Abstract
Background
In thyroid-associated ophthalmopathy (TAO), orbital decompression is a critical surgical approach for functional and aesthetic reasons. Meanwhile, the presence of surgical complications, especially the new onset of primary gaze diplopia, also influences postoperative patient satisfaction. This research investigates the effectiveness and potential risks associated with different orbital decompression in patients with TAO.
Methods
Systematic searches were conducted to identify pertinent studies from PubMed, Embase, and the Cochrane Library databases. The search was completed on October 11, 2023. And after retrieval, the publication dates of the articles included in the analysis ranged from January 1, 2008, to February 22, 2023. The overall postoperative outcomes were determined using random-effects meta-analyses with corresponding 95% confidence intervals (CI). A network meta-analysis was performed to integrate both direct and indirect evidence. The primary outcomes were defined as the status of exophthalmos and the new onset of primary gaze diplopia.
Results
From 1,538 identified records, 87 studies were selected, encompassing 5102 patients and 8,779 procedures. The studies reported varying degrees of exophthalmos reduction based on different surgical techniques: -3.46 mm (95% CI -3.76 to -3.15 mm) for fat removal orbital decompression, -4.02 mm (95% CI -5.14 to -2.89 mm) for the medial wall technique, -3.89 mm (95% CI -4.22 to -3.55 mm) for the lateral wall technique, -5.23 mm (95% CI -5.69 to -4.77 mm) for the balanced wall technique, -3.91 mm (95% CI -4.37 to -3.46 mm) for the infero-medial wall technique, and − 5.80 mm (95% CI -6.47 to -5.13 mm) for the three-wall technique. The incidence of new-onset primary gaze diplopia was reported in 31 studies involving 214 out of 2001 patients, resulting in a weighted proportion of 0.11 (95% CI 0.06–0.14). Notably, the lowest rates were associated with the lateral approach and fat removal orbital decompression, with pooled proportion (95% CI) rates of 3% (1–6) and 3% (2–4), respectively, suggesting that these two techniques may be more effective in preventing the occurrence of this complication during the postoperative period.
Conclusions
This meta-analysis establishes that orbital decompression is a beneficial and safe surgical approach. While this study enhances the evidence hierarchy for orbital decompression in treating TAO, it requires further validation through larger, prospective, and randomized studies with long-term follow-up periods.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-024-03749-3.
Keywords: Thyroid-associated ophthalmopathy, Orbital decompression, Exophthalmos, Diplopia, Meta-analysis
Background
Thyroid-associated ophthalmopathy (TAO), an organ-specific autoimmune disorder, is the most prevalent adult orbital disease [1]. TAO, also named Graves’ orbitopathy and Graves’ ophthalmopathy, is the most frequent extrathyroidal manifestation of Graves’ disease. It is characterized by the infiltration of inflammatory cells in the retrobulbar and periorbital tissues, leading to a range of symptoms, including eyelid retraction, edema of the periorbital tissues and conjunctivae, exophthalmos, ocular surface irritation symptoms, such as grittiness and watering, and the occurrence of restrictive strabismus and diplopia caused by the involvement of the extraocular muscles [2–4]. In cases of severe disease progression, TAO can lead to vision-threatening conditions such as exposure keratopathy and dysthyroid optic neuropathy (DON), which can result in irreversible vision loss and potential disfigurement, significantly impacting the patient’s quality of life and mental well-being [5–10].
Depending on the activity and severity of TAO, treatment options include drug therapy, orbital radiotherapy, surgery, or a combination [11]. Surgical interventions for TAO include orbital decompression, strabismus correction, and blepharoplasty. Among these, orbital decompression surgery stands out as the cornerstone of surgical rehabilitation [12]. The orbital walls are divided into four segments: medial, lateral, orbital roof, and floor. Owing to suboptimal outcomes and the potential for severe intracranial complications, the excision of the orbital roof is not commonly favored. Orbital decompression is accomplished by removing the bony wall (typically medial, inferior, lateral, or combination), orbital fat, or both to decrease the orbital content and increase orbital volume [13]. Orbital decompression is performed urgently in cases of sight-threatening optic nerve compression to relieve optic nerve pressure, reduce retrobulbar pressure, restore venous outflow, increase orbital perfusion, and improve vision [14–16]. However, except for such emergencies, rehabilitative surgery is limited to the inactive phase of the disease, aiming to improve visual function and cosmetic appearance [17].
A severe complication of orbital decompression surgery is the worsening of preexisting diplopia or the development of new-onset diplopia [18]. Therefore, orbital decompression surgery still faces the challenge of reducing eye protrusion effectively while simultaneously reducing the risk of surgical complications as much as possible. Preoperative diplopia in TAO is primarily due to extraocular muscle fibrosis [3]. In the preliminary stages of the disease, these muscles exhibit edema and inflammatory cell infiltration, progressing to fibrosis and stiffening in the later stages [19]. All forms of orbital decompression surgery face the risk of exacerbating pre-existing diplopia or inducing new instances. Potential causes include the vulnerability of extraocular muscle balance, disruption of tissue planes, intraorbital tissue adhesions outside the extraocular muscles postoperative inflammatory responses leading to inconsistent resolution of soft tissue swelling, or reactivation of the patient’s immune response [20–22]. In cases where diplopia persists long-term post-stabilization of the condition, strabismus correction surgery may be considered [23].
This research uses a meta-analysis approach to assess the effectiveness of various interventions for orbital decompression surgery in treating TAO. It also summarizes information on potential complications, such as the new onset of primary gaze diplopia. The patients were categorized into six groups based on the surgical technique: fat removal orbital decompression, the medial wall only, the lateral wall only, the balanced (medial and lateral) wall, the infero-medial wall, and the three-wall (medial, lateral, and inferior). This meta-analysis was structured using the ‘PICO’ framework, focusing on patients with TAO (P) undergoing orbital decompression surgery (I). The study compares various surgical methods (C) to determine the most effective approach in reducing exophthalmos and minimizing the incidence of new-onset primary gaze diplopia (O).
Methods
Study design
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered on the Prospero platform with the registration number CRD42023478618 (Link:https://www.crd.york.ac.uk/prospero/#myprospero) .
The primary outcomes of this meta-analysis were (1) an evaluation of proptosis reduction, quantified in millimeters and (2) the incidence of new-onset primary gaze diplopia. Secondary outcomes included: (1) visual acuity, assessed using logMAR best-corrected visual acuity (BCVA), which refers to the best possible vision a patient can achieve with optimal optical correction [24]; (2) changes in intraocular pressure (IOP), measured in mmHg; (3) alterations in upper eyelid margin distance to the corneal reflex (MRD1) and lower eyelid margin distance to the corneal reflex (MRD2), recorded in millimeters; (4) changes in visual field mean deviation (VF-MD), quantified in decibels (dB); and (5) the assessment of other sequelae or complications.
Search strategy
The search was completed on October 11, 2023, and the preliminary inclusion encompassed all literature from the three databases from their inception up to this date. The publication dates of the articles included in the analysis ranged from January 1, 2008, to February 22, 2023. During our literature search, we strictly adhered to the Cochrane principles, meticulously designed our search strategy, and comprehensively searched relevant studies from the PubMed, Embase, and Cochrane Library (CENTRAL) from their inception, focusing exclusively on articles published in English. Emtree/MeSH terms such as “Graves Ophthalmopathy” and “Decompression, Surgical” were used in the search algorithm, supplemented by relevant free terms tailored to each database. The management of these studies and removing duplicates were facilitated using Endnote X9.32.
Inclusion criteria
The primary criteria for including citations in this study were as follows: (1) Studies focused on patients with TAO treated through orbital decompression, including emergency operation for sight-threatening DON or rehabilitative surgery for mild-to-moderate patients; (2) Studies that used purely surgical decompression without combination with steroids or other ophthalmic operations; (3) All randomized and nonrandomized controlled studies, as well as prospective or retrospective case series of TAO adults; (4) Selected studies must report at least one primary outcome and one or more secondary outcome parameters; and (5) Studies published in English or with an English translation.
Exclusion criteria
The following studies were excluded: (1) Case reports, systematic reviews, conference proceedings, comments, and letters; (2) Studies published before 2007; (3) No relevant outcomes; (4) Studies with duplicate data; and (5) Studies lacking a clear definition of the surgical technique used.
Study selection
Data extraction was carried out by a single reviewer (W.G.) and cross-verified for accuracy by a second reviewer (L.J.G.). Uncertain cases were assessed for eligibility by reviewing the full texts. Any disagreements were resolved through discussion and finally resolved by the senior author (D.M.L.). Information regarding the research design, study period, demographic data, and interventional data was meticulously recorded.
Quality assessment
The quality of both direct and network meta-analysis evidence was evaluated using the Newcastle–Ottawa Quality Assessment Scale (NOS). This scale has a maximum score of 9 points, and studies scoring above five were included in the meta-analysis. The final quality rating was established based on mutual agreement between the reviewers.
Statistical analysis
Statistical analyses were performed using STATA, version 14.2 (StataCorp, College Station, TX, USA). We conducted both traditional pairwise and network meta-analyses concurrently. Using a random-effects model, we calculated the standardized mean difference (SMD) for continuous outcomes, along with its 95% confidence interval (CI), and the odds ratio (OR) for dichotomous outcomes, also with its 95% CI, to serve as the pooled effect sizes. Besides, a traditional pairwise meta-analysis using random effects was executed for each intervention separately, utilizing the ‘mean’ command in STATA. The surface under the cumulative ranking curve (SUCRA) was utilized to rank each outcome. Additionally, a matrix was constructed to compare all interventions and determine if the SUCRA difference between each pair of interventions reached a statistically significant level. The threshold for statistical significance was set at p < 0.05.
