Abstract
Background
There are a myriad of ways patient partners can enact their roles on research teams. International guidelines emphasize the need for a collaborative approach to determining these roles to try to improve research impact and positive patient partner experience. The aims of this review were to: (1) describe how patient partners’ roles as co-researchers in health research are determined; and (2) identify factors that influence how these decisions are made.
Methods
A scoping review was conducted. Four databases were searched plus citation searching occurred. Descriptions of English language studies of any design and commentaries of studies that report on patient partners’ or researchers’ reflections on their decision-making processes for engagement were included. Two reviewers completed screening and data extraction, with a third to resolve disagreements. Results were summarized and then content analysis was undertaken to synthesize the findings. Two patient partners contributed to the protocol development, screening, data interpretation, and manuscript writing at varying times during the process.
Results
A total of 45 papers (25 commentaries, 19 studies and 1 both a study and commentary) were included in this review. Most papers were from the United Kingdom (n = 15) and Canada (n = 12). Most patient partners had experiences related to chronic conditions rather than acute or time-limited illnesses. The synthesis yielded two categories. The first category, the research and research team attributes shape patient partner roles, encompassed patient partner, researcher and activity related factors that influenced patient partner engagement in activities. The second category, shared and ongoing decision-making, described the decision-making process to determine patient partner engagement, timing of these decisions, and tools to support these decisions.
Conclusion
A dynamic, systematic and shared decision-making approach to determining patient partners’ roles in the research process has the potential to support meaningful engagement and maximize benefits. Because the research process may evolve over time and patient partners situations can change, there may be a need to renegotiate the patient partner’s role.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40900-024-00664-1.
Keywords: Decision making, Patient engagement, Patient participation, Research methods, Review, Stakeholder participation
Plain language summary
Patient partners can undertake various roles in the research process. International guidelines recommend patient partners and researchers work together to decide how patient partners will be involved in the research and there are many activities patient partners can do to enact their role. This review describes how these decisions are made and what shapes them. We reviewed 45 English-language research studies and commentaries on the views of patient partners and researchers that described patient partners’ and researchers’ approaches to determining patient partners’ roles in the research process. Most of these studies were from the United Kingdom and Canada, with patient partners generally having chronic illness experience. We found that patient partner roles evolved throughout the study, with many factors affecting this process. Determining the patient partner role was dynamic, with reflection, discussion and negotiation occurring throughout the research process. The review suggests the need for both patient partners and researchers to together make decisions about their respective roles.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40900-024-00664-1.
Introduction
Engaging patients and the public as co-researchers can reduce research waste, increase research value, and improve health outcomes and systems [1, 2]. ‘Patient and public engagement’ is known by many terms including ‘patient and public involvement’ and ‘consumer and community involvement’ and will be referred to as ‘engagement’ in this manuscript [3]. It is characterized by carrying out research ‘with’ or ‘by’ patients and the public [4], where they contribute to study activities at discrete points during the research, or from study conception through to dissemination [3]. Consistent with several international organizations [5, 6] the term ‘patient partner’ is used to describe patients, their caregivers, and members of the public [7], who partner in the research process, with shared power and responsibility for decisions about research activities [2, 8].
Engagement, the deliberate interactions between patient partners and researchers, is often researcher-led. For example, in a study of Patient Centered Outcomes Research Institute (PCORI) funded projects, only 6% engaged patients as leaders, where it is the patient who initiates, designs, and undertakes the research process, while in other projects more superficial and tick-box approaches were used [9]. Ultimately, the benefits of engagement in research may not be realized if patients are not legitimate partners in the process. General guidance for making decisions about engagement including a focus on joint decision-making are available [10, 11], but an understanding of how these broad principles are operationalized into specific roles and activities is lacking [12].
Determining patient partners’ role in research projects is challenging because there are a multitude of activities they can become involved in [13]. For example, during the research planning phase, patient partners may be involved in determining the research focus, the methods such as how participants are recruited, the development of surveys and interview guides and how and what data is collected [14, 15]. During execution phase, they may assist with recruitment, data collection and analysis [14, 15]. During the dissemination process patient partners may help with interpretation of the findings, writing of the paper and plain language statements and presenting results at conferences and other events [15, 16]. Their level of engagement in these activities can range from passive to more engaged roles [17]. If power dynamics between the patient partner and researcher are not considered, it is likely the researchers will shape and control the research and the patient partners’ role in it [18]. Without transparent discussions between patient partners and researchers, each may become dissatisfied, either wanting more or less engagement. This can lead to patient partners resigning from the research study [19], and might leave researchers unconvinced of the appropriateness and value of engagement [20]. Given the emerging evidence that engagement can enhance outcomes by making research more rigorous, ethical, and understandable [1, 21], there is an imperative to unveil how best to engage patient partners in discrete research activities. Therefore, the aims of this review were to: (1) describe how patient partners’ roles as co-researchers in health research are determined; and (2) identify factors that influence how these decisions are made. By providing a clear and transparent description of this process, our review may act as a guide for others in their planning and enacting the patient partner role in health research and ultimately improve practice resulting in more beneficial research.
Methods
Design
We conducted a scoping review, reflecting the seminal methodological guidance by Arksey and O’Malley [22] and updated guidance by Peters et al. [23]. Scoping reviews, which can incorporate stakeholder engagement [24], are used to broadly map the breadth of the evidence. They are systematic and include a wide range of study designs but do not generally assess the quality of these studies [22–24]. The review protocol was registered a priori on Figshare (https://figshare.com/articles/preprint/MAKING_DECISIONS_FOR_PATIENT_ENGAGEMENT_IN_HEALTH_RESEARCH_A_SCOPING_REVIEW_PROTOCOL/21569025). The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist was used to guide reporting [25]. This scoping review had six stages.
Stage 1: Identification of research questions
We used the Population, Phenomenon of Interest, and Context (PICo) framework to develop the research questions. This review focused on patient partner and researcher perspectives (P) on decision making processes and factors influencing this (I) in health research projects (Co). We defined health research as being studies where the goal was to improve health treatment, practices and outcomes across any discipline. As per the Canadian Institutes of Health Research, the following types of health research were included: biomedical, clinical, and health services research were included but social, cultural, environmental, and population research studies were excluded to keep the review manageable [6]. Commentaries focused on reporting health research with patient partners were also included.
Thus, the research questions guiding this scoping review were:
How are decisions made about roles and activities patient partners undertake in health research?
What factors influence decisions about roles and activities patient partners undertake in health research?
Stage 2: Identification of relevant studies
Four biomedical citation databases, MEDLNE, Embase, CINAHL and Scopus, were searched from 2011 to 2024. An expert health librarian helped devise the search strategy. We used a systematic literature search approach guided by the PICo framework. Examples of terms for ‘population’ included patient, public and consumer to reflect international differences. For ‘phenomenon of interest’ examples included participate, involve, engage and partner. Finally, examples of terms for ‘context’ included health and research. The search strategy is available in Supplementary File 1.
