Abstract
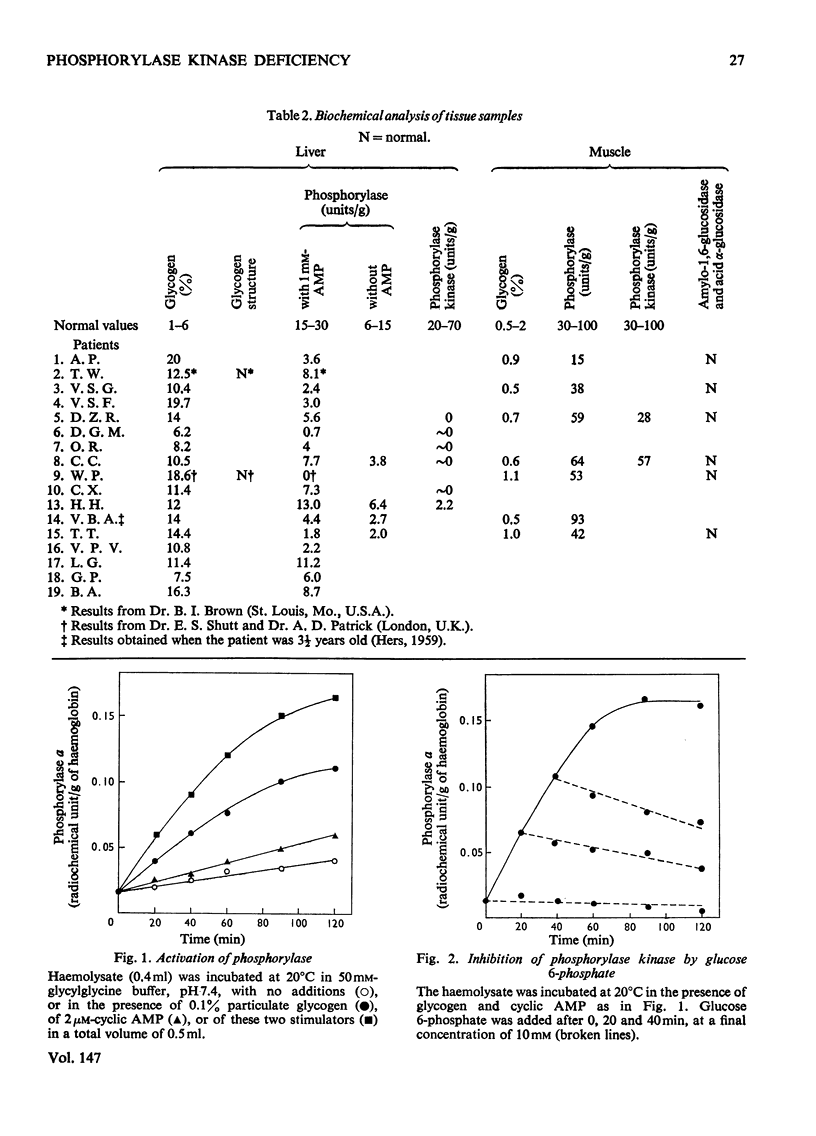
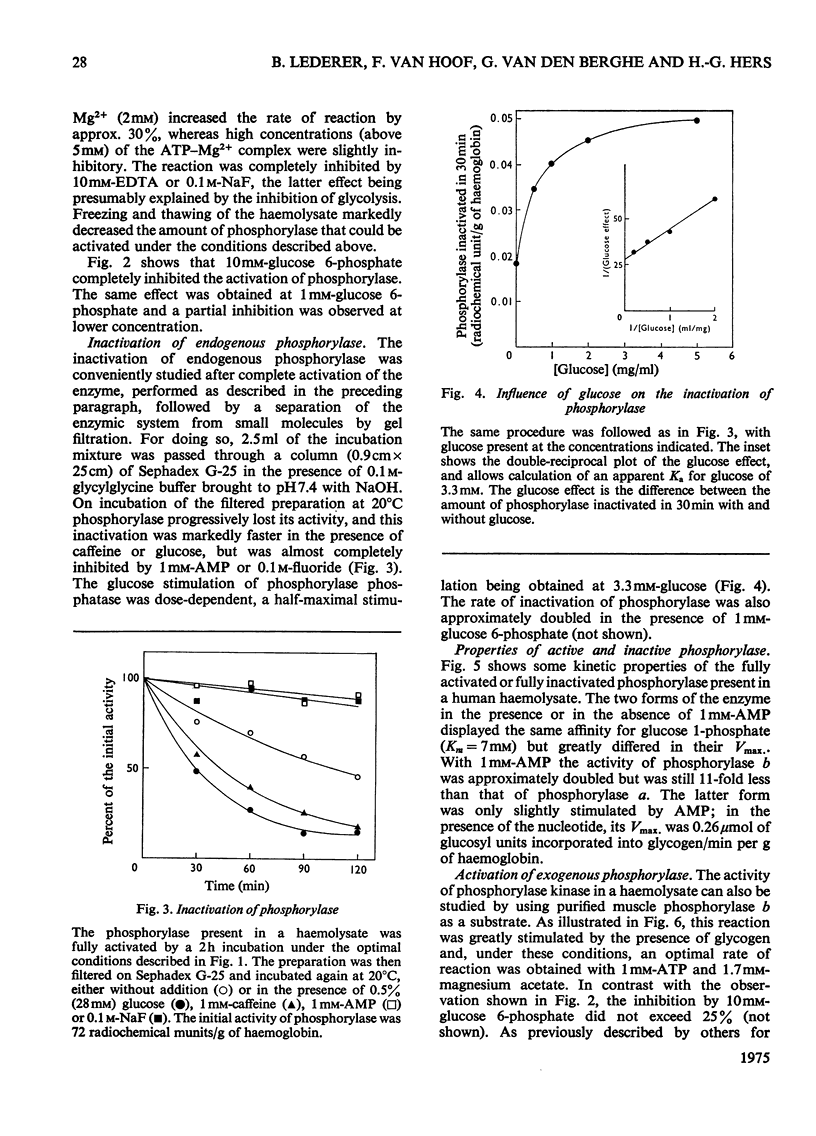
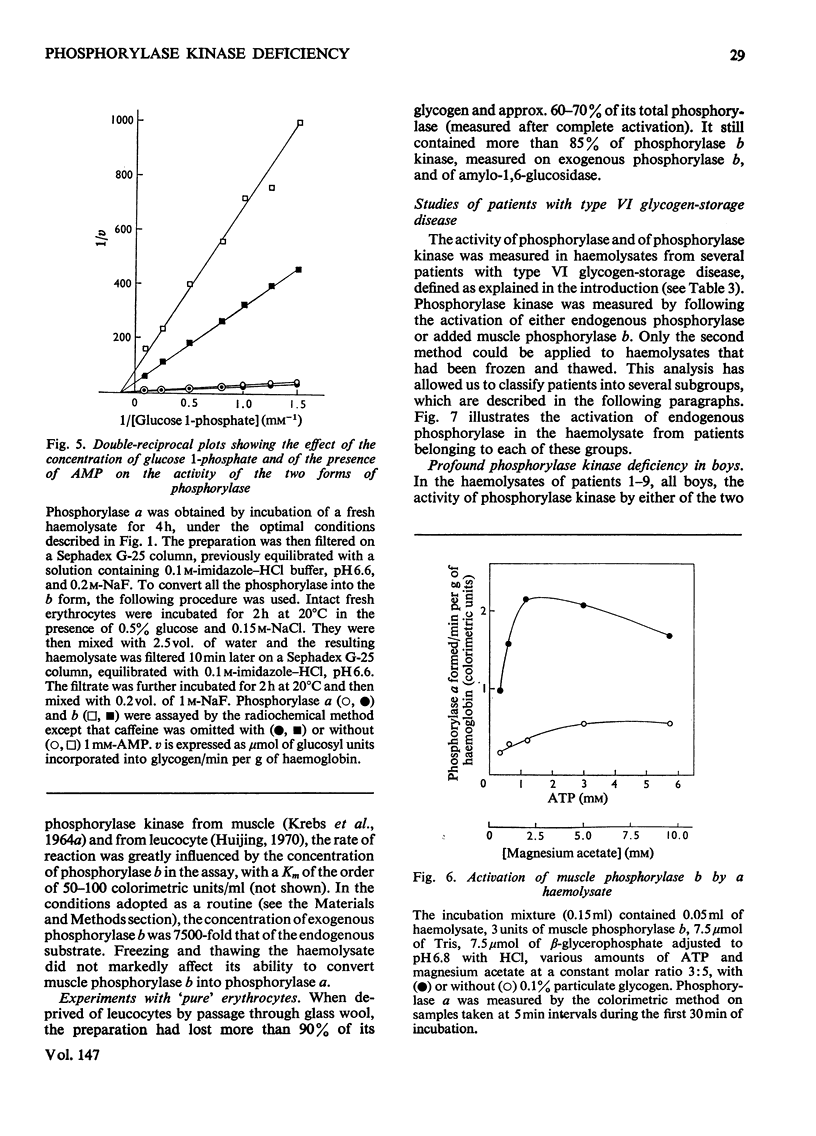
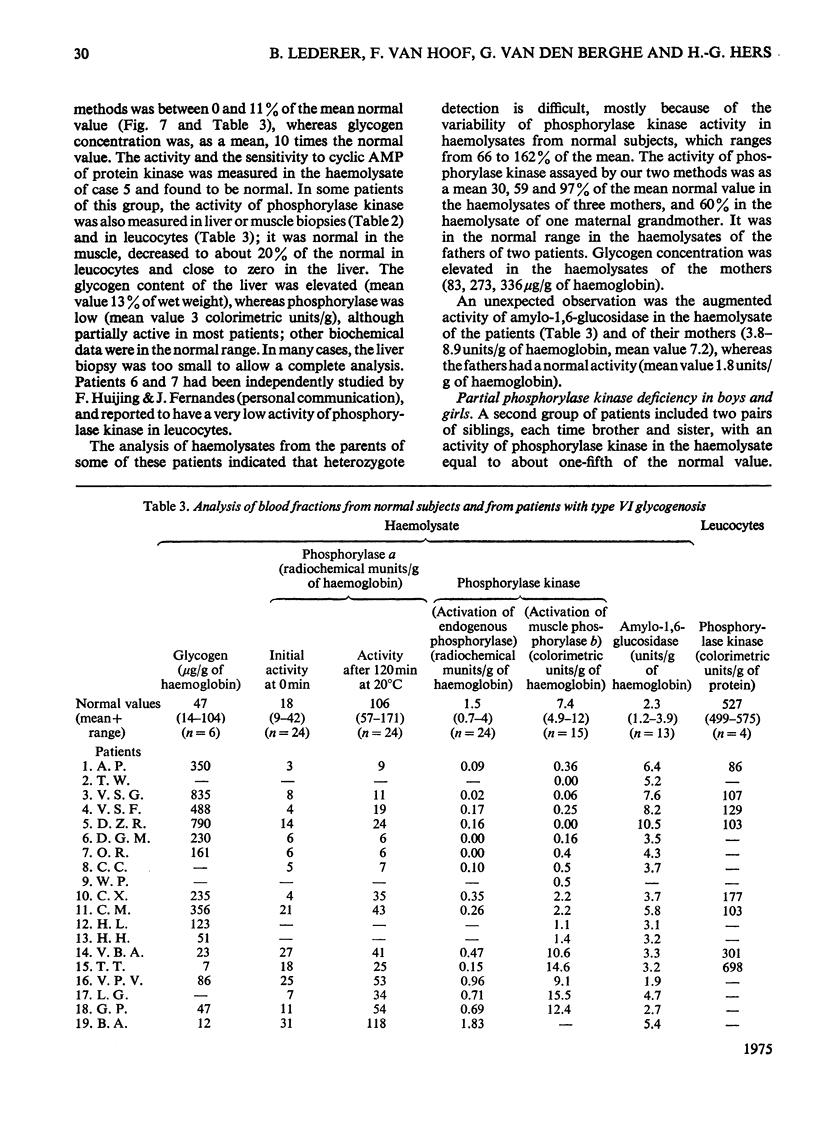
1. The properties of phosphorylase a, phosphorylase b, phosphorylase kinase and phosphorylase phosphatase present in a human haemolysate were investigated. The two forms of phosphorylase have the same affinity for glucose 1-phosphate but greatly differ in Vmax. Phosphorylase b is only partially stimulated by AMP, since, in the presence of the nucleotide, it is about tenfold less active than phosphorylase a. In a fresh human haemolysate phosphorylase is mostly in the b form; it is converted into phosphorylase a by incubation at 20degreesC, and this reaction is stimulated by glycogen and cyclic AMP. Once activated, the enzyme can be inactivated after filtration of the haemolysate on Sephadex G-25. This inactivation is stimulated by caffeine and glucose and inhibited by AMP and fluoride. The phosphorylase kinase present in the haemolysate can also be measured by the rate of activation of added muscle phosphorylase b, on addition of ATP and Mg2+. 2. The activity of phosphorylase kinase was measured in haemolysates obtained from a series of patients who had been classified as suffering from type VI glycogenosis. In nine patients, all boys, an almost complete deficiency of phosphorylase kinase was observed in the haemolysate and, when it could be assayed, in the liver. A residual activity, about 20% of normal, was found in the leucocyte fraction, whereas the enzyme activity was normal in the muscle. These patients suffer from the sex-linked