Abstract
The presence of microflora in the digestive tract promotes the development of the intestinal immune system. In this study, to evaluate the roles of two types of indigenous microbe, segmented filamentous bacteria (SFB) and clostridia, whose habitats are the small and large intestines, respectively, in this immunological development, we analyzed three kinds of gnotobiotic mice contaminated with SFB, clostridia, and both SFB and clostridia, respectively, in comparison with germfree (GF) or conventionalized (Cvd) mice associated with specific-pathogen-free flora. In the small intestine, the number of αβ T-cell receptor-bearing intraepithelial lymphocytes (αβIEL) increased in SFB-associated mice (SFB-mice) but not in clostridium-associated mice (Clost-mice). There was no great difference in Vβ usage among GF mice, Cvd mice, and these gnotobiotic mice, although the association with SFB decreased the proportion of Vβ6+ cells in CD8β− subsets to some extent, compared to that in GF mice. The expression of major histocompatibility complex class II molecules on the epithelial cells was observed in SFB-mice but not in Clost-mice. On the other hand, in the large intestine, the ratio of the number of CD4− CD8+ cells to that of CD4+ CD8− cells in αβIEL increased in Clost-mice but not in SFB-mice. On association with both SFB and clostridia, the numbers and phenotypes of IEL in the small and large intestines changed to become similar to those in Cvd mice. In particular, the ratio of the number of CD8αβ+ cells to that of CD8αα+ cells in αβIEL, unusually elevated in the small intestines of SFB-mice, decreased to the level in Cvd mice on contamination with both SFB and clostridia. The number of immunoglobulin A (IgA)-producing cells in the lamina propria was more elevated in SFB-mice than in Clost-mice, not only in the ileum but also in the colon. The number of IgA-producing cells in the colons of Clost-mice was a little increased compared to that in GF mice. Taken together, SFB and clostridia promoted the development of both IEL and IgA-producing cells in the small intestine and that of only IEL in the large intestine, respectively, suggesting the occurrence of compartmentalization of the immunological responses to the indigenous bacteria between the small and large intestines.
IEL (intraepithelial lymphocytes), immunoglobulin A (IgA)-producing cells in the lamina propria, and intestinal epithelial cells are key players that determine the nature of the immunological responses to antigens or pathogens ingested. Although the precise functions of IEL remain obscure, they are postulated to take part in the mechanism of defense against pathogens such as Cryptosporidium (34), Toxoplasma (7), and Listeria (14) spp. IEL, in particular γδIEL, have been shown to be closely associated with regulation of the proliferation of epithelial cells (28). Intestinal epithelial cells have also been shown to be engaged in antigen presentation, suggesting the involvement of major histocompatibility complex (MHC) molecules expressed on the epithelial cells in this process (33). Interleukin-7 (IL-7), IL-6, and transforming growth factor β are also produced in epithelial cells in various situations (38). It is clear that the apparatus and tools of the immunological responses of conventional animals differ greatly from those of germfree (GF) animals based on previous studies. In GF animals, the number of IEL, in particular αβ T-cell receptor (αβTCR)-bearing T cells (αβIEL), is greatly reduced and their Thy-1 expression and cytolytic activity are very low (31, 49). IgA production is also rare in GF mice, compared to that in conventional or specific-pathogen-free (SPF) animals (45). Macroscopically, Peyer’s patches in GF animals are small and poorly developed in comparison with those in conventional animals (42). The intestinal flora is essential for the generation of intestinal mucosal lymphocytes in severe combined immunodeficiency mice reconstituted with thymus-derived T cells (8). Thus, a large amount of evidence accumulated suggests the premature immune responsiveness of GF animals. This is consistent with differences between the physiological characteristics of the digestive tracts, such as intestinal motility and digestive enzyme activities, of GF and conventional animals (23). Association of a kind of intestinal indigenous microbe, i.e., segmented filamentous bacteria (SFB), with GF mice or rats was shown to activate the immunological characteristics of the small intestine to near the levels in conventional mice or rats (49). However, the immunological and physiological characteristics of SFB-monoassociated mice (SFB-mice) are far from those of conventional mice, except in the small intestine. SFB cannot be cultivated and therefore are only identified based on their 16S rRNA gene (rDNA) sequence (25, 46). Under SPF breeding conditions, SFB colonize the surfaces of small intestinal epithelial cells but not those of the large intestine (27).
Recently, it was reported that mice with some kinds of gene-targeted knockouts involving the TCR-α (36), IL-2 (43), or IL-10 (30) gene and some mutant mice, such as C3HJBir (47) and SAMP1/Yit (32), develop a colitis similar to inflammatory bowel disease. However, when these mice are kept under GF conditions they no longer develop colitis or the disease is ameliorated (10, 13). Together, these results strongly suggest that the presence of the commensal bacteria is closely associated with some steps in the development of colitis or enteritis. In the colitis model, the anaerobes in the large intestine are assumed to be candidates for the agent causing the pathogenesis (9, 16).
Although it is clear that commensal bacteria are closely associated with the development of the immune system or with the pathogenesis of inflammatory bowel disease, the precise underlying mechanisms remain obscure. In this study, we aimed to clarify how the indigenous microbes in the small and large intestines, respectively, affect the development of IEL and IgA production in both parts of the intestine. We selected SFB and clostridia as typical indigenous bacteria in the small and large intestines, respectively, based on the results of previous studies (26, 48).