The reference lists of included studies were checked, and the studies were searched in Scopus for additional studies which cited the included studies. One reviewer searched, and the search results were transferred to Covidence where duplicates were removed.
Stage 3: Study selection
Study selection was based on the following inclusion criteria:
Published studies with any type of design, including commentaries of previous empirical health research;
Study reports on patient partner engagement at the International Association for Public Participation (IAP2) Public Participation Spectrum level of involve, collaborate or empower [26];
Study reports data about researchers’ or patient partners’ reflections/perceptions of the decision-making process of how patient partners engaged in research activities OR factors that influenced decisions for how patient partners engaged in research activities;
Studies published in English only, because translation resources were limited and there are limitations such as potential inaccuracies when freely available translation services when technical terms are used.
The exclusion criteria were:
Reviews, quality improvement projects, protocols, conference abstracts and theses;
Studies reporting a list of research activities patient partners were engaged in, without details of the decision-making process or factors influencing decisions;
Studies published before 2011, when the first GRIPP reporting tool was released [27].
Two reviewers used the selection criteria to independently screen titles and abstracts to identify potentially relevant studies. Full texts were retrieved where exclusion could not be determined from the study’s title/abstract. Full texts were screened against the selection criteria by two reviewers. Any discrepancies were resolved through discussion. A third reviewer was available if agreement could not be met through discussion, however, this was not required.
Stage 4: Charting the data
The purpose of the data extraction tool was to provide contextual details about studies. Throughout the review process, types of data that were extracted evolved based on elements that the research team identified as important, which is common in scoping reviews. The final tool included author and year, country, purpose of study, setting, sample, patient partner experience. For commentaries, details about the primary results papers being reflected upon were also extracted including the study design, aim and number of patient partners. One reviewer extracted data, and a second reviewer checked its accuracy. Reviewers met to discuss discrepancies. A third reviewer was available to judge any discrepancies that could not be resolved. As part of quality control, the reviewers met with the project lead for data checks if required.
Stage 5: Collating, summarising and reporting the results
Data extraction was presented in a table. Data in the table were used to develop a descriptive summary and bar graph of the contextual details extracted from studies to highlight patterns across study characteristics (see Findings section).
Next, both inductive and deductive qualitative content analysis was undertaken by the lead researcher to provide a summary of data that addressed each research question [28]. First, a deductive analytic matrix was created in NVivo 11, where two categories were created, one for each research question. Next, the included studies were uploaded in NVivo 11 where line by line coding occurred (i.e. inductive analysis). For example, sentences that addressed the research questions (i.e. information on factors that influence decisions, and how decisions were made) in the methods, findings/results sections, or appendices/supplementary files of studies, were given codes, which described the topic of the sentence. Data from quantitative studies were converted from numbers into written descriptions prior to coding, and these written descriptions were coded [29]. Codes were then placed into the deductive analytic matrix, and later grouped inductively into sub-categories based on similar characteristics. The entire research team scrutinized and confirmed the generated sub-categories. Finally, the relationships between sub-categories and categories were examined. One researcher deliberated over the categories and drew many versions of mind maps until reaching a diagram that made sense (see Findings section). The diagram was scrutinized by the research team.
To recognize that our individual experiences can influence the research, we identified that as researchers we had varying experiences and expertise in involving patient partners in our research. We also acknowledged we were from two countries whose research funding bodies prioritize patient partners’ authentic contribution to the grants and we were motivated to learn how to do this better, meaning we were really engaged in answering our review questions. While we did not explicitly document our individual assumptions and experiences a priori, our approach may have helped our reflexivity. That is, we had a diverse team develop the review protocol and scrutinize the sub-categories, categories and diagram, questioning assumptions about the emerging findings, both during team meetings and individually via email. As a team we discussed how this feedback would be incorporated into our written work. Taking this approach reflects ‘collective reflexivity’, which has been described as the group reflecting on and interacting to shape the research outcomes [30].
Stage 6: Consult with experts
Despite Arksey and O’Malley [22] terming Stage 6 ‘consultation with experts’, we aimed for higher levels of engagement as per the IAP2 Public Participation Spectrum [17]. A patient partner was invited to participate in this review at the level ‘collaborate’ or ‘involve’ on each activity for this scoping review, as per the IAP2 Public Participation Spectrum [17]. Then after they had to withdraw because of personal circumstances a second patient partner was invited into the team who partnered at a similar level as the first patient partner. Both patient partners were identified through our research team’s networks. The lead researcher had an initial meeting with each patient partner and discussed factors like their skills, previous research experiences, time available, and health experiences. We also discussed the methodology of the current research, and briefly outlined some possible activities patient partners could take part in. As we did with other members of the research team, we negotiated patient partner engagement in each research activity as the timing of the activity drew closer, providing details of what the activity entailed and the time commitment via email or in meetings. Patient partners were provided time to consider their engagement in each activity before informing us of their decision. Our patient partners had the same status as all other members of the research team irrespective of the specific activities they chose to be part of. This negotiation process and other details about patient partner backgrounds and training are provided in more depth in Supplementary File 2 as per the GRIPP2 reporting checklist [31].
Findings
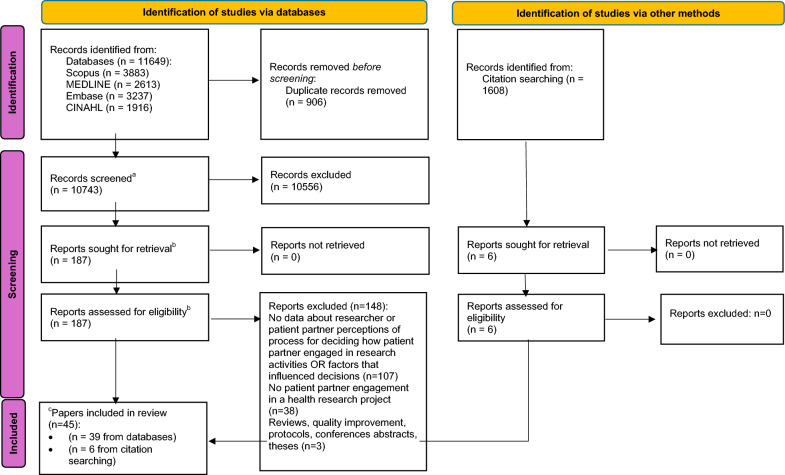
The search was conducted in December 2023. In total, 13,257 papers were found through database and citation searching (See Fig. 1). In total, 187 full-text papers were reviewed, of which 39 met eligibility criteria. Six full-text papers were reviewed during citation searching; all were included. Thus, 45 papers were included in the review.
Fig. 1.