phosphorylase kinase deficiency previously described by others. Two pairs of siblings, each time brother and sister, displayed a partial deficiency of phosphorylase kinase in the haemolysate and leucocytes and an almost complete deficiency in the liver. This is considered as being the autosomal form of phosphorylase kinase deficiency. Other patients were characterized by a low activity of total (a+b) phosphorylase and a normal or high activity of phosphorylase kinase in their haemolysate.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman M. M., Krebs E. G., Fischer E. H. Purification and properties of inactive liver phosphorylase. Biochemistry. 1966 Jun;5(6):2101–2107. doi: 10.1021/bi00870a043. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- De Wulf H., Stalmans W., Hers H. G. The effect of glucose and of a treatment by glucocorticoids on the activation in vitro of liver glycogen synthetase. Eur J Biochem. 1970 Jul;15(1):1–8. doi: 10.1111/j.1432-1033.1970.tb00967.x. [DOI] [PubMed] [Google Scholar]
- Drummond G. I., Hardwick D. F., Israels S. Liver glycogen phosphorylase deficiency. Can Med Assoc J. 1970 Apr 11;102(7):740–742. [PMC free article] [PubMed] [Google Scholar]
- Er-el Z., Zaidenzaig Y., Shaltiel S. Hydrocarbon-coated sepharoses. Use in the purification of glycogen phosphorylase. Biochem Biophys Res Commun. 1972 Oct 17;49(2):383–390. doi: 10.1016/0006-291x(72)90422-6. [DOI] [PubMed] [Google Scholar]
- Guibaud P., Mathieu M. Hétèrogénéite de la glycogénose type VI. Etude de l'activité de la phosphorylase leucocytaire dans deux familles. Arch Fr Pediatr. 1972 Dec;29(10):1043–1057. [PubMed] [Google Scholar]
