Abstract
Background
Previous studies have established a correlation between elevated levels of remnant cholesterol (RC) and the occurrence of type 2 diabetes mellitus (T2D) as well as insulin resistance (IR); however, the precise nature of these associations remains incompletely elucidated. This study aimed to evaluate the relationships between RC and IR, as well as RC and T2D, and to determine the extent to which IR mediated the relationship between RC and T2D.
Methods
This was an observational study that utilized cross-sectional methods to examine the general population in the National Health and Nutrition Examination Survey (NHANES) 1999–2020. The participants were divided into 4 groups according to the RC quartiles. The outcome was the prevalence of IR and T2D. Survey-weighted binary logistic regression analysis was used to analyze the associations, and the restricted cubic spline (RCS) curve was used to further analyze the nonlinear relationship. Receiver operating characteristic (ROC) curves were generated to evaluate the diagnostic performance, and the areas under the curves (AUC) of RC, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were compared using the DeLong test. The mediating effect of IR on the relationship between RC and T2D was evaluated through mediation analysis.
Results
A total of 23,755 participants (46.02 ± 18.48 years, 48.8% male) were included in our study. Higher RC levels were significantly associated with increased prevalence of both IR and T2D. After adjusting for potential confounders, logistic regression analysis showed that higher RC quartiles were associated with the increased prevalence of IR [Quartile 4 vs. Quartile 1: odds ratio (OR) (95% confidence interval, CI): 1.65 (1.41–1.94), p < 0.001] and T2D [Quartile 4 vs. Quartile 1: OR (95% CI): 1.24 (1.03–1.50), p = 0.024]. RCS analysis revealed two distinct nonlinear relationships: one between RC levels and the prevalence of IR (nonlinear p < 0.001), and another between RC levels and the prevalence of T2D (nonlinear p < 0.001). ROC curve analysis demonstrated that RC had the highest discriminative ability, significantly outperforming LDL-C, HDL-C, and TG in predicting both IR and T2D risk (all P < 0.001 by DeLong test). Mediation analysis revealed that IR significantly mediated the relationship between RC and T2D, with approximately 54.1% of the effect of RC on T2D being indirect through IR.
Conclusions
Higher RC level was associated with increased prevalence of IR and T2D. IR mediated 54.1% of the association between RC and T2D, suggesting that managing IR could be crucial in reducing the risk of T2D in individuals with elevated RC levels.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02393-6.
Keywords: Remnant cholesterol, Insulin resistance, Type 2 diabetes mellitus, Mediation analysis, NHANES
Introduction
Insulin resistance (IR), characterized by a diminished efficacy of insulin action, plays a significant role in the onset and progression of metabolic dysregulation, such as type 2 diabetes mellitus (T2D) [1]. T2D is a chronic metabolic condition characterized by persistently high blood sugar levels due to abnormalities in glucose metabolism [2]. Amid advancements in the socioeconomic landscape, lifestyle transformations, and a surge in obesity rates, the prevalence of T2D is escalating swiftly, with a noticeable trend towards younger demographics globally, particularly in developing nations [3]. This surge positions diabetes as the third leading cause of mortality worldwide. In 2021, the International Diabetes Federation reported that roughly 536.6 million people worldwide, aged between 21 and 79, were living with diabetes [4]. Consequently, the immediate implementation of preventive screening and management strategies for individuals with early-onset T2D is critically necessary.
Recent research has identified sedentary lifestyles, excess body weight, and dyslipidemia as key potential risk factors for T2D [5, 6]. Among these, glucose and lipid metabolism disorder played a critical role in the pathogenesis of T2D and T2D combined cardiovascular disease [7, 8]. Lately, significant research efforts have been made to understand the relationship between both traditional and non-traditional lipid profiles and the risk of diabetes and its complications [9, 10]. RC, a non-conventional lipid, is part of triglyceride-rich lipoproteins (TRLs), including intermediate-density lipoprotein (IDL) and very-low-density lipoprotein (VLDL) in the fasting state or chylomicron remnants when not fasting, essentially constituting cholesterol [11]. RC has attracted growing attention due to its potential role in metabolic diseases. Several studies have highlighted that elevated RC levels are associated with an increased risk of IR, T2D, and cardiovascular diseases [12–14] For instance, RC has been shown to be a better predictor of new-onset diabetes and cardiovascular events compared to traditional lipid markers such as low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) [15]. Studies have suggested that RC contributes to insulin resistance through inflammatory pathways and by influencing lipid metabolism in insulin-sensitive tissues [12]. Unlike LDL-C, which is mainly involved in cholesterol delivery, RC is derived from triglyceride-rich lipoproteins, which may play a distinct role in insulin resistance and metabolic dysfunction [16]. These findings highlight the need for further investigation of RC’s role in T2D pathogenesis and its potential as a therapeutic target.
However, further investigation is warranted to establish a comprehensive understanding of the intricate relationship between RC and IR, as existing research has only provided limited evidence suggesting an elevated concentration of RC in patients with early-onset T2D, thereby establishing a connection with the severity of IR [17]. Despite the established role of traditional lipid measures such as LDL-C and TG in T2D risk, RC has not been as extensively studied in relation to IR and T2D. Furthermore, existing studies have not fully explored the relationship between elevated RC levels and the severity of IR in individuals with early-onset T2D. These gaps in the literature highlight the need for further research into RC’s potential role in T2D pathogenesis. To further understand the intricate relationship between RC, IR, and T2D, we carried out a comprehensive cross-sectional analysis using the National Health and Nutrition Examination Survey (NHANES) database, aiming to uncover a clinically viable measure for monitoring IR among the general population in the USA.
Methods
Data source
This investigation utilized a cross-sectional approach, analyzing data collected by the continuous NHANES from 1999 to 2020. NHANES, a project of the National Center for Health Statistics (NCHS), surveys a broad section of the U.S. civilian, non-institutionalized population through a complex, multistage, stratified sampling methodology that operates on a biennial basis [18]. Since its inception in 1999, NHANES has systematically gathered extensive data covering demographic characteristics, socioeconomic status, dietary patterns, and health-related information from a carefully selected pool of participants. Participant involvement was based on the provision of informed consent, with the study’s procedures receiving clearance from the NCHS Research Ethics Review Board. Detailed information regarding the survey’s methodology and participant response rates is publicly available on the NHANES website [19].