PRISMA flowchart. aRefers to titles and abstracts screened; bRefers to full-text papers retrieved and screened; of the 45 papers, c19 were studies, 25 were commentaries and 1 was both a study and commentary
Summary of study characteristics
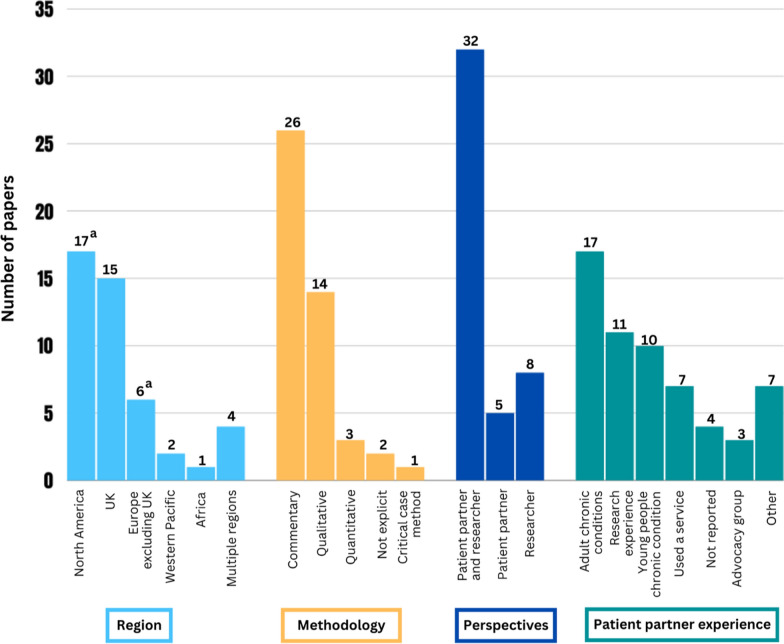
Figure 2 provides a summary of key characteristics of the papers. Of the 45 papers, 17 were from North America (12 from Canada; 4 from the USA; 1 from both Canada and the USA), 15 were from the UK, 6 from Europe excluding the UK (1 each from Denmark, The Netherlands, Norway, Ireland, Germany and 1 from both Norway and the Netherlands), 2 from the Western Pacific (both from Australia) and one from Africa (South Africa). Four papers were from ≥ 2 countries in ≥ 2 regions of the world. The study methodology of over half the papers were commentary in nature (n = 25), whereby authors reflected on a study or studies they had conducted in the past, providing an account of and reflections on the engagement process. A total of 19 papers were empirical research studies, of which the most common study design was qualitative (n = 14). One paper [32] was both a commentary and qualitative study. There were no instances where a commentary and study presented data about the same study. Finally, across all types of studies, patient partners most often had experience with an adult or youth chronic condition (n = 27; n = 17 adult; n = 10 youth) or previous research experience (n = 11). More detailed data extraction is available in Supplementary Files 3 and 4.
Fig. 2.
Characteristics of included papers. aOne study was conducted in two different countries in the same region. Methodologies and patient partner experiences add up to > n = 45 because: some papers were both a commentary and study; and more than one experience was reported for patient partners in some studies
Synthesis of findings
The deductive and inductive analysis yielded two categories including: The research and research team attributes shape patient partner roles; and Shared and ongoing decision-making. A total of six sub-categories were inductively identified (See Table 1). Some studies included both patient partner and researcher reflections; when we were unable to attribute verbatim quotes to either a patient partner or researcher we labelled the quotes as coming from ‘patient partner and researcher’.
Table 1.
Categories and sub-categories
| Categories | Sub-categories |
|---|---|
| The research and research team attributes shape patient partner roles | Understanding patient partners’ characteristics when making decisions about engagement |
| Examining possible research activities when making decisions about engagement | |
| Accounting for researcher circumstances in patient partner engagement decisions | |
| Shared and ongoing decision-making | Undertaking the negotiation process |
| Making timely decisions when determining patient partner engagement | |
| Using tools to facilitate the agreement |
Category 1: The research and research team attributes shape patient partner roles
Many factors facilitated or hindered patient partner roles, which were related to the individuals involved in the decision (patient partner and researcher), and the activity they were deciding upon.
Understanding patient partners’ characteristics when making decisions about engagement
Many unique patient partner factors influenced the activities patient partners engaged in, including their previous professional career experience [33–37], research experiences [34, 38–42], lived experiences [40, 43–45], and perceived skills [33, 36, 38, 46, 47]:
“The public advisor really took to NVivo (qualitative data analysis software package)…It’s moving people out from just seeing a patient as a generalised statistic of a person to individuals who have their own skills and experiences” (Researcher, United Kingdom) [36].
Other factors were patient partner interest in engaging in the activity [17, 35, 39–44, 46–55] and their motivations and goals [48, 49, 55], such as skill-building [37, 40, 46, 49, 56]:
“I was looking to add things to my professional résumé… it was professional development, but also, if anything, I thought I would be able to help more patients and if I were going to go to work on digital-related things, I could use those for later jobs.” (Patient partner, United States)”.
Patient partner availability was another factor [33, 38, 46, 48, 51, 52, 55, 57–59] including consideration of their other life responsibilities that were competing priorities [38, 42, 45, 48, 55]. Finally, patients’ health status [33, 42, 49, 52, 59], including their physical [46, 55, 59], emotional [55] and mental health [33, 46, 55, 60, 61] influenced decisions about engagement in activities.
Examining possible research activities when making decisions about engagement
The nature of the research activity also affected patient partner engagement. Projects had different methodologies with distinct activities for patient partners [33, 46, 51, 52, 56, 62], views on each activity could influence decisions about how patient partners were involved. For example, in some cases analysis [35] and dissemination of findings [45], were perceived as appropriate and desirable activities for patient partners, while in other studies, they were viewed by participants as too technical, stressful, not expected practices for patient partners or too time-intensive [33, 47, 62, 63]:
“…we were not able to identify a patient partner role throughout conduct of the retrospective study…due to the highly technical nature of the …cell product development, it was challenging to ensure all members of our multidisciplinary team understood the complex process involved…engagement throughout this component primarily focused on informing and information sharing…” (Patient partner and researcher, Canada) [51].
Ethical considerations and study site regulations precluded some activities such as data collection, or made them more challenging for participants [38, 46, 53, 61]:
“During our study, carer co‐researchers reported that at times they wanted to step away from the constraints of the interviewer role and offer advice to the people they were interviewing. The urge to alleviate participants’ anxiety raised specific tensions for carer co‐researcher D, who struggled to balance the objective researcher stance with their experience of empathy as a fellow carer. This case raised moral and methodological questions.” (Researcher, United Kingdom) [46].
