, requisition form, a hot compress and Testing Materials: a) Blood Collection Materials: 2 Lancets, 1 Microtainer Tube, 2 Bandages, 2 Gauze Pads, 2 Alcohol Swabs, b) Swab Collection Materials: 2 Multicollects (Vaginal and Rectal Swab), one pair of Gloves, and c) Extra Materials: 2 Condoms (latex or non-latex), a Desiccant bag, Specimen Labels, Biohazard Bag. Items are illustrated in Fig. 3.
Fig. 3.
Contents of study packages
Informed consent
Interested participants will review information about the study before screening and will indicate their interest to screen via REDCap. At the enrollment visit written informed e-consent for study participation is collected, providing details about study procedures, risks, benefits, site contact information, confidentiality, and voluntary participation. The consent process also details the trial and study compensation.
Enrollment
Participants who screen eligible are scheduled to meet with a study team member via videoconference call. This ensures the integrity and success of the study because:
We can eliminate fraud by verifying participants’ identities via video conference—fraud being a potential problem in online research; and
We establish rapport with our study participants, which has resulted in very high retention rates related to this rapport building between our staff and study participants, which will be augmented by electronic retention strategies in this study.
Prior to signing the e-consent form, participants are asked to participate in a confirmatory screening visit via videoconferencing during their baseline phone call to confirm race, sex, and age. If participants are deemed ineligible, we let them know that we cannot continue with the visit because eligibility has changed.
To cross-check age, participants are asked their date of birth.
Participants are required to show a form of photo ID during the initial video conference to verify their identity. If a participant does not have a government or school issued ID, we will ask them to furnish a report card/transcript with their legal name, age, and sex.
During the enrollment visit, participants will be asked to provide an address and contact information so that study materials can be sent, and staff can follow up with them throughout the study. We will collect each participant’s cell phone number, email address, as well as encourage them to share their social media handles (e.g., Snapchat, Instagram, Twitter Facebook, WhatsApp, and/or Skype usernames). Study staff will not send messages or leave voicemail messages unless expressly permitted to do so by the participant. If permission is given to leave voice messages, site staff will assure participants that messages left will not include any protected health information or information related to study participation. Contact information will be maintained using the same confidential data management practices used for all study data.
Once screened eligible, participants will complete the baseline assessment comprised of the study measures listed in Table 1. We will use REDCap and Qualtrics for survey data collection; the benefits [13] for large multisite studies include direct electronic data capture and interactive data capture checks, allowing for secure and consistent data capture across sites. Participants scoring above 15 on the General Anxiety Disorder-7 or Center for Epidemiologic Studies-Depression-10 scales for anxiety and depression will receive a popup message in Qualtrics with mental health resources. All participants receive the same list of resources upon enrollment in the study.
Table 1.
AWARE study measures
| Demographic Characteristics | |
| Height; Weight; Gender identity; Ethnicity | |
| PRAPARE [39] | |
| Reproductive health | |
| Religion | |
| Menstrual period; Birth control; Pregnancy | |
| Pregnancy discrimination | |
| HIV Risk Behavior Knowledge | |
| HIV Transmission Knowledge [40] | |
| Self-efficacy for Safe Sex | |
| Sexual Self-Efficacy Scale [41] | |
| STI | |
| DoxyPep | |
| Reproductive Autonomy | |
| Reproductive Autonomy Scale [42] | |
| Pregnancy Coercion/Birth Control Sabotage | |
| Reproductive Coercion Scale [43] | |
| BioIndividual Level Abortion Stigma Scale | |
| Abortion stigma scale [44] | |
| Substance/Drug/Alcohol Use | |
| TAPS Tool [45] | |
| AUDIT-C [46] | |
| Partner Characteristics and Risk Behaviors | |
| Brief AIDS Risk Behavioral Assessment (ARBA) [47] | |
| PrEP Routing | |
| Current PrEP Use | |
| PrEP Stigma | |
| Intimate Partner Violence | |
| Composite Abuse Scale Short Form (CASR_SF) [48] | |
| Depression | |
| Center for Epidemiologic Studies Depression Scale Revised (CESD-R-10) [49] | |
| Anxiety | |
| General Anxiety Disorder-7 (GAD-7) [50] | |
| Stressful Life Experiences [51] | |
| Health History [52] | |
| Douching | |
| Discrimination [53] | |
| Traumatic Life Events [54] | |
| Adverse Childhood Experience [55] | |
| Review of Systems | |
| Social Support | |
| Health Literacy | |
| Newest Vital Sign [56] |
HIV testing
At baseline visits and follow-up timepoints, participants will receive a box (in plain unmarked packaging) containing self-collection kits (affixed with unique, matching barcoded stickers to enable specimen identification upon return) and written instructions with color images. Participants will be given the option to have a direct signature required on shipped materials. They will also receive a link to video instructions via email or text message. Participants will be instructed to package their specimens in a biohazard bag and prepaid envelope and return them directly to the study lab at Emory University in Atlanta, Georgia. Finger-stick blood samples will be screened for HIV. If positive, the lab will perform an additional Asante Recency test to determine when the infection may have occurred. Participants who are unable to give sufficient blood samples for HIV and syphilis testing will be offered a re-sampling Zoom visit with our team. Participants who are scheduled for re-sampling may use Tasso + devices to collect blood. The Tasso + is a blood lancet that collects whole liquid blood samples. Participants who test HIV-positive will be referred to care. Linkage to care strategies will include a variety of modalities. Active approaches will involve one-on-one follow-up phone calls by trained study staff who will provide referral information for ongoing HIV treatment in the participant’s area of residence. Participants who test HIV negative will be sent a secure email through REDCap with their negative test results.
