Abstract
OBJECTIVE
The objective of this study was to evaluate whether volumetric measurements on early cranial ultrasound (CUS) in high-grade germinal matrix hemorrhage–intraventricular hemorrhage (GMH-IVH) are associated with hydrocephalus and neurodevelopmental metrics.
METHODS
A retrospective case series analysis of infants with high-grade GMH-IVH admitted to the St. Louis Children’s Hospital neonatal intensive care unit between 2007 and 2015 who underwent neurodevelopmental testing using the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-III) at 2 years of corrected age was performed. GMH volume, periventricular hemorrhagic infarction volume, and frontotemporal horn ratio were obtained from direct review of neonatal CUS studies. Univariate and multivariable regression models were used to evaluate the association between hemorrhage volumes and hydrocephalus requiring permanent CSF diversion with ventricular shunt or endoscopic third ventriculostomy with or without choroid plexus cauterization and composite Bayley-III cognitive, language, and motor scores.
RESULTS
Forty-three infants (29 males, mean gestational age 25 weeks) met the inclusion criteria. The mean age at time of the CUS with the largest hemorrhage volume or first diagnosis of highest grade was 6.2 days. Nineteen patients underwent treatment for hydrocephalus with permanent CSF diversion. In multivariable analyses, larger GMH volume was associated with worse estimated Bayley-III cognitive (left-sided GMH volume: p = 0.048, total GMH volume: p = 0.023) and motor (left-sided GMH volume: p = 0.010; total GMH volume: p = 0.014) scores. Larger periventricular hemorrhagic infarction volume was associated with worse estimated Bayley-III motor scores (each side p < 0.04). Larger left-sided (OR 2.55, 95% CI 1.10–5.88; p = 0.028) and total (OR 1.35, 95% CI 1.01–1.79; p = 0.041) GMH volumes correlated with hydrocephalus. There was no relationship between early ventricular volume and hydrocephalus or neurodevelopmental outcomes.
CONCLUSIONS
Location-specific hemorrhage volume on early CUS may be prognostic for neurodevelopmental and hydrocephalus outcomes in high-grade GMH-IVH.
Keywords: germinal matrix hemorrhage, neurodevelopment, ultrasound, hydrocephalus, neonatal
Germinal matrix hemorrhage–intraventricular hemorrhage (GMH-IVH) is the most common cause of brain injury in very preterm infants and is responsible for the worst neurocognitive outcomes of all preterm brain injuries.1 This condition affects up to 25% of low-birth-weight (< 1500 g) preterm infants, and its incidence correlates inversely with gestational age (GA).2,3 GMH-IVH severity is standardly graded based on a diagnostic cranial ultrasound (CUS),4 with the worst outcomes occurring in neonates with high-grade GMH-IVH (Papile grades III and IV).5 As the germinal matrix is the site of ongoing neuro- and gliogenesis,6,7 it is imperative to understand how hemorrhage within this area is associated with outcome.
At the time of high-grade GMH-IVH diagnosis, there are three potential regional hemorrhagic components: GMH (usually within the ganglionic eminence),4,8 IVH, and periventricular hemorrhagic infarction (PVHI). These pathologies are frequently grouped together for the purpose of outcome studies, although the pathophysiology underlying each is different. Early ultrasound features of high-grade GMH-IVH, especially bilateral pathology, have been associated with cognitive and language outcomes.9–12 While ultrasound features of PVHI have been associated with motor and cognitive outcomes, the majority of these studies are restricted to linear measurements and/or lack detailed neurodevelopmental testing.13–15 Furthermore, no studies have evaluated the volume of hemorrhage within the progenitor-rich germinal matrix or PVHI and their relationship to neurodevelopmental outcomes. Posthemorrhagic hydrocephalus (PHH) occurs in up to 25%–30% of infants with high-grade GMH-IVH and is defined as elevated intracranial pressure with clinical signs of enlarging head circumference, bulging anterior fontanelle, splayed cranial sutures, poor feeding, irritability, and lethargy with accompanying radiological evidence of progressive ventricular dilation.16 PHH may require surgical intervention with permanent CSF diversion, such as ventriculoperitoneal shunting, which requires lifelong monitoring and may further worsen neurocognitive outcomes.1,17–20 However, there are no metrics for assessing the risk for hydrocephalus in high-grade GMH-IVH. Furthermore, while animal models support IVH as a key factor in the pathophysiology of hydrocephalus,21 how each hemorrhagic component within high-grade GMH-IVH contributes to the development of hydrocephalus remains unknown.
This study is the first to address the relationship between volumetric measurements of GMH and PVHI components in high-grade GMH-IVH on CUS with neurodevelopmental and hydrocephalus outcomes. These measurements are readily obtainable using 2D CUS studies acquired on a routine clinical basis, which has important implications for early outcome prediction, particularly in settings with limited access to higher-resolution imaging, and for understanding the pathophysiology of poor neurodevelopmental and hydrocephalus outcomes in high-grade GMH-IVH.
Methods
Participants
All aspects of this retrospective study of a prospectively recruited, longitudinal cohort were approved by the IRB at the Washington University in St. Louis. This larger prospectively recruited cohort included patients born at ≤ 30 weeks of GA without evidence of chromosomal abnormality or suspected/proven congenital infection admitted to the St. Louis Children’s Hospital neonatal intensive care unit from 2007 to 2015. Neurodevelopmental testing using the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-III) at 2 years of corrected age1 was performed on all participants as a part of the research program (i.e., not obtained clinically). Those with grade III and/or IV GMH-IVH diagnosed on CUS within the 1st month of life were selected for analysis in this study. Patients with grade II GMH-IVH (without higher contralateral grade and without subsequent progression to higher grade) were also identified from the same cohort for comparison of GMH volume and ventricular size. Clinical information was used to generate a clinical medical risk index, as previously published, which includes factors related to prematurity-related complications such as intrauterine growth restriction, prolonged oxygen supplementation, antenatal steroid exposure, necrotizing enterocolitis, sepsis, patent ductus arteriosus, retinopathy of prematurity, relative weight, and duration of parenteral nutrition.22 Parents or legal guardians provided standardized sociodemographic information that was used to calculate sociodemographic stressor index scores, as previously published.23–26 Informed consent was obtained from parents or legal guardians prior to inclusion in the study.