Population selection
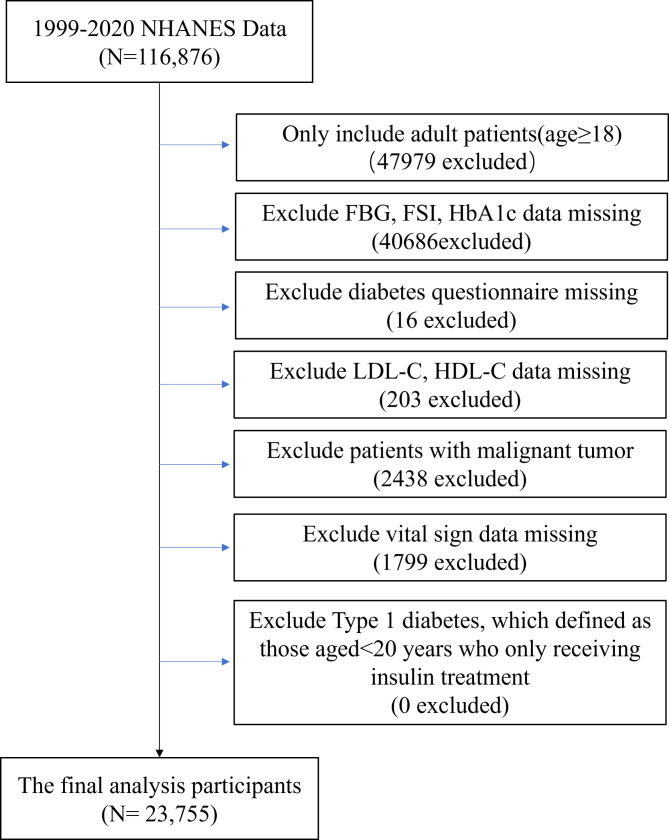
To broaden our study’s sample size and enhance data comprehensiveness, we sourced our data from ten cycles of the NHANES database (involving 116,876 participants). The exclusion criteria were: [1] age < 18 years old; [2] fasting blood glucose (FBG), fasting serum insulin (FSI), glycated haemaglobin A1c (HbA1c) data missing; [3] diabetes questionnaire missing; [4] LDL-C, high-density lipoprotein cholesterol (HDL-C) data missing; [5] patients with malignant tumor; [6] vital sign data missing; [7] possible type 1 diabetes (defined as those aged < 20 years who only receiving insulin treatment). Finally, 23,755 participants with complete data were included (Fig. 1).
Fig. 1.
Flow chart of study population. Abbreviation: FBG: Fasting Blood Glucose; FSI: Fasting Serum Insulin; HbA1c: Glycated Hemoglobin; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol
Data collection and definition
The following data were included: age, sex, education level (less than high school, completed high school, and more than high school), race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other), vital signs [systolic blood pressure (SBP) (mmHg), diastolic blood pressure (DBP) (mmHg), heart rate (beats/min), body mass index (BMI) (kg/m²)], laboratory parameters [TG (mmol/L), total cholesterol (TC) (mmol/L), LDL-C (mmol/L), HDL-C (mmol/L), creatinine (µmol/L), blood nitrogen urea (mmol/L), alanine aminotransferase (ALT) (U/L), aspartate aminotransferase (AST) (U/L), FBG (mmol/L), FSI(pmol/L), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), HbA1c (%), uric acid (µmol/L), sodium (mmol/L), potassium (mmol/L)], and medical history.
RC (mmol/L) was determined using the standard lipid panel of patients in a fasting state, calculated as TC (mmol/L) minus LDL-C (mmol/L) minus HDL-C (mmol/L) [20]. The HOMA-IR was used to indicate IR by calculating FSI (µU/mL) × FBG (mmol/L)/22.5 [21]. Consistent with other research findings, a HOMA-IR > 2.6 was recognized as indicative of IR among the general U.S. population [22], and this threshold was adopted as a selection criterion in our investigation. T2D in this research was identified based on fasting plasma glucose (FPG) levels ≥ 7.0 mmol/L (126 mg/dL), 2-hour plasma glucose levels ≥ 11.1 mmol/L (200 mg/dL) following an oral glucose tolerance test, HbA1c levels ≥ 6.5%, a self-reported diagnosis of T2D, or current use of glucose-lowering medications [23, 24].
Grouping and outcomes
Study participants were segmented into four groups, arranged into quartiles according to their RC levels. The specific boundaries for each quartile were as follows: Quartile 1 (Q1) (RC < 0.36 mg/dL), Quartile 2 (Q2) (0.361 ≤ RC < 0.541 mg/dL), Quartile 3 (Q3) (0.541 ≤ RC < 0.801 mg/dL), and Quartile 4 (Q4) (RC ≥ 0.801 mg/dL). The outcomes were the prevalence of IR and T2D.
Statistical analysis
To account for the complex, multistage sampling design of NHANES, appropriate sample weights (Mobile Examination Center (MEC) weights) were applied to the data, ensuring nationally representative estimates by adjusting for oversampling, nonresponse, and noncoverage, as described in detail on the NHANES website: https://wwwn.cdc.gov/nchs/nhanes/tutorials/Weighting.aspx.
The missing data were handled as follows: for key variables directly related to the primary outcomes (e.g. LDL-C, HDL-C, TG, TC), cases with missing values were excluded to ensure data integrity and minimize potential biases in the association analysis. For other variables with missing values, multiple imputation was applied to retain sample size and reduce possible biases. The ‘mice’ package in R was used to perform multiple imputations, generating several complete datasets and combining the results to enhance the robustness of the final analysis.