Sample self-collection for STI testing
We will follow the same procedure for baseline visits and follow-up visits for STI testing. Participants will be given time to perform the self-collections during baseline visits, remaining on a secure video call but turning video and camera off. Study staff will be available to answer questions and provide instructions for returning specimens to the lab.
PrEP use
Participants who self-report current PrEP use at any study visit will be asked to collect an urine sample that will be tested using a lateral flow tenofovir immunoassay developed by the University of California, San Francisco (UCSF) Analytical Laboratory, in collaboration with Abbott Rapid Diagnostics [57]. The assay has been validated against the gold standard of LC–MS/MS to accurately measure tenofovir uptake [58].
Network study procedures
A subsample of 200 women will be enrolled into the network sub-study. We will create a network sampling frame by stratifying cohort participants to ensure representation by jurisdictional HIV hotspots (defined as ZIP codes with HIV prevalence > 5%) and behavioral HIV risk mode (85% heterosexual vs. 15% PWID). All potential participants for the network interview will be electronically screened through REDCap for eligibility using a standardized screening instrument; if eligible and willing to participate, they will provide e-consent in the same manner described for the main cohort. Selected participants will complete a 1 hour, interviewer-administered virtual network survey, deployed and managed on Network Canvas [59, 60], a user-friendly, interactive software suite designed to facilitate complex data collection. Participants will be asked to name people in their social, sexual, and drug use networks (name generators), describe the demographics and behaviors of these (male and female) network members (name interpreters), and indicate how each of these network members are connected (sociogram).
Cohort members who complete the network survey (i.e., index respondents) will be given five coupons linked to their cohort study ID and asked to refer their sexual and/or drug use network members to participate. Referred participants must be linked to an index participant by presenting a coupon or knowing the participant’s name and be either a sexual or drug use connection. A cohort participant’s study data will be used as attributes in the social network analysis; network members who are recruited into the network survey will complete an attribute survey in addition to the relational social network survey. The attribute survey will contain measures of demographics and sexual and drug use behaviors. Each index participant can refer up to 5 referrals. Referred participants will also complete STI testing with the staff member on a Zoom call. Female participants will provide blood samples to be tested for HIV and syphilis, vaginal swab samples to be tested for chlamydia, gonorrhea, and trichomoniasis, and rectal swab samples to be tested for chlamydia and gonorrhea. Male participants will provide blood samples to be tested for HIV and syphilis, rectal swabs to be tested for chlamydia and gonorrhea, and urine and urogenital samples to be tested for chlamydia, gonorrhea, and trichomoniasis.
Follow-up visits
Participants are sent a REDCap form to update their address every 6 months. Participants are also sent a survey with a battery of questions listed in Table 1. After completing the address form, study staff mail a package to each participant which contains a self-sampling kit and a return pre-paid package which is sent to the laboratory at Emory University. Follow-up survey and testing is completed every 6 months until the 24-month timepoint.