Result
The characteristics of the included studies
A total of 1538 studies were initially retrieved. After removing duplicates, 1143 papers remained eligible for screening by title and abstract. Subsequently, 197 studies were evaluated for inclusion based on their full texts. Two investigators rigorously screened and selected 87 studies for inclusion. The search and screening process used to identify relevant studies is described in Fig. 1.
Fig. 1.
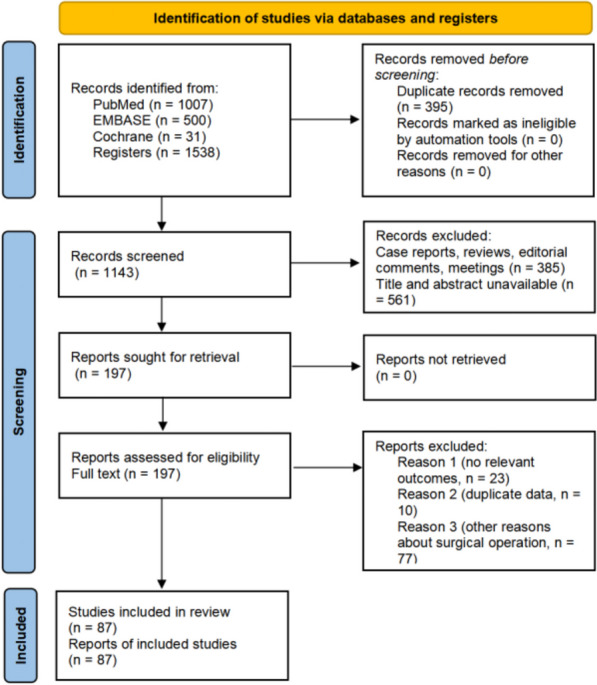
Flow Diagram of Study Selection Process
A total of 87 studies, including 5102 patients and 8779 procedures, were deemed eligible for analysis. These studies, published in English-language journals between 2008 and 2023, focused on orbital decompression surgery. The investigations detailed six different surgical methods. All included studies were either prospective or retrospective observational studies. Table 1 provides a brief description of these 87 studies.
Table 1.
Baseline characteristics of patients with thyroid-Associated Ophthalmopathy (TAO) included in the Meta-Analysis
| Study | Country | Study design | Period of data collection | Patients(n) | Sex(F/M) | Orbits(n) | Mean age(y) | OD technique(Orbits) | Mean follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Alsuhaibani et al.2011 [25] | Saudi Arabia | RA | 2003–2007 | 20 | 16:4 | 38 | 45 | Medial–lateral | 21(3–48) |
| Antisdel et al. 2013 [26] | USA | RA | 2004–2010 | 50 | 39:11 | 86 | 48.6 ± 12.9 | Three-wall | 0.25 |
| Baril et al. 2014 [27] | Spain | RA | 2000–2010 | 34 | NR | 59 | 57.80 ± 11.89 | Medial–lateral | 24–120 |
| Barkhuysen et al. 2009 [28] | Netherlands | RA | 2003–2004 | 7 | 7:0 | 14 | 48.1 ± 14.2 | Three-wall | 24 ± 16(5–52) |
| Bengoa-González et al. 2019 [29] | Spain | RA | 2015–2017 | 35 | 26:9 | 58 | 52.6 ± 13.9 | Lateral | >6 |
| Boulanouar et al.2020 [30] | France | RA | 1995–2016 | 136 | 113:23 | 272 | 44.8 | Infero-medial | 2–12 |
| Byeon et al. 2023 [31] | Korea | RA | 2017–2020 | 217 | 173:44 | 420 | 35.85 ± 10.06 | Inferno-medial | 15.6(3–30) |
| Chang et al. 2008 [32] | USA | RA | 2004–2005 | 33 | 21:12 | 65 | 41 | Lateral | 9(3–18) |
| Chang et al. 2013 [33] | Korea | RA | 2004–2008 | 33 | 23:10 | 33 | 45.0 |
FROD(13) Medial–lateral(20) |
36 |
| Cheng et al. 2018 [34] | USA | RA | 2003–2014 | 845 | 633:212 | 1604 | 39.6 ± 11.6 | FROD | 37.9 ± 24.4 |
| Cheng et al. 2021 [35] | China | RA | 2013–2019 | 37 | 28:9 | 52 | 48.27 ± 10.59 |
Medial–lateral(31) Three-wall(21) |
22 ± 17(3–71) |
| Cho et al. 2010 [36] | USA | RA | 2005–2008 | 22 | NR | 36 | NR | Lateral | 2.3 |
| Choe et al. 2011 [37] | USA | RA | 2003–2008 | 17 | 15:2 | 28 | NR |
Medial(18) Lateral(10) |
22.3(1.5–52.9) |
| Choe et al. 2011 [38] | Korea | RA | 2011–2013 | 24 | 19:5 | 48 | 34.08 ± 7.03 | Medial–lateral | 11.46 ± 6.55 |
| Chu et al. 2009 [39] | USA | RA | 2001–2008 | 48 | NR | 80 | NR |
Infero-medial(32) Three-wall(48) |
4–5 |
| Cubuk et al. 2018 [40] | Turkey | RA | 1994–2014 | 149 | 89:60 | 248 | 42.3 ± 13.2 |
Lateral(13) Medial-lateral(181) Infero-medial(15) Three-wall(39) |
24–240 |
| Curragh et al. 2019 [41] | Australia | RA | 2011–2018 | 19 | 12:7 | 19 | 57.4 | Medial | 15.79 ± 9.32(5–40) |
| Dallan et al. 2022 [42] | Italy | RA | 2015–2020 | 8 | NR | 14 | NR | Three-wall | 1 |
| Dubin et al. 2008 [43] | USA | RA | 1999–2002 | 24 | 17:7 | 45 | 52.7 ± 10.4 | Medial–lateral | 20.4 ± 16.8(1.4–72) |
| Fichter et al. 2015 [22] | Switzerland | RA | 1999–2011 | 111 | 87:24 | 164 | 48.8 ± 11.7 | Lateral | 16.4 ± 20.4(3-126) |
| Finn et al. 2017 [44] | USA | RA | 2012–2015 | 26 | 20:6 | 45 | 58.3 |
Infero-medial(11) Three-wall(34) |
0.66–20.36 |
| Fu et al. 2022 [45] | China | RA | 2019–2021 | 22 | 11:11 | 30 | 43.17 ± 10.11 | Medial | >3 |
| Gong et al. 2018 [46] | China | RA | 2016–2017 | 38 | 20:18 | 41 | 41.95 ± 12.65 | Medial–lateral | >3 |
| Gu et al. 2023 [47] | China | PCS | 2021–2022 | 23 | 16:7 | 34 | 45.1 ± 11.1 | Lateral | 6 |
| Guo et al. 2021 [48] | China | RA | 2016–2018 | 54 | 28:26 | 54 | 51.7 ± 12.5 |
Lateral(15) Medial-lateral(18) Three-wall(21) |
4.2 ± 0.9(4–9) |
| Gupta et al. 2018 [49] | USA | RA | 2010–2014 | 48 | NR | 75 | 46.5 | Medial–lateral | 6 |
| Hernández-García et al. 2017 [50] | Spain | RA | 2004–2014 | 20 | 14:6 | 36 | 51.7 | Medial–lateral | 44(18–84) |
| Hill et al. 2012 [51] | USA | RA | 1995–2007 | 16 | 14:2 | 16 | 42.6 | Medial | NR |
| Hu et al. 2017 [52] | China | RA | 2011–2016 | 55 | 31:24 | 77 | 52.10 ± 5.22 | Three-wall | 6 |
| Juniat et al. 2019 [53] | UK | RA | 2011–2018 | 47 | NR | 70 | 53.8 |
Medial(24) Infero-medial(31) Three-wall(15) |
15.7 ± 14.8(4–85) |
| Kakizaki et al. 2011 [54] | Japan | RA | 2008–2010 | 32 | 20:12 | 47 | NR |
Lateral(24) Medial-lateral(23) |
>6 |
| Kim et al.2015 [55] | Korea | RA | 2011–2014 | 21 | 13:8 | 42 | NR |
Medial-lateral(25) Three-wall(8) |
3 |
| Kim et al.2021 [56] | Korea | RA | 2015–2018 | 71 | 55:16 | 123 | 35 |
Medial (68) Infero-medial(55) |
6 |
| Kingdom et al.2015 [57] | USA | RA | 2002–2013 | 77 | 52:25 | 114 | 57.2 | Three-wall | 31.3(1-126) |
| Kitaguchi et al. 2019 [58] | Japan | RA | 2013–2016 | 43 | 41:2 | 43 | 38 ± 10 | Lateral | >3 |
| Korkmaz et al. 2016 [59] | Turkey | RA | 2004–2010 | 42 | 17:25 | 68 | 53.5 |
Medial-lateral(41) Three-wall(27) |
39.3 ± 15(12–72) |
| Lal et al. 2013 [60] | India | RA | 2002–2010 | 12 | 2:10 | 24 | 36.5 | Infero-medial | 6 |
| Lee et al. 2014 [61] | Korea | RA | 2009–2012 | 55 | NR | 90 | 39.8 |
FROD(29) Medial(15) Infero-medial(46) |
>6 |
| Li et al. 2015 [20] | China | RA | NR | 11 | 8:3 | 21 | 45.2 ± 11.8 | FROD | 6 |
| Liao et al. 2011 [62] | Taiwan, China | PCS | 2006–2007 | 22 | 13:9 | 44 | 37.9 ± 12.0 | FROD | 6 |