- HERS H. G. Etudes enzymatiques sur fragments hépatiques; application à la classification des glycogénoses. Rev Int Hepatol. 1959;9(1):35–55. [PubMed] [Google Scholar]
- Hayakawa T., Perkins J. P., Walsh D. A., Krebs E. G. Physiochemical properties of rabbit skeletal muscle phosphorylase kinase. Biochemistry. 1973 Feb;12(4):567–573. doi: 10.1021/bi00728a001. [DOI] [PubMed] [Google Scholar]
- Holmes P. A., Mansour T. E. Glucose as a regulator of glycogen phosphorylase in rat diaphragm. II. Effect of glucose and related compounds on phosphorylase phosphatase. Biochim Biophys Acta. 1968 Mar 11;156(2):275–284. doi: 10.1016/0304-4165(68)90256-0. [DOI] [PubMed] [Google Scholar]
- Hug G., Schubert W. K., Chuck G. Deficient activity of dephosphophosphorylase kinase and accumulation of glycogen in the liver. J Clin Invest. 1969 Apr;48(4):704–715. doi: 10.1172/JCI106028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug G., Schubert W. K., Chuck G. Phosphorylase kinase of the liver: deficiency in a girl with increased hepatic glycogen. Science. 1966 Sep 23;153(3743):1534–1535. doi: 10.1126/science.153.3743.1534. [DOI] [PubMed] [Google Scholar]
- Hug G., Schubert W. K. Type VI glycogenosis: biochemical demonstration of liver phosphorylase deficiency. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1178–1184. doi: 10.1016/0006-291x(70)90210-x. [DOI] [PubMed] [Google Scholar]
- Huijing F., Fernandes J. Liver glycogenosis and phosphorylase kinase deficiency. Am J Hum Genet. 1970 Jul;22(4):484–485. [PMC free article] [PubMed] [Google Scholar]