MATERIALS AND METHODS
Mice.
Throughout this study, male GF BALB/c mice kept at our institute were used. The preparation of the first generation of gnotobiotic SFB-mice or clostridium-associated mice (Clost-mice) is described elsewhere (26, 48). In brief, a 3% chloroform-treated preparation of small intestinal epithelial cells was orally administered to GF mice. Thereafter, through repeated passages through GF mice, SFB-mice were selected. Although SFB cannot be cultivated in vitro, the homogeneity of the contaminating bacteria was confirmed based on the 16S rDNA sequence and microscopy of the intestinal contents of these mice (13). In Clost-mice, 46 strains of clostridia isolated singly from conventional mice were inoculated into GF mice after preinoculation of mouse-derived Escherichia coli to reduce the redox potential in the luminal environment. Mice associated with both SFB and clostridia were prepared by being housed in the same cage in a vinyl isolator. Conventionalized (Cvd) mice were prepared by administration of a suspension of feces freshly isolated from SPF mice to GF mice. For immunological analysis, we used the second generation of the established gnotobiotic mice or Cvd mice together with age-matched GF mice.
IEL preparation and flow cytometry.
IEL were prepared as described elsewhere (48, 49). Briefly, the epithelial cell fraction obtained on EDTA treatment was subjected to Percoll density gradient centrifugation after filtration through nylon mesh and a nylon column. IEL were recovered at the 44-to-70% Percoll interface (Pharmacia, Uppsala, Sweden). IEL of the large intestine were prepared from both the cecum and the colon. An aliquot of the IEL suspension was stained with fluorescein isothiocyanate (FITC)–anti-αβTCR (H57-597), phycoerythrin (PE)–anti-Lyt-2, and biotin–anti-L3T4, followed by streptavidin–Cy-chrome, FITC–γδTCR (GL3), and biotin–anti-Thy1.2 (30-H12), followed by streptavidin–Cy-chrome, or with PE–anti-Lyt-2 (53-6.7), FITC–Lyt-3 (53-5.8), and biotin–anti-αβTCR, followed by streptavidin–Cy-chrome. For analysis of Vβ usage, PE-labelled antibodies against Vβ6, Vβ8.1+8.2, Vβ4, Vβ7, Vβ8.3, Vβ10b, Vβ11, or Vβ12 (Caltag, Burlingame, Calif.) were used in combination with Cy-chrome–anti-αβTCR (H57-597) and FITC–anti-Lyt-3. All reagents not specified here were purchased from Pharmingen (San Diego, Calif.).
Immunohistochemistry.
Thin sections of ileum (5 to 10 cm from the ileocecal valve) of GF, Cvd, and gnotobiotic mice were stained with an admixture of monoclonal biotinylated antibodies against I-Ad and I-Ed (Pharmingen) and avidin-peroxidase (Cappel, West Chester, Pa.) as the second antibody. To stain IgA-producing cells, biotinylated anti-mouse IgA monoclonal antibodies (Pharmingen) were used in combination with streptavidin-peroxidase. For estimation of the number of IgA-producing cells in the lamina propria, the immunoreactive cells in a 1-mm-wide area of a section cut longitudinally from the tip of a villus or the flat surface, in the ileum or the colon, respectively, to the bottom of a crypt were counted by using two sections per mouse.
RESULTS
Microbiological profiles of the small and large intestines of gnotobiotic mice.
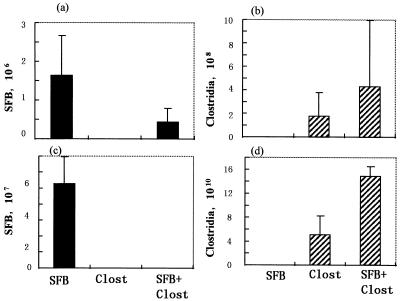
In this study, we established three kinds of gnotobiotes which were contaminated with SFB or clostridia, residents of the small and large intestine, respectively, or with both bacterial populations. The microbiological profiles of the small and large intestines were investigated by microscopy using smear preparations of the ileal contents and fresh feces, respectively. As shown in Fig. 1a and c, a great amount of SFB was present not only in the ilea but also in the feces of SFB-mice. Many SFB adhered to ileal epithelial cells of the small intestine. Large amounts of clostridia were also present in both the ilea and feces of Clost-mice (Fig. 1b and d). In mice associated with both SFB and clostridia, both bacteria were recognized microscopically in the ileum (Fig. 1a and b) while only clostridia were recognized in the feces (Fig. 1c and d). The clostridia used here comprised 46 strains which were isolated from the feces of conventional mice and were well characterized microbiologically (26). In conventional mice, SFB and clostridia were confined to the small and large intestines, respectively.
FIG. 1.
Microbiological status of the small intestines (ileum) and large intestines (feces) of SFB-mice, Clost-mice, and mice associated with both SFB and clostridia. The bacterial numbers were determined by microscopy using smear preparation of the ileal contents (a and b) and feces (c and d). The data are numbers of bacteria per gram of ileal contents or feces.
IEL in the small intestines of gnotobiotic mice.