| Lipski et al. 2011 [63] | Germany | RA | NR | 15 | 14:1 | 30 | 56.2 ± 10.9 | Three-wall | 30 ± 13 |
| Lv et al. 2024 [64] | China | PCS | 2018–2022 | 112 | 57:55 | 112 | 50.88 ± 10.51 | Medial | >1 |
| Lv et al. 2016 [65] | China | RA | 2006–2013 | 43 | 31:12 | 72 | 45 | Medial | 9 ± 3(6–18) |
| Maalouf et al. 2008 [66] | France | RA | 1999–2001 | 19 | 17:2 | 36 | 48.4 ± 13.3 | Infero-medial | 43.5 ± 12 |
| Mainville et al. 2014 [67] | Canada | RA | 1999–2008 | 119 | NR | 212 | NR | Infero-medial | 19.2(1–90) |
| Malik et al. 2008 [68] | UK | RA | 1996–2002 | 15 | 13:2 | 20 | 51 | Infero-medial | 13(2–30) |
| Mehta et al. 2011 [69] | UK | RA | 2007–2009 | 17 | 12:5 | 21 | 50 | Lateral | 11.8(9–12) |
| Millar et al. 2009 [70] | Australia | RA | NR | 7 | NR | 7 | NR | Medial-lateral | 6 |
| Murta et al. 2021 [71] | UK | PCS | 2015–2017 | 33 | 22:11 | 54 | NR |
Lateral(39) Medial-lateral(3) Three-wall(12) |
>3 |
| Nguyen et al. 2014 [72] | USA | RA | 2006–2013 | 69 | NR | 108 | 50.4 ± 11.9 | Medial-lateral | 5.35 |
| Norris et al. 2012 [73] | UK | PCS | NR | 33 | 22:11 | 52 | 52.5 ± 9.4 |
FROD(6) Lateral(13) Medial-lateral(26) Three-wall(7) |
3 |
| Onaran et al. 2014 [74] | Turkey | RA | 2002–2008 | 36 | 20:16 | 72 | 49.3 ± 12.5 | Medial-lateral | 6 |
| Park et al. 2015 [75] | Korea | RA | NR | 7 | 3:4 | 9 | 54.1 | Infero-medial | 6 |
| Pereira et al. 2022 [76] | Brazil | PCS | 2016–2019 | 42 | 31:11 | 84 | NR |
Medial-lateral(42) Infero-medial(42) |
6 |
| Pieroni Goncalves et al.2017 [77] | Brazil | RA | 2011–2013 | 57 | 40:17 | 105 | 53.6 ± 12.7 |
FROD(5) Medial(2) Lateral(40) Medial-lateral(58) |
>3 |
| Prat et al. 2015 [78] | USA | RA | 1990–2010 | 109 | NR | 217 | 44 | FROD | 3 |
| Prevost et al. 2020 [79] | France | RA | 1997–2017 | 191 | 153:38 | 350 | NR | Infero-medial | 16.1 ± 23.1 |
| Rajabi et al. 2021 [80] | Iran | PCS | 2013–2019 | 20 | 11:9 | 20 | 30.3 | Lateral | 6 |
| Ramesh et al. 2019 [81] | USA | PCS | 2009–2011 | 36 | 8:28 | 60 | 46.5 | Medial-lateral | 6 |
| Rocchi et al. 2012 [19] | Italy | RA | 2002–2009 | 247 | 184:63 | 485 | NR |
Lateral(97) Medial-lateral(388) |
>3 |
| Sagili et al. 2008 [82] | UK | RA | NR | 10 | 9:1 | 18 | 49 |
FROD(6) Medial-lateral(7) Three-wall(5) |
3 |
| Schiff et al. 2015 [83] | USA | RA | NR | 9 | NR | 12 | NR | Infero-medial | 3.4 ± 2.5 |
| Seibel et al. 2017 [84] | Germany | RA | 2012–2014 | 20 | 16:4 | 34 | 54.8 |
Medial(12) Medial-lateral(22) |
12 |
| Sellari-Franceschini et al.2018 [85] | Italy | PCS | 2012–2014 | 38 | 31:7 | 76 | NR |
Lateral(38) Medial-lateral(38) |
6 |
| Seo et al. 2019 [86] | Korea | PCS | NR | 40 | 28:12 | 40 | 36.83 ± 2.15 | Infero-medial | 3 |
| She et al. 2014 [87] | Taiwan, China | RA | 1996–2010 | 25 | 11:14 | 42 | 51.2 | Infero-medial | 3 |
| Shi et al. 2015 [88] | China | RA | 2010–2014 | 6 | 2:4 | 12 | 42 ± 12 | Three-wall | 18(12–28) |
| Singh et al. 2019 [89] | India | RA | 2011–2018 | 17 | 11:6 | 17 | 69 | Infero-medial | 12(6–40) |
| Sobti et al. 2024 [90] | UK | RA | 2013–2017 | 30 | NR | 56 | NR | Lateral | 3 |
| Stähr et al. 2019 [91] | Germany | RA | 2014–2016 | 174 | NR | 318 | NR | Medial-lateral | 7.4 |
| Takahashi et al. 2014 [92] | Japan | RA | 2010–2012 | 40 | NR | 78 | NR |
Lateral(61) Medial-lateral(17) |
3 |
| Thorne et al. 2020 [93] | USA | RA | 2012–2016 | 86 | NR | 131 | 46.6 | Lateral | 3 |
| Tu et al. 2022 [94] | China | PCS | 2019–2020 | 22 | 10:12 | 39 | 42.0 ± 15.7 | Lateral | 3.4 ± 0.7(3–5) |
| Ueland et al. 2016 [95] | Norway | RA | 1999–2013 | 84 | 76:8 | 144 | 50 | Lateral | 124(13–188) |
| Wang et al. 2022 [96] | China | PCS | 2021 | 10 | 10:0 | 18 | 30.0 ± 6.8 | FROD | 3 |
| Wang et al. 2017 [97] | China | RA | 2013–2015 | 18 | 15:3 | 28 | 30 |
Infero-medial(10) Three-wall(18) |
3 |
| Woo et al. 2017 [98] | Korea | RA | 2011–2014 | 59 | 50:9 | 118 | NR |
FROD(36) Medial(22) Infero-medial (60) |
3–50 |
| Woods et al. 2020 [99] | Ireland | RA | 2004–2017 | 22 | 15:7 | 35 | 52 | Infero-medial | 3 |
| Wu et al. 2008 [100] | Taiwan, China | RA | 2003–2006 | 120 | 89:31 | 222 | 37.8 ± 10.3 | FROD | 10.9 ± 5.1(6–37) |
| Wu et al. 2016 [101] | USA | RA | 1999–2014 | 53 | 34:19 | 80 | 56.60 ± 15.4 | Medial-lateral | 49.89 ± 39.2 |
| Wu et al. 2015 [102] | China | RA | 2006–2013 | 108 | 74:34 | 206 | 37.66 ± 9.5 | Medial | 16.0 ± 4.2(12–24) |
| Xu et al. 2020 [103] | China | PCS | 2016–2019 | 60 | NR | 84 | NR |
Medial-lateral(33) Infero-medial (51) |
6 |
| Yao et al. 2016 [104] | USA | RA | 2007–2014 | 73 | NR | 115 | 53.8 ± 12.9 | Three-wall | 3 |
| Ye et al. 2023 [105] | China | RA | 2020–2022 | 34 | 20:14 | 55 | 38.62 | Medial | >3 |
| Yeo et al. 2017 [106] | Korea | RA | 2014–2016 | 54 | 35:19 | 108 | 34.59 ± 9.72 |
Medial(28) Medial-lateral(48) Three-wall(32) |
3 |
| Yinghong et al.2023 [107] | China | RA | 2021–2022 | 9 | 7:2 | 15 | 53.86 ± 10.05 | Three-wall | 8(3–13) |
| Zhang et al. 2019 [108] | China | RA | 2015–2017 | 50 | 34:16 | 75 | 40.2 ± 13.3 |
Lateral(18) Medial-lateral(24) Three-wall(33) |
3 |
Quality assessment
The literature quality is conducive to supporting the meta-analysis, with all studies attaining Newcastle–Ottawa Quality Assessment Scale (NOS) scores of 5 or higher. This indicates that the studies included in this research are based on moderate- to high-quality evidence. The NOS scores for the included controlled studies are depicted in Supplementary Table 1.
Direct meta-analysis
Primary outcomes
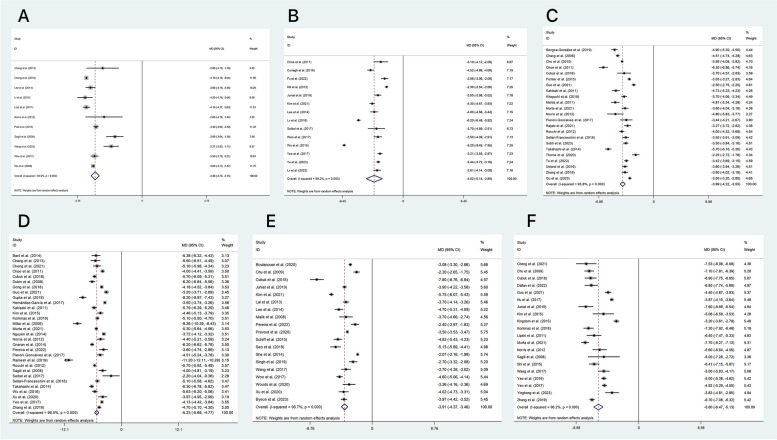
In our study, we used a random-effect model to analyze the outcomes of proptosis (as shown in Fig. 2) and the incidence of new-onset primary gaze diplopia (illustrated in Fig. 3).