- Huijing F., Fernandes J. X-chromosomal inheritance of liver glycogenosis with phosphorylase kinase deficiency. Am J Hum Genet. 1969 May;21(3):275–284. [PMC free article] [PubMed] [Google Scholar]
- Huijing F. Glycogen-storage disease Type VIa: low phosphorylase kinase activity caused by a low enzyme-substrate affinity. Biochim Biophys Acta. 1970 Apr 22;206(1):199–201. doi: 10.1016/0005-2744(70)90102-6. [DOI] [PubMed] [Google Scholar]
- Huijing F. Phosphorylase kinase deficiency in mice. FEBS Lett. 1970 Oct;10(5):328–332. doi: 10.1016/0014-5793(70)80465-3. [DOI] [PubMed] [Google Scholar]
- Hurd S. S., Teller D., Fischer E. H. Probable formation of partially phosphorylated intermediates in the interconversions of phosphorylase A and B. Biochem Biophys Res Commun. 1966 Jul 6;24(1):79–84. doi: 10.1016/0006-291x(66)90413-x. [DOI] [PubMed] [Google Scholar]
- KREBS E. G., LOVE D. S., BRATVOLD G. E., TRAYSER K. A., MEYER W. L., FISCHER E. H. PURIFICATION AND PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHORYLASE B KINASE. Biochemistry. 1964 Aug;3:1022–1033. doi: 10.1021/bi00896a003. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Fernandes J., Slee R. G., van Berkel T. J., Hülsmann W. C. Hepatic phosphorylase deficiency: a biochemical study. Biochem Biophys Res Commun. 1973 Jul 2;53(1):282–290. doi: 10.1016/0006-291x(73)91431-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYON J. B., Jr, PORTER J. The relation of phosphorylase to glycogenolysis in skeletal muscle and heart of mice. J Biol Chem. 1963 Jan;238:1–11. [PubMed] [Google Scholar]