CUS Image Analysis
Quantification of GMH and PVHI
CUS studies for all participants were obtained according to clinical protocols at our institution.4 Preset settings for frequency, scan depth (field of view), and initial dynamic range, as set by hospital standards, were used for image acquisition and were similar between all scans (frequency 8–10 MHz, dynamic range 70, depth 7–10 cm). CUS images were directly reviewed by one trained physician blinded to the outcome (P.H.Y.). All CUSs starting from the initial CUS to the first CUS with resolving hemorrhage were reviewed. The earliest CUS scan with the highest grade or, if the hemorrhage enlarged, the CUS scan with the largest hemorrhage volume was used for analysis. Measurements and locations for both the GMH and PVHI components were determined based on hyperechoic signal consistent with blood products and estimated to have spherical or ellipsoid geometries. SmallMeasure software27 was used to perform linear measurements of hemorrhage in pixels, and then each image was scaled using embedded depth calibration markers. For each location of hemorrhage (GMH [Fig. 1A] or PVHI [Fig. 1B]), the coronal and sagittal views with the largest hemorrhage diameters were used to calculate volume:28 Volume = (a × b × c)/2, where a, b, and c are the left-to-right, superior-to-inferior, and anterior-to-posterior lengths, respectively. For hemorrhages with indistinct borders between GMH and PVHI, only the component confined to the anatomical limits of the subependymal germinal matrix within or adjacent to the caudothalamic groove was considered as GMH, while any hemorrhage outside this anatomical region was considered PVHI.
FIG. 1.
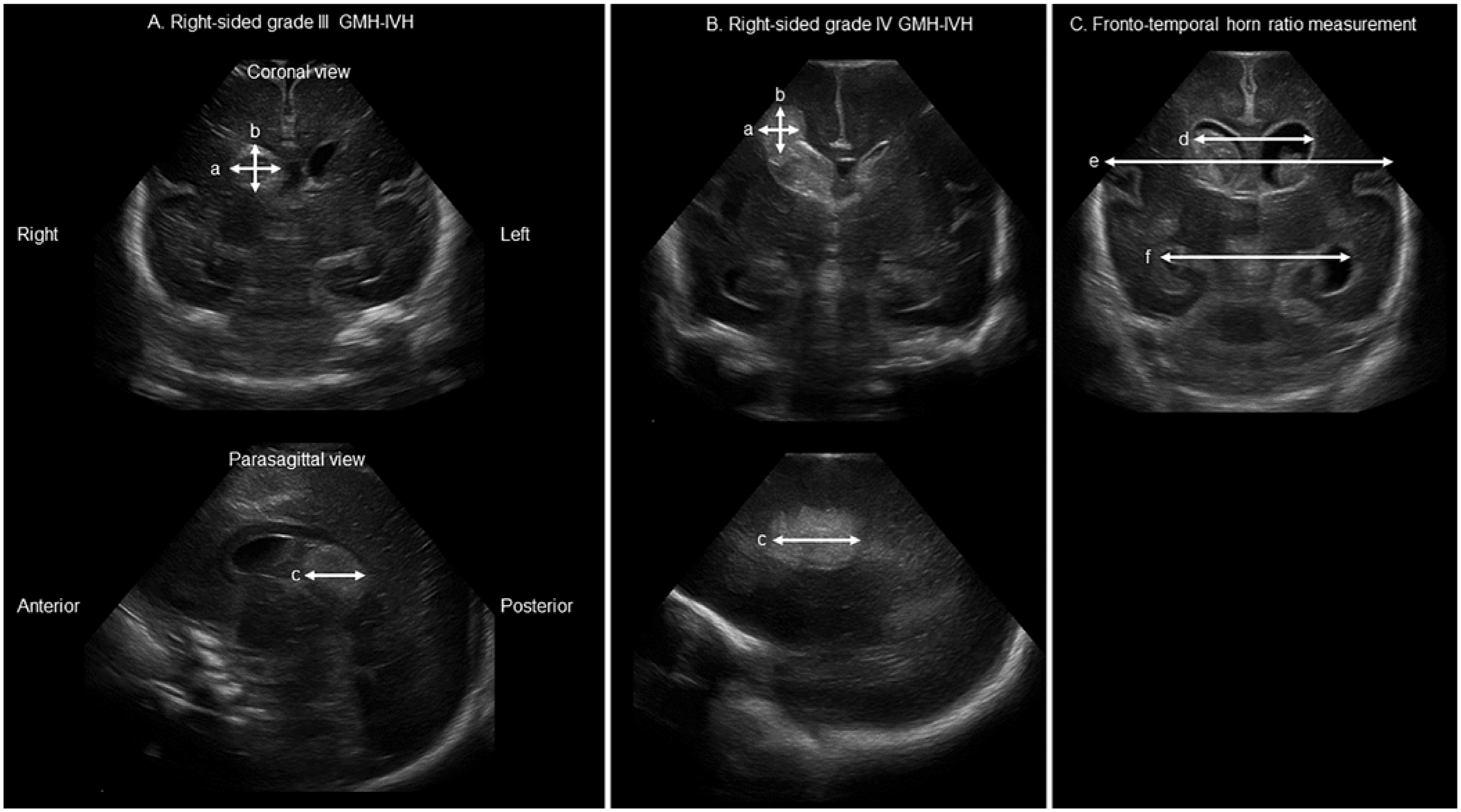
CUS measurements of hemorrhage volumes and ventricular size. A: Measurements obtained along the three major axes of a right-sided GMH. B: Measurements obtained along the three major axes of a right-sided PVHI. C: Measurements obtained to calculate FTHR.
An analysis of linear versus volumetric GMH and PVHI measurements used the longest of the three linear measurements described above to correlate with outcome.
Quantification of Ventricular Size
The frontotemporal horn ratio (FTHR)29 was obtained using coronal images at the level of the foramina of Monro (Fig. 1C), defined as FTHR = (d + e)/(2 × f), where d, e, and f are the bifrontal distance, bitemporal distance, and biparietal distance, respectively.
Neurodevelopmental and Behavioral Testing
All subjects underwent assessments using Bayley-III testing30 at 2 years of age, corrected for prematurity, by blinded psychometricians. Composite scores for the motor, language, and cognitive domains were reported.
Statistical Analysis
Two-sample t-tests, one-way ANOVAs, or Wilcoxon rank-sum tests were used to assess the differences of continuous variables as appropriate. Chi-square tests or Fisher’s exact tests were used to assess the relationship between categorical variables. Unadjusted and adjusted linear regression models were performed to test associations between clinical factors and Bayley-III scores. Unadjusted and adjusted logistic regression models were performed to assess the effect of risk factors on shunt placement. Pearson’s correlation analysis was conducted to detect correlations between continuous variables. Data were analyzed using IBM SPSS Statistics version 29.0.0.0 (IBM Corp.). A p value < 0.05 was considered statistically significant. FTHR, estimated GA, sex, and clinical variables with p < 0.05 in univariate analysis were used as covariates in multivariable analyses.