Fig. 2.
Forest Plot Displaying Proptosis Reduction(mm) Outcome in Patients with TAO Following Orbital Decompression The groups are denoted as follows: A: fat removal orbital decompression, B: medial wall, C: lateral wall, D: balanced wall, E: infero-medial wall, and F: three-wall
Fig. 3.
Forest Plot Illustrating the Incidence of New-Onset Primary Gaze Diplopia in Patients with TAO Following Orbital Decompression The groups are denoted as follows: A: fat removal orbital decompression, B: medial wall, C: lateral wall, D: balanced wall, E: infero-medial wall, F: three-wall
For the proptosis outcomes, we found each of the following interventions to be significantly effective (P < 0.001): the three-wall (MD=-5.80 mm, 95% CI -6.47 to -5.13, 20 studies), the balanced wall (MD=-5.23 mm, 95% CI -5.69 to -4.77, 30 studies), the medial wall (MD=-4.02 mm, 95% CI -5.14 to -2.89, 14 studies), the infero-medial wall (MD=-3.91 mm, 95% CI -4.37 to -3.46, 19 studies), the lateral wall (MD=-3.89 mm, 95% CI -4.22 to -3.55, 23 studies), and fat removal orbital decompression (MD=-3.46 mm, 95% CI -3.76 to -3.15, 11 studies). Heterogeneity values were high in all analyses.
The rate of new-onset primary gaze diplopia was reported in 31 studies. Of those without diplopia before surgery, the pooled proportion (95% CI) of the rate of new-onset primary gaze diplopia was 11% (6–14), which exhibited heterogeneous outcomes (I2 = 85.3%, P < 0.001, Z = 7.01, P < 0.001). The lowest rates were associated with the lateral approach and fat removal orbital decompression, with pooled proportion (95% CI) rates of 3% (1–6) and 3% (2–4), respectively. The results were both homogeneous (I2 = 0.0%, P = 0.68, Z = 2.33, P = 0.02, I2 = 0.0%, P = 0.79, Z = 5.18, P<0.001).
Secondary outcomes
Eighteen of 87 studies, comprising 1072 of 8779 eyes, provided detailed data on ΔBCVA (measured in logMAR) values with standard deviations. The ΔBCVA was improved by 0.35 (95% CI 0.21 to 0.50) LogMAR in the balanced wall group, surpassing the other groups (as illustrated in Supplementary Fig. 1). The ranking of the other groups in terms of visual acuity enhancement, in decreasing order of effectiveness, was as follows: the three-wall group, the infero-medial wall group, the medial wall group, and finally, the lateral wall group.
The pooled proportion (95% CI) ΔIOP across 12 studies was − 2.15mmHg (95% CI -2.93 to -1.37). Notably, the improvement in IOP was more pronounced in the group undergoing the balanced wall decompression (I2 = 56.7%, p = 0.042, Z = 7.45, P<0.001), as shown in Supplementary Fig. 2.
Through our analysis, the average change in MRD1 was determined to be -0.41 mm (95% CI -0.69 to -0.13), as illustrated in Supplementary Fig. 3. Additionally, our meta-analysis also revealed a decrease in MRD2 following orbital decompression surgery. The average change in MRD2 was − 1.12 mm (95% CI -1.49 to -0.76), as shown in Supplementary Fig. 4, indicating a more notable postoperative alteration than MRD1.
Supplementary Fig. 5 depicts that among the 87 studies included in our analysis, only five provided data on the standard deviation of ΔVF-MD, with a pooled average ΔVF-MD of 6.92dB (95% CI 3.97–9.86), highlighting a significant improvement in visual field parameters post-surgery.
Other complications encountered in the analyzed studies, which necessitated medical intervention, included hemorrhage, infection, sensory nerve damage, chemosis, oscillopsia, new-onset strabismus, epiphora, cerebrospinal fluid leaks, dural tears, temporal hollowing, chewing alterations, and sinonasal issues. We used a random-effect model to analyze the incidence of permanent infraorbital nerve hypoesthesia—defined as symptoms persisting for more than six months or having a long-term presence—as illustrated in Supplementary Fig. 6. Additionally, we also analyzed the incidence of cerebrospinal fluid leaks, as detailed in Supplementary Fig. 7.
Network meta-analysis
Pairwise analysis of eligible comparisons
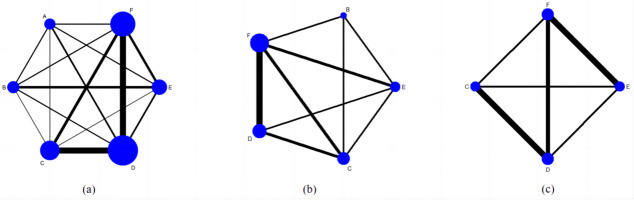
Figure 4 depicts the mesh diagrams. The nodes’ size is proportional to the number of trials that assessed the same intervention, and the thickness of the lines corresponds to the number of trials with a direct comparison.
Fig. 4.
Network Map of Surgical Comparisons for Outcomes and Complications in TAO Treatment It includes proptosis (a), logMAR BCVA (b), and the rate of new-onset primary gaze diplopia (c). The groups are denoted as follows: A: fat removal orbital decompression, B: medial wall, C: lateral wall, D: balanced wall, E: infero-medial wall, F: three-wall
The forest plots of various surgical groups are shown in Supplementary Fig. 8. Notably, the three-wall approach represented a significant advantage in exophthalmos improvement. Similarly, compared with other surgical methods except fat removal orbital decompression, the lateral wall approach had a significant disadvantage in terms of improving logMAR BCVA. Furthermore, some network meta-analyses comparing two surgical methods in postoperative efficacy and complications revealed no statistically significant differences.
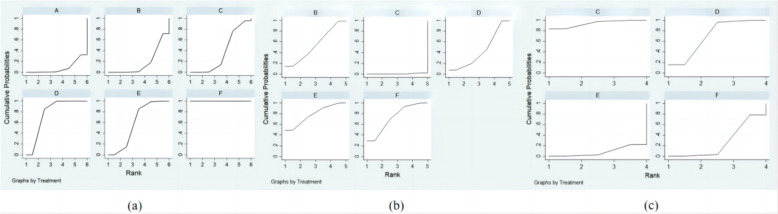
SUCRA ranking analysis
Upon ranking the efficacy of all surgical interventions using the SUCRA probabilities, it was evident that the three-wall approach had the highest likelihood of being the best intervention to improve proptosis, with a SUCRA score of 100%, and the least effective was fat removal orbital decompression, with a SUCRA probability of only 8.1%, as illustrated in Fig. 5(a). The improvement in the logMAR BCVA of all surgical interventions was ranked with SUCRA probabilities in Fig. 5(b). It clearly showed that the infero-medial wall approach ranked first, with a SUCRA score of 78.3%. Simultaneously, the lateral wall approach ranked the best in minimizing the risk of new-onset diplopia, with an SUCRA score of 93.7%, as described in Fig. 5(c).
Fig. 5.
The SUCRA for Different Surgical Comparisons about Outcomes and Complications It includes proptosis (a), logMAR BCVA (b), and the rate of new-onset primary gaze diplopia (c). The groups are denoted as follows: A: fat removal orbital decompression, B: medial wall, C: lateral wall, D: balanced wall, E: infero-medial wall, F: three-wall
Median values of standardized mean differences with 95% confidence intervals (column vs row) of the outcomes of different surgical interventions were exhibited in the lower left part of Table 2.. In comparison, standardized mean differences with 95% confidence intervals using the ‘metan’ command were exhibited on the upper right of the table. Numbers in bold with darker shades showed statistically significant results.
Table 2.