- Lyon J. B., Jr The X-chromosome and the enzymes controlling muscle glycogen: phosphorylase kinase. Biochem Genet. 1970 Feb;4(1):169–185. doi: 10.1007/BF00484028. [DOI] [PubMed] [Google Scholar]
- Morishita Y., Nishiyama K., Yamamura H., Kodama S., Negishi H. Glycogen phosphorylase kinase deficiency: a survey of enzymes in phosphorylase activating system. Biochem Biophys Res Commun. 1973 Oct 1;54(3):833–841. doi: 10.1016/0006-291x(73)90769-9. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Proux D., Dreyfus J. C. Phosphorylase isoenzymes in tissues: prevalence of the liver type in man. Clin Chim Acta. 1973 Oct 12;48(2):167–172. doi: 10.1016/0009-8981(73)90362-8. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- STETTEN D., Jr, STETTEN M. R. Glycogen metabolism. Physiol Rev. 1960 Jul;40:505–537. doi: 10.1152/physrev.1960.40.3.505. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., WOSILAIT W. D. The relationship of epinephrine and glucagon to liver phosphorylase. I. Liver phosphorylase; preparation and properties. J Biol Chem. 1956 Jan;218(1):459–468. [PubMed] [Google Scholar]
- Schimke R. N., Zakheim R. M., Corder R. C., Hug G. Glycogen storage disease type IX: benign glycogenosis of liver and hepatic phosphorylase kinase deficiency. J Pediatr. 1973 Dec;83(6):1031–1034. doi: 10.1016/s0022-3476(73)80544-x. [DOI] [PubMed] [Google Scholar]
- Stalmans W., De Wulf H., Lederer B., Hers H. G. The effect of glucose and of a treatment by glucocorticoids on the inactivation in vitro of liver glycogen phosphorylase. Eur J Biochem. 1970 Jul;15(1):9–12. doi: 10.1111/j.1432-1033.1970.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Stalmans W., de Wulf H., Hers H. G. The control of liver glycogen synthetase phosphatase by phosphorylase. Eur J Biochem. 