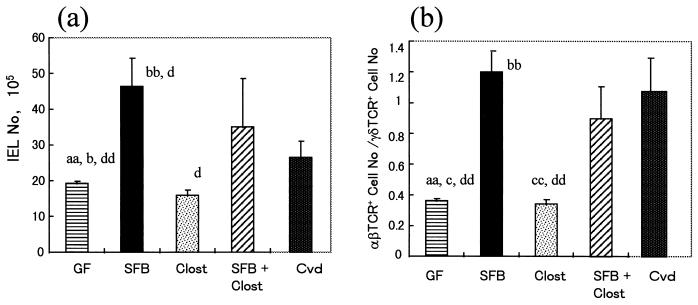
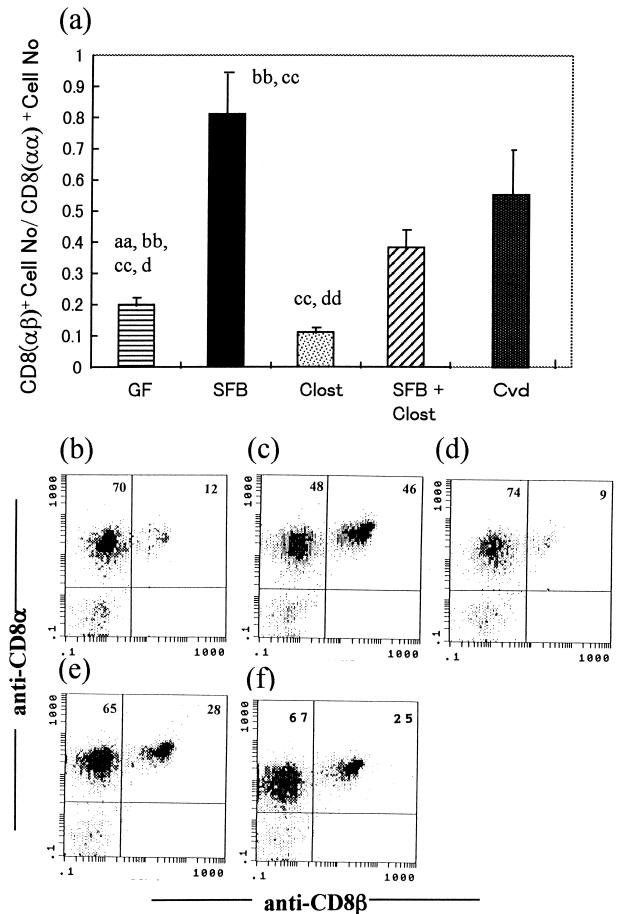
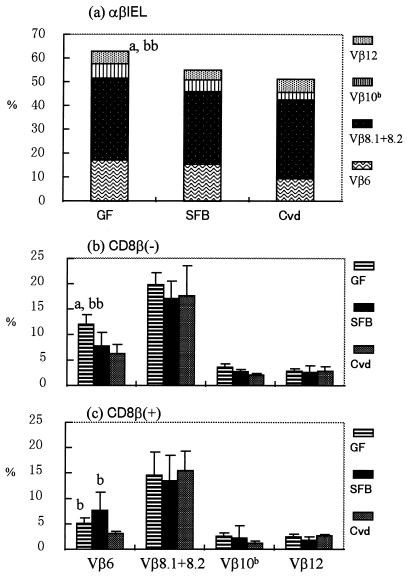
The total number of IEL in the small intestine was much higher in SFB-mice than in Clost-mice (Fig. 2a). The IEL number was hardly increased by the association of clostridia with GF mice. There was little difference in the number of IEL between SFB-mice and mice associated with both SFB and clostridia. Almost all of the IEL increase in SFB-mice consisted of αβIEL (Fig. 2b). It was noted that the ratio of CD8+ cells with the αβ heterodimer to those with the αα homodimer was much larger in SFB-mice than in the other groups (Fig. 3). The Vβ usage in IEL isolated from GF mice, Cvd mice, and these gnotobiotic mice was estimated, based on the percentage in total αβIEL, by using monoclonal antibodies against Vβ4, Vβ6, Vβ7, Vβ8.1+8.2, Vβ8.3, Vβ10b, Vβ11, and Vβ12. More than 60% of the Vβ comprised Vβ6, Vβ8.1+8.2, Vβ10b, and Vβ12 in GF mice, as shown in Fig. 4a, while the percentages of the four Vβ subsets were below 60% in SFB-mice and Cvd mice. The percentages of other Vβ subsets in αβIEL in any group were below 2%. In both the CD8β+ and CD8β− IEL subsets, the proportion of Vβ6+ was smaller in SFB-mice or Cvd mice than in GF mice (Fig. 4b and c). Overall, there was little difference in Vβ usage among these gnotobiotes and GF and Cvd mice.
FIG. 2.
Total numbers of IEL (a) and ratios of the number of αβIEL to that of γδIEL (b) in the small intestines of GF mice, SFB-mice, Clost-mice, mice associated with both SFB and clostridia (SFB + Clost), and Cvd mice. The gnotobiotic mice were analyzed at 16 weeks of age in the second generation and compared with age-matched GF mice. The data represent means plus the standard deviations (n = 3 or 4) and were also reproducible in the experiment involving the first generation. The letters a, b, c, and d near the columns indicate statistically significant differences from the SFB, Clost, SFB + Clost, and Cvd groups, respectively. Single letter, P < 0.05; double letters, P < 0.01.