Matrix of Pairwise Comparisons Among Different Surgical Methods for Efficacy and Safety (shown as mean difference and 95% confidence intervals), including proptosis (a), logMAR BCVA (b), and the rate of new-onset primary gaze diplopia (c). The groups are denoted as follows: A: fat removal orbital decompression, B: medial wall, C: lateral wall, D: balanced wall, E: infero-medial wall, F: three-wall
| F | D | E | C | B | A | |
|---|---|---|---|---|---|---|
| SUCRA (%) | 100 | 77.0 | 59.7 | 37.1 | 18.2 | 8.1 |
| F | 0 | 1.69(1.02, 2.37) | 2.16(1.29 3.03) | 2.70(1.90, 3.50) | 3.16(2.19, 4.14) | 3.47(2.37, 4.58) |
| D | -1.69(-2.37, -1.02) | 0 | 0.46(-0.40, 1.33) | 1.00(0.34, 1.67) | 1.47(0.52, 2.42) | 1.78(0.74, 2.82) |
| E | -2.16(-3.03, -1.29) | -0.46(-1.33, 0.40) | 0 | 0.54(-0.44, 1.52) | 1.01(0.08, 1.93) | 1.32(0.22, 2.41) |
| C | -2.70(-3.50, -1.90) | -1.00(-1.67, -0.34) | -0.54(-1.52, 0.44) | 0 | 0.46(-0.58, 1.51) | 0.78(-0.38, 1.93) |
| B | -3.16(-4.14, -2.19) | -1.47(-2.42, -0.52) | -1.01(-1.93, -0.08) | -0.46(-1.51, 0.58) | 0 | 0.31(-0.82, 1.44) |
| A | -3.47(-4.58, -2.37) | -1.78(-2.82, -0.74) | -1.32(-2.41, -0.22) | -0.78(-1.93, 0.38) | -0.31(-1.44, 0.82) | 0 |
| (a) | ||||||
| E | F | B | D | C | ||
| SUCRA (%) | 78.3 | 72.9 | 54.9 | 43.4 | 0.6 | |
| E | 0 | 0.02(-0.14, 0.17) | 0.06(-0.11, 0.23) | 0.09(-0.12, 0.29) | 0.25(0.06, 0.44) | |
| F | -0.02(-0.17, 0.14) | 0 | 0.04(-0.12, 0.20) | 0.07(-0.07, 0.21) | 0.24(0.10, 0.37) | |
| B | -0.06(-0.23, 0.11) | -0.04(-0.20, 0.12) | 0 | 0.03(-0.16, 0.21) | 0.20(0.03, 0.36) | |
| D | -0.09(-0.29, 0.12) | -0.07(-0.21, 0.07) | -0.03(-0.21, 0.16) | 0 | 0.17(0.04, 0.30) | |
| C | -0.25(-0.44, -0.06) | -0.24(-0.37, -0.10) | -0.20(-0.36, -0.03) | -0.17(-0.30, -0.04) | 0 | |
| (b) | ||||||
| C | D | F | E | |||
| SUCRA (%) | 93.7 | 70.7 | 27.3 | 8.2 | ||
| C | 0 | 2.54(0.40,16.00) | 9.67(1.15, 81.07) | 18.51(1.52, 225.30) | ||
| D | 0.39(0.06, 2.47) | 0 | 3.80(1.05, 13.80) | 7.28(1.15, 46.16) | ||
| F | 0.10(0.01, 0.87) | 0.26(0.07, 0.95) | 0 | 1.91(0.36, 10.30) | ||
| (c) | ||||||
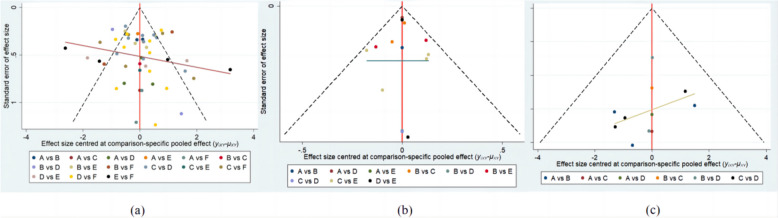
Publication bias
The funnel plot in Fig. 6 performs publication bias for included studies, revealing that most of the scatter points are located on both sides of the vertical line. Therefore, it suggested this was a reliable analysis.
Fig. 6.
Funnel Plot of Different Surgical Comparisons for Outcomes and Complications in TAO Treatment It includes proptosis (a), logMAR BCVA (b), and the rate of new-onset primary gaze diplopia (c). The groups are denoted as follows: A: fat removal orbital decompression, B: medial wall, C: lateral wall, D: balanced wall, E: infero-medial wall, F: three-wall
Discussion
Orbital decompression surgery is typically indicated for TAO patients with severe vision-threatening conditions or those unresponsive to pharmacotherapy. With the progressive expansion of surgical indications, an increasing number of patients with mild to moderate TAO exhibiting exophthalmos are also seeking surgical intervention to enhance their appearance [109]. Current prevalent surgical methods involve the resection of the medial, lateral, and/or inferior orbital walls, either singularly or in combination [110]. Concurrent with the removal of the orbital walls, selective excision of orbital fat may also be undertaken. Alternatively, fat removal orbital decompression can be performed so that the injury is relatively small, and the recovery is fast, although the surgical effect is limited [85].
Our current meta-analysis included 8779 procedures of orbital decompression managed with six surgical approaches, and the findings of this study indicated that various orbital decompression surgeries could effectively reduce exophthalmos and improve patients’ appearance. Consistent with prior research [111], our study corroborated that a greater extent of orbital wall removal correlates with an increased reduction in exophthalmos. The three-wall decompression technique maximally ameliorated the degree of exophthalmos in patients, and the average proptosis reduction for the three-wall decompressions was 5.80 mm (95% CI 5.13 to 6.47).
It is noteworthy that previous research indicated a positive correlation between the amount of orbital fat removal and the degree of reduction in exophthalmos, with each milliliter of fat removed correlating to reduction in exophthalmos of approximately 0.5 to 1.0 mm [102]. Willaert et al. [112] systematically reviewed the efficacy of fat removal orbital decompression in improving exophthalmos, revealing a weighted mean difference in Hertel score of − 3.81 mm (95% CI -4.21 to -3.41), surpassing the surgical outcomes of the medial wall decompression (MD=-3.47 mm, 95% CI -5.81 to -1.12) reported in Gioacchini et al. [113] systematic review. Our study, however, found that medial orbital wall decompression (MD=-4.02 mm, 95% CI -5.14 to -2.89) surpassed the efficacy of fat removal orbital decompression (MD=-3.46 mm, 95% CI -3.76 to -3.15) in reducing exophthalmos. Fat removal orbital decompression does not include any bone removed, but the removal of one or more bony orbital walls typically involves the selective removal of adipose tissue based on individual patient requirements. Therefore, a direct comparison between the medial orbital wall approach without fat removal and fat removal orbital decompression is warranted in subsequent studies to assess their respective advantages.
Orbital decompression surgery also should endeavor to minimize associated complications, such as the new-onset primary gaze diplopia. The incidence of new-onset diplopia reported in the literature ranged between 10% and 20% [17], aligning with the findings of our study. Among patients without preoperative diplopia, the pooled proportion (95% CI) of new-onset primary gaze diplopia was 11% (6–14). The lowest rates were associated with the lateral approach and fat removal orbital decompression, with pooled proportion (95% CI) rates of 3% (1–6) and 3% (2–4), respectively. Our study also indicated that the incidence of new-onset diplopia was notably greater with approaches that were inferior and/or medial, as diplopia primarily arose from centrifugal (outward from the orbital axis) displacement of the inferior rectus muscle path (towards the orbital floor) and the medial rectus muscle towards the ethmoidal sinus [113]. Studies [44, 114, 115] highlighted the critical importance of preserving the junction between the ethmoid and maxillary bones, which is regarded as the inferomedial support of the orbit (orbital strut). This particular bony structure served as a barrier against the inferomedial shifting of the eyeball, thereby lessening the occurrences of hypoglobus and preventing iatrogenic diplopia [116, 117].
This meta-analysis presents a novel perspective on the efficacy of diverse types of orbital decompression procedures in ameliorating secondary outcome indicators such as BCVA, IOP, MRD1, MRD2, or VF-MD among patients with TAO. The meta-analysis reported that the balanced wall technique demonstrated the most significant improvement in improving BCVA and IOP. The ΔBCVA was improved by 0.35 (95% CI 0.21 to 0.50) LogMAR, and the pooled proportion (95% CI) ΔIOP was − 2.15mmHg (95% CI -2.93 to -1.37). In terms of the improvement of BCVA, the three-wall approach was the second most effective approach. Meanwhile, the comprehensive network comparison also offers the same insights into the outcomes of these two surgical interventions for improving BCVA related to TAO. Although the BCVA showed similar improvement between the balanced wall and three-wall groups in previous studies [35, 40, 59], it was clinically more marked in the three-wall decompression group.
In our study, the relatively less favorable visual improvement observed in the three-wall decompression group compared to the balanced decompression group may be attributed to two potential factors. Firstly, patients chosen for three-wall decompression in clinical practice may have worse visual function, resulting in irreversible visual deficits. Secondly, within one of the included studies, one patient whose visual acuity worsened postoperatively underwent three-wall decompression and suffered from subhyaloid hemorrhage presumed to be caused by globe pressure during lateral wall decompression. After excluding this particular study [41], it was discerned that the three-wall decompression(ΔBCVA, MD=-0.39, 95% CI -0.51 to -0.27) exhibited a more favorable impact on visual improvement compared to the balanced decompression (ΔBCVA, MD=-0.35, 95% CI -0.50 to -0.21) in the subsequent meta-analysis.
Prior research [118] has demonstrated that the high IOP and excessive fluctuation of IOP may be risk factors for developing VF defects in patients with DON. MD represented the non-specific generalized loss on the visual field, and increased retrobulbar pressure might lead to the deterioration of MD [59]. Our meta-analysis revealed that orbital decompression could lead to a reduction in IOP with a pooled average ΔIOP of -2.15mmHg (95% CI -2.93 to -1.37), meanwhile, a significant improvement in visual field parameters post-surgery with a pooled average ΔVF-MD of 6.92dB (95% CI 3.97–9.86). Thus, our study provided theoretical support for the possibility of orbital decompression surgery to improve patients’ visual field deficits by reducing intraocular pressure.
Meanwhile, Al-Qadi et al. [119] recently published a systematic review of the efficacy of orbital decompression for the effect of upper eyelid retraction in TAO, and the weighted mean difference of MRD1 was − 0.35 mm (95% CI -0.63 to -0.08), which, although statistically significant, represented a meager change in clinical practice. Similarly, our meta-analysis also revealed a decrease in MRD1 and MRD2 following orbital decompression surgery, and the average change was determined to be -0.41 mm (95% CI -0.69 to -0.13) in MRD1 and − 1.12 mm (95% CI -1.49 to -0.76) in MRD2, indicating that MRD2 has a more notable postoperative alteration than MRD1. This information may prove valuable for surgeons when counseling patients regarding the necessity of concurrent or subsequent eyelid surgery following orbital decompression.