Laboratory procedures
Blood samples are tested using the OraQuick Advance HIV-1/2 Rapid Antibody Test for the presence of HIV 1 and 2 antibodies. Utilizing a proprietary lateral flow immunoassay procedure, this test allows for rapid diagnosis. Samples are reported as Non-Reactive or Reactive. Rectal and vaginal swabs are tested using the Abbott Real Time CT/NG assay, which is an FDA cleared real-time polymerase chain reaction (PCR) assay for the direct, qualitative detection of a region of the cryptic plasmid DNA of Chlamydia trachomatis (CT) and a region of the Opa gene of Neisseria gonorrhoeae (NG). The CT/NG assay is used for dual detection of C. trachomatis and N. gonorrhoeae. CT: Sensitivity 95.2% Specificity 99.3%; NG: Sensitivity 97.5% Specificity 99.7% Samples are reported as Negative or Positive. Vaginal samples are tested for the presence of Trichomonas vaginalis using Taq-Man PCR. The limit of detection for the TV assay is < 0.2 organisms per reaction or 40 copies per mL. The sensitivity/specificity of the TV assay is 100% and 99.6%. The product is detected with an internal probe which fluoresces upon cleavage by exonuclease activity of Taq polymerase. Samples are reported as Negative or Positive. The traditional diagnostic algorithm for syphilis testing is used. A screening test for syphilis serology is completed with the ASI RPR (rapid plasma reagin) Card Test—a qualitative and semiquantitative nontreponemal flocculation test for the detection of reagin antibodies in human serum and plasma. The result of this antigen–antibody reaction is macroscopic flocculation. Samples are reported as Non-Reactive or Reactive plus a titer. Reactive samples with enough volume are confirmed with a T pallidum IgG/IgM EIA immunoassay, which is performed at Emory Medical Laboratories. All test results are provided to participants within a period of 7 days of receipt by the laboratory.
Data management and monitoring
All study data will be stored in password-protected computers or file cabinets in locked offices. All research team members are completing the protection of human subjects and HIPAA research exams and sign a protocol-specific conflict of interest. Risks will be minimized by not including personal identifying information on the forms, when possible, and by conducting collection of personal information in a private setting. All data will be collected using unique patient identification codes. All laboratory specimens, evaluation forms, reports, and other records will be identified by a coded number to maintain participant confidentiality. Study data will be collected and managed using REDCap, a secure web application designed to support data capture for research studies, providing user-friendly, web-based case report forms, real-time data entry validation (e.g., for data types and range checks), audit trails, and deidentified data export mechanisms to common statistical packages (SPSS, SAS, Stata, R/SPlus). Study data will be collected and managed using REDCap. All study data will be harmonized into a single database. Individual self-report and biomedical cohort data will be accessed via REDCap and network data will be accessed via Network Canvas. Big Data variables will be merged with individual participant residential location using Geographic Information System (GIS) technology on a local secure desktop (i.e., not cloud-based). The residential address of each participant will be geocoded using Esri ArcPro and Street Map Premium. The geocoding process transforms an address to its corresponding point location on the earth’s surface. Using GIS the county and census tract boundaries that the geocoded point lies within are identified and a unique geo-identifier for each administrative boundary is added to the individual point locations as an attribute. To maintain confidentiality and participant privacy, the geocoded residential location of each participant will only be used for the purpose of aggregating the number of participants to a larger geography which anonymizes the locations within the county and census tract. Individual participants will not be mapped for manuscript or presentation display.
Cohort statistical analysis
Descriptive characteristics of all participants will be estimated using means (standard deviations) and frequencies (percentages). We will define HIV and STI incidence as the presence of a positive test after a previous negative test. Overall HIV and STI prevalence will be defined as having a positive test during the study period. STI prevalence will be examined in two ways. First, overall STI prevalence will be estimated using the entire follow-up period for each participant and indicated by any positive test at any time. Raw frequencies and percentages and 95% confidence intervals will be calculated. To examine relationships with other factors, specifically race/ethnicity, age, and region, Modified Poisson regression models will be used to estimate risk ratios adjusting for potential confounders (e.g., marital status) for STI prevalence. Additionally, we will model each STI separately. Lastly, we will count the number of STIs during follow up and will model that count using Negative Binomial regression with an offset of person-time as described previously for both overall STIs and by each STI separately. We will examine STI incidence longitudinally using generalized linear mixed models allowing us to examine factors related to STI incidence at each follow-up over time. PrEP use will also be modeled longitudinally with generalized linear mixed models to identify factors associated with PrEP initiation as well as time trends for PrEP use. For all models described above, we will examine individual survey items and contextual data separately and jointly. As some measures are likely correlated, e.g., poverty and unemployment rates, we will investigate multicollinearity and remove any factor that has a variance inflation factor greater than 10 from multiple models with the assumption that we examine each factor unadjusted. While the above models will permit examination of associations with HIV risk outcomes, we will also test predictive models for each outcome using extreme gradient boosting, a type of applied machine learning [61]. This method has become more prominent than other predictive modeling techniques due to computation speed and model performance [62] and has been used in predicting other HIV related factors [63, 64]. Additionally, it allows for integration of multiple imputations to maximize the number of participants included. 5-fold cross-validation will be employed to assess model performance. Feature importance, i.e., factor importance, will be used to identify which variables, such as age and race/ethnicity, were most useful in the modeling process.