Results
Clinical Characteristics
Fifty-eight patients with grade III and/or IV GMH-IVH were identified from a prospectively studied cohort of 213 very preterm infants. Fifteen patients were excluded due to missing/incomplete Bayley-III scores or CUS imaging unavailable for direct review in the case of outborn infants. A total of 43 infants (29 males, mean 25.0 weeks estimated GA, mean birth weight 835 g) met the inclusion criteria (Table 1). Nine had bilateral grade III GMH-IVH, 6 had bilateral grade IV, 3 had unilateral grade III, 5 had unilateral grade IV, and 20 had some combination of bilateral grade III and IV. Nine with grade III or IV GMH-IVH had either no contralateral GMH-IVH or grade I GMH-IVH. Two had unilateral subcentimeter cerebellar hemorrhages. The mean age at GMH-IVH diagnosis was 3.0 days (range 0–13 days). The mean age at the time of the CUS with the largest hemorrhage volume or initial highest grade was 6.2 days (range 1–20 days). Nineteen patients underwent temporary CSF diversion with ventricular reservoir (n = 17) or subgaleal shunt (n = 2) placement (mean 27.5 days after birth, SD 13.4 days). One patient underwent permanent CSF diversion without temporization. One patient with temporary CSF diversion did not require permanent CSF diversion. Therefore, 19 patients underwent permanent CSF diversion surgery; of these, 2 patients initially underwent endoscopic third ventriculostomy with choroid plexus cauterization (ETV-CPC) and 1 had ETV without CPC, but all 3 returned with hydrocephalus and ventricular shunts were placed. Therefore, all 19 patients who had permanent CSF diversion had ventricular shunts. There was no difference in baseline clinical characteristics, neurodevelopment, or shunt outcomes between patients with grade III and grade IV GMH-IVH (Table 1). A higher medical risk index was associated with worse motor outcomes (p = 0.027), and a greater number of general anesthesia events in the study period was associated with worse cognitive (p = 0.030) and motor (p = 0.006) outcomes (Table 2).
TABLE 1.
Clinical and radiological characteristics of patients with high-grade GMH-IVH
| Clinical Characteristic | High-Grade (n = 43) | Grade III (n = 12) | Grade IV (n = 31) | p Value* |
|---|---|---|---|---|
| Estimated GA, wks | 25.0 (1.9) | 25.2 (1.5) | 24.9 (2.1) | 0.69 |
| Male sex, n (%) | 29 (67.4) | 10 (83.3) | 19 (61.3) | 0.28 |
| Birth weight, g | 835.2 (178.9) | 852.5 (132.8) | 828.6 (195.3) | 0.70 |
| FTHR | 0.52 (0.08) | 0.50 (0.06) | 0.52 (0.09) | 0.48 |
| Medical risk index, median (IQR) | 3 (2–5) | 3 (2.5–6) | 3 (2–5) | 0.50 |
| Sociodemographic stressor index, median (IQR) | 2 (0–3) | 2 (0–3) | 2 (0–3) | 0.94 |
| General anesthesia events | 3.8 (3.6) | 4.6 (2.9) | 3.5 (3.8) | 0.32 |
| Bayley-III score | ||||
| Cognition | 75.9 (12.7) | 81.7 (11.9) | 73.7 (12.5) | 0.07 |
| Language | 73.7 (25.5) | 82.8 (13.5) | 70.2 (28.3) | 0.06 |
| Motor | 68.6 (17.0) | 68.4 (24.4) | 68.7 (13.6) | 0.98 |
| CP diagnosis, n (%) | 30 (69.8) | 9 (75.0) | 21 (67.7) | 0.73 |
| Needs assistance w/ ADLs, n (%)† | 11 (28.2) | 2 (20.0) | 9 (31.0) | 0.69 |
| Walks freely w/o assistance, n (%) | 32 (74.4) | 10 (83.3) | 22 (71.0) | 0.70 |
| Symmetric gait, n (%) | 26 (60.5) | 10 (83.3) | 16 (51.6) | 0.08 |
| Toe-walking, n (%)† | 20 (51.3) | 6 (54.5) | 14 (50.0) | 0.99 |
| Shunt placement, n (%) | 19 (44.2) | 6 (50.0) | 13 (41.9) | 0.74 |
| Hemorrhage vol, cm3 | ||||
| Lt GMH vol | 0.14 (0.14) | 0.07 (0.06) | 0.17 (0.16) | 0.006 |
| Rt GMH vol | 0.29 (0.42) | 0.09 (0.10) | 0.36 (0.47) | 0.004 |
| Lt PVHI vol | —‡ | 0.98 (1.07) | — | |
| Rt PVHI vol | — | 1.68 (2.22) | — |
CP = cerebral palsy.
Values are given as mean (SD) unless otherwise indicated. Boldface type indicates statistical significance (p < 0.05).
Calculated using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables.
Excludes 4 patients with missing data.
Not applicable as grade III by definition does not have PVHI.
TABLE 2.