Accounting for researcher circumstances in patient partner engagement decisions
The circumstances surrounding the individual researcher also influenced decisions about patient engagement, those with higher skills and abilities were more likely to engage patient partners in more activities [36, 47]: “Knowledge was highlighted as a barrier to involving public and patient partners in numerical aspects of trials…”(Patient partner and researcher, United Kingdom) [47]. Researchers’ time and budget to support and train patient partners [17, 33, 38, 43, 46, 48, 52, 55, 57–59], and budget to remunerate patient partners to engage in each activity [42, 61] could also influence activities patient partners engage in:
“…it was often necessary to limit the involvement of the ‘Research Buddies’ due to resources and time constraints. For example, one ‘Research Buddy’ was keen to involve other sites and extend recruitment to include patients, which was not feasible for this doctoral study.” (Researcher, United Kingdom) [64].
Category 2: Shared and ongoing decision-making
A shared decision-making approach was generally described when negotiating what activities patient partners took part in, which could occur at the start or throughout the project. Tools could be used to facilitate a shared decision-making process. This process was dynamic, with the patient partners and researchers negotiating the patient partner role iteratively. Thus, reflection, new discussions and negotiation were used to determine the patient role occurred throughout the project.
Undertaking the negotiation process
In most studies, a shared decision-making approach was described where patient partners were offered an array of activities they could choose from [17, 36, 38, 42, 43, 54, 61, 65–67] and add to [17, 57], which differed based on the research methodology [33, 46, 51, 52, 56, 62]. Patient partners were provided clear details about what each activity entailed [59, 64], including the time commitment [17, 38, 41, 43, 55, 59, 61, 68, 69]:
“In another example, a P2P awardee laid out all of the tasks that needed to be accomplished on the project – outreach and recruitment, communication, a literature review, and data collection- and asked partners, “Where do you see yourself in this?” (Researcher, United States) [54].
Next, discussions occurred between the patient partner and researcher to help patient partners decide on their roles [17, 33–35, 39, 56]. For example, “adjusting” engagement in activities to higher or lower levels based on factors like current health status [33, 35, 42, 49, 52, 59, 62, 63]; determining the resources needed to engage a patient partner on an activity and comparing this to the value added to the activity and project overall [17, 38, 43, 45, 46, 54, 57, 70, 71], and ‘matching’ patient factors like skills to fitting activities [17, 33, 34, 36, 38–41, 43, 44, 47, 49, 52–56, 59, 61, 63, 65, 70–72]:
“[Just] giving us the freedom to get involved with the bits we want to and feel able to and allowing us to avoid those things we are less comfortable with or able to be involved with due to clashes, mental health or other reasons [and therefore it has] not being overly demanding or making the co-researchers feel they are individually ‘responsible’ for a whole project.” (Patient partner, United Kingdom) [61].
Ultimately, the shared decision-making approach enabled patient partners to exercise power in making final decisions about what activities they engaged in [17, 36, 38, 39, 42, 43, 46, 53, 54, 59, 66, 73–77].
However, a smaller number of studies showed divergence in this sub-category. There were instances where researchers did not engage patient partners in the decision-making process, made assumptions about patient partner factors [46, 60, 62], or invited patient partners onto activities that were decided by the researcher [32, 53, 64]:
“One of the things that has surprised me that most is how much is depends on my own abilities. Do I know how to frame what I want from them and help them get involved? I make many decisions about when to involve them…”(Researcher, Denmark) [32].
However, researchers were often reflective, and realised the importance of not making assumptions about what activities patients could undertake in future research [55, 63], as patient partners had surprised researchers in terms of the range of activities they were able to meaningfully contribute to [36].
Making timely decisions when determining patient partner engagement
There was tension between the need to define patient partner activities early versus organic decisions that evolved over time about these activities. Decisions were made early due to the need to detail patient partner activities in grants [38, 42, 56] or ethics applications [61], which precluded patient partners from activities like setting the research question when no funding was available [38, 53, 61]. Decisions were also set early due to the general principle that patient partners should be engaged in all study activities from the start [38, 59, 70, 77]. However, there was a lack of clarity for both patient partners and researchers about what this meant [36] and this standardised approach could result in tokenism [56]:
“Potentially, we do a bit of a disservice sometimes when we just include them, without thinking about why, and I’ve definitely been in a room before, including on my own project, when there have been PPI [patient and public involvement] people in the room but I’m not really sure what the purpose is...it really hasn’t been beneficial.” (Researcher, United Kingdom) [56].
Yet, having structure from the start was viewed as important [17, 48], as patient partners wanted a sense of how they would be involved [38, 56].
On the other hand, it was highlighted that the activities patient partners took part in changed throughout the study [32, 33, 36, 38–40, 44, 48, 55, 63, 71, 76]: “My role evolved over the period of the study. I was given the opportunity to opt in or out of involvement with each activity, but took on more of the workload and responsibilities as time went on.” (Patient partner, United Kingdom) [38]. Agreed-upon patient partner activities were discussed regularly and adjusted as needed [32, 41, 55, 59]; at least annually [17, 76] and after grants were won and patient partners were on board [38, 50, 52, 76]. Additionally, it was suggested that researchers should seek and be open to new opportunities for patient partner activities that arise [17, 40, 44, 50, 55].
Using tools to facilitate the agreement
Tools from the INVOLVE organisation [51], the Involvement Matrix [59, 67], or frameworks [66], were identified as aids for decision-making between patient partners and researchers:
“The Involvement Matrix could be used as a conversation tool to discuss the roles that young people would like to have in research (e.g., listener, co-thinker, advisor, partner, or decision-maker) for tasks in different stages of preparation, execution and implementation in the research project.” (Patient partner and researcher, South Africa) [67].
In some cases, activity planning sheets [46] or templates were given to patient partners to complete, so they could self-disclose factors such as interests: “The template itself functioned as a survey to allow patient partners an organized and formal method of identifying the project activities they were most interested in and at what level they wished to engage with those activities.” (Patient partner and researcher, Canada) [17].
Once decisions were made, they were formally documented. For example, at the start of projects, patient partner activities were documented in grants [52, 71, 76, 77] and recruitment documents [36, 49, 55]. In other studies, patient engagement plans, role description documents, or terms of reference were developed later to outline patient partner roles [17, 41, 44, 50–53, 75, 76].
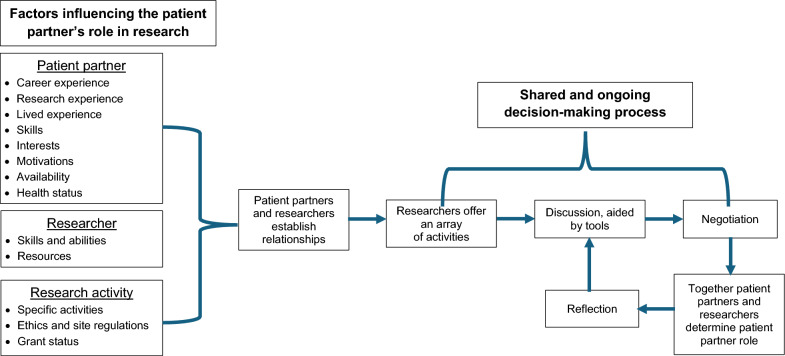
Relationship between categories and sub-categories
The conceptualised relationship between categories and sub-categories is shown in Fig. 3. Influencing factors can positively or negatively influence how decision-making occurs. The decision-making process is shared between the patient partner and researcher, whereby options for the patient partner role are discussed and negotiated. The process is dynamic, with reflection occurring and the patient partner role is re-negotiated when required.