Network data analysis
Once data collection has been completed at each timepoint, we will attempt to create a macro network by merging individuals who appear across multiple egocentric networks [65]. We will use bivariate analyses to assess demographic associations (i.e., region, age, and risk mode) with network characteristics including density, homophily (similarity), multiplexity (overlap in sexual and social networks), and tie strength. Both individual- and network- level factors will be included in an exponential random graph model to test whether any of these variables are associated with the presence of more connections than would be expected by chance. Summary network variables will be incorporated into the knowledgebase for multi-level analysis.
Protection of human subjects
All participants are informed of the risks of participation, including and not limited to lost packages and mis-delivery of packages. All participants must provide e-consent to participate. A waiver of parental consent has been granted by the IRB since participants 14 years of age and older are considered adults regarding HIV and STI testing. All participants are compensated for their time to participate in the study activities; $50 for the baseline study visit, $60 for the 6- month visit; $75 for the 12-month visit (including completing the survey, HIV test, and STI self-collection); $90 at the 18-month visit and $100 at the 24-month visit. Women who complete urine testing for PrEP receive an additional $40. The study protocol and its amendments, informed consent forms, and recruitment materials were approved by the Columbia University Institutional Board under protocol # AAAU2650. All participants provided written informed e-consent for study screening and participation, and HIV and STI testing. Positive test results are reported to local and state level health departments based on the requirements of each municipality of where the participant resides.
Discussion
AWARE aims to build a knowledge base of integrated data, including data from an epidemiologic cohort of women, disease surveillance, social determinants of health, and network data. This cohort study will use innovative electronic methods (e.g., social media with community-informed advertisements) to recruit and retain a large (N = 1,800), diverse national sample of women who meet inclusion criteria or women that are or could be disproportionately impacted by HIV who are 14 years of age and older to better understand the correlates of HIV-related sexual risk and HIV incidence within the context of a theoretically-grounded social-ecological framework.
Establishing and maintaining a cohort digitally requires minimal engagement with study participants. This low-interaction strategy provides researchers opportunities to describe trends in STI and HIV infections, while minimizing participant burden (sampling bias) [66] that has been seen in other cohort studies. This approach also has the advantage of identifying women across the US without relying on discrete recruitment sites. Further, in prior studies, digital approaches have facilitated recruitment into research studies irrespective of geographic location, improving the inclusion of people in rural and underserved areas, as well as marginalized individuals who experience substantial stigma and discrimination. The proposed study is therefore well positioned to help identify factors related to HIV risk among women who are often hard-to-reach [67–75].
Previous cohort studies enrolling women vulnerable to HIV infection and assessing behavioral risk factors associated with HIV risk include the Women’s HIV SeroIncidence Study (i.e., HIV Prevention Trials Network (HPTN) study 064) which enrolled women from geographic areas with high rates of poverty and HIV prevalence to understand behaviors associated with the risk of HIV [11]. Enrollees reported a range of individual and partner-level sexual and drug use risk behaviors. Researchers found HIV incidence rates that were substantially higher than the 2009 U.S. national general population estimate from the CDC among Black women of similar age (0.05%) [76] that were comparable to the adult HIV incidence rates of sub-Saharan African countries at that time [77]. However, no specific individual-level sexual behavior was predictive of increased risk for HIV acquisition, supporting the need for further investigation of factors predicting HIV risk among women. In a study focused on HIV risk among Black women living in lower-income communities, socioeconomic factors (e.g., homelessness and receipt of Medicaid), older age (> 35 years old), and sex partner characteristics, rather than sexual behavior, were associated with HIV acquisition [12]. Findings from these studies highlight the lack of a direct correlation between individual-level behavior and HIV incidence and support the need to better understand sexual networks and community characteristics, which so far have been understudied in women vulnerable to HIV. Furthermore, despite two decades of research on social network characteristics and interventions among populations at high risk for HIV acquisition, there is a dearth of research among populations disproportionately impacted by HIV.
Our study has several limitations. The anticipated challenges inherent in studying at-risk populations include high participant attrition and low enrollment, as well as high rates of loss to follow-up. To address these challenges, we have built a robust protocol that minimizes attrition through comprehensive collection of information on how to locate and contact participants, active tracking and engagement of participants between appointments, and graduated incentives to encourage retention. Knowledge gained through this study will inform strategies that are most effective in retaining women for HIV risk studies.
Acknowledgements
Not applicable.