The meta-analysis has certain limitations. Initially, the scarcity of randomized controlled studies is noteworthy. Much of the included literature comprises retrospective analyses and case series, which heighten the risk of bias. Furthermore, most clinical studies show long temporal spans, and the follow-up time of patients is inconsistent. This phenomenon serves to obscure the potential influence of follow-up duration on research outcomes. Thirdly, the baseline characteristics across various studies such as patients’ inclusion criteria, surgical indications, surgical techniques, and assessment parameters, are not entirely congruent, which contributes to quite high heterogeneity for most of the investigated outcomes. Finally, the search is based on a limited number of publications in several bibliographic databases, which might have resulted in missing potential eligible studies.
Conclusions
In conclusion, despite the potential presence of certain biases in this study’s results, based on the existing data, it can still be inferred that orbital decompression surgery is effective in alleviating proptosis, and the most efficacious procedure is the three-wall technique. While the surgery may lead to new-onset primary gaze diplopia, the incidence remained low, especially in the lateral approach and fat removal orbital decompression. Therefore, the choice of surgical approach necessitates a reasonable balance between the extent of fat and bone removal, the degree of exophthalmos reduction, and the risk of postoperative diplopia. Nevertheless, conclusive evidence will require large-scale prospective clinical studies with long-term follow-up in the future.
Supplementary Information
Acknowledgements
Not applicable.
Peer review
Peer review reports can be found at supplementary file 2.
Abbreviations
- TAO
thyroid-associated ophthalmopathy
- CI
confidence intervals
- DON
dysthyroid optic neuropathy
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- BCVA
best-corrected visual acuity
- IOP
intraocular pressure
- MRD
margin-reflex distance
- VF-MD
visual field mean deviation
- NOS
Newcastle–Ottawa Quality Assessment Scale
- SMD
standardized mean difference
- OR
odds ratio
- SUCRA
the surface under the cumulative ranking curve
- PCS
prospective cohort study
- RA
retrospective analysis
- NR
not reported
Authors’ contributions
W. G. designed the study and developed the retrieve strategy. W. G. and J. G. executed the systematic evaluation as the first and second reviewers, searching and screening the summaries and titles, assessing the inclusion and exclusion criteria, generating data collection forms, and extracting data, and evaluating the quality of the study. W. G. and D. L. performed the meta-analysis. W. G. drafted the article, which was reviewed and revised by D. L. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82071005) and the Sanming Project of Medicine in Shenzhen (No. SZSM202311018).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taylor PN, Zhang L, Lee RWJ, Muller I, Ezra DG, Dayan CM, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat Rev Endocrinol. 2020;16(2):104–16. [DOI] [PubMed] [Google Scholar]
- 2.Weiler DL. Thyroid eye disease: a review. Clin Exp Optometry. 2017;100(1):20–5. [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moledina M, Damato EM, Lee V. The changing landscape of thyroid eye disease: current clinical advances and future outlook. Eye. 2024;38(8):1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolman PJ. Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Investig. 2021;44(3):421–9. [DOI] [PubMed] [Google Scholar]
- 6.Saeed P, Tavakoli Rad S, Bisschop P. Dysthyroid optic neuropathy. Ophthalmic plastic and reconstructive surgery. 2018;34(4S Suppl 1):S60–s7. [DOI] [PubMed] [Google Scholar]
- 7.Dolman PJ. Evaluating Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):229–48. [DOI] [PubMed] [Google Scholar]
- 8.Wong Y, Dickinson J, Perros P, Dayan C, Veeramani P, Morris D, et al. A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom. Eye. 2018;32(10):1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91(4):455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5(1):9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartalena L, Tanda ML. Current concepts regarding Graves’ orbitopathy. J Intern Med. 2022;292(5):692–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall AJH, Topliss DJ. Medical and surgical treatment of thyroid eye disease. Intern Med J. 2022;52(1):14–20. [DOI] [PubMed] [Google Scholar]
- 13.Baeg J, Choi HS, Kim C, Kim H, Jang SY. Update on the surgical management of Graves’ orbitopathy. Front Endocrinol. 2022;13:1080204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giaconi JA, Kazim M, Rho T, Pfaff C. CT scan evidence of dysthyroid optic neuropathy. Ophthal Plast Reconstr Surg. 2002;18(3):177–82. [DOI] [PubMed] [Google Scholar]
- 15.Otto AJ, Koornneef L, Mourits MP, Deen-van Leeuwen L. Retrobulbar pressures measured during surgical decompression of the orbit. Br J Ophthalmol. 1996;80(12):1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riemann CD, Foster JA, Kosmorsky GS. Direct orbital manometry in patients with thyroid-associated orbitopathy. Ophthalmology. 1999;106(7):1296–302. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein A, Schittkowski M, Esser J. Surgical treatment of Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):339–58. [DOI] [PubMed] [Google Scholar]
- 18.Stähr K, Daser A, Oeverhaus M, Hussain T, Lang S, Eckstein A, et al. Proposing a surgical algorithm for graduated orbital decompression in patients with Graves’ orbitopathy. Eur Arch Otorhinolaryngol. 2022;279(5):2401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocchi R, Lenzi R, Marinò M, Latrofa F, Nardi M, Piaggi P, et al. Rehabilitative orbital decompression for Graves’ orbitopathy: risk factors influencing the new onset of diplopia in primary gaze, outcome, and patients’ satisfaction. Thyroid: Official J Am Thyroid Association. 2012;22(11):1170–5. [DOI] [PubMed] [Google Scholar]
- 20.Li EY, Kwok TY, Cheng AC, Wong AC, Yuen HK. Fat-removal orbital decompression for disfiguring proptosis associated with Graves’ ophthalmopathy: safety, efficacy and predictability of outcomes. Int Ophthalmol. 2015;35(3):325–9. [DOI] [PubMed] [Google Scholar]
- 21.Li YW, Yang SQ, Zhang W, Guo X. [The surgical effect of secondary esotropia with diplopia after orbital decompression for thyroid-associated ophthalmopathy]. Zhonghua Yan Ke Za Zhi. 2020;56(3):183–8. [DOI] [PubMed] [Google Scholar]
- 22.Fichter N, Guthoff RF. Results after en bloc lateral wall decompression surgery with orbital fat resection in 111 patients with Graves’ Orbitopathy. Int J Endocrinol. 2015;2015:860849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honglertnapakul W, Cavuoto KM, McKeown CA, Capó H. Surgical treatment of strabismus in thyroid eye disease: characteristics, dose-response, and outcomes. J AAPOS: Official Publication Am Association Pediatr Ophthalmol Strabismus. 2020;24(2):72. [DOI] [PubMed] [Google Scholar]
- 24.Glassman AR, Elmasry MA, Baskin DE, Brigell M, Chong V, Davis Q, et al. Visual function measurements in eyes with diabetic retinopathy: an expert opinion on available measures. Ophthalmol Sci. 2024;4(5):100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsuhaibani AH, Carter KD, Policeni B, Nerad JA. Orbital volume and eye position changes after balanced orbital decompression. Ophthalmic Plast Reconstr Surg. 2011;27(3):158–63. [DOI] [PubMed]
- 26.Antisdel JL, Gumber D, Holmes J, Sindwani R. Management of sinonasal complications after endoscopic orbital decompression for Graves' orbitopathy. Laryngoscope. 2013;123(9):2094–8. [DOI] [PubMed]
- 27.Baril C, Pouliot D, Molgat Y. Optic neuropathy in thyroid eye disease: results of the balanced decompression technique. Can J Ophthalmol. 2014;49(2):162–6. [DOI] [PubMed]
- 28.Barkhuysen R, Nielsen CC, Klevering BJ, Van Damme PA. The transconjunctival approach with lateral canthal extension for three-wall orbital decompression in thyroid orbitopathy. J Craniomaxillofac Surg. 2009;37(3):127–31. [DOI] [PubMed]