FIG. 3.
Ratios of the number of CD8αβ-bearing αβIEL to that of CD8αα-bearing αβIEL in GF mice, SFB-mice, Clost-mice, mice associated with both SFB and clostridia (SFB + Clost), and Cvd mice. The data in panel a are means plus standard deviations (n = 3 or 4) and were reproducible in the experiment involving the first generation. The letters a, b, c, and d near the columns indicate statistically significantly differences from the SFB, Clost, SFB + Clost, and Cvd groups, respectively. Single letter, P < 0.05; double letters, P < 0.01. (b to f) Representative data for the quadrants on three-color analysis gating of αβIEL in the GF (b), SFB (c), Clost (d), SFB + Clost (e), and Cvd (f) groups.
FIG. 4.
Vβ usage in GF mice, SFB-mice, and Cvd mice. (a) Data showing the sum of Vβ6+, Vβ8.1+8.2+, Vβ10b+, and Vβ12+ cells in each group. (b and c) Vβ6+, Vβ8.1+8.2+, Vβ10b+, and Vβ12+ cells in CD8β− (b) and CD8β+ (c) subsets determined by three-color analysis of GF mice, SFB-mice, and Cvd mice. The data are means plus standard deviations (n = 4 to 7). The letters a and b near the columns indicate statistically significant differences from the SFB and Cvd groups, respectively. Single letter, P < 0.05; double letters, P < 0.01.
MHC class II expression in intestinal epithelial cells of gnotobiotic mice.
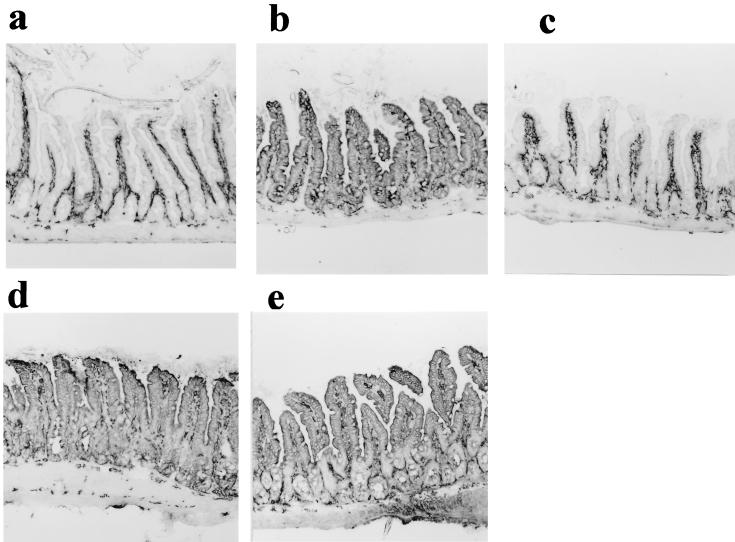
It is well known that MHC class II molecules are expressed on the small intestinal epithelial cells of conventional mice but are absent in GF mice. They were expressed in gnotobiotic SFB-mice or mice associated with both SFB and clostridia but not in Clost-mice (Fig. 5). The intensity of immunohistochemical staining of epithelial cells of SFB-mice was not homogeneous throughout a section, which was different from that in Cvd mice. In particular, the staining intensity of the tip portion of the villus was stronger than that of the base portion.
FIG. 5.
MHC class II molecule expression in ileal epithelial cells of GF mice (a), SFB-mice (b), Clost-mice (c), mice associated with both SFB and clostridia (d), and Cvd mice (e). Shown are longitudinal sections of the ileum stained with an admixture of biotinylated anti-I-Ad and anti-I-Ed antibodies, followed by peroxidase-labelled streptavidin. The data are representative of the mice in each group and were reproducible in the experiment involving the first generation. Magnification, ×85.
IEL in the large intestines of gnotobiotic mice.
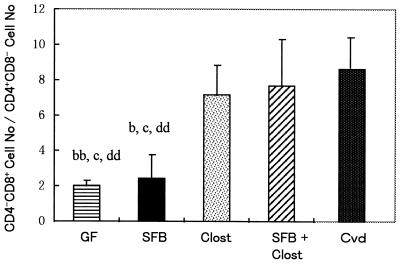
The number of IEL in the large intestines, including the ceca and colons, of Cvd mice was not very different from that in GF mice. The numbers of IEL were a little greater in gnotobiotic SFB-mice, Clost-mice, and mice associated with both bacteria than in GF mice. However, the percentages of CD4+ CD8−, CD4− CD8+, CD4+ CD8+ (DP), and CD4− CD8− (DN) cells in the αβIEL populations were considerably different among these gnotobiotic mice. In particular, an increase in the ratio of the number of CD8+ cells to that of CD4+ cells in αβIEL was evident in Cvd mice. This ratio increased in Clost-mice or mice associated with both SFB and clostridia compared to that in GF mice. However, the increase in this ratio was not so evident in SFB-mice (Fig. 6).
FIG. 6.