Abbreviations
- AWARE
American Women: Assessing Risk Epidemiologically Cohort Study
- HIV
Human immunodeficiency virus
- U.S.
United States
- STI
Sexually Transmitted Infection
- YMSM
Young men who have sex with men
- PWID
People who inject drugs
- CBOs
Community-Based Organizations
- QR codes
Quick-response code
- PrEP
Pre-exposure prophylaxis
- UCSF
University of California, San Francisco
- LC–MS/MS
Liquid Chromatography with tandem mass spectrometry
- CT
Chlamydia trachomatis
- NG
Neisseria gonorrhoeae
- FDA
U.S. Food and Drug Administration
- PCR
Polymerase chain reaction
- DNA
Deoxyribonucleic acid
- RPR
Rapid plasma reagin
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- EIA
Enzyme immunoassay
- HIPAA
Health Insurance Portability and Accountability Act
- GIS
Geographic Information System
- CDC
Centers for Disease Control and Prevention
Authors’ contributions
RS, MCK, GP, JD, and AKJ conceptualized and designed the study. RS, DML, AFN wrote the study protocol. RS drafted the manuscript. RS, MCK, GP, JD, GW, DML, RK, TLH, JL, AFN, JLC, AKJ authors read and approved the final version of the manuscript for publication.
Funding
Research reported in this publication is supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institute of Health under award number R01 AI172469. This work was supported in part by the Emory University Center for AIDS Research (P30AI050409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study protocol and its amendments, informed consent forms, and recruitment materials were approved by the Columbia University Institutional Board under protocol # AAAU2650. Written informed consent will be obtained from study participants for study screening and participation, and HIV and STI testing. Positive test results are reported to local and State level health departments based on the requirements of each municipality of where the participant resides.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Linley L, Johnson AS, Song R, Wu B, Hu S, Gant Z, et al. Estimated HIV incidence and prevalence in the United States, 2014–2018. 2020.
- 2.Kalichman SC. HIV transmission risk behaviors of men and women living with HIV-AIDS: Prevalence, predictors, and emerging clinical interventions. Clin Psychol Sci Pract. 2000;7(1):32–47. [Google Scholar]
- 3.McCree DH, Sutton M, Bradley E, Harris N. Changes in the disparity of HIV diagnosis rates among black women - United States, 2010–2014. MMWR Morb Mortal Wkly Rep. 2017;66(4):104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulkind J, Mbonye M, Watts C, Seeley J. The social context of gender-based violence, alcohol use and HIV risk among women involved in high-risk sexual behaviour and their intimate partners in Kampala, Uganda. Cult Health Sex. 2016;18(7):770–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaimie P, Meyer SAS, Frederick L. Altice. Substance abuse, violence, and HIV in Women: a literature review of the syndemic. J Womens Health. 2011;20(7):991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne FA, Wechsberg WM. The intersecting risks of substance use and HIV risk among substance-using South African men and women. Curr Opin Psychiatry. 2010;23(3):205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla N, Sarkar S. Defining, “high-risk sexual behavior” in the context of substance use. J Psychosex Health. 2019;1(1):26–31. [Google Scholar]
- 8.Duby Z, Jonas K, McClinton Appollis T, Maruping K, Vanleeuw L, Kuo C, et al. From survival to glamour: motivations for engaging in transactional sex and relationships among adolescent girls and young women in South Africa. AIDS Behav. 2021;25(10):3238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranganathan M, Kilburn K, Stoner MCD, Hughes JP, MacPhail C, Gomez-Olive FX, et al. The mediating role of partner selection in the association between transactional sex and HIV incidence among young women. J Acquir Immune Defic Syndr. 2020;83(2):103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Transactional sex among women in Soweto, South Africa: prevalence, risk factors and association with HIV infection. Soc Sci Med. 2004;59(8):1581–92. [DOI] [PubMed] [Google Scholar]
- 11.Hodder SL, Justman J, Hughes JP, Wang J, Haley DF, Adimora AA, et al. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med. 2013;158(1):10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivy W 3rd, Miles I, Le B, Paz-Bailey G. Correlates of HIV infection among African American women from 20 cities in the United States. AIDS Behav. 2014;18 Suppl 3(Suppl 3):266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crime UNOoDa. Women who inject drugs and HIV: addressing specific needs. 2014.