Continuous variables with a normal distribution were expressed as weighted mean ± SD, and comparisons between groups were made using weighted one-way analysis of variance (ANOVA). For continuous variables that did not follow a normal distribution, data were presented as weighted median [interquartile range, IQR], and differences between groups were assessed using the weighted Kruskal-Wallis test. Categorical variables were expressed as weighted number (percentage), and comparisons between groups were performed using the weighted Chi-square test. Survey-weighted binary logistic regression analysis was performed to explore the association between RC and IR, as well as the association between RC and T2D. The results were expressed by the odds ratio (OR) and the 95% confidence interval (CI). In Model 1, no variables were adjusted. In Model 2, age, sex, and ethnicity were adjusted. In addition, Model 3 was adjusted for age, sex, ethnicity, education levels, hypertension, self-reported of diabetes, hypercholesterolemia, chronic pulmonary disease, heartrate, BMI, TG, TC, LDL-C, creatinine, ALT, AST, FBG, uric acid, sodium. The confounding variables in Model 3 were obtained using stepwise method with removal at p > 0.05. Based on Model 3, restricted cubic spline (RCS) analysis was carried out to distinctly investigate the associations between RC as a continuous variable and the prevalence of both IR and T2D. RCS analysis was selected for its superior flexibility in modeling complex relationships between variables, particularly when nonlinear patterns are present. Model comparison using Akaike Information Criterion (AIC) supported this choice, with the RCS model demonstrating better fit (AIC = 31031.98) compared to the conventional logistic regression model (AIC = 31144.56). The discriminative ability of RC compared with traditional lipid biomarkers (LDL-C, HDL-C, and TG) for predicting IR and T2D risk was evaluated using ROC curve analyses, and the AUCs were compared using the DeLong test. In mediation analysis, a mediating variable (M) was assumed to mediate the relationship between independent variables (X) and dependent variables (Y) [25]. An indirect effect and a direct effect of RC on T2D were evaluated separately. The independent variable was RC (X), the outcome variable was T2D (Y), and the mediating variable (M) was IR. Statistical significance was set at P < 0.05. Statistical analyses were performed using R software (R-project ®; R Foundation for Statistical Computing, Vienna, Austria, ver. 4.2.1).
Result
Subjects and baseline characteristics
Table 1 presented the baseline demographic and clinical characteristics of participants categorized by RC levels. A total of 23,755 participants were included. The median and mean RC levels were 0.54 and 0.62 mmol/L, respectively. Among all participants, 3,879 (16.3%) had T2D, and 10,935 (46.0%) exhibited IR. Participants in the RC Q4 group, compared to those in the lower RC group, were more likely to be male, of Mexican American, and to have higher education levels. They also exhibited higher levels of SBP, DBP, heart rate, BMI, TG, TC, LDL-C, HDL-C, ALT, AST, FBG, FSI, HOMA-IR, HbA1c, and uric acid (all P < 0.05).
Table 1.
Characteristics of patients stratified by RC quartiles
| Characteristics | Total (n = 23755) |
Quartiles of RC | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| RC < 0.361 (n = 6090) |
0.361 ≤ RC < 0.541 (n = 5797) |
0.541 ≤ RC < 0.801 (n = 5937) |
RC ≥ 0.801 (n = 5931) |
|||
| Age (years) | 46.02 ± 18.48 | 42.10 ± 18.44 | 45.19 ± 18.81 | 48.29 ± 18.41 | 48.60 ± 17.49 | < 0.001 |
| sex, n (%) | < 0.001 | |||||
| Male | 11,588 (48.8) | 2722 (44.7) | 2730 (47.1) | 2984 (50.3) | 3152 (53.1) | |
| Female | 12,167 (51.2) | 3368 (55.3) | 3067 (52.9) | 2953 (49.7) | 2779 (46.9) | |
| Race, n (%) | < 0.001 | |||||
| Mexican American | 4512 (19.0) | 843 (13.8) | 965 (16.6) | 1201 (20.2) | 1503 (25.3) | |
| Other Hispanic | 2117(8.9) | 428(7.0) | 520(9.0) | 576(9.7) | 593 (10.0) | |
| Non-Hispanic White | 9527 (40.1) | 2010 (33.0) | 2289 (39.5) | 2588 (43.6) | 2640 (44.5) | |
| Non-Hispanic Black | 5054 (21.3) | 2179 (35.8) | 1405 (24.2) | 935 (15.7) | 535(9.0) | |
| Other Race | 2545 (10.7) | 630 (10.3) | 618 (10.7) | 637 (10.7) | 660 (11.1) | |
| Education levels, n(%) | < 0.001 | |||||
| < high school | 6380 (26.9) | 1414 (23.2) | 1468 (25.3) | 1659 (27.9) | 1839 (31.0) | |
| =high school | 6151 (25.9) | 1629 (26.7) | 1539 (26.5) | 1470 (24.8) | 1513 (25.5) | |
| > high school | 11,224 (47.2) | 3047 (50.0) | 2790 (48.1) | 2808 (47.3) | 2579 (43.5) | |
| SBP (mmHg) | 122.67 ± 18.68 | 119.89 ± 18.27 | 121.70 ± 18.51 | 123.81 ± 19.01 | 125.33 ± 18.47 | < 0.001 |
| DBP (mmHg) | 70.13 ± 12.22 | 69.00 ± 11.95 | 69.66 ± 12.18 | 70.40 ± 12.13 | 71.46 ± 12.49 | < 0.001 |
| Heart rate (beats/min) | 71.04 ± 11.86 | 69.77 ± 11.38 | 70.44 ± 11.61 | 70.96 ± 11.81 | 73.01 ± 12.36 | < 0.001 |
| BMI (kg/m2) | 28.76 ± 6.88 | 27.27 ± 6.78 | 28.24 ± 6.95 | 29.30 ± 7.02 | 30.25 ± 6.39 | < 0.001 |