- 29.Bengoa-González Á, Galindo-Ferreiro A, Mencía-Gutiérrez E, Sánchez-Tocino H, Martín-Clavijo A, Lago-Llinás MD. Deep Lateral Wall Partial Rim-Sparing Orbital Decompression with Ultrasonic Bone Removal for Treatment of Thyroid-Related Orbitopathy. J Ophthalmol. 2019;2019:9478512. [DOI] [PMC free article] [PubMed]
- 30.Boulanouar L, Grunenwald S, Imbert P, Khalifa J, Dekeister C, Boutault F, et al. Effect of orbital radiotherapy on the outcome of surgical orbital decompression for thyroid-associated orbitopathy (TAO): a retrospective study in 136 patients. Endocrine. 2020;67(3):605–12. [DOI] [PubMed]
- 31.Byeon HJ, Ko J, Kikkawa DO, Yoon JS. Preoperative Risk Factors for Proptosis Recurrence After Rehabilitative Orbital Decompression in Graves' Orbitopathy Patients. Am J Ophthalmol. 2024;258:110–8. [DOI] [PubMed]
- 32.Chang EL, Piva AP. Temporal fossa orbital decompression for treatment of disfiguring thyroid-related orbitopathy. Ophthalmology. 2008;115(9):1613–9. [DOI] [PubMed]
- 33.Chang M, Baek S, Lee TS. Long-term outcomes of unilateral orbital fat decompression for thyroid eye disease. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):935–9. [DOI] [PubMed]
- 34.Cheng AM, Wei YH, Tighe S, Sheha H, Liao SL. Long-term outcomes of orbital fat decompression in Graves' orbitopathy. Br J Ophthalmol. 2018;102(1):69–73. [DOI] [PubMed]
- 35.Cheng SN, Yu YQ, You YY, Chen J, Pi XH, Wang XH, et al. Comparison of 2-wall versus 3-wall orbital decompression against dysthyroid optic neuropathy in visual function: a retrospective study in a Chinese population. Medicine. 2021;100(8):e24513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho RI, Choe CH, Elner VM. Ultrasonic bone removal versus high-speed burring for lateral orbital decompression: comparison of surgical outcomes for the treatment of thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2010;26(2):83-7. [DOI] [PubMed]
- 37.Choe CH, Cho RI, Elner VM. Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2011;27(1):4–11. [DOI] [PubMed]
- 38.Choe CH, Kim KW, Lee JK. Surgical Outcomes of Balanced Deep Lateral and Medial Orbital Wall Decompression in Korean Population: Clinical and Computed Tomography-based Analysis. Korean J Ophthalmol. 2016;30(2):85–91. [DOI] [PMC free article] [PubMed]
- 39.Chu EA, Miller NR, Grant MP, Merbs S, Tufano RP, Lane AP. Surgical treatment of dysthyroid orbitopathy. Otolaryngol Head Neck Surg. 2009;141(1):39–45. [DOI] [PubMed]
- 40.Cubuk MO, Konuk O, Unal M. Orbital decompression surgery for the treatment of Graves’ ophthalmopathy: comparison of different techniques and long-term results. Int J Ophthalmol. 2018;11(8):1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juniat V, Abbeel L, McGilligan JA, Curragh D, Selva D, Rajak S. Endoscopic orbital decompression by oculoplastic surgeons for proptosis in thyroid eye disease. Ophthal Plast Reconstr Surg. 2019;35(6):590–3. [DOI] [PubMed] [Google Scholar]
- 42.Dallan I, Cristofani-Mencacci L, Fiacchini G, Benettini G, Picariello M, Lanzolla G, et al. Functional outcomes and complications in refractory dysthyroid optic neuropathy management: Experience with 3 different surgical protocols. Am J Otolaryngol. 2022;43(3):103451. [DOI] [PubMed]
- 43.Dubin MR, Tabaee A, Scruggs JT, Kazim M, Close LG. Image-guided endoscopic orbital decompression for Graves' orbitopathy. Ann Otol Rhinol Laryngol. 2008;117(3):177–85. [DOI] [PubMed]
- 44.Finn AP, Bleier B, Cestari DM, Kazlas MA, Dagi LR, Lefebvre DR, et al. A retrospective review of orbital decompression for thyroid orbitopathy with endoscopic preservation of the inferomedial orbital bone strut. Ophthal Plast Reconstr Surg. 2017;33(5):334–9. [DOI] [PubMed] [Google Scholar]
- 45.Fu W. Changes of Volume Parameters in the Treatment of Graves Ophthalmopathy by Endoscopic Transethmoidal Decompression of the Orbital Inner Wall Combined with Fat Decompression. Scanning. 2022;2022:8149247. [DOI] [PMC free article] [PubMed] [Retracted]
- 46.Gong Y, Yin J, Tong B, Li J, Zeng J, Zuo Z, et al. Original endoscopic orbital decompression of lateral wall through hairline approach for Graves' ophthalmopathy: an innovation of balanced orbital decompression. Ther Clin Risk Manag. 2018;14:607–16. [DOI] [PMC free article] [PubMed]
- 47.Gu KM, Chen XH, Dai BZ, Lu TJ, Yu XM, Dai Y. Effect of orbital decompression on choroidal thickness in patients with thyroid associated ophthalmopathy. Int Eye Sci. 2023;23(5):823–6.
- 48.Guo J, Li X, Ma R, Qian J. Correlation between uniocular deviation and duction changes following different decompression surgeries in thyroid eye disease. BMC Ophthalmol. 2021;21(1):134. [DOI] [PMC free article] [PubMed]
- 49.Gupta A, Nobori A, Wang Y, Rootman D, Goldberg R. Lateral Rectus Muscle Expands More Than Medial Rectus Following Maximal Deep Balanced Orbital Decompression. Ophthalmic Plast Reconstr Surg. 2018;34(2):140–2. [DOI] [PubMed]
- 50.Hernández-García E, San-Román JJ, González R, Nogueira A, Genol I, Stoica B, et al. Balanced (endoscopic medial and transcutaneous lateral) orbital decompression in Graves' orbitopathy. Acta Otolaryngol. 2017;137(11):1183–7. [DOI] [PubMed]
- 51.Hill RH, Czyz CN, Bersani TA. Transcaruncular medial wall orbital decompression: an effective approach for patients with unilateral graves ophthalmopathy. ScientificWorldJournal. 2012;2012:312361. [DOI] [PMC free article] [PubMed]
- 52.Hu SZ, Chen Z, Dong WJ. Clinical efficacy of orbital decompression in patients with Graves Ophthalmopathy. Int J Ophthalmol. 2017;17(10):1963–5.
- 53.Juniat V, Abbeel L, McGilligan JA, Curragh D, Selva D, Rajak S. Endoscopic Orbital Decompression by Oculoplastic Surgeons for Proptosis in Thyroid Eye Disease. Ophthalmic Plast Reconstr Surg. 2019;35(6):590–3. [DOI] [PubMed]
- 54.Kakizaki H, Takahashi Y, Ichinose A, Iwaki M, Selva D, Leibovitch I. The importance of rim removal in deep lateral orbital wall decompression. Clin Ophthalmol. 2011;5:865–9. [DOI] [PMC free article] [PubMed]
- 55.Kim SA, Jung SK, Paik JS, Yang SW. Effect of Orbital Decompression on Corneal Topography in Patients with Thyroid Ophthalmopathy. PLoS One. 2015;10(9):e0133612. [DOI] [PMC free article] [PubMed]
- 56.Kim SH, Kang SM. Changes in Eyelid Parameters after Orbital Decompression according to the Surgical Approach in Thyroid Eye Disease. Korean J Ophthalmol. 2021;35(6):421–8. [DOI] [PMC free article] [PubMed]
- 57.Kingdom TT, Davies BW, Durairaj VD. Orbital decompression for the management of thyroid eye disease: An analysis of outcomes and complications. Laryngoscope. 2015;125(9):2034–40. [DOI] [PubMed]
- 58.Kitaguchi Y, Takahashi Y, Kakizaki H. Computed Tomography-Based Prediction of Exophthalmos Reduction After Deep Lateral Orbital Wall Decompression for Graves' Orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257(12):2759–67. [DOI] [PubMed]
- 59.Korkmaz S, Konuk O. Surgical treatment of dysthyroid optic neuropathy: long-term visual outcomes with comparison of 2-wall versus 3-wall orbital decompression. Curr Eye Res. 2016;41(2):159–64. [DOI] [PubMed] [Google Scholar]
- 60.Lal P, Thakar A, Tandon N. Endoscopic orbital decompression for Graves' orbitopathy. Indian J Endocrinol Metab. 2013;17(2):265–70. [DOI] [PMC free article] [PubMed]
- 61.Lee KH, Jang SY, Lee SY, Yoon JS. Graded decompression of orbital fat and wall in patients with Graves' orbitopathy. Korean J Ophthalmol. 2014;28(1):1–11. [DOI] [PMC free article] [PubMed]
- 62.Liao SL, Huang SW. Correlation of retrobulbar volume change with resected orbital fat volume and proptosis reduction after fatty decompression for Graves ophthalmopathy. Am J Ophthalmol. 2011;151(3):465–9.e1. [DOI] [PubMed]
- 63.Lipski A, Eckstein A, Esser J, Loesch C, Mann K, Mohr C, et al. Course of pattern-reversed visual evoked cortical potentials in 30 eyes after bony orbital decompression in dysthyroid optic neuropathy. Br J Ophthalmol. 2011;95(2):222–6. [DOI] [PubMed]
- 64.Lv X, Gao Y, Ma Y, Li C, Ren Y, Zhang Z, et al. Comparison of surgical effect in active and inactive Dysthyroid Optic Neuropathy after Endoscopic Transnasal Medial Orbital Decompression. Graefes Arch Clin Exp Ophthalmol. 2024;262(1):281–93. [DOI] [PubMed]
- 65.Lv Z, Selva D, Yan W, Daniel P, Tu Y, Wu W. Endoscopical Orbital Fat Decompression with Medial Orbital Wall Decompression for Dysthyroid Optic Neuropathy. Curr Eye Res. 2016;41(2):150–8. [DOI] [PubMed]