Ratio of the number of CD4 CD8 cells to that of CD4− CD8+ cells among αβBIEL in the large intestines of GF mice, SFB-mice, Clost-mice, mice associated with both SFB and clostridia (SFB + Clost), and Cvd mice. The data are means plus the standard deviations (n = 3 or 4) and were reproducible in the experiment involving the first generation. The letters b, c, and d indicate statistically significant differences from the Clost, SFB + Clost, and Cvd groups, respectively. Single letter, P < 0.05; double letters, P < 0.01.
IgA production in the small and large intestines of gnotobiotic mice.
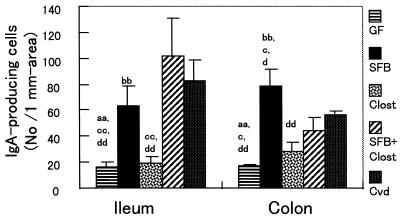
IgA production in the small and large intestines was estimated immunohistochemically by measuring the number of IgA-producing cells in the ileal lamina propria and colonic lamina propria (Fig. 7). In the small intestine, the number of IgA-producing cells was much higher in SFB-mice than that in Clost-mice. The numbers of IgA-producing cells in the colonic lamina propria in these gnotobiotic mice were close to those obtained from the ileum. However, the number of IgA-producing cells in the colonic tissue of Clost-mice was a little higher than that in GF mice, which is different from the ileum data.
FIG. 7.
Numbers of IgA-producing cells in the ileal lamina propria and colonic lamina propria of GF mice, SFB-mice, Clost-mice, mice associated with both SFB and clostridia (SFB + Clost), and Cvd mice. The data are means plus the standard deviations (n = 3 or 4) and were reproducible in the experiment involving the first generation. The letters a, b, c, and d indicate statistically significant differences from the SFB, Clost, SFB + Clost, and Cvd groups, respectively. Single letter, P < 0.05; double letters, P < 0.01.
DISCUSSION
It is uncertain what kinds of microbes are necessary for the development of the gut immune system and how intestinal microbes affect this process, because many kinds of intestinal bacteria are present and their occurrence greatly differs between the small and large intestines. Our previous studies strongly suggested that a kind of indigenous microbe in the small intestine, SFB, is essential for the development of the function of the small intestine (48, 50). SFB are well known to strongly attach to epithelial cells in the small intestine since their discovery in a wide range of animals, including humans. Although SFB have not been cultivated in vitro in spite of much effort, they can be identified based on the 16S rDNA sequence. Moreover, for this study, we chose clostridia as the indigenous bacteria in the large intestine because they are dominant there and are able to normalize cecal size when associated with GF mice them (26). Mouse-derived E. coli, used to facilitate colonization by clostridia, was confirmed not to affect the IEL profile or their cytolytic activity or physiological characteristics substantially (data not shown).
The numbers of IEL, in particular αβIEL, and IgA-producing cells in the small intestine increased to near the levels in Cvd mice, and MHC class II molecules on epithelial cells were expressed in SFB-mice as observed in Cvd mice. However, these characteristics of Clost-mice remained similar to those of GF mice and agreed with the findings on IgA-producing cells in the duodenal mucosa of ex-GF C3H mice associated with indigenous clostridia (37). In the large intestine, the IEL profile of Clost-mice changed to that of Cvd mice but not that of SFB-mice, while the numbers of IgA-producing cells the colonic lamina propria were still larger in SFB-mice than in Clost-mice. Numbers of IgA-producing cells in the colonic tissue of Clost-mice were a little higher than in GF mice.
In SFB-mice or Clost-mice, SFB or clostridia were not confined to the small or large intestine, respectively, as shown in Fig. 1. In mice associated with both SFB and clostridia, these immunological responses were slightly augmented in both parts of the intestine compared to those in SFB-mice or Clost-mice. Based on these results, it seems that the presence of clostridia in the small intestine or of SFB in the large intestine causes enhancement of the immunological responses and at least there is no interference with the immunological responses evoked by the residents of the small or large intestine, i.e., SFB or clostridia, respectively.
How the immune systems of the small and large intestines primarily sense SFB and clostridia, respectively, is an important question. It was suggested that bacterial translocation producing systemic effects might be excluded in the case of SFB and clostridia because these bacteria were not recovered in the blood or spleen after intragastric challenge (data not shown). Taking into the consideration the difference between the immunological responses to the two bacterial populations in the small and large intestines, there may be a difference between the natures of the stimuli produced on colonization by SFB and by clostridia. SFB have been shown to bind epithelial cells in the small intestine but not in the large intestine, although they colonize both parts of the intestine in SFB-mice. Electron microscopy shows the accumulation of electron-dense actin-like materials under the site of insertion of SFB into the microvillus membrane (12, 29). We have evidence that SFB isolated from rats can induce IEL conversion and IgA production in rats, but not in mice, in which they can colonize the intestine but not adhere to epithelial cells (data not shown). On the other hand, clostridia secrete a great amount of metabolites in the large intestine, such as short-chain fatty acids and secondary bile acids (data not shown), although they are not likely to be bound to the epithelial cells in either part of the intestine. Butyrate, a major product, has been reported to be metabolized preferentially in colonic epithelial cells and to stimulate their proliferation (11). If the metabolites are associated with the initiation of signaling, as described above, other bacteria with metabolic activities similar to those of clostridia may be exchangeable with these bacteria. Accordingly, the epithelial cells of the small intestine may sense the adhesion of commensal bacteria while those of the large intestine may sense bacterial metabolites or their gradient, as speculated in the case of the interaction of epithelial cells and Bacteroides thetaiotaomicron in α(1→2)fucosyltransfease induction (6). It is not difficult to envisage pathways that link epithelial cells and the development of immunological characters once epithelial cells have sensed the two bacterial populations. For example, IL-7 secreted by epithelial cells can activate IL-7 receptor-bearing IEL or their progenitors, in particular γδIEL, which are deleted in IL-7R gene knockout mice (15, 51). IL-6 (35) or transforming growth factor β (3) produced by the epithelia during infection can stimulate the development of Peyer’s patches and IgA production (4). Although our results do not rule out the possibility that the initial event(s) in the immune response to commensal bacteria, in particular, IgA production, occurs in Peyer’s patches or lymph nodes after engulfment of the microbes by M cells (3, 5), it is possible to explain both the IEL and IgA responses to commensal bacteria by cross talk between epithelial and immune cells.