| TG (mmol/L) | 1.34 ± 0.76 | 0.70 ± 0.25 | 0.98 ± 0.28 | 1.34 ± 0.34 | 2.33 ± 0.73 | < 0.001 |
| TC (mmol/L) | 4.94 ± 1.07 | 4.46 ± 0.94 | 4.74 ± 0.93 | 5.05 ± 0.99 | 5.52 ± 1.12 | < 0.001 |
| LDL-C (mmol/L) | 2.93 ± 0.93 | 2.67 ± 0.84 | 2.84 ± 0.86 | 3.04 ± 0.90 | 3.17 ± 1.01 | < 0.001 |
| HDL-C (mmol/L) | 1.39 ± 0.40 | 1.54 ± 0.42 | 1.45 ± 0.39 | 1.36 ± 0.38 | 1.20 ± 0.35 | < 0.001 |
| Creatinine (µmol/L) | 76.82 ± 38.36 | 75.24 ± 39.22 | 77.08 ± 36.97 | 77.70 ± 37.15 | 77.29 ± 39.91 | 0.002 |
| Blood nitrogen urea (mmol/L) | 4.74 ± 2.01 | 4.64 ± 1.78 | 4.72 ± 1.99 | 4.80 ± 2.03 | 4.78 ± 2.22 | < 0.001 |
| ALT (U/L) | 20 [15, 28] | 18 [14, 24] | 19 [15, 26] | 21 [16, 28] | 23 [17, 32] | < 0.001 |
| AST (U/L) | 22 [18, 27] | 21 [17, 25] | 21 [18, 26] | 22 [19, 27] | 23 [19, 28] | < 0.001 |
| FBG (mmol/L) | 5.92 ± 1.87 | 5.56 ± 1.27 | 5.70 ± 1.40 | 6.02 ± 1.91 | 6.38 ± 2.53 | < 0.001 |
| FSI (pmol/L) | 9.7 [6.2, 15.6] | 7.7 [5.1, 11.7] | 8.7 [5.7, 13.7] | 10.3 [6.6, 16.3] | 13.0 [8.4, 20.4] | < 0.001 |
| HOMA-IR | 3.75 ± 6.00 | 2.61 ± 3.96 | 3.16 ± 4.83 | 3.96 ± 6.40 | 5.28 ± 7.75 | < 0.001 |
| HbA1c (%) | 5.66 ± 1.05 | 5.49 ± 0.77 | 5.55 ± 0.86 | 5.74 ± 1.12 | 5.88 ± 1.31 | < 0.001 |
| Uric acid (µmol/L) | 322.12 ± 85.26 | 301.35 ± 80.26 | 315.89 ± 81.67 | 327.17 ± 83.54 | 344.46 ± 89.42 | < 0.001 |
| Sodium (mmol/L) | 139.44 ± 2.38 | 139.62 ± 2.39 | 139.50 ± 2.35 | 139.49 ± 2.36 | 139.14 ± 2.40 | < 0.001 |
| Potassium (mmol/L) | 4.04 ± 0.34 | 4.04 ± 0.34 | 4.03 ± 0.34 | 4.05 ± 0.34 | 4.05 ± 0.34 | 0.115 |
| Medical history, n(%) | ||||||
| Hypertension | 7365 (31.0) | 1547 (25.4) | 1663 (28.7) | 1994 (33.6) | 2161 (36.4) | < 0.001 |
| Self-reported of diabetes | 2528 (10.6) | 440(7.2) | 496(8.6) | 729 (12.3) | 863 (14.6) | < 0.001 |
| Hypercholesterolemia | 6731 (28.3) | 1193 (19.6) | 1408 (24.3) | 1846 (31.1) | 2284 (38.5) | < 0.001 |
| Congestive heart failure | 610(2.6) | 122(2.0) | 134(2.3) | 165(2.8) | 189(3.2) | < 0.001 |
| CAD | 775(3.3) | 143(2.3) | 185(3.2) | 210(3.5) | 237(4.0) | < 0.001 |
| Chronic pulmonary disease | 1586(6.7) | 305(5.0) | 380(6.6) | 432(7.3) | 469(7.9) | < 0.001 |
| Stroke | 696(2.9) | 152(2.5) | 165(2.8) | 187(3.1) | 192(3.2) | 0.067 |
The specific RC boundaries for each quartile were as follows: Quartile 1 (Q1) (RC < 0.36 mg/dL), Quartile 2 (Q2) (0.361 ≤ RC < 0.541 mg/dL), Quartile 3 (Q3) (0.541 ≤ RC < 0.801 mg/dL), and Quartile 4 (Q4) (RC ≥ 0.801 mg/dL). Values were presented as means ± standard deviations for continuous variables and as counts (percentages) for categorical variables. Statistical significance was determined using one-way ANOVA for normally distributed continuous variables, the Kruskal-Wallis test for non-normally distributed continuous variables, and the Chi-square test for categorical variables. Abbreviation: RC: remnant cholesterol; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; BMI: Body Mass Index; TG: Triglycerides; TC: Total Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; FBG: Fasting Blood Glucose; FSI: Fasting Serum Insulin; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; HbA1c: Glycated Hemoglobin; CAD: Coronary Artery Disease
Association between RC and outcomes
Table 2 showed the association between RC quartiles and outcomes. With RC quartiles increasing, the prevalence of IR increased significantly (Quartile 4 vs. Quartile 1: 65.1% vs. 30.3%, p < 0.001). Similarly, high RC quartiles were associated with a higher prevalence of T2D (Quartile 4 vs. Quartile 1: 23.2% vs. 10.5%, p < 0.001).
Table 2.
Outcomes of patients stratified by RC quartiles
| Outcomes | Total | Quartiles of RC | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| IR | 10,935 (46.0) | 1846 (30.3) | 2253 (38.9) | 2974 (50.1) | 3862 (65.1) | < 0.001 |
| T2D | 3879 (16.3) | 639 (10.5) | 748 (12.9) | 1115 (18.8) | 1377 (23.2) | < 0.001 |
Values were presented as counts (percentages) for categorical outcomes across RC quartiles. Statistical significance for differences between quartiles was assessed using the Chi-square test. Abbreviations: RC, remnant cholesterol; IR, insulin resistance; T2D, type 2 diabetes
The independent association of RC with the prevalence of IR was verified using binary logistic regression models (Table 3). In Model 1, higher RC quartiles were associated with increased prevalence of IR [Quartile 4 vs. Quartile 1: OR (95% CI): 4.29 (3.98, 4.63), p < 0.001)]. After adjusting for age, sex, and ethnicity in Model 2, similar results were observed for the association between higher RC quartiles and increased prevalence of IR [Quartile 4 vs. Quartile 1: OR (95% CI):4.60 (4.25, 4.99), p < 0.001]. In Model 3, which incorporated additional confounding variables, higher RC quartiles were still associated with increased prevalence of IR [Quartile 4 vs. Quartile 1: OR (95% CI): 1.65 (1.41, 1.94), p < 0.001]. When examining as a continuous variable, the presence of elevated RC levels was associated with a significantly increased prevalence of IR [OR per 1-SD increase in RC (95% CI): 1.53 (1.22, 1.93) p < 0.001].
Table 3.