- 14.El-Bassel N, Strathdee SA. Women who use or inject drugs: an action agenda for women-specific, multilevel, and combination HIV prevention and research. J Acquir Immune Defic Syndr. 2015;69 Suppl 2(Suppl 2):S182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strathdee SA, West BS, Reed E, Moazen B, Azim T, Dolan K. Substance use and HIV among female sex workers and female prisoners: risk environments and implications for prevention, treatment, and policies. J Acquir Immune Defic Syndr. 2015;69 Suppl 2(01):S110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinstead O, Comfort M, McCartney K, Koester K, Neilands T. Bringing it home: design and implementation of an HIV/STD intervention for women visiting incarcerated men. AIDS Educ Prev. 2008;20(4):285–300. [DOI] [PubMed] [Google Scholar]
- 17.Adams JW, Lurie MN, King MRF, Brady KA, Galea S, Friedman SR, et al. Decreasing HIV transmissions to African American women through interventions for men living with HIV post-incarceration: an agent-based modeling study. PLoS One. 2019;14(7):e0219361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GrinsteadReznick O, Comfort M, McCartney K, Neilands TB. Effectiveness of an HIV prevention program for women visiting their incarcerated partners: the HOME project. AIDS Behav. 2011;15(2):365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buot ML, Docena JP, Ratemo BK, Bittner MJ, Burlew JT, Nuritdinov AR, et al. Beyond race and place: distal sociological determinants of HIV disparities. PLoS One. 2014;9(4):e91711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechsberg WM, Luseno W, Riehman K, Karg R, Browne F, Parry C. Substance use and sexual risk within the context of gender inequality in South Africa. Subst Use Misuse. 2008;43(8–9):1186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parry C, Petersen P, Carney T, Dewing S, Needle R. Rapid assessment of drug use and sexual HIV risk patterns among vulnerable drug-using populations in Cape Town, Durban and Pretoria, South Africa. SAHARA J. 2008;5(3):113–9. [DOI] [PubMed] [Google Scholar]
- 22.Loue S, Sajatovic M, Mendez N. Substance use and HIV risk in a sample of severely mentally Ill Puerto Rican women. J Immigr Minor Health. 2011;13(4):681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iversen J, Page K, Madden A, Maher L. HIV, HCV, and health-related harms among women who inject drugs: implications for prevention and treatment. J Acquir Immune Defic Syndr. 2015;69 Suppl 2(0 1):S176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sopheab H, Chhea C, Tuot S, Muir JA. HIV prevalence, related risk behaviors, and correlates of HIV infection among people who use drugs in Cambodia. BMC Infect Dis. 2018;18(1):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(CDC) CfDCaP. HIV and women: prevention challenges. 2021.
- 26.Frew PM, Parker K, Vo L, Haley D, O’Leary A, Diallo DD, et al. Socioecological factors influencing women’s HIV risk in the United States: qualitative findings from the women’s HIV SeroIncidence study (HPTN 064). BMC Public Health. 2016;16(1):803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojikutu BO, Mayer KH. Hidden in plain sight: identifying women living in the United States who could benefit from HIV preexposure prophylaxis. J Infect Dis. 2020;222(9):1428–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RR, Crane JS, López J, Chan PA, Liu AY, Tooba R, et al. Pre-exposure prophylaxis for HIV prevention preferences among young adult African American men who have sex with men. PLoS One. 2018;13(12):e0209484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reback CJ, Clark K, Fletcher JB, Holloway IW. A multilevel analysis of social network characteristics and technology use on HIV risk and protective behaviors among transgender women. AIDS Behav. 2019;23(5):1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walters SM, Reilly KH, Neaigus A, Braunstein S. Awareness of pre-exposure prophylaxis (PrEP) among women who inject drugs in NYC: the importance of networks and syringe exchange programs for HIV prevention. Harm Reduct J. 2017;14(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risher K, Mayer K, Beyrer C. The HIV treatment cascade in men who have sex with men, people who inject drugs and sex workers. Curr Opin HIV AIDS. 2015;10(6):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neblett RC, Davey-Rothwell M, Chander G, Latkin CA. Social network characteristics and HIV sexual risk behavior among urban African American women. J Urban Health. 2011;88(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shushtari ZJ, Hosseini SA, Sajjadi H, Salimi Y, Latkin C, Snijders TAB. Social network and HIV risk behaviors in female sex workers: a systematic review. BMC Public Health. 2018;18(1):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyer TV, Khan MR, Sandoval M, Acheampong A, Regan R, Bolyard M, et al. Drug use and sexual HIV transmission risk among Men Who have Sex with Men and Women (MSMW), Men Who have Sex with Men only (MSMO), and Men Who have Sex with Women Only (MSWO) and the female partners of MSMW and MSWO: a network perspective. AIDS Behav. 2017;21(12):3590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Z, He N, Duan S, Jiang Q, Ye R, Pu Y, et al. HIV infection, sexual behaviors, sexual networks, and drug use among rural residents in Yunnan province, China. AIDS Behav. 2011;15(5):1017–25. [DOI] [PubMed] [Google Scholar]
- 36.Doherty IA, Schoenbach VJ, Adimora AA. Sexual mixing patterns and heterosexual HIV transmission among African Americans in the southeastern United States. J Acquir Immune Defic Syndr. 2009;52(1):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson KM, Alarcón J, Watts DM, Rodriguez C, Velasquez C, Sanchez J, et al. Sexual networks of pregnant women with and without HIV infection. AIDS. 2003;17(4):605–12. [DOI] [PubMed] [Google Scholar]
- 38.Aaron E, Blum C, Seidman D, Hoyt MJ, Simone J, Sullivan M, et al. Optimizing delivery of HIV preexposure prophylaxis for women in the United States. AIDS Patient Care STDS. 2018;32(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Protocol for Responding to and ASessing Patients’ Assets R, and Experiences. PRAPARE: Protocal for REsponding to and assessing patient assets, risks, and experiences. In: PRAPARE-English, editor. 2016.