Fig. 3.
The dynamic and negotiated decision making approach to determining patient partners’ roles in research
Discussion
Our review of 45 papers identified the decision-making process regarding patient partners’ roles in research is ongoing and shared and that a myriad of factors related to the patient partner, the researcher, and the research activity influenced this process. This review is the first to provide a detailed description of this complex process; identifying factors that influence it, and showing negotiating patient partner roles can occur throughout the research process. Over half of the papers in our review were commentaries, suggesting that for them to be published, the authors had to reflect on their experiences, possibly providing more insights into how patient partner engagement was enacted. However, this claim cannot be verified.
We found many patient partner factors can influence patient partner roles in the research. In a multinational study that involved people with lived experience in healthcare and health researchers across nine countries, a conversation during patient partner recruitment was viewed as the simplest way to explore factors influencing patient partner engagement [78]. Similar to our review, they recommended that patient factors, like skills, willingness, time capacity, technical knowledge, lived experiences, and training needs should be discussed. On the other hand, more formal methods could be used to assess these influencing factors. McCarron et al. [79] created and tested a survey for patient partners to assess their underlying motivations for being patient partners on health research projects; a factor uncovered in our review. Ultimately, understanding patient factors appears to be a critical first step; with this knowledge decision-making practices found in this review like ‘matching’ patient factors to activities will likely be facilitated.
Our review underscored researcher factors were critical when determining patient partner engagement in activities. Like other research, we found issues such as researchers’ resources and a lack of time influenced the type of activities patient partners took part in [80]. Others found that time commitments for researchers include scheduling meetings, engaging patient partners in shared decision-making, incorporating patient partner feedback and communicating with patient partners for research training [81], can be difficult to manage against competing academic priorities [16, 80]. In addition, the time required for training was a determining resource factor in our review. Consistent with previous research, lack of adequate funding was another resource implication that influenced engagement, which can include costs of staff time and patient partner remuneration [82, 83]. Researchers have shown that the United Kingdom and Europe have higher engagement, plus well-established organisations to provide funding [84]. This suggests resource availability is an important driver of engagement [84] and explains our findings related to early decisions being made without patient partner input, when no funding was available to remunerate patient partners. Overall, researchers need to consider the resources they have available including funding, time and their own expertise prior to embarking on engaging with patient partners in their research endeavors.
Creating a plan for patient engagement can be daunting, due to the multitude of options [85]; our review uncovered that tools may facilitate this process. In the clinical trials context, “usable” decision aids were developed to assist with patient partner engagement [86]. Decision aids were used for assessing priorities (i.e. where in the research process patient partners want to be engaged), understanding patient partner motivations, and determining time/costs of patient partner participation, which helped reach decisions [86]. Another resource to facilitate shared decision-making could be the use of matrices. Researchers have previously synthesised expert opinion and research resources from four countries into practical guidance; they suggested that a matrix that outlines all possible research activities for patient partner engagement, with descriptions of the activities, can facilitate decisions [85]. Given that decision aids typically provide options, a matrix approach could enhance decision aids used for engagement. In summary, clearly documenting the plan for patient partner engagement, using various tools in this process, may help to ensure patient partners are engaged in the most meaningful activities.
In our review shared decision-making did not always occur. Shared decision-making approaches may be advantageous for planning patient partner engagement, because clear expectations are set and hierarchical structures may be minimised [87]. Patient partners have identified that unconscious biases held by researchers can underestimate patient partner capabilities to engage in research, exacerbating power imbalances in the patient partner-researcher relationship [88]. However, akin to the findings in our review, others have found that after reflection, researchers’ assumptions and their traditional scientific approaches to how patient partners are engaged were challenged [82]. Drawing on qualitative approaches like ‘reflexivity’, whereby researchers undertake exercises to make their own influence and biases explicit to themselves and others, could help develop awareness of power dynamics and strengthen engagement practices [65]. Given our review showed that some decisions about patient partner engagement were not shared, reflexive practices could be used to consider how decisions about patient partners’ engagement in research activities were made [89]. Thus, to make the most out of patient partners’ contribution to the research process, the whole research team may benefit from regular reflection and discussion about how their individual characteristics can better shape the research process.
Recommendations
Based on our findings, we suggest several recommendations, as described in Table 2. Generally, we recommend that patient partners and researchers who are working together discuss factors that influence patient partner engagement in research activities and incorporate these learnings into a shared decision-making approach when determining patient partner roles. To enact this shared decision-making approach ongoing efforts may be needed to break down researchers’ paternalistic barriers. We propose that researcher reflexivity could be used to minimise paternalism but because time constraints are common, feasible reflexivity activities require further exploration. Furthermore, we recommend renegotiating patient partners’ roles throughout the research process. Tools could be used to aid the shared decision-making process although there is limited evidence on the effects of tool use on engagement.
Table 2.
Challenges and recommendations for patient partners’ roles
| Challenges | Recommendations |
|---|---|
| Many factors influence patient partner research roles and activities, which can change throughout the research |
Recognise, discuss and assess individual patient partner, researcher and activity factors to effectively negotiate patient partner contribution Renegotiate this contribution as required |
| Making a plan for patient partner engagement can be daunting |
Various tools may assist in this planning Co-creating new tools and approaches that are more context specific may help planning |
| Shared decision-making can be stymied when researchers choose to control the research process | Researchers could include various approaches to reflexivity to aid a more shared approach to decision-making |
Limitations and strengths
We deviated from the published protocol, deciding to present our categories in a figure, rather than the ‘Patterns, Advances, Gaps, Evidence for practice and Research recommendations’ (PAGER) framework [90]. We believe this provided a deeper overview of patterns than using the framework approach. In addition, we had intended to extract data about the level of IAP2 Public Participation Spectrum for each study, however, these data were not made explicit by many of the authors in original papers, and determining the level of engagement was too subjective. Additionally, we did not search the grey literature and excluded non-English publications. Given the broad scope of the review, we quickly found there was potential to be overwhelmed by the large number of studies included through our search strategy; instead, we only included peer-reviewed literature, which ensured quick access to high-quality information. Further, during content analysis there were adequate data to develop categories, confirming that including peer-review literature was sufficient.