The association between RC and IR in logistic analysis model
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.46 [1.35, 1.58] | 1.51 [1.40, 1.63] | 1.20 [1.09, 1.33] |
| Quartile 3 | 2.31 [2.14, 2.49] | 2.42 [2.24, 2.62] | 1.45 [1.31, 1.62] |
| Quartile 4 | 4.29 [3.98, 4.63] | 4.60 [4.25, 4.99] | 1.65 [1.41, 1.94] |
| RC | 4.50 [4.16, 4.86] | 4.17 [3.85, 4.51] | 1.53 [1.22, 1.93] |
Models were derived from binary logistic regression analysis. Model 1: unadjusted. Model 2: adjusted for age, sex, ethnicity. Model 3: adjusted for age, sex, ethnicity, education levels, hypertension, self-reported of diabetes, hypercholesterolemia, chronic pulmonary disease, heartrate, BMI, TG, TC, LDL-C, creatinine, ALT, AST, FBG, uric acid, sodium. Abbreviations: RC, remnant cholesterol; IR, insulin resistance; T2D, type 2 diabetes; BMI, body mass index; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FBG, fasting blood glucose; OR, odds ratio; CI, confidence interval
Table 4 demonstrated the independent association between RC and the prevalence of T2D. In Model 1, a significant positive relationship between RC and T2D was observed, with higher RC quartiles being related to an increased prevalence of T2D [Quartile 4 vs. Quartile 1: OR (95% CI): 2.58 (2.33, 2.86), p < 0.001)]. After adjusting for age, sex, and ethnicity in Model 2, similar results were observed for the association between higher RC quartiles and increased prevalence of T2D [Quartile 4 vs. Quartile 1: OR (95% CI): 2.16 (1.94, 2.41), p < 0.001]. In Model 3, higher RC quartiles were still associated with increased prevalence of T2D [Quartile 4 vs. Quartile 1: OR (95% CI): 1.24 (1.03, 1.50), P = 0.013)]. When examining as a continuous variable, the presence of elevated RC levels was linked to a significantly increased prevalence of T2D [OR per 1-SD increase in RC (95% CI): 1.23 (1.02, 1.49) p = 0.032].
Table 4.
The association between RC and T2D in logistic analysis model
| Model 1 | Model 2 | Model 2 | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.26 [1.13, 1.41] | 1.10 [0.98, 1.24] | 1.07 [0.90, 1.27] |
| Quartile 3 | 1.97 [1.78, 2.19] | 1.58 [1.41, 1.76] | 1.19 [1.00, 1.41] |
| Quartile 4 | 2.58 [2.33, 2.86] | 2.16 [1.94, 2.41] | 1.24 [1.03, 1.50] |
| RC | 2.37 [2.18, 2.58] | 2.21 [2.02, 2.43] | 1.23 [1.02, 1.49] |
Models were derived from binary logistic regression analysis. Model 1: unadjusted. Model 2: adjusted for age, sex, ethnicity. Model 3: adjusted for age, sex, ethnicity, DBP, heart rate, BMI, HOMA-IR, TC, LDL-C, creatinine, blood nitrogen urea, AST, FBG, sodium, potassium. Abbreviations: DBP, diastolic blood pressure; BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; FBG, fasting blood glucose, OR, odds ratio; CI, confidence interval
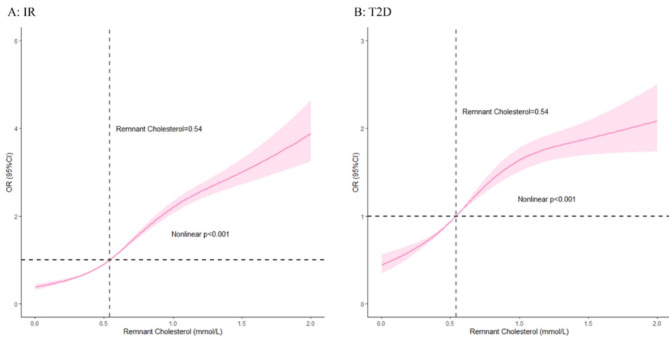
In Fig. 2, we conducted RCS analysis to analyze the nonlinear relationship between RC and outcomes as a continuous variable. After the potential confounders were considered, a nonlinear was observed between RC values and the prevalence of IR (Nonlinear p < 0.001), and T2D (Nonlinear p < 0.001). Generally, as RC levels increased, the prevalence of IR and T2D tended to increase.
Fig. 2.
RCS model showing the association between the RC and outcomes. A: IR; B: T2D. RCS model was adjusted for age, sex, ethnicity, education levels, hypertension, self-reported of diabetes, hypercholesterolemia, chronic pulmonary disease, heartrate, BMI, TG, TC, LDL-C, creatinine, ALT, AST, FBG, uric acid, sodium. Abbreviations: RCS, restricted cubic spline; RC, remnant cholesterol; IR, insulin resistance; T2D, type 2 diabetes; BMI, body mass index; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FBG, fasting blood glucose
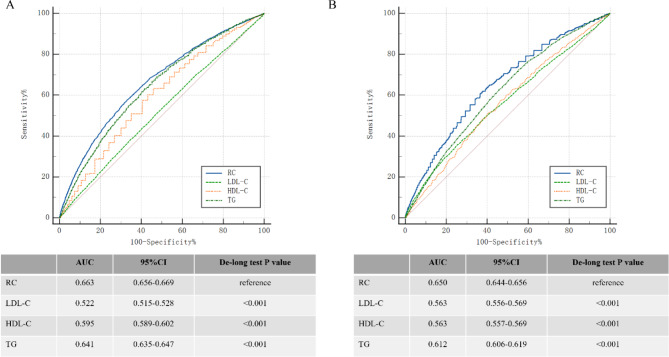
The ROC curves (Fig. 3) demonstrated that RC had moderate discriminative ability for IR and T2D, with AUCs of 0.663 and 0.650, respectively. RC exhibited superior predictive performance compared to LDL-C, HDL-C, and TG for both IR and T2D (all P < 0.001, DeLong test).
Fig. 3.
ROC curves comparing the predictive performance of RC, HDL-C, LDL-C, and triglycerides for (A) insulin resistance (IR) and (B) type 2 diabetes (T2D). Abbreviations: ROC, receiver operating characteristic; AUC, area under the curve; RC, remnant cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides
The potential mediating effects of IR on the association between RC and T2D were presented in Table 5, with RC as the independent variable, T2D as the dependent variable, and IR as the mediator. Our mediation analysis confirmed all necessary conditions: RC significantly influenced IR levels, IR significantly affected T2D occurrence, and RC demonstrated significant direct effects on T2D. Further analysis verified no significant interaction between IR and T2D (detailed results shown in supplementary file). The results revealed that IR exerted significant indirect effects on the RC-T2D relationship, accounting for approximately 54.9% of the total effect [OR:1.07; 95% CI (1.06, 1.07)]. After adjustment for covariates, although the mediating effect of IR slightly decreased to 54.1%, its indirect effect remained statistically significant [OR:1.06; 95% CI (1.05, 1.06)]. These findings suggested that IR played a crucial mediating role in the association between RC and T2D.