- 40.Psaros C, Goodman GR, McDonald VW, Ott C, Blyler A, Rivas A, et al. Protocol for WeExPAnd: a prospective, mixed-methods pilot demonstration study to increase access to pre-exposure prophylaxis among women vulnerable to HIV infection in the Southern USA. BMJ Open. 2023;13(6):e075250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assarzadeh R, Bostani Khalesi Z, Jafarzadeh-Kenarsari F. Sexual self-efficacy and associated factors: a review. Shiraz E-Medical Journal. 2019;In Press.
- 42.Upadhyay UD, Dworkin SL, Weitz TA, Foster DG. Development and validation of a reproductive autonomy scale. Stud Fam Plann. 2014;45(1):19–41. [DOI] [PubMed] [Google Scholar]
- 43.McCauley HL, Silverman JG, Jones KA, Tancredi DJ, Decker MR, McCormick MC, et al. Psychometric properties and refinement of the reproductive coercion scale. Contraception. 2017;95(3):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cockrill K, Upadhyay UD, Turan J, Greene FD. The stigma of having an abortion: development of a scale and characteristics of women experiencing abortion stigma. Perspect Sex Reprod Health. 2013;45(2):79–88. [DOI] [PubMed] [Google Scholar]
- 45.McNeely J, Wu LT, Subramaniam G, Sharma G, Cathers LA, Svikis D, et al. Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) tool for substance use screening in primary care patients. Ann Intern Med. 2016;165(10):690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 47.Donenberg GR, Emerson E, Bryant FB, Wilson H, Weber-Shifrin E. Understanding AIDS-risk behavior among adolescents in psychiatric care: links to psychopathology and peer relationships. J Am Acad Child Adolesc Psychiatry. 2001;40(6):642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford-Gilboe M, Wathen CN, Varcoe C, MacMillan HL, Scott-Storey K, Mantler T, et al. Development of a brief measure of intimate partner violence experiences: the Composite Abuse Scale (Revised)-Short Form (CASR-SF). BMJ Open. 2016;6(12):e012824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 50.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 51.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the life experiences survey. J Consult Clin Psychol. 1978;46(5):932–46. [DOI] [PubMed] [Google Scholar]
- 52.Health Information National Trends Survey: National Cancer Institute. HINTS 5 Cycle 2 (2018) hints.cancer.gov: NIH; 2018. Available from: https://hints.cancer.gov/view-questions/question-detail.aspx?PK_Cycle=11&qid=1073.
- 53.Harvard University. Everyday Discrimination Scale scholar.harvard.edu: Harvard; 2017. Available from: https://scholar.harvard.edu/davidrwilliams/node/32397.
- 54.PTSD: National Center for PTSD. Stressful Life Events Screening Questionnaire (SLESQ): U.S. Department of Veterans Affairs; 2018. Available from: https://www.ptsd.va.gov/professional/assessment/te-measures/stress-life-events.asp#obtain.
- 55.Murphy A, Steele H, Steele M, Allman B, Kastner T, Dube SR. The Clinical Adverse Childhood Experiences (ACEs) Questionnaire: Implications for trauma-informed behavioral healthcare. Integrated early childhood behavioral health in primary care: A guide to implementation and evaluation. Cham: Springer International Publishing/Springer Nature; 2016. p. 7–16. [Google Scholar]
- 56.Pzifer. Newest Vital Sign (NVS) Pzifer: Pfizer; 2021. Available from: https://cdn.pfizer.com/pfizercom/health/nvs_flipbook_english_final.pdf.