Clinical and radiologic factors and their association with neurodevelopmental outcomes
| Bayley-III Score* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognition | Language | Motor | |||||||
| Estimated Change | SE | p Value | Estimated Change | SE | p Value | Estimated Change | SE | p Value | |
| Unadjusted linear regression model | |||||||||
| Estimated GA, wks | +0.93 | 1.04 | 0.37 | −0.319 | 1.334 | 0.812 | +1.353 | 1.077 | 0.216 |
| Male sex | −2.33 | 4.18 | 0.58 | −3.012 | 5.523 | 0.589 | +2.893 | 4.392 | 0.514 |
| Birth weight, g | +0.02 | 0.01 | 0.18 | 0.014 | 0.015 | 0.336 | +0.022 | 0.011 | 0.057 |
| Medical risk index | −1.98 | 0.99 | 0.054 | −2.494 | 1.248 | 0.053 | −2.348 | 1.024 | 0.027 |
| Sociodemographic stressor index | −0.38 | 1.29 | 0.77 | −1.694 | 1.713 | 0.329 | −0.092 | 1.367 | 0.946 |
| No. of general anesthesia events | −1.19 | 0.53 | 0.030 | −0.804 | 0.698 | 0.257 | −1.541 | 0.534 | 0.006 |
| FTHR | −13.7 | 24.0 | 0.57 | −4.6 | 48.3 | 0.92 | −14.8 | 32.1 | 0.65 |
| GMH vol, cm3 | |||||||||
| Lt | −36.45 | 13.32 | 0.009 | −27.087 | 18.185 | 0.145 | −42.403 | 13.611 | 0.003 |
| Rt | −7.941 | 4.802 | 0.106 | −6.469 | 6.282 | 0.310 | −7.532 | 4.900 | 0.133 |
| Total | −9.061 | 3.998 | 0.029 | −8.069 | 5.247 | 0.132 | −10.382 | 4.148 | 0.017 |
| PVHI vol, cm3 | |||||||||
| Lt | −2.224 | 3.085 | 0.481 | −4.620 | 4.505 | 0.322 | −3.844 | 2.824 | 0.192 |
| Rt | −2.302 | 1.202 | 0.072 | −2.260 | 1.616 | 0.181 | −3.265 | 1.403 | 0.033 |
| Multivariable model | |||||||||
| GMH vol, cm3 | |||||||||
| Lt† | −28.8 | 14.0 | 0.048 | −21.9 | 19.3 | 0.268 | −35.8 | 13.1 | 0.010 |
| Rt† | −7.30 | 4.89 | 0.145 | −8.76 | 6.78 | 0.206 | −6.23 | 4.61 | 0.186 |
| Total‡ | −9.85 | 4.17 | 0.023 | −11.0 | 5.54 | 0.054 | −10.8 | 4.21 | 0.014 |
| PVHI vol, cm3 | |||||||||
| Lt§ | −2.35 | 3.20 | 0.474 | −4.39 | 4.69 | 0.367 | −3.80 | 2.94 | 0.215 |
| Rt§ | −2.23 | 1.19 | 0.080 | −2.19 | 1.65 | 0.204 | −3.21 | 1.43 | 0.039 |
| Lt¶ | −4.30 | 3.26 | 0.206 | −7.02 | 5.05 | 0.188 | −6.36 | 2.80 | 0.038 |
| Rt¶ | −2.03 | 1.22 | 0.114 | −2.23 | 1.69 | 0.208 | −2.90 | 1.40 | 0.055 |
| Lt** | −2.53 | 2.70 | 0.363 | −4.57 | 2.46 | 0.85 | −4.09 | 2.60 | 0.137 |
| Rt** | −2.48 | 1.05 | 0.031 | −2.34 | 1.64 | 0.173 | −3.46 | 1.26 | 0.014 |
| Lt†† | −0.244 | 3.16 | 0.939 | −2.99 | 4.83 | 0.547 | −2.26 | 2.94 | 0.461 |
| Rt†† | −1.60 | 1.20 | 0.203 | −1.97 | 1.75 | 0.278 | −2.48 | 1.42 | 0.099 |
Boldface type indicates statistical significance (p < 0.05).
Estimated change of composite score with 1 unit increasing in the continuous factors or estimated score difference between two groups (yes minus no) for categorical factors.
Controlling for medical risk index, number of general anesthesia events, and contralateral GMH volume.
Controlling for medical risk index and number of general anesthesia events.
Controlling for presence of contralateral GMH volume.
Controlling for presence of contralateral PVHI volume.
Controlling for medical risk index.
Controlling for number of general anesthesia events.
Hemorrhage Quantification
The mean GMH volume was 0.14 cm3 on the left and 0.29 cm3 on the right (Table 1). GMH volumes were larger in patients with grade IV GMH-IVH compared with those with grade III GMH-IVH (0.17 cm3 vs 0.07 cm3 on the left [p = 0.006] and 0.36 cm3 vs 0.09 cm3 on the right [p = 0.004]). The mean PVHI volume was 1.68 cm3 on the right and 0.98 cm3 on the left. There was a positive correlation between right-sided GMH volume and PVHI volume (p = 0.02) and between left-sided GMH volume and FTHR (p = 0.04) (Supplementary Table 2).
Hemorrhage Volume and Neurodevelopmental Outcomes
In the unadjusted linear regression analysis, larger left-sided and total GMH volumes were associated with lower Bayley-III cognition (left-sided p = 0.009, total p = 0.029) and motor (left-sided p = 0.003, total p = 0.017) scores (Table 2). For every cubic centimeter increase in total GMH volume, the cognitive score decreased by 9 points and the motor score decreased by 10 points. The motor score decreased by 3.3 points with every cubic centimeter increase in right PVHI volume (p = 0.03). The estimated GA, sex, birth weight, sociodemographic stressor index, and ventricular size (FTHR) were not associated with Bayley-III scores.
After controlling for medical risk index, number of general anesthesia events, and contralateral grade III hemorrhage, we found that greater left-sided GMH volume remained associated with Bayley-III cognitive scores (p = 0.048), in which there was a decrease by 28.8 points with every cubic centimeter increase in left-sided GMH volume. Greater left-sided GMH volume was associated with Bayley-III motor scores (p = 0.010), in which there was a decrease by 35.8 points with every cubic centimeter increase in left-sided GMH volume. Greater total GMH volume was independently associated with worse cognition (p = 0.023) and motor (p = 0.014) scores when controlling for medical risk index and number of general anesthesia events.
Larger PVHI volume was associated with worse motor outcomes when controlling for contralateral hemorrhage volume (right PVHI when controlling for left GMH volume: p = 0.039, left PVHI when controlling for right PVHI volume: p = 0.038). However, the impact of PVHI volume on motor outcomes was smaller than the impact of left-sided and total GMH volumes.
In addition to Bayley-III scores, we also evaluated several clinical outcome metrics that relate to global performance and found similar results (Supplementary Table 1). Information regarding gross motor function and cerebral palsy was similarly collected retrospectively in this prospectively curated database. In the multivariable analysis, larger left GMH volume was associated with asymmetrical gait (p = 0.012) and toe-walking (p = 0.041), larger total GMH volume was associated with needing assistance with activities of daily living (ADLs) (p = 0.038), and right PVHI volume was associated with the inability to walk freely without assistance (p = 0.042). Cerebral palsy diagnosis was not associated with hemorrhage volume on multivariable analysis.