Table 5.
Effects of RC on T2D, mediated through IR
| Causal mediation analysis | OR (95% CI) | Proportion (%) |
|---|---|---|
| Model 1 | ||
| Indirect effect | 1.07 [1.06, 1.07] | 54.3% |
| Direct effect | 1.06 [1.04, 1.06] | 45.7% |
| Total effect | 1.13 [1.11, 1.14] | 100% |
| Model 2 | ||
| Indirect effect | 1.06 [1.05, 1.06] | 54.1% |
| Direct effect | 1.05 [1.04, 1.06] | 45.9% |
| Total effect | 1.11 [1.10, 1.12] | 100% |
Mediation analysis results were presented to illustrate the effects of RC on T2D, mediated through IR. The OR and 95% CI were shown for the indirect effect (mediated by IR), direct effect, and total effect of RC on T2D. Model 1 was unadjusted; Model 2 was adjusted for age, gender, and ethnicity. Abbreviations: RC, remnant cholesterol; T2D, type 2 diabetes; IR, insulin resistance; OR, odds ratio; CI, confidence interval
Discussion
This study was the first large-scale investigation to explore the association between RC and the prevalence of IR and T2D in the general population. The prevalence of IR and T2D was higher in the higher RC quartiles. After adjusting for potential confounding variables, higher RC levels were related to a higher prevalence of IR and T2D, with RC demonstrating moderate predictive value for both outcomes. The RCS analysis revealed a progressively non-linear relationship between RC values and the prevalence of IR and T2D. Mediation analysis showed that 54.1% of the association between RC and T2D prevalence was mediated by IR.
RC includes VLDL, which is associated with the release of aldosterone and an increase in circulating blood volume [26]. VLDL can stimulate aldosterone synthesis in adrenocortical cells by increasing StAR and CYP11B2 expression, an event likely mediated by a calcium-initiated signaling cascade [27]. Available evidence suggested that both hyperactivity of the renin–angiotensin–aldosterone system and IR are potential mechanisms for diabetes [28, 29]. RC has attracted attention for its potential pathogenic role due to a recently proposed concept—that of cholesterol toxicity—and this has led to new insights into the relationship between RC and diabetes development [30]. Previous cohort studies of the general population and coronary artery disease patients suggested that RC was a predictor of new-onset hyperglycemia, superior to other traditional lipid parameters [15, 31]. Another study conducted in a kidney transplant cohort also demonstrated a significant association between baseline RC levels and new-onset diabetes after transplantation [32]. Compared to traditional lipid measures such as LDL-C and TG, RC is considered a potentially more sensitive biomarker for diabetes risk. While LDL-C has long been associated with cardiovascular diseases, research suggests that RC, as a component of triglyceride-rich lipoproteins, may provide additional insights into metabolic dysfunction that LDL-C alone cannot fully capture [33]. Some studies have indicated that RC shows a stronger correlation with T2D than LDL-C or TG, suggesting that RC could be a more accurate marker for assessing metabolic risk [34, 35]. Consistent with these findings, our ROC analysis demonstrated that RC had superior predictive performance for both outcomes compared to other lipid parameters.
A nationwide cohort study identified RC as an independent factor linked to T2D [13]. RC plays a significant role in the inflammatory processes that contribute to both insulin resistance and β-cell dysfunction. Elevated RC levels have been shown to activate the renin-angiotensin-aldosterone system (RAAS), a key mediator of systemic inflammation [36]. Through RAAS activation, RC promotes inflammatory responses that worsen insulin resistance and β-cell damage [37]. RC breakdown and metabolism by lipoprotein lipase produce free fatty acids and monoacylglycerols, which induce an inflammatory response linked to diabetes [38]. Additionally, a kidney transplant cohort study found a significant link between baseline RC levels and new-onset diabetes post-transplantation [32]. Various studies have also demonstrated that β-cell dysfunction severity in T2D correlates with the level of inflammatory cell infiltration in pancreatic islets during diabetes development [39]. Islet-resident macrophages and islet-cell inflammation, driven by cytokine and reactive oxygen species (ROS) overproduction, play critical roles in the development and progression of human T2D [40, 41]. Xiangming Hu et al. previously reported that elevated RC levels were associated with increased white blood cells (WBC) and high-sensitivity c-reactive protein (hs-CRP), indicating a pro-inflammatory state. Mediation analyses showed that WBC count and CRP levels mediate the relationship between RC and diabetes, though the mediating effects of each factor were relatively weak. Consequently, it was suggested that the systemic pro-inflammatory response induced by high RC levels could play a role in the onset and progression of T2D [42].
IR is a major risk factor for cardiovascular and metabolic diseases (34–35). RC, mainly composed of TRLs, such as VLDL, undergoes peripheral metabolism leading to free fatty acid accumulation [43], which plays a key role in IR development [44].
Excess lipid accumulation in insulin-sensitive tissues (adipose tissue, liver, and muscle) disrupts metabolic homeostasis and impairs insulin sensitivity [45]. Recent evidence suggests that lipid accumulation in adipocytes activates inflammatory pathways and alters adipokine (leptin and adiponectin) regulation, contributing to systemic insulin resistance. Furthermore, arterial retention of residual lipoproteins enhances IR, associated with hyperglycemia [36, 37].
Studies on modern lipoprotein subclasses demonstrate that VLDL and LDL-C particle characteristics are linked to IR in prediabetic individuals [38–40]. These particles metabolize into free fatty acids and monoacylglycerols, accumulating in insulin-sensitive tissues [46] and impairing insulin receptor function and downstream signaling molecules (IRS and PI3K) [47], ultimately leading to reduced glucose uptake and IR development.