- 57.Gandhi M, Glidden DV, Chakravarty D, Wang G, Biwott C, Mogere P, et al. Impact of a point-of-care urine tenofovir assay on adherence to HIV pre-exposure prophylaxis among women in Kenya: a randomised pilot trial. Lancet HIV. 2024;11(8):e522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gandhi M, Bacchetti P, Spinelli MA, Okochi H, Baeten JM, Siriprakaisil O, et al. Brief report: validation of a urine tenofovir immunoassay for adherence monitoring to PrEP and ART and establishing the cutoff for a point-of-care Test. J Acquir Immune Defic Syndr. 2019;81(1):72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janulis P, Phillips G 2nd, Melville J, Hogan B, Banner K, Mustanski B, et al. Network canvas: an open-source tool for capturing social and contact network data. Int J Epidemiol. 2023;52(4):1286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birkett M, Melville J, Janulis P, Phillips G 2nd, Contractor N, Hogan B. Network Canvas: key decisions in the design of an interviewer assisted network data collection software suite. Soc Networks. 2021;66:114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen T, Guestrin C, editors. Xgboost: A scalable tree boosting system. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining; 2016.
- 62.Chen X, Wang ZX, Pan XM. HIV-1 tropism prediction by the XGboost and HMM methods. Sci Rep. 2019;9(1):9997. Epub 2019/07/12. 10.1038/s41598-019-46420-4. PubMed [DOI] [PMC free article] [PubMed]
- 63.Mutai CK, McSharry PE, Ngaruye I, Musabanganji E. Use of machine learning techniques to identify HIV predictors for screening in sub-Saharan Africa. BMC Med Res Methodol. 2021;21(1):159. Epub 2021/08/02. 10.1186/s12874-021-01346-2. PubMed [DOI] [PMC free article] [PubMed]
- 64.Orel E, Esra R, Estill J, Marchand-Maillet S, Merzouki A, Keiser O. Machine learning to identify socio-behavioural predictors of HIV positivity in East and Southern Africa. medRxiv. 2020:2020.01.27.20018242. 10.1101/2020.01.27.20018242.
- 65.Phillips Ii G, Rodriguez-Ortiz AE, Adewumi OM, Banner K, Adetunji A, Awolude OA, Olayinka OA, Simons LM, Hultquist JF, Ozer EA, Kapogiannis B, Kuhns LM, Garofalo R, Taiwo B, Birkett M, Lorenzo-Redondo R. Social/sexual networks of people newly diagnosed with HIV in Ibadan, Nigeria. AIDS Behav. 2024;28(1):300-9. 10.1007/s10461-023-04200-2. [DOI] [PMC free article] [PubMed]
- 66.Cochran WG. Sampling techniques. Hoboken: Wiley; 2007.
- 67.Sullivan P, Jones J, Kishore N, Stephenson R. The roles of technology in primary HIV prevention for men who have sex with men. Curr HIV/AIDS Rep. 2015;12(4):481–8. [DOI] [PubMed] [Google Scholar]
- 68.Kirk GD, Himelhoch SS, Westergaard RP, Beckwith CG. Using mobile health technology to improve HIV care for persons living with HIV and substance abuse. AIDS Res Treat. 2013;2013:194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.CTIA-The Wireless Association. Wirless Quick Facts 2014. Available from: http://www.ctia.org/your-wireless-life/how-wireless-works/wireless-quick-facts.
- 70.Center PR. Internet project survey. 2014.
- 71.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32(1):56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guse K, Levine D, Martins S, Lira A, Gaarde J, Westmorland W, et al. Interventions using new digital media to improve adolescent sexual health: a systematic review. J Adolesc Health. 2012;51(6):535–43. [DOI] [PubMed] [Google Scholar]
- 73.Bauermeister JA, Golinkoff JM, Muessig KE, Horvath KJ, Hightow-Weidman LB. Addressing engagement in technology-based behavioural HIV interventions through paradata metrics. Curr Opin HIV AIDS. 2017;12(5):442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schnall R, Travers J, Rojas M, Carballo-Diéguez A. eHealth interventions for HIV prevention in high-risk men who have sex with men: a systematic review. J Med Internet Res. 2014;16(5):e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bull S, Pratte K, Whitesell N, Rietmeijer C, McFarlane M. Effects of an Internet-based intervention for HIV prevention: the Youthnet trials. AIDS Behav. 2009;13(3):474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.(UNAIDS) JUNPoHA. Global Report: UNAIDS Report on the GLobal AIDS Epidemic 2010. 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.