Because assessing the quality of research is not part of the scoping review process, the results of the review must be interpreted with caution as the methodological quality of included studies and accuracy of the commentaries are unknown. In addition, given most papers included in our review were from high income regions of the world such as North America, the UK and other parts of Europe, researchers must determine their applicability to other settings. Finally, although we included diverse study types, which could be seen as a limitation, content analysis aided in creating a cohesive overall picture of answers to the research questions.
Conclusions
In summary, our review showed that making decisions about patient partners’ roles in research is a dynamic and negotiated process. Patient partner engagement involves reflection and renegotiation throughout the research project. Factor such as patient partners’ lived experiences and motivation, researcher skills and abilities and the types of research activities being undertaken influence how patient partners are engaged during a research project. Overall, this sharing of decision making may lead to more meaningful engagement. However, our findings must be interpreted within their context. We included research primarily from high income, western regions of the world, and patient partners often had lived experience of chronic illness and had been engaged in research projects.
Supplementary Information
Acknowledgements
Acknowledgements: Thank you to Debra Perger the patient partner who was part of the team up until “Stage 5: Collating, summarising and reporting the results” and was remunerated for her contribution, however was not an author on the paper because she determined she was unable to contribute to manuscript drafting and approval. Thank you to Bonnie Dixon, the Discipline Librarian, for assisting with the search strategy development.
Author contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work (GT, BMG, JC, RM, JR, CSW, TLM, KM, SJ, WC); and Drafting the work or reviewing it critically for important intellectual content (GT, BMG, JC, RM, JR, CSW, TLM, KM, SJ, WC); and Final approval of the version to be published (GT, BMG, JC, RM, JR, CSW, TLM, KM, SJ, WC); and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (GT, BMG, JC, RM, JR, CSW, TLM, KM, SJ, WC).
Funding
GT and CSW salary funded by the NHMRC Centre of Research Excellence in Wiser Wound Care (APP1196436).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chudyk AM, Stoddard R, McCleary N, Duhamel TA, Shimmin C, Hickes S, et al. Activities and impacts of patient engagement in CIHR SPOR funded research: a cross-sectional survey of academic researcher and patient partner experiences. Research Involvement and Engagement. 2022;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarron TL, Clement F, Rasiah J, Moran C, Moffat K, Gonzalez A, et al. Patients as partners in health research: a scoping review. Health Expect. 2021;24(4):1378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoddinott P, Pollock A, O’Cathain A, Boyer I, Taylor J, MacDonald C, et al. How to incorporate patient and public perspectives into the design and conduct of research. F1000Research. 2018;7:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Research (NIHR). About Us 2024. Available from: https://www.invo.org.uk/.
- 5.Institute PCOR. Engagement FAQs: PCORI; 2023. Available from: https://www.pcori.org/funding-opportunities/applicant-and-awardee-resources/frequently-asked-questions/engagement-faqs#:~:text=PCORI%20uses%20patients%20and%20patient,represent%20the%20population%20of%20interest.
- 6.Canadian Institutes of Health Research. What is health research? 2023. Available from: https://cihr-irsc.gc.ca/e/53146.html.
- 7.Carlini J, Robertson J. Consumer partnerships in research (CPR) checklist: a method for conducting market research with vulnerable consumers. Int J Mark Res. 2022;65(2–3):215–36. [Google Scholar]
- 8.Manafo E, Petermann L, Mason-Lai P, Vandall-Walker V. Patient engagement in Canada: a scoping review of the ‘how’ and ‘what’ of patient engagement in health research. Health Res Policy Syst. 2018;16(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsythe LP, Ellis LE, Edmundson L, Sabharwal R, Rein A, Konopka K, et al. Patient and stakeholder engagement in the PCORI pilot projects: description and lessons learned. J Gen Intern Med. 2016;31(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partnership Upisd. UK Standards for Public Involvement. 2019.
- 11.NHMRC. Statement on consumer and community involvement in health and medical research 2016. Available from: https://www.nhmrc.gov.au/about-us/publications/statement-consumer-and-community-involvement-health-and-medical-research
- 12.Nielssen I, Santana M, Pokharel S, Strain K, Kiryanova V, Zelinsky S, et al. Operationalizing the principles of patient engagement through a Patient Advisory Council: lessons and recommendations. Health Expect. 2024;27(1): e13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ball S, Harshfield S, Carpenter A, Bertscher A, Marjanovic S. Patient and public involvement and engagement in research: enabling meaningful contributions. Santa Monica, CA; 2019.
- 14.Karlsson AW, Kragh-Sorensen A, Borgesen K, Behrens KE, Andersen T, Kidholm ML, et al. Roles, outcomes, and enablers within research partnerships: a rapid review of the literature on patient and public involvement and engagement in health research. Res Involv Engag. 2023;9(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhati DK, Fitzgerald M, Kendall C, Dahrouge S. Patients’ engagement in primary care research: a case study in a Canadian context. Res Involv Engag. 2020;6(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacker KE, Smith AB. Engaging stakeholders and patient partners. Surg Oncol Clin N Am. 2018;27(4):665–73. [DOI] [PubMed] [Google Scholar]
- 17.Etchegary H, Pike A, Patey AM, Gionet E, Johnston B, Goold S, et al. Operationalizing a patient engagement plan for health research: sharing a codesigned planning template from a national clinical trial. Health Expect. 2022;25(2):697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locock L, Boylan AM, Snow R, Staniszewska S. The power of symbolic capital in patient and public involvement in health research. Health Expect. 2017;20(5):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison M, Palmer R. Exploring patient and public involvement in stroke research: a qualitative study. Disabil Rehabil. 2015;37(23):2174–83. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison K, Rogers W, Entwistle VA. Addressing deficits and injustices: the potential epistemic contributions of patients to research. Health Care Anal. 2017;25(4):386–403. [DOI] [PubMed] [Google Scholar]
- 21.Marshall DA, Suryaprakash N, Lavallee DC, McCarron TL, Zelinsky S, Barker KL, et al. Studying how patient engagement influences research: a mixed methods study. The Patient - Patient-Centered Outcomes Research. 2024. [DOI] [PMC free article] [PubMed]
- 22.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 23.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–6. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien KK, Colquhoun H, Levac D, Baxter L, Tricco AC, Straus S, et al. Advancing scoping study methodology: a web-based survey and consultation of perceptions on terminology, definition and methodological steps. BMC Health Serv Res. 2016;16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Association for Public Participation. IAP2 Public Participation Spectrum 2019. Available from: https://iap2.org.au/resources/spectrum/.
- 27.Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care. 2011;27(4):391–9. [DOI] [PubMed] [Google Scholar]
- 28.Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–15. [DOI] [PubMed] [Google Scholar]
- 29.Nzabonimpa JP. Quantitizing and qualitizing (im-)possibilities in mixed methods research. Methodol Innov. 2018;11(2):2059799118789021. [Google Scholar]
- 30.Cayir E, Felder TM, Nkwonta CA, Jackson JR, Dawson R. Discovering new connections: insights from individual and collective reflexivity in a mixed methods study. Int J Qual Methods. 2022;21.