Considering these findings, our study aimed to delve deeper into the relationship between RC, IR, and T2D. Our analysis demonstrated that as RC quartiles increased, the prevalence of IR rose significantly. Similarly, higher RC quartiles were linked to a greater prevalence of T2D. Additionally, we discovered that IR mediated 54.1% of the association between RC and T2D. This suggests that the relationship between RC and T2D may, in part, be influenced by IR levels. RC is known to be associated with metabolic disturbances, including IR [42]. Elevated levels of RC contribute to the accumulation of lipids in insulin-sensitive tissues, which is associated with impaired insulin signaling and IR [48].This relationship underscores the metabolic burden imposed by RC and its contribution to the development of IR. Furthermore, IR is a pivotal factor in the development of T2D. IR impairs glucose uptake in peripheral tissues, which is associated with hyperglycemia and compensatory hyperinsulinemia [49]. Over time, the pancreatic β-cells may fail to compensate for the increased insulin demand, contributing to the onset of T2D [50]. Therefore, the mediating role of IR in the RC-T2D pathway emphasizes the importance of addressing IR in strategies aimed at preventing T2D. This mediation analysis highlights the necessity of early detection and management of IR in individuals with elevated RC levels. This approach aligns with the growing emphasis on personalized medicine, where understanding individual risk factors and their interconnections can result in more effective and tailored interventions.
The study underscored the critical role of IR as a mediator in the relationship between RC and T2D. The significant relationship between RC and T2D through IR not only clarified the association between these variables but also highlighted the potential for targeted interventions aimed at managing IR. These findings provided a valuable framework for future research and clinical strategies aimed at addressing T2D risk through comprehensive management of lipid abnormalities and IR. Moreover, RCS analysis revealed a nonlinear relationship between RC levels and the prevalence of both IR and T2D. Generally, as RC levels increased, so did the prevalence of IR and T2D. As a study of the general population, RC emerged as a novel biomarker that has been scarcely explored in previous research. Our study offered a new perspective for identifying individuals at high risk of T2D.
Limitations
This study had several limitations: [1] Due to the cross-sectional nature of the study design, causal conclusions regarding the pathogenesis of RC, IR, and T2D cannot be drawn. Future longitudinal cohort studies are needed to establish causality and examine the temporal sequence of these associations [2]. Although multiple covariates were adjusted for in our analysis, the potential influence of unmeasured confounding factors cannot be ruled out. Future investigations should incorporate comprehensive assessments of potential confounders, particularly genetic factors and lifestyle variables. Notably, our study lacked detailed data on important lifestyle factors, including dietary patterns, physical activity levels, and smoking behaviors. These unmeasured variables may introduce residual confounding effects. Therefore, future research incorporating these elements would enhance the robustness of the observed associations between RC and metabolic outcomes [3]. The representativeness of the study’s sample across diverse ethnic and socioeconomic groups is limited, potentially affecting the generalizability of the results. Future studies should include populations from diverse ethnic and socioeconomic backgrounds to enhance the applicability of the findings [4]. This study did not include follow-up data, limiting our understanding of the long-term effects of RC, IR, and T2D. Longitudinal studies and interventional trials are needed to assess the effectiveness of targeting RC in managing IR and T2D and to explore the impact of dietary and pharmacological interventions [5]. The biochemical pathways underlying the associations observed in this study remain unclear. Mechanistic studies are required to elucidate the specific biochemical and molecular mechanisms involved.
Conclusions
Higher RC level was associated with increased prevalence of IR and T2D. IR mediated 54.1% of the association between RC and T2D, suggesting that managing IR could be crucial in reducing the risk of T2D in individuals with elevated RC levels. However, further research is necessary to validate the underlying mechanisms identified in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
Y.L.: Conceptualization, Methodology, Writing – original draftQ.Z.: Data curation, Formal analysis, SoftwareD.P.: Formal analysis, Validation, Writing – review & editingP.H.: Investigation, Data curation, ResourcesJ.L.: Writing – review & editing, VisualizationK.Z.: Methodology, Formal analysis, Writing – review & editingY.Y., R.S.: Data curation, VisualizationJ.X., S.L.: Project administration, ResourcesJ.F., J.L.: Formal analysis, Writing – review & editingY.H.: Supervision, Writing – review & editing.
Funding
None.
Data availability
Data are accessible in a public, open access repository. Open access data can be found on the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval
This study includes human participants, and the data were obtained from NHANES. NHANES was approved by the National Center for Health Statistics Research Ethics Review Board under Continuation of Protocol. All subjects signed the informed consent during the recruitment period.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macdonald IA. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur J Nutr. 2016;55(Suppl 2):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB et al. Erratum to IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045 [Diabetes Res. Clin. Pract. 183 (2022) 109119]. Diabetes Res Clin Pract. 2023;204:110945. [DOI] [PubMed]
- 5.Hu P, Zheng M, Duan X, Zhou H, Huang J, Lao L, et al. Association of healthy lifestyles on the risk of hypertension, type 2 diabetes mellitus, and their comorbidity among subjects with dyslipidemia. Front Nutr. 2022;9:1006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si R, Xiao J, Zheng K, Yin Y, Li Y. Association between the hepatic steatosis index and risk of Incident Type 2 diabetes Mellitus in the Normoglycemic Population:a longitudinal prospective study in Japan. Diabetes Metab Syndr Obes. 2024;17:2317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Xie LF, Chen K, Yang GY, Xing XY, Zhao JJ, et al. Initiating characteristics of early-onset type 2 diabetes Mellitus in Chinese patients. Chin Med J (Engl). 2016;129(7):778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Li Y, Pei J, Wang X, Zheng K, Zhao Z, et al. Elevated glucose on Admission was an independent risk factor for 30-Day major adverse Cardiovascular events in patients with STEMI but not NSTEMI. Rev Cardiovasc Med. 2024;25(2):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan Y, Wang Q, Zhang Y, Tong X, Pu S, Xu Y, et al. High remnant cholesterol level is relevant to diabetic retinopathy in type 2 diabetes mellitus. Lipids Health Dis. 2022;21(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng G, Kuang M, Yang R, Zhong Y, Zhang S, Zou Y. Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of diabetes in a non-diabetic population. J Transl Med. 2022;20(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem. 2016;62(4):593–604. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Zhang Q, Qin B. Association between remnant cholesterol and insulin resistance levels in patients with metabolic-associated fatty liver disease. Sci Rep. 2024;14(1):4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huh JH, Roh E, Lee SJ, Ihm SH, Han KD, Kang JG. Remnant cholesterol is an independent predictor of type 2 diabetes: a Nationwide Population-based Cohort Study. Diabetes Care. 2023;46(2):305–12. [DOI] [PubMed] [Google Scholar]