Hemorrhage Volume and Hydrocephalus Outcomes
In unadjusted logistic regression analysis, both left-sided (OR 2.42, 95% CI 1.22–4.79; p = 0.011) and total (OR 1.31, 95% CI 1.03–1.673; p = 0.030) GMH volumes were associated with permanent CSF diversion (Table 3). Estimated GA, sex, weight, medical risk index, and sociodemographic stressor index were not associated with the need for permanent CSF diversion. There was no association between early FTHR and shunted hydrocephalus in high-grade GMH-IVH patients; however, there was a positive correlation between left GMH volumes and ventricular size (p = 0.04) (Supplementary Table 2). After controlling for FTHR, GA, sex, and contralateral GMH volume, we found that left-sided GMH volume remained associated with the need for permanent CSF diversion (OR 2.55, 95% CI 1.10–5.88; p = 0.028) (Table 3). The total GMH volume was associated with permanent CSF diversion after controlling for FTHR, GA, and sex (OR 1.35, 95% CI 1.01–1.79; p = 0.041).
TABLE 3.
Clinical and radiologic factors and their association with hydrocephalus outcomes
| Shunt (n = 19) | No Shunt (n = 24) | OR (95% CI)* | p Value | |
|---|---|---|---|---|
| Unadjusted logistic regression model | ||||
| Estimated GA, wks | 24.8 (1.5) | 25.1 (2.2) | 0.903 (0.638–1.279)a | 0.566 |
| Male sex, n (%) | 15 (79.0) | 14 (58.3) | 2.679 (0.681–10.534) | 0.158 |
| Birth weight, g | 845.0 (173.1) | 827.5 (186.7) | 1.01 (0.97–1.04)a | 0.748 |
| GMH vol, cm3 | ||||
| Lt | 0.22 (0.18) | 0.09 (0.07) | 2.42 (1.22–4.79)b | 0.011 |
| Rt | 0.45 (0.58) | 0.19 (0.18) | 1.22 (0.97–1.519)b | 0.083 |
| Total | 0.64 (0.62) | 0.26 (0.18) | 1.31 (1.03–1.673)b | 0.030 |
| PVHI vol, cm3 | ||||
| Lt | 1.15 (1.08) | 0.85 (1.10) | 1.03 (0.940–1.125)b | 0.544 |
| Rt | 2.51 (2.9) | 0.94 (1.06) | 1.05 (0.980–1.121)b | 0.175 |
| FTHR | 0.53 (0.06) | 0.51 (0.10) | 1.04 (0.958–1.12)c | 0.384 |
| Medical risk index, median (IQR) | 3 (2–5) | 3 (1–4.5) | 1.168 (0.844–1.618)a | 0.349 |
| Sociodemographic stressor index, median (IQR) | 2 (0–3) | 2 (0–3) | 1.074 (0.722–1.597)a | 0.724 |
| Multivariable model | ||||
| GMH vol, cm3 | ||||
| Lt† | 0.22 (0.18) | 0.09 (0.07) | 2.55 (1.10–5.88)b | 0.028 |
| Rt† | 0.45 (0.58) | 0.19 (0.18) | 1.18 (0.923–1.52)b | 0.183 |
| Total‡ | 0.64 (0.62) | 0.26 (0.18) | 1.35 (1.01–1.79)b | 0.041 |
| PVHI vol, cm3 | ||||
| Lt§ | 1.15 (1.08) | 0.85 (1.10) | 1.02 (0.932–1.12)b | 0.646 |
| Rt§ | 2.51 (2.9) | 0.94 (1.06) | 1.05 (0.980–1.12)b | 0.173 |
| Lt¶ | 1.15 (1.08) | 0.85 (1.10) | 1.08 (0.962–1.21)b | 0.194 |
| Rt¶ | 2.51 (2.9) | 0.94 (1.06) | 1.05 (0.976–1.13)b | 0.186 |
Values are given as mean (SD) unless otherwise indicated. Boldface type indicates statistical significance (p < 0.05).
The ORs of continuous variables have different units: a) per 1-unit increase, b) per 0.1-unit increase, and c) per 0.01-unit increase. The ORs of categorical variables are male versus female (reference group) or yes versus no (reference group).
Controlling for FTHR, GA, sex, and contralateral GMH volume.
Controlling for FTHR, GA, and sex.
Controlling for presence of contralateral GMH.
Controlling for presence of contralateral PVHI.
To further investigate the relationship between GMH volume, ventricular size, and hydrocephalus outcomes, we evaluated a separate cohort of patients with grade II GMH-IVH, a population in which PHH rates are known to be low (Supplementary Table 3).31,32 Fifteen patients (9 with bilateral GMH and 6 with unilateral GMH, totaling 12 right-sided GMHs and 12 left-sided GMHs) in the recruited cohort had grade II GMH-IVH without contralateral higher-grade or progression to higher-grade hemorrhage, and none underwent CSF diversion surgery. As expected, FTHR was lower in patients with grade II GMH-IVH than in high-grade GMH-IVH patients. Interestingly, right- and left-sided GMH volumes were also smaller in grade II patients (p < 0.001 and p = 0.001, respectively). Because both GMH volume and FTHR were lower in grade II patients, one cannot definitively say that hydrocephalus outcomes are strictly due to one or the other.
Shunt Complications and Neurodevelopmental Outcomes
We assessed whether complications related to hydrocephalus treatment within the study period (number of revision surgeries and infections by 2 years of corrected age) were associated with neurodevelopmental outcomes (Supplementary Table 4), which may offer an alternative explanation for the differences seen in Bayley-III scores. There were 2 shunt infections out of the 19 patients with shunted hydrocephalus. There was no statistically significant association between number of shunt revisions or shunt infections with Bayley-III cognition, language, or motor scores.
Comparison of Volumetric and Linear Hemorrhage Measurements With Outcome
To compare our findings with prior reports using linear measurements of PVHI size,33 we compared Bayley-III scores and hydrocephalus outcomes with both volumetric and linear measurement techniques. Our linear measurement approach revealed a negative correlation between total GMH length, left- and right-sided PVHI lengths, and motor outcomes in the unadjusted linear regression model (Supplementary Table 5). In the multivariable analysis, after controlling for medical risk index, number of general anesthesia events, and contralateral GMH length, we found that left GMH length was negatively correlated with motor scores (p = 0.028) and right GMH length was negatively correlated with cognitive (p = 0.024) and language (p = 0.031) scores. Total GMH length was negatively correlated with language (p = 0.030) and motor (p = 0.013) scores. Left- and right-sided PVHI lengths were negatively associated with motor scores when controlling for contralateral hemorrhage, and right PVHI lengths were associated with motor scores (p = 0.005) when controlling for medical risk index. Finally, GMH and PVHI lengths were not correlated with hydrocephalus outcomes in the multivariable analysis (Supplementary Table 6).