Although there is some disagreement as to the effects of the microflora on Vβ usage in the small-intestinal IEL of mice (7) and rats (21), there is a consensus that the small-intestinal IEL expand oligoclonally in mice, rats, and humans (2, 41). In this study, Vβ usage in αβIEL was not so different among these gnotobiotic mice, GF mice, and Cvd mice. However, the proportion of Vβ6+ cells in IEL, with the CD8αβ heterodimer or the CD8αα homodimer, was biased by association with SFB or the whole intestinal flora. It seems that there was little difference in Vβ usage between SFB-mice and Cvd mice. These results suggest that stimuli evoked by the association of SFB with GF mice were not different from those evoked by association with the whole intestinal flora, although there is a difference in the ratio of the number of CD8αβ+ cells to that of CD8αα+ cells between the two groups. The selection of Vβ in both CD8αα+ and CD8αβ+ αβIEL subsets may occur via antigen-driven selection or subset-specific expansion but not differential migration, as suggested by Lefrancois et al. (1). Generally, microbial association seems to be a growth factor-like stimulus for the development of pan-IEL in the digestive tract. We have already established that bacterial colonization of GF mice induces the proliferation of both CD8αα+ and CD8αβ+ αβIEL subsets in bromodeoxyuridine uptake experiments (24). It is not known whether or not these microbes induce the diversification of immunoglobulin genes in Peyer’s patches of rodents, as observed in the bursa of Fabricius in the chick or in ileal Peyer’s patches of sheep (17).
It is also very interesting that the ratio of the number of CD8αβ+ cells to that of CD8αα+ cells in αβIEL of SFB-associated mice exceeded those in the other groups. When clostridia were added to SFB-mice or the whole intestinal flora was associated with GF mice, the CD8αβ+/CD8αα+ cell ratio was decreased to that in conventional mice. Generally, CD8αβ and CD8αα molecules bearing αβIEL are thought to be derived from thymus-dependent and thymus-independent cell lineages, respectively (18, 19), and to differentially respond to TCR stimulation (40). It is difficult to explain why there were more αβIEL with the CD8αβ heterodimer in SFB-mice than in the other groups. As it is assumed that the development of αβIEL is more advanced in Cvd mice than in SFB-mice, αβIEL with CD8αβ molecules are likely to emerge in an intermediate form on the way to those with CD8αα molecules, as observed in the sequential development of neonatal rat IEL expressing CD8αβ (22).
Among the immunological characteristics, the compartmentalization of IEL responses and MHC class II expression on epithelial cells between the two microbe populations in the small and large intestines was clearer than in IgA-producing cells. Therefore, different mechanisms are assumed to underlie the IEL, MHC class II, and IgA responses. IgA-producing cells in the lamina propria are well known to migrate there by homing after their precursor cells have been primed in the Peyer’s patches. On the other hand, it has been suggested that the progenitor cells of IEL, lacking markers of mature T cell, are present in the crypt patches (44) and the IEL themselves (20, 39). Therefore, IEL responses to indigenous bacteria may occur locally in the intestinal mucosa and it is possible that IgA-producing cells, after their precursor cells have been primed in the small intestines of SFB-mice, migrate into the lamina propria of not only the small intestine but also the large intestine via the circulation. In this situation, it is likely that compartmentalization between the two parts of the intestine will be less evident in IgA responses than in IEL responses. As shown in this report, in GF mice, the IEL phenotypes were clearly different in the two parts of the intestine, suggesting that the compartmentalization of IEL responses between the two parts also occurs in the absence of living bacteria.
In this study, two populations of indigenous microbes, SFB and clostridia, were shown to cooperatively contribute to the immune system in the intestine. Definitive compartmentalization of the immunological responses to these microbes, in particular those of IEL, between the small and large intestine was observed. Moreover, it is important to stress that not only MHC class II expression, but also increases in the proliferation rate and the fucosylation of the asialoGM1 glycolipids occurred in the epithelial cells, coincident with the development of the immunological characteristics (data not shown). The immunologic effects of the commensal bacteria are positively exerted in healthy animals with no immunodeficiency, as described in this report. However, when abnormal immunological responses occur due to mutations, as observed in inflammatory bowel disease model mice, the commensal bacteria may play a negative role in disease development.