- 14.Castaner O, Pinto X, Subirana I, Amor AJ, Ros E, Hernaez A, et al. Remnant cholesterol, not LDL cholesterol, is Associated With Incident Cardiovascular Disease. J Am Coll Cardiol. 2020;76(23):2712–24. [DOI] [PubMed] [Google Scholar]
- 15.Xie G, Zhong Y, Yang S, Zou Y. Remnant cholesterol is an independent predictor of New-Onset diabetes: a single-Center Cohort Study. Diabetes Metab Syndr Obes. 2021;14:4735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baratta F, Cocomello N, Coronati M, Ferro D, Pastori D, Angelico F et al. Cholesterol remnants, Triglyceride-Rich Lipoproteins and Cardiovascular Risk. Int J Mol Sci. 2023;24(5). [DOI] [PMC free article] [PubMed]
- 17.Dong W, Yan S, Chen H, Zhao J, Zhang Z, Gu W. Association of remnant cholesterol and newly diagnosed early-onset type 2 diabetes mellitus in Chinese population: a retrospective cross-sectional study. J Diabetes. 2024;16(2):e13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel CJ, Pho N, McDuffie M, Easton-Marks J, Kothari C, Kohane IS, et al. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci Data. 2016;3:160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 20.Qian S, You S, Sun Y, Wu Q, Wang X, Tang W, et al. Remnant cholesterol and common carotid artery intima-media thickness in patients with ischemic stroke. Circ Cardiovasc Imaging. 2021;14(4):e010953. [DOI] [PubMed] [Google Scholar]
- 21.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Gong R, Luo G, Li J, Li Q, Yang L, et al. Associations of Triglycerides/High-Density lipoprotein cholesterol ratio with insulin resistance, impaired glucose tolerance, and diabetes in American adults at different vitamin D3 levels. Front Endocrinol (Lausanne). 2021;12:735736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Liu F, Kong R, Han X. Association between Globulin and Diabetic Nephropathy in Type2 Diabetes Mellitus patients: a cross-sectional study. Front Endocrinol (Lausanne). 2022;13:890273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes A. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MM, Huang X, Xu C, Song XH, Liu YM, Yao D, et al. High remnant cholesterol level potentiates the development of hypertension. Front Endocrinol (Lausanne). 2022;13:830347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y, Rainey WE, Apolzan JW, Francone OL, Harris RB, Bollag WB. Adrenal cell aldosterone production is stimulated by very-low-density lipoprotein (VLDL). Endocrinology. 2012;153(2):721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Li CX, Lin YN, Zhang LY, Li SQ, Zhang L, et al. The role of Aldosterone in OSA and OSA-Related hypertension. Front Endocrinol (Lausanne). 2021;12:801689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsimihodimos V, Gonzalez-Villalpando C, Meigs JB, Ferrannini E. Hypertension and diabetes Mellitus: Coprediction and Time Trajectories. Hypertension. 2018;71(3):422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y, Liu J, Zhao K, Gao L, Zhao J. Cholesterol-induced toxicity: an integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021;33(10):1911–25. [DOI] [PubMed] [Google Scholar]
- 31.Hadi Alijanvand M, Aminorroaya A, Kazemi I, Amini M, Aminorroaya Yamini S, Mansourian M. Prevalence and predictors of prediabetes and its coexistence with high blood pressure in first-degree relatives of patients with type 2 diabetes: a 9-year cohort study. J Res Med Sci. 2020;25:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szili-Torok T, Sokooti S, Oste MCJ, Gomes-Neto AW, Dullaart RPF, Bakker SJL, et al. Remnant lipoprotein cholesterol is associated with incident new onset diabetes after transplantation (NODAT) in renal transplant recipients: results of the TransplantLines Biobank and cohort studies. Cardiovasc Diabetol. 2022;21(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bashir B, Schofield J, Downie P, France M, Ashcroft DM, Wright AK, et al. Beyond LDL-C: unravelling the residual atherosclerotic cardiovascular disease risk landscape-focus on hypertriglyceridaemia. Front Cardiovasc Med. 2024;11:1389106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huh JH, Han KD, Cho YK, Roh E, Kang JG, Lee SJ, et al. Remnant cholesterol and the risk of cardiovascular disease in type 2 diabetes: a nationwide longitudinal cohort study. Cardiovasc Diabetol. 2022;21(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XH, Zhang BL, Cheng Y, Fu SK, Jin HM. Association of remnant cholesterol with risk of cardiovascular disease events, stroke, and mortality: a systemic review and meta-analysis. Atherosclerosis. 2023;371:21–31. [DOI] [PubMed] [Google Scholar]
- 36.Kong L, Wu Y, Yang H, Guo M, Zhong Y, Li J, et al. Nonlinear association between remnant cholesterol and reversion from impaired fasting glucose to normoglycemia: a multicenter cohort study. Lipids Health Dis. 2024;23(1):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winneke G, Kastka J. Comparison of odour-annoyance data from different industrial sources: problems and implications. Dev Toxicol Environ Sci. 1987;15:129–40. [PubMed] [Google Scholar]
- 38.Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42(47):4791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat Rev Endocrinol. 2020;16(2):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boni-Schnetzler M, Meier DT. Islet inflammation in type 2 diabetes. Semin Immunopathol. 2019;41(4):501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Marco E, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci (Lond). 2015;129(2):199–216. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Liu Q, Guo X, Wang W, Yu B, Liang B, et al. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc Diabetol. 2022;21(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021;50:101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. [DOI] [PubMed] [Google Scholar]
- 45.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94(2):206–18. [DOI] [PubMed] [Google Scholar]
- 46.Dixon ED, Nardo AD, Claudel T, Trauner M. The role of lipid sensing Nuclear receptors (PPARs and LXR) and metabolic lipases in obesity, diabetes and NAFLD. Genes (Basel). 2021;12(5). [DOI] [PMC free article] [PubMed]
- 47.Khalid M, Alkaabi J, Khan MAB, Adem A. Insulin Signal Transduction Perturbations in insulin resistance. Int J Mol Sci. 2021;22(16). [DOI] [PMC free article] [PubMed]
- 48.Wu Y, Wei Q, Li H, Yang H, Wu Y, Yu Y, et al. Association of remnant cholesterol with hypertension, type 2 diabetes, and their coexistence: the mediating role of inflammation-related indicators. Lipids Health Dis. 2023;22(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, An X, Yang C, Sun W, Ji H, Lian F. The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol (Lausanne). 2023;14:1149239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rachdaoui N, Insulin. The friend and the foe in the development of type 2 diabetes Mellitus. Int J Mol Sci. 2020;21(5). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are accessible in a public, open access repository. Open access data can be found on the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.