Discussion
Preterm infants with high-grade GMH-IVH are often clinically unstable, resulting in difficulty acquiring high-resolution cross-sectional cranial images at the time of GMH-IVH diagnosis, which has contributed to the use of categorical metrics of easily identifiable hemorrhagic components for outcome prediction.9–12,20 In this study, we show the feasibility of using volumetric measurements of hemorrhagic components within the brain parenchyma on routine clinical 2D CUS to predict neurodevelopmental and hydrocephalus outcomes, expanding on prior studies that used linear measurements.13,34 Importantly, we also demonstrate the utility of volumetric measurements and control for predictors of outcome when assessing the independent prognostic value of individual hemorrhagic components, including GMH volume which to our knowledge has not been previously assessed before in this context.2,3 Our findings suggest that hemorrhage burden within the progenitor-rich germinal matrix and developing subcortical regions may play an important role in the pathophysiological mechanisms of brain injury after neonatal GMH-IVH, with implications for early outcome prediction.
Role of GMH and PVHI Volume in Cognition
The germinal matrix (including the ganglionic eminence) is a site of progenitor cells for the developing brain that lies subjacent to the brain ventricles and harbors neuronal and glial precursor cells essential for healthy brain development.6 Blood products in these progenitor-rich regions have been shown to suppress oligodendrocyte progenitor cell proliferation and subventricular zone maturation35 via gliosis and microglia infiltration.6,36 Larger hemorrhages within the germinal matrix and surrounding structures may therefore produce a greater degree of suppression of cellular proliferation, cellular growth, and myelination, leading to a proportional disruption in brain development, and result in worse cognitive outcomes. Thus, we chose to evaluate GMH in the clinical setting.
Specifically, larger left-sided and total GMH volumes correlated with worse cognitive outcomes in our study. Prior work has shown that a high PVHI severity score—for which one criterion is involvement of more than one lobar region—is associated with cognitive deficits.18 Our study is not directly comparable to this prior investigation as different outcome scales were used and the PVHI severity score did not include volumetric measurements. However, the results from these studies suggest that the extent of injury to the subcortical brain parenchyma contributes to early brain development and ultimately to cognitive outcomes in GMH-IVH. To our knowledge, this is the first study to correlate hemorrhage volume within the germinal matrix to neurodevelopmental outcomes, including cognition, language, and motor function.
Role of GMH Volume in Hydrocephalus Outcomes
Hydrocephalus requiring permanent CSF diversion develops in approximately 30% of infants with high-grade IVH.37 We showed that left-sided and total GMH volumes, but not PVHI volume or early ventricular size, were associated with permanent CSF diversion. In our study, 19 of 43 patients ultimately required permanent CSF diversion with ventricular shunting (3 patients initially underwent endoscopic third ventriculostomy but later required ventricular shunting). Our findings are consistent with prior work in adults showing an independent association between thalamic hemorrhage and permanent shunting, outside of IVH burden alone.38 These findings have implications for the pathophysiology of hydrocephalus, in which germinal matrix injury specifically may impact CSF circulation due to specific injury to cells that mediate CSF circulation. Recently, whole-exome sequencing of neurosurgically treated infants with congenital hydrocephalus revealed de novo mutations in key regulators of neurogliogenesis, suggesting that altered early brain development is a major contributor to the development of hydrocephalus.39 Hemorrhage within the germinal matrix may also disrupt maturation of nearby ventricular ependymal cells, contributing to impaired CSF circulation and to the pathogenesis of PHH.40 Further study in preclinical models is needed to evaluate these pathways.
The observation that GMH volume was associated with the development of hydrocephalus, while controlling for ventricular size, prompted us to evaluate GMH volumes in grade II GMH-IVH, which, by definition,5 differs from grade III GMH-IVH only by ventricle size and patients are much less likely to develop hydrocephalus.31,32 In this analysis, similar GMH volumes between grade II and high-grade GMH-IVH would have suggested that the difference in early ventricular size alone accounts for the difference in rates of permanent CSF diversion. Interestingly, we found that GMH volumes were smaller in patients with grade II GMH-IVH. This finding indicates that there may be additional factors beyond ventricular size, such as region-specific injury within the germinal matrix, that contribute to impaired CSF circulation and later development of hydrocephalus.
Evaluating IVH Burden on 2D CUS
The extent of IVH and its role in neurodevelopment and hydrocephalus outcomes was not evaluated in our study. Our method for estimating hemorrhage volumes based on 2D CUS images relies on the assumption of spherical or ellipsoid shapes of the object of interest, making it possible to measure GMH and PVHI. However, IVH can often contour to the C-shape of the ventricles, resulting in irregular shapes and precluding the use of our volume estimation technique. To overcome these challenges, 3D CUS systems have been developed and have reliably produced 3D quantifications of the neonatal ventricular system containing hemorrhage,41,42 but this modality is not standard of care and the associations between these IVH volumes and clinical outcomes require further investigation. We asked whether 2D CUS measurements could be used to estimate IVH burden. In our study, FTHR was measured at an early time point, prior to maximum ventricular dilation, and when the hemorrhage burden was the most significant. Given the minimal CSF within the ventricle at the time, we used FTHR as an estimate of ventricle size. We found that FTHR was not associated with neurodevelopmental and hydrocephalus outcomes. Assuming that the FTHR measures in our study were proportional to IVH burden, it may be reasoned that IVH burden is not directly associated with these outcomes, a conclusion consistent with prior work.38 However, this approach assumes a simple linear relationship between early ventricular size and IVH burden, which must be further validated.
Role of GMH and PVHI Volume in Motor Outcome
Impairment in motor outcomes is seen in approximately two-thirds of PVHI survivors.14 While preclinical models of GMH-IVH have implicated striatal gray matter injury in impaired motor coordination,43 the specific contribution of each hemorrhagic component to motor outcomes has not been clearly demonstrated. Here we show that left-sided and total GMH volumes and bilateral PVHI volumes were correlated with worse motor outcomes. PVHI characteristics have been associated with neurodevelopmental outcome, particularly in conjunction with grade III GMH-IVH.9–12,20 Bilaterality of PVHI has been associated with poor neurological outcomes at 12 months of age, including increased seizure risk and motor deficits.15,33 Patients with PVHI also exhibit abnormal MR diffusion parameters within the white matter, such as the internal capsule and corpus callosum, that correlate with motor scores, suggesting that PVHI may disrupt functionally connected brain regions.44,45 PVHI may therefore impair the developing corticospinal tracts and other motor and premotor pathways depending on volume and extent of injury.