REFERENCES
- 1.Badiner G, Goodman T G, Lefrancois L. Selection of intestinal intraepithelial lymphocyte T cell receptors: evidence for a dynamic tissue specific process. Int Immunol. 1993;5:223–226. doi: 10.1093/intimm/5.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Balk S P, Ebert E C, Blumenthal R L, Mcdermott F V, Wucherpfenning K W, Landau S B, Blumberg R S. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991;253:1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- 3.Barnard J A, Warwick G J, Gold L I. Localization of transforming growth factor β isoforms in the normal murine small intestine and colon. Gastroenterology. 1993;105:67–73. doi: 10.1016/0016-5085(93)90011-z. [DOI] [PubMed] [Google Scholar]
- 4.Beagley K W, Eldridge J H, Kiyono H, Everson M P, Koopman J P, Hirano Y, Kishimoto T, McGhee J R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg R D, Savage D C. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975;11:320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bry L, Falk P G, Midvedt T, Gordon G I. A model of host-microbial interactions in an open ecosystem. Science. 1996;273:1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 7.Buzoni-Gatel D, Lepage A C, Dimier-Poisson H, Bout D T, Kasper L H. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J Immunol. 1997;158:5883–5889. [PubMed] [Google Scholar]
- 8.Camerini V, Sydora B C, Aaranda R, Nguyen C, MacLean C, McBride W H, Kronenberg M. Generation of intestinal mucosal lymphocytes in scid mice reconstituted with mature, thymus-derived T cells. J Immunol. 1998;160:2608–2618. [PubMed] [Google Scholar]
- 9.Cong Y, Brandwein S L, McCabe R P, Lazenby A, Birkenmeier E H, Sundberg J P, Elson C O. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contractor N V, Bassiri H, Reya T, Park A Y, Baumgart D C, Wasik M A, Emerson S G, Carding S R. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development on colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1998;160:385–394. [PubMed] [Google Scholar]
- 11.Cumming J H, Rombeau J L, Sakata T, editors. Physiological and clinical aspects of short-chain fatty acids. Cambridge, England: Cambridge University Press; 1995. [Google Scholar]
- 12.Davis C P, Savage D C. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974;10:948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dianda L, Hanby M, Wright N A, Sebesteny A, Hayday A C, Owen J C. T cell receptor-αβ-deficient mice fail to develop colitis in the absence of microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 14.Emoto M, Neuhaus O, Emoto Y, Kaufmann S H E. Influence of β2-microglobulin expression on gamma interferon secretion and target cell lysis by intraepithelial lymphocytes during intestinal Listeria monocytogenes infection. Infect Immun. 1996;64:569–575. doi: 10.1128/iai.64.2.569-575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujihashi K, McGhee J R, Yamamoto M, Peschon J, Kiyono H. An interleukin-7 internet for intestinal intraepithelial T cell development: knock out ligand or receptor reveal differences in the immunodeficient state. Eur J Immunol. 1997;27:2133–2138. doi: 10.1002/eji.1830270903. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Salas A, Forcada P, Laguarda M, Gavalda J, Beana J A, Vilaseca J, Malabelada J R. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am J Physiol. 1997;272:G10–G15. doi: 10.1152/ajpgi.1997.272.1.G10. [DOI] [PubMed] [Google Scholar]
- 17.Griebel, P. J., and W. R. Hein. 1996. Expanding the role of Peyer’s patches in B-cell ontogeny. Immunol. Today 1730–1739. [DOI] [PubMed]
- 18.Guy-Grand D, Cref-Benussan N, Malissen B, Malassis-Series M, Briottet C, Vassali P. Two gut intraepithelial lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy-Grand D, Rocha B, Mintz P, Malassis-Series M, Selz F, Malissen B, Vassali P. Different use of T cell receptor transducing molecules on two populations of gut intraepithelial lymphocytes are related to distinct pathways of T cell differentiation. J Exp Med. 1994;180:673–679. doi: 10.1084/jem.180.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamad M, Whestsell M, Wang J, Klein R. T cell progenitors in the murine small intestine. Dev Comp Immunol. 1997;21:435–442. doi: 10.1016/s0145-305x(97)00018-9. [DOI] [PubMed] [Google Scholar]
- 21.Helgeland L, Vaage J T, Rolstad B, Midvedt T, Brandtzaeg P. Microbial colonization influences composition and T-cell receptor Vβ repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 1996;89:494–501. doi: 10.1046/j.1365-2567.1996.d01-783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helgeland L, Brandtzaeg P, Rolstad B, Vaage J T. Sequential development of intraepithelial lymphocytes γδ and αβ T lymphocytes expressing CD8 αβ in neonatal rat intestine: requirement for the thymus. Immunology. 1997;92:447–456. doi: 10.1046/j.1365-2567.1997.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henegan J B. Physiology of the alimentary tract. In: Coates M E, Gustaffson B E, editors. The germ-free animal in biomedical research. London, England: Laboratory Animals Ltd.; 1984. pp. 169–191. [Google Scholar]
- 24.Imaoka A, Matsumoto S, Setoyama H, Okada Y, Umesaki Y. Proliferative recruitment of intestinal intraepithelial lymphocytes after microbial colonization of germ-free mice. Eur J Immunol. 1996;26:945–948. doi: 10.1002/eji.1830260434. [DOI] [PubMed] [Google Scholar]