- 31.Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358: j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jørgensen CR, Eskildsen NB, Johnsen AT. User involvement in a Danish project on the empowerment of cancer patients—experiences and early recommendations for further practice. Res Involv Engag. 2018;4(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayes M, Moulam L, Meredith S, Whittle H, Lynch Y, Goldbart J, et al. Making public involvement in research more inclusive of people with complex speech and motor disorders: The I-ASC project. Qual Health Res. 2021;31(7):1260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Lorito C, Godfrey M, Dunlop M, Bosco A, Pollock K, van der Wardt V, et al. Adding to the knowledge on patient and public involvement: reflections from an experience of co-research with carers of people with dementia. Health Expect. 2020;23(3):691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aries AM, Bailey P, Hunter SM. The mutual benefits of patient and public involvement in research: an example from a feasibility study (MoTaStim-Foot). Res Involv Engag. 2021;7(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saini P, Hassan SM, Morasae EK, Goodall M, Giebel C, Ahmed S, et al. The value of involving patients and public in health services research and evaluation: a qualitative study. Res Involv Engag. 2021;7(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutman T, Kelly A, Scholes-Robertson N, Craig JC, Jesudason S, Tong A. Patient and caregiver experiences and attitudes about their involvement in research in chronic kidney disease. Clin J Am Soc Nephrol. 2022;17(2):215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marks S, Mathie E, Smiddy J, Jones J, da Silva-Gane M. Reflections and experiences of a co-researcher involved in a renal research study. Res Involv Engag. 2018;4(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould DJ, Glanville-Hearst M, Bunzli S, Choong PFM, Dowsey MM. Research Buddy partnership in a MD–PhD program: lessons learned. Res Involv Engag. 2023;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prebeg M, Patton M, Desai R, Smith M, Krause K, Butcher N, et al. From participants to partners: reconceptualising authentic patient engagement roles in youth mental health research. Lancet Psychiatry. 2023;10(2):139–45. [DOI] [PubMed] [Google Scholar]
- 41.Bisson M, Aubrey-Bassler K, Chouinard MC, Doucet S, Ramsden VR, Dumont-Samson O, et al. Patient engagement in health implementation research: a logic model. Health Expect. 2023;26(5):1854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Røssvoll TB, Rosenvinge JH, Liabo K, Hanssen TA, Pettersen G. Patient and public involvement in health research from researchers’ perspective. Health Expect. 2023;26(6):2525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Beinum A, Talbot H, Hornby L, Fortin MC, Dhanani S. Engaging family partners in deceased organ donation research-a reflection on one team’s experience. Can J Anaesth. 2019;66(4):406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curran JA, Bishop A, Chorney J, MacEachern L, Mackay R. Partnering with parents to advance child health research. Healthc Manag Forum. 2018;31(2):45–50. [DOI] [PubMed] [Google Scholar]
- 45.Headrick K, Thornton M, Hogan A, Deramore Denver B, Drake G, Wallen M. Consumer involvement in research – parent perceptions of partnership in cerebral palsy research: a qualitative study. Disabil Rehabil. 2023;45(3):483–93. [DOI] [PubMed] [Google Scholar]
- 46.Poland F, Charlesworth G, Leung P, Birt L. Embedding patient and public involvement: managing tacit and explicit expectations. Health Expect. 2019;22(6):1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goulao B, Camille P, Katie G. Patient and public involvement in numerical aspects of trials: a mixed methods theory-informed survey of trialists’ current practices, barriers and facilitators. BMJ Open. 2021;11(3): e046977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pozniak K, Cross A, Babic R, Cavalieros V, Martens R, Rosenbaum P, et al. Co-development of the ENVISAGE-Families programme for parents of children with disabilities: Reflections on a parent-researcher partnership. Aust Occup Ther J. 2022;69(6):653–61. [DOI] [PubMed] [Google Scholar]
- 49.McCarron TL, Clement F, Rasiah J, Moffat K, Wasylak T, Santana MJ. Co-designing strategies to support patient partners during a scoping review and reflections on the process: a commentary. Res Involv Engag. 2021;7(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etherington C, Lê M, Proulx L, Boet S. Bringing the patient voice into the operating room: engaging patients in surgical safety research with the Operating Room Black Box®. Res Involv Engag. 2022;8(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster M, Fergusson DA, Hawrysh T, Presseau J, Kekre N, Schwartz S, et al. Partnering with patients to get better outcomes with chimeric antigen receptor T-cell therapy: towards engagement of patients in early phase trials. Res Involv Engag. 2020;6(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gesell SB, Klein KP, Halladay J, Bettger JP, Freburger J, Cummings DM, et al. Methods guiding stakeholder engagement in planning a pragmatic study on changing stroke systems of care. J Clin Transl Sci. 2017;1(2):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwok A, Cheung D, Gordon M, Mudryk E, Manns PJ. Stroke survivors partner in research: a case example of collaborative processes. Res Involv Engag. 2022;8(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayne AI, Dullabh P, Skillman M, Ubri P, Rotondo C, Zainulbhai S, et al. Engaging patients and stakeholders in preresearch: findings from the Pipeline to Proposal Awards Initiative. J Comp Eff Res. 2020;9(10):721–36. [DOI] [PubMed] [Google Scholar]
- 55.Markle-Reid M, Ganann R, Ploeg J, Heald-Taylor G, Kennedy L, McAiney C, et al. Engagement of older adults with multimorbidity as patient research partners: Lessons from a patient-oriented research program. J Multimorbidity Comorbidity. 2021;11:2633556521999508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson H, Ogden M, Brighton LJ, Etkind SN, Oluyase AO, Chukwusa E, et al. Patient and public involvement in palliative care research: What works, and why? A qualitative evaluation. Palliative Med. 2021;35(1):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aase I, Ree E, Johannessen T, Holen-Rabbersvik E, Thomsen LH, Strømme T, et al. Strategies and lessons learnt from user involvement in researching quality and safety in nursing homes and homecare. Int J Health Governance. 2021;26(4):384–96. [Google Scholar]
- 58.Patterson S, Trite J, Weaver T. Activity and views of service users involved in mental health research: UK survey. Br J Psychiatry. 2014;205(1):68–75. [DOI] [PubMed] [Google Scholar]
- 59.van Schelven F, van der Meulen E, Kroeze N, Ketelaar M, Boeije H. Patient and public involvement of young people with a chronic condition: lessons learned and practical tips from a large participatory program. Res Involv Engag. 2020;6(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waite J, Poland F, Charlesworth G. Facilitators and barriers to co-research by people with dementia and academic researchers: findings from a qualitative study. Health Expect. 2019;22(4):761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fraser C, Carrington B, Crooks J, Diffey J, Evans N, Kirk S, et al. A Blueprint for involvement: Reflections of lived experience co-researchers and academic researchers on working collaboratively. Res Involv Engag. 2022;8(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crockett LK, Shimmin C, Wittmeier KDM, Sibley KM. Engaging patients and the public in Health Research: experiences, perceptions and training needs among Manitoba health researchers. Res Involv Engag. 2019;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson L, Newton J, Dawson P. Professionals and the public: power or partnership in health research? J Eval Clin Pract. 2012;18(2):276–82. [DOI] [PubMed] [Google Scholar]