1971 Feb;18(4):582–587. doi: 10.1111/j.1432-1033.1971.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Start C., Newsholme E. A. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. doi: 10.1042/bj1070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARUI S., OKUNO G., IKURA Y., TANAKA T., SUDA M., NISHIKAWA M. PHOSPHOFRUCTOKINASE DEFICIENCY IN SKELETAL MUSCLE. A NEW TYPE OF GLYCOGENOSIS. Biochem Biophys Res Commun. 1965 May 3;19:517–523. doi: 10.1016/0006-291x(65)90156-7. [DOI] [PubMed] [Google Scholar]
- Tu J. I., Graves D. J. Inhibition of the phosphorylase kinase catalyzed reaction by glucose-6-P. Biochem Biophys Res Commun. 1973 Jul 2;53(1):59–65. doi: 10.1016/0006-291x(73)91400-9. [DOI] [PubMed] [Google Scholar]
- Van Hoof F. Amylo-1,6-glucosidase activity and glycogen content of the erythrocytes of normal subjects, patients with glycogen storage disease and heterozygotes. Eur J Biochem. 1967 Oct;2(3):271–274. doi: 10.1111/j.1432-1033.1967.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Yunis A. A., Arimura G. K. Enzymes of glycogen metabolism in white blood cells. II. Activation and inactivation of glycogen phosphorylase of rat chloroma. Biochim Biophys Acta. 1966 May 5;118(2):325–334. doi: 10.1016/s0926-6593(66)80041-3. [DOI] [PubMed] [Google Scholar]
- Yunis A. A., Arimura G. K. Isoenzymes of glycogen phosphorylase in human leukocytes and platelets: relation to muscle phosphorylase. Biochem Biophys Res Commun. 1968 Oct 10;33(1):119–128. doi: 10.1016/0006-291x(68)90265-9. [DOI] [PubMed] [Google Scholar]