- 25.Imaoka A, Okada Y, Matsumoto S, Setoyama H, Umesaki Y. 16S ribosomal DNA sequence of segmented filamentous bacteria with special reference to inter-species and within-species variation of host animals. Syst Appl Microbiol. 1997;20:418–422. [Google Scholar]
- 26.Itoh K, Mitsuoka T. Characterization of clostridia isolated from faces of limited flora mice and their effect on caecal size when associated germ-free mice. Lab Anim. 1985;19:111–118. doi: 10.1258/002367785780942589. [DOI] [PubMed] [Google Scholar]
- 27.Klassen H L B, Koopman J P, Poelma F G J, Beynen A C. Intestinal, segmented, filamentous bacteria. FEMS Microbiol Rev. 1992;88:165–180. doi: 10.1111/j.1574-6968.1992.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 28.Komano H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, Momberts P, Tonegawa S, Yamamoto H, Itohara S, Nanno M, Ishikawa H. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koopman J P, Stadhouders A M, Kennis H M, De Boer H. The attachment of filamentous segmented micro-organisms to the distal ileum wall of the mouse: a scanning and transmission electron microscopy study. Lab Anim. 1989;21:48–52. doi: 10.1258/002367787780740743. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn I, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 31.Lefrancois L, Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-γδ+ intraepithelial lymphocytes. Science. 1989;243:1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- 32.Matsumototo S, Okabe Y, Setoyama H, Funahashi H, Imaoka, Okada Y, Umesaki Y. Inflammatory bowel disease-like enteritis and cecitis in a senescence-accelerated mouse (SAM) P1/Yit strain. Gut. 1998;43:71–78. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer L. Current concepts in mucosal immunity. I. Antigen presentation in the intestine: new rules and regulations. Am J Physiol. 1998;274:G7–G9. doi: 10.1152/ajpgi.1998.274.1.G7. [DOI] [PubMed] [Google Scholar]
- 34.McDonald V, Robinson H A, Kelly J P, Bancroft G J. Immunity to Cryptosporidium muris infection in mice is expressed through gut CD4+ intraepithelial lymphocytes. Infect Immun. 1996;64:2556–2562. doi: 10.1128/iai.64.7.2556-2562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGee D W, Beagley K W, Aicher W K, McGhee J R. Transforming growth factor-β enhances interleukin-6 secretion by intestinal epithelial cells. Immunology. 1992;77:7–12. [PMC free article] [PubMed] [Google Scholar]
- 36.Momberts P, Mizoguchi E, Grusby M J, Glimchner L H, Bhan A K, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:275–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 37.Moreau N C, Ducluzeau R, Guy-Grand D, Muller M C. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21:532–539. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mowat A M, Viney J L. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 39.Page S T, Bogatzki L Y, Hamerman J A, Sweenie C H, Hogarth P J, Malissen V, Perlmutter R M, Pullen A M. Intestinal intraepithelial lymphocytes include precursors committed to the T cell receptor αβ lineage. Proc Natl Acad Sci USA. 1998;95:9459–9464. doi: 10.1073/pnas.95.16.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poussier P, Teh H S, Julius M. Thymus-independent positive and negative selection of T cells expressing a major histocompatibility complex class I restricted transgenic T cell receptor α/β in the intestinal epithelium. J Exp Med. 1993;178:1947–1957. doi: 10.1084/jem.178.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regnault A, Levraud J-P, Lim A, Six A, Moreau C, Cumano A, Kourilsky P. The expansion and selection of T cell receptor αβ intestinal epithelial T cell clones. Eur J Immunol. 1996;26:914–921. doi: 10.1002/eji.1830260429. [DOI] [PubMed] [Google Scholar]
- 42.Rothkotter H J, Pabst R. Lymphocyte subsets in jejunal and ileal Peyer’s patches of normal and gnotobiotic minipigs. Immunology. 1989;67:103–108. [PMC free article] [PubMed] [Google Scholar]
- 43.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 44.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut crypt patches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 45.Shroff K H, Meslin K, Cebra J J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snel J, Block H J, Ludwig W, Poelma F G J, Koopman J P, Akkermans A D L. Phylogenetic characterization of Clostridium related segmented bacteria in mice based on 16S ribosomal analysis. Syst Appl Microbiol. 1994;17:172–179. [Google Scholar]
- 47.Sundberg J P, Elson C O, Bedigian H, Birkenmeir E H. Spontaneous heritable colitis in a new substrain of C3H/Hej mice. Gastroenterology. 1994;107:1726–1735. doi: 10.1016/0016-5085(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 48.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of αβ T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79:32–37. [PMC free article] [PubMed] [Google Scholar]
- 49.Umesaki Y, Okada Y, Matsumoto S, Setoyama H. Segmented filamentous bacteria are indigenous bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in ex-germ-free mouse. Microbiol Immunol. 1995;39:555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 50.Umesaki Y, Okada Y, Imaoka A, Setoyama H, Matsumoto S. Interaction between epithelial cells and bacteria, normal and pathogenic. Science. 1997;276:964–965. doi: 10.1126/science.276.5314.964. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe M, Ueno Y, Yajima T, Iwao Y, Tsuchiya M, Ishikawa H, Aiso S, Hibi T, Ishii H. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Investig. 1995;95:2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]