- 64.Coupe N, Mathieson A. Patient and public involvement in doctoral research: impact, resources and recommendations. Health Expect. 2020;23(1):125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phoenix M, Nguyen T, Gentles SJ, VanderKaay S, Cross A, Nguyen L. Using qualitative research perspectives to inform patient engagement in research. Res Involv Engag. 2018;4(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schöpf-Lazzarino AC, Böhm P, Garske U, Schlöffel M, Stoye A, Lamprecht J, et al. Involving patients as research partners exemplified by the development and evaluation of a communication-skills training programme (KOKOS-Rheuma). Z Rheumatol. 2021;80(2):132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dada S, May A, Bastable K, Samuels A, Tönsing K, Wilder J, et al. The involvement matrix as a framework for involving youth with severe communication disabilities in developing health education materials. Health Expect. 2022;25(3):1004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen T, Palisano RJ, Graham I. Perspectives and experiences with engaging youth and families in research. Phys Occup Ther Pediatr. 2019;39(3):310–23. [DOI] [PubMed] [Google Scholar]
- 69.Manikandan M, Foley K, Gough J, Harrington S, Wall É, Weldon F, et al. Public and patient involvement in doctoral research during the covid-19 pandemic: reflections on the process, challenges, impact and experiences from the perspectives of adults with cerebral palsy and the doctoral researcher. Front Rehabil Sci. 2022;3: 874012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pratt B. What should engagement in health research look like? Perspectives from people with lived experience, members of the public, and engagement managers. Camb Q Healthcare Ethics. 2022;31(2):263–74. [DOI] [PubMed] [Google Scholar]
- 71.Buck D, Gamble C, Dudley L, Preston J, Hanley B, Williamson PR, et al. From plans to actions in patient and public involvement: qualitative study of documented plans and the accounts of researchers and patients sampled from a cohort of clinical trials. BMJ Open. 2014;4(12): e006400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eaton England AL, Ritchie CS, Mickler A, Perissinotto CM, Garrigues SK, Leff B, et al. Attitudes of homebound older adults and their caregivers toward research and participation as research advisors. Gerontologist. 2021;61(8):1202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cross A, Rosenbaum P, Grahovac D, Brocklehurst J, Kay D, Baptiste S, et al. A web-based knowledge translation resource for families and service providers (The “F-Words” in childhood disability knowledge hub): developmental and pilot evaluation study. JMIR Rehabil Assist Technol. 2018;5(2): e10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cross A, Rosenbaum P, Grahovac D, Kay D, Gorter JW. Knowledge mobilization to spread awareness of the “F-words” in childhood disability: lessons from a family-researcher partnership. Child Care Health Dev. 2015;41(6):947–53. [DOI] [PubMed] [Google Scholar]
- 75.Hruslinski J, Menio DA, Hymes RA, Jaffe JD, Langlois C, Ramsey L, et al. Engaging patients as partners in a multicentre trial of spinal versus general anaesthesia for older adults. Br J Anaesth. 2021;126(2):395–403. [DOI] [PubMed] [Google Scholar]
- 76.Evans BA, Carson-Stevens A, Cooper A, Davies F, Edwards M, Harrington B, et al. Implementing public involvement throughout the research process-Experience and learning from the GPs in EDs study. Health Expect. 2022;25(5):2471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Browne T, Swoboda A, Ephraim PL, Lang-Lindsey K, Green JA, Hill-Briggs F, et al. Engaging patients and family members to design and implement patient-centered kidney disease research. Res Involv Engag. 2020;6(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parker R, Tomlinson E, Concannon TW, Akl E, Petkovic J, Welch VA, et al. Factors to consider during identification and invitation of individuals in a multi-stakeholder research partnership. J Gen Intern Med. 2022;37(16):4047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCarron TL, Noseworthy T, Moffat K, Wilkinson G, Zelinsky S, White D, et al. Understanding the motivations of patients: A co-designed project to understand the factors behind patient engagement. Health Expect. 2019;22(4):709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boylan A-M, Locock L, Thomson R, Staniszewska S. “About sixty per cent I want to do it”: Health researchers’ attitudes to, and experiences of, patient and public involvement (PPI)—A qualitative interview study. Health Expect. 2019;22(4):721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heckert A, Forsythe LP, Carman KL, Frank L, Hemphill R, Elstad EA, et al. Researchers, patients, and other stakeholders’ perspectives on challenges to and strategies for engagement. Res Involv Engag. 2020;6(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carlini J, Muir R, McLaren-Kennedy A, Grealish L. Researcher perceptions of involving consumers in health research in Australia: a qualitative study. Int J Environ Res Public Health. 2023;20(10):5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryan L, Wenke R, Carlini J, Weir KA, Shapiro M, Baglot N, et al. Exploring barriers and solutions to consumer involvement in health service research using a nominal group technique. Res Involv Engag. 2024;10(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lang I, King A, Jenkins G, Boddy K, Khan Z, Liabo K. How common is patient and public involvement (PPI)? Cross-sectional analysis of frequency of PPI reporting in health research papers and associations with methods, funding sources and other factors. BMJ Open. 2022;12(5): e063356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Concannon TW, Grant S, Welch V, Petkovic J, Selby J, Crowe S, et al. Practical guidance for involving stakeholders in health research. J Gen Intern Med. 2019;34(3):458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parry M, Bjørnnes AK, Toupin-April K, Najam A, Wells D, Sivakumar A, et al. Patient engagement partnerships in clinical trials: development of patient partner and investigator decision aids. Patient Patient-Centered Outcomes Res. 2020;13(6):745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaisler RE, Missbach B. Co-creating a patient and public involvement and engagement ‘how to’ guide for researchers. Res Involv Engag. 2020;6(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richards DP, Poirier S, Mohabir V, Proulx L, Robins S, Smith J. Reflections on patient engagement by patient partners: how it can go wrong. Res Involv Engag. 2023;9(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rolfe DE, Ramsden VR, Banner D, Graham ID. Using qualitative health research methods to improve patient and public involvement and engagement in research. Res Involv Engag. 2018;4(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradbury-Jones C, Aveyard H, Herber OR, Isham L, Taylor J, O’Malley L. Scoping reviews: the PAGER framework for improving the quality of reporting. Int J Soc Res Methodol. 2022;25(4):457–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.