Furthermore, we evaluated the association between hemorrhage volume and global performance metrics, such as cerebral palsy diagnosis, requiring assistance with ADLs, walking with assistance, gait asymmetry, and toe-walking, and found that GMH and PVHI volumes were independently associated with several of these outcomes. While Bayley-III scores offer a measure against normative controls, the above metrics offer insight into overall functional status and impact on quality of life.
Limitations
This was a single-center study with a small sample size. The inclusion criteria required all patients to undergo standardized neurodevelopmental testing, and therefore potentially selected for patients with better outcomes (i.e., no mortality). Future studies should focus on volumetric IVH calculations, which was not possible given the irregular shape of IVH and the use of 2D CUS in our study. The amount of intraventricular blood may play a role in hydrocephalus and neurodevelopmental outcomes.46 Cerebellar hemorrhage was not quantified given the constraints of CUS through the anterior fontanelle to image the posterior fossa;47 however, this finding was limited to 2 patients with subcentimeter cerebellar hemorrhages in our cohort. Future clinical studies may expand on this work by using early MRI or 3D CUS, when feasible, to quantify and map hemorrhage after GMH-IVH.
Conclusions
To our knowledge, this is the first study to associate volumetric measurements of the germinal matrix and periventricular hemorrhagic components with neurodevelopmental and hydrocephalus outcomes using routine bedside CUS. As CUS has several advantages, including safety and efficacy in the diagnosis and monitoring of GMH-IVH as well as familiarity in routine neonatal care, these findings may help in early prognostication for neonates with GMH-IVH.
Supplementary Material
Acknowledgments
This work was funded by NIH grant R01 NS110793 (to J.M.S.). Dr. Lean reported grants from the National Institute of Mental Health (K01 MH122735 and R01 MH113570) and National Institute of Neurological Disorders and Stroke (U01 NS107486) during the conduct of the study.
Disclosures
Dr. Lean reported receiving the 2019 NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation. Dr. Limbrick reported being the CMO of Rhaeos Inc. outside the submitted work.
ABBREVIATIONS
- ADL
activity of daily living
- Bayley-III
Bayley Scales of Infant and Toddler Development, 3rd Edition
- CUS
cranial ultrasound
- FTHR
frontotemporal horn ratio
- GA
gestational age
- GMH-IVH
germinal matrix hemorrhage–intraventricular hemorrhage
- PHH
posthemorrhagic hydrocephalus
- PVHI
periventricular hemorrhagic infarction
Footnotes
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Supplementary Tables 1–6. https://thejns.org/doi/suppl/10.3171/2024.3.PEDS22376.
Previous Presentations
This work was previously presented as an oral presentation at the American Association of Neurological Surgeons Annual Scientific Meeting, Philadelphia, Pennsylvania, April 29–May 2, 2022.
References
- 1.Strahle JM, Triplett RL, Alexopoulos D, et al. Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury. Neuroimage Clin. 2019; 22: 101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens R Intraventricular hemorrhage in the premature neonate. Neonatal Netw. 2005; 24(3): 55–71. [DOI] [PubMed] [Google Scholar]
- 3.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005; 115(4): 997–1003. [DOI] [PubMed] [Google Scholar]
- 4.Inder TE, Perlman JM, Volpe JJ. Preterm intraventricular hemorrhage/posthemorrhagic hydrocephalus. In: Volpe JJ, Inder TE, Darras BT, et al. , eds. Volpe’s Neurology of the Newborn. 6th ed. Elsevier; 2018: 637–698.e21. [Google Scholar]
- 5.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92(4): 529–534. [DOI] [PubMed] [Google Scholar]
- 6.Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain. 2011; 134(Pt 5): 1344–1361. [DOI] [PubMed] [Google Scholar]
- 7.Vasileiadis GT, Gelman N, Han VK, et al. Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics. 2004; 114(3): e367–e372. [DOI] [PubMed] [Google Scholar]
- 8.Gould SJ, Howard S, Hope PL, Reynolds EOR. Periventricular intraparenchymal cerebral haemorrhage in preterm infants: the role of venous infarction. J Pathol. 1987; 151(3): 197–202. [DOI] [PubMed] [Google Scholar]
- 9.Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014; 133(1): 55–62. [DOI] [PubMed] [Google Scholar]
- 10.Sherlock RL, Anderson PJ, Doyle LW. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005; 81(11): 909–916. [DOI] [PubMed] [Google Scholar]
- 11.Payne AH, Hintz SR, Hibbs AM, et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 2013; 167(5): 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merhar SL, Tabangin ME, Meinzen-Derr J, Schibler KR. Grade and laterality of intraventricular haemorrhage to predict 18–22 month neurodevelopmental outcomes in extremely low birthweight infants. Acta Paediatr. 2012; 101(4): 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cizmeci MN, de Vries LS, Ly LG, et al. Periventricular hemorrhagic infarction in very preterm infants: characteristic sonographic findings and association with neurodevelopmental outcome at age 2 years. J Pediatr. 2020; 217: 79–85.e1. [DOI] [PubMed] [Google Scholar]
- 14.Bassan H, Limperopoulos C, Visconti K, et al. Neurodevelopmental outcome in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007; 120(4): 785–792. [DOI] [PubMed] [Google Scholar]
- 15.Maitre NL, Marshall DD, Price WA, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009; 124(6): e1153–e1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilard V, Chadie A, Ferracci FX, et al. Post hemorrhagic hydrocephalus and neurodevelopmental outcomes in a context of neonatal intraventricular hemorrhage: an institutional experience in 122 preterm children. BMC Pediatr. 2018; 18(1): 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam HP, Heilman CB. Ventricular access device versus ventriculosubgaleal shunt in post hemorrhagic hydrocephalus associated with prematurity. J Matern Fetal Neonatal Med. 2009; 22(11): 1097–1101. [DOI] [PubMed] [Google Scholar]
- 18.Bir SC, Konar S, Maiti TK, Kalakoti P, Bollam P, Nanda A. Outcome of ventriculoperitoneal shunt and predictors of shunt revision in infants with posthemorrhagic hydrocephalus. Childs Nerv Syst. 2016; 32(8): 1405–1414. [DOI] [PubMed] [Google Scholar]
- 19.Dorner RA, Allen MC, Robinson S, et al. Early neurodevelopmental outcome in preterm posthemorrhagic ventricular dilatation and hydrocephalus: Neonatal ICU Network Neurobehavioral Scale and imaging predict 3–6-month motor quotients and Capute Scales. J Neurosurg Pediatr. 2019; 25(3): 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008; 152(5): 648–654. [DOI] [PubMed] [Google Scholar]
- 21.Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012; 3(suppl 1): 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lean RE, Paul RA, Smyser CD, Rogers CE. Maternal intelligence quotient (IQ) predicts IQ and language in very preterm children at age 5 years. J Child Psychol Psychiatry. 2018; 59(2): 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hack M, Breslau N, Aram D, Weissman B, Klein N, Borawski-Clark E. The effect of very low birth weight and social risk on neurocognitive abilities at school age. J Dev Behav Pediatr. 1992; 13(6): 412–420. [PubMed] [Google Scholar]
- 24.Whitaker AH, Feldman JF, Van Rossem R, et al. Neonatal cranial ultrasound abnormalities in low birth weight infants: relation to cognitive outcomes at six years of age. Pediatrics. 1996; 98(4 Pt 1): 719–729. [PubMed] [Google Scholar]
- 25.Lean RE, Gerstein ED, Smyser TA, Smyser CD, Rogers CE. Socioeconomic disadvantage and parental mood/affective problems links negative parenting and executive dysfunction in children born very preterm. Dev Psychopathol. 2023; 35(3): 1092–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liljenwall H, Lean RE, Smyser TA, Smyser CD, Rogers CE. Parental ADHD and ASD symptoms and contributions of psychosocial risk to childhood ADHD and ASD symptoms in children born very preterm. J Perinatol. 2023; 43(4): 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skrommel. SmallMeasure. Skrommel’s One Hour Software; 2017. Accessed March 12, 2024. https://www.dcmembers.com/skrommel/download/smallmeasure/ [Google Scholar]
- 28.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27(8): 1304–1305. [DOI] [PubMed] [Google Scholar]
- 29.Radhakrishnan R, Brown BP, Kralik SF, et al. Frontal occipital and frontal temporal horn ratios: comparison and validation of head ultrasound-derived indexes with MRI and ventricular volumes in infantile ventriculomegaly. AJR Am J Roentgenol. 2019; 213(4): 925–931. [DOI] [PubMed] [Google Scholar]
- 30.Bayley N Bayley-III: Bayley Scales of Infant and Toddler Development. Pearson; 2005. [Google Scholar]
- 31.Ahmann PA, Lazzara A, Dykes FD, Brann AW Jr, Schwartz JF. Intraventricular hemorrhage in the high-risk preterm infant: incidence and outcome. Ann Neurol. 1980; 7(2): 118–124. [DOI] [PubMed] [Google Scholar]
- 32.Burstein J, Papile LA, Burstein R. Intraventricular hemorrhage and hydrocephalus in premature newborns: a prospective study with CT. AJR Am J Roentgenol. 1979; 132(4): 631–635. [DOI] [PubMed] [Google Scholar]
- 33.Bassan H, Benson CB, Limperopoulos C, et al. Ultrasonographic features and severity scoring of periventricular hemorrhagic infarction in relation to risk factors and outcome. Pediatrics. 2006; 117(6): 2111–2118. [DOI] [PubMed] [Google Scholar]
- 34.Beijst C, Dudink J, Wientjes R, et al. Two-dimensional ultrasound measurements vs. magnetic resonance imaging-derived ventricular volume of preterm infants with germinal matrix intraventricular haemorrhage. Pediatr Radiol. 2020; 50(2): 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juliet PA, Frost EE, Balasubramaniam J, Del Bigio MR. Toxic effect of blood components on perinatal rat subventricular zone cells and oligodendrocyte precursor cell proliferation, differentiation and migration in culture. J Neurochem. 2009; 109(5): 1285–1299. [DOI] [PubMed] [Google Scholar]
- 36.Xue M, Balasubramaniam J, Buist RJ, Peeling J, Del Bigio MR. Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J Neuropathol Exp Neurol. 2003; 62(11): 1154–1165. [DOI] [PubMed] [Google Scholar]
- 37.Radic JA, Vincer M, McNeely PD. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J Neurosurg Pediatr. 2015; 15(6): 580–588. [DOI] [PubMed] [Google Scholar]
- 38.Zacharia BE, Vaughan KA, Hickman ZL, et al. Predictors of long-term shunt-dependent hydrocephalus in patients with intracerebral hemorrhage requiring emergency cerebrospinal fluid diversion. Neurosurg Focus. 2012; 32(4): E5. [DOI] [PubMed] [Google Scholar]
- 39.Jin SC, Dong W, Kundishora AJ, et al. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat Med. 2020; 26(11): 1754–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domínguez-Pinos MD, Páez P, Jiménez AJ, et al. Ependymal denudation and alterations of the subventricular zone occur in human fetuses with a moderate communicating hydrocephalus. J Neuropathol Exp Neurol. 2005; 64(7): 595–604. [DOI] [PubMed] [Google Scholar]
- 41.Kishimoto J, de Ribaupierre S, Lee DS, Mehta R, St Lawrence K, Fenster A. 3D ultrasound system to investigate intraventricular hemorrhage in preterm neonates. Phys Med Biol. 2013; 58(21): 7513–7526. [DOI] [PubMed] [Google Scholar]
- 42.Qiu W, Chen Y, Kishimoto J, et al. Automatic segmentation approach to extracting neonatal cerebral ventricles from 3D ultrasound images. Med Image Anal. 2017; 35: 181–191. [DOI] [PubMed] [Google Scholar]
- 43.Jinnai M, Koning G, Singh-Mallah G, et al. A model of germinal matrix hemorrhage in preterm rat pups. Front Cell Neurosci. 2020; 14: 535320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roze E, Benders MJ, Kersbergen KJ, et al. Neonatal DTI early after birth predicts motor outcome in preterm infants with periventricular hemorrhagic infarction. Pediatr Res. 2015; 78(3): 298–303. [DOI] [PubMed] [Google Scholar]
- 45.Nieuwets A, Cizmeci MN, Groenendaal F, et al. Post-hemorrhagic ventricular dilatation affects white matter maturation in extremely preterm infants. Pediatr Res. 2022; 92(1): 225–232. [DOI] [PubMed] [Google Scholar]
- 46.Garton T, Keep RF, Wilkinson DA, et al. Intraventricular hemorrhage: the role of blood components in secondary injury and hydrocephalus. Transl Stroke Res. 2016; 7(6): 447–451. [DOI] [PubMed] [Google Scholar]
- 47.Parodi A, Rossi A, Severino M, et al. Accuracy of ultrasound in assessing cerebellar haemorrhages in very low birthweight babies. Arch Dis Child Fetal Neonatal Ed. 2015; 100(4): F289–F292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