- 66.Maalouf T, Vedrine PO, Coffinet L, George JL. Mid-term rhinosinusal consequences of bony orbital decompression in Graves' disease: a retrospective study. Orbit. 2008;27(3):169–73. [DOI] [PubMed]
- 67.Mainville NP, Jordan DR. Effect of orbital decompression on diplopia in thyroid-related orbitopathy. Ophthalmic Plast Reconstr Surg. 2014;30(2):137–40. [DOI] [PubMed]
- 68.Malik R, Cormack G, MacEwen C, White P. Endoscopic orbital decompression for dyscosmetic thyroid eye disease. J Laryngol Otol. 2008;122(6):593–7. [DOI] [PubMed]
- 69.Mehta P, Durrani OM. Outcome of deep lateral wall rim-sparing orbital decompression in thyroid-associated orbitopathy: a new technique and results of a case series. Orbit. 2011;30(6):265–8. [DOI] [PubMed]
- 70.Millar MJ, Maloof AJ. The application of stereotactic navigation surgery to orbital decompression for thyroid-associated orbitopathy. Eye (Lond). 2009;23(7):1565–71. [DOI] [PubMed]
- 71.Murta F, Hyer JN, Haridas A, Rose GE, Ezra DG. Quantitative Assessment of Orbital Decompression Surgery Using Photogrammetric Stereoimaging. Ophthalmic Plast Reconstr Surg. 2021;37(5):420–3. [DOI] [PubMed]
- 72.Nguyen J, Fay A, Yadav P, MacIntosh PW, Metson R. Stereotactic microdebrider in deep lateral orbital decompression for patients with thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2014;30(3):262–6. [DOI] [PubMed]
- 73.Norris JH, Ross JJ, Kazim M, Selva D, Malhotra R. The effect of orbital decompression surgery on refraction and intraocular pressure in patients with thyroid orbitopathy. Eye (Lond). 2012;26(4):535–43. [DOI] [PMC free article] [PubMed]
- 74.Onaran Z, Konuk O, Oktar SÖ, Yücel C, Unal M. Intraocular pressure lowering effect of orbital decompression is related to increased venous outflow in Graves orbitopathy. Curr Eye Res. 2014;39(7):666–72. [DOI] [PubMed]
- 75.Park SM, Nam SB, Lee JW, Song KH, Choi SJ, Bae YC. Quantitative Assessment of Orbital Volume and Intraocular Pressure after Two-Wall Decompression in Thyroid Ophthalmopathy. Arch Craniofac Surg. 2015;16(2):53–7. [DOI] [PMC free article] [PubMed]
- 76.Pereira TS, Leite CA, Kuniyoshi CH, Gebrim EMMS, Monteiro MLR, Pieroni Gonçalves AC. A randomized comparative study of inferomedial vs. balanced orbital decompression. Analysis of changes in orbital volume, eyelid parameters, and eyeball position. Eye (Lond). 2022;36(3):547–54. [DOI] [PMC free article] [PubMed]
- 77.Pieroni Goncalves AC, Gupta S, Monteiro MLR, Douglas RS. Customized Minimally Invasive Orbital Decompression Surgery Improves Lower Eyelid Retraction and Contour in Thyroid Eye Disease. Ophthalmic Plast Reconstr Surg. 2017;33(6):446–51. [DOI] [PubMed]
- 78.Prat MC, Braunstein AL, Dagi Glass LR, Kazim M. Orbital fat decompression for thyroid eye disease: retrospective case review and criteria for optimal case selection. Ophthalmic Plast Reconstr Surg. 2015;31(3):215–8. [DOI] [PubMed]
- 79.Prevost A, Dekeister C, Caron P, Imbert P, Cavallier Z, Lauwers F, et al. Outcomes of orbital decompression using surgical navigation in thyroid-associated ophthalmopathy. Int J Oral Maxillofac Surg. 2020;49(10):1279–85. [DOI] [PubMed]
- 80.Rajabi MT, Tabary M, Baharnoori S, Salabati M, Mahmoudzadeh R, Hosseinzadeh F, et al. Orbital anatomical parameters affecting outcome of deep lateral orbital wall decompression. Eur J Ophthalmol. 2021;31(4):2069–75. [DOI] [PubMed]
- 81.Ramesh S, Nobori A, Wang Y, Rootman D, Goldberg RA. Orbital Expansion in Cranial Vault After Minimally Invasive Extradural Transorbital Decompression for Thyroid Orbitopathy. Ophthalmic Plast Reconstr Surg. 2019;35(1):17–21. [DOI] [PubMed]
- 82.Sagili S, Desousa JL, Malhotra R. Intraocular pressure and refractive changes following orbital decompression with intraconal fat excision. Open Ophthalmol J. 2008;2:73–6. [DOI] [PMC free article] [PubMed]
- 83.Schiff BA, McMullen CP, Farinhas J, Jackman AH, Hagiwara M, McKellop J, et al. Use of computed tomography to assess volume change after endoscopic orbital decompression for Graves' ophthalmopathy. Am J Otolaryngol. 2015;36(6):729–35. [DOI] [PubMed]
- 84.Seibel I, Hofmann VM, Sönmez H, Schönfeld S, Jumah MD, Lenarz M, et al. Medial and mediolateral orbital decompression in intractable Graves' Orbitopathy. Auris Nasus Larynx. 2017;44(4):428–34. [DOI] [PubMed]
- 85.Sellari-Franceschini S, Rocchi R, Marinò M, Bajraktari A, Mazzi B, Fiacchini G, et al. Rehabilitative orbital decompression for Graves’ orbitopathy: results of a randomized clinical trial. J Endocrinol Investig. 2018;41(9):1037–42. [DOI] [PubMed] [Google Scholar]
- 86.Seo Y, Shin WB, Bae HW, Yoon JS. Effects of Orbital Decompression on Lamina Cribrosa Depth in Patients with Graves' Orbitopathy. Korean J Ophthalmol. 2019;33(5):436–45. [DOI] [PMC free article] [PubMed]
- 87.She YY, Chi CC, Chu ST. Transnasal endoscopic orbital decompression: 15-year clinical experience in Southern Taiwan. J Formos Med Assoc. 2014;113(9):648–55. [DOI] [PubMed]
- 88.Shi W, Sun F, Tang D, Zhang Y, Jian T. [Endoscopic transnasal orbital balanced decompression technique for severe Graves' orbitopathy]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;50(11):904–8. [PubMed]
- 89.Singh S, Curragh DS, Selva D. Augmented endoscopic orbital apex decompression in dysthyroid optic neuropathy. Eye (Lond). 2019;33(10):1613–8. [DOI] [PMC free article] [PubMed]
- 90.Sobti M, Brogan K, Patel R, Miller D, Chadha V, Cauchi P. Impact of sphenoid trigone size and extraocular muscle thickness on the outcome of lateral wall orbital decompression for thyroid eye disease. Oral Maxillofac Surg. 2024;28(1):307–13. [DOI] [PubMed]
- 91.Stähr K, Eckstein A, Holtmann L, Schlüter A, Dendy M, Lang S, et al. A comparative analysis of piezosurgery and oscillating saw for balanced orbital decompression. Orbit. 2019;38(6):433–9. [DOI] [PubMed]
- 92.Takahashi Y, Nakamura Y, Ichinose A, Kakizaki H. Intraocular pressure change with eye positions before and after orbital decompression for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2014;30(1):47–50. [DOI] [PubMed]
- 93.Thorne AW, Rootman DB. Influence of surgical approach for decompression on lower eyelid position in thyroid eye disease. Orbit. 2020;39(2):84–6. [DOI] [PubMed]
- 94.Tu Y, Wu S, Pan Z, Hu X, Zhou G, Shi J, et al. Endoscopic Transconjunctival Deep Lateral Wall Decompression for Thyroid-associated Orbitopathy: A Minimally Invasive Alternative: Transconjunctival Endoscopic with Wall Decompression for TAO. Am J Ophthalmol. 2022;235:71–9. [DOI] [PubMed]
- 95.Ueland HO, Haugen OH, Rødahl E. Temporal hollowing and other adverse effects after lateral orbital wall decompression. Acta Ophthalmol. 2016;94(8):793–7. [DOI] [PubMed]
- 96.Wang Y, Sun J, Liu X, Li Y, Fan X, Zhou H. Robot-Assisted Orbital Fat Decompression Surgery: First in Human. Transl Vis Sci Technol. 2022;11(5):8.Erratum in: Transl Vis Sci Technol. 2022;11(9):13. [DOI] [PMC free article] [PubMed]
- 97.Wang Y, Yang N, Li YY, Xiao LH. [Multi-wall orbital decompression for disfiguring proptosis in patients with mild or moderate thyroid eye disease]. Zhonghua Yan Ke Za Zhi. 2017;53(2):128–35. [DOI] [PubMed]
- 98.Woo YJ, Yoon JS. Changes in pupillary distance after fat versus bony orbital decompression in Graves' orbitopathy. Can J Ophthalmol. 2017;52(2):186–91. [DOI] [PubMed]
- 99.Woods RSR, Pilson Q, Kharytaniuk N, Cassidy L, Khan R, Timon CVI. Outcomes of endoscopic orbital decompression for graves' ophthalmopathy. Ir J Med Sci. 2020;189(1):177–83. [DOI] [PubMed]
- 100.Wu CH, Chang TC, Liao SL. Results and predictability of fat-removal orbital decompression for disfiguring graves exophthalmos in an Asian patient population. Am J Ophthalmol. 2008;145(4):755–9. [DOI] [PubMed]
- 101.Wu CY, Stacey AW, Kahana A. Simultaneous Versus Staged Balanced Decompression for Thyroid-Related Compressive Optic Neuropathy. Ophthalmic Plast Reconstr Surg. 2016;32(6):462–7. [DOI] [PubMed]
- 102.Wu W, Selva D, Bian Y, Wang X, Sun MT, Kong Q, et al. Endoscopic medial orbital fat decompression for proptosis in type 1 graves orbitopathy. Am J Ophthalmol. 2015;159(2):277–84. [DOI] [PubMed] [Google Scholar]
- 103.Xu H, Wu T, Sun FY, Tang DR, Shi SS, Zhao L. Comparison of the effects of balanced orbital decompression and endoscopic transnasal inferomedial wall decompression with the high orbital pressure. Chin J Exp Ophthalmol. 2020;38(11):967–72.
- 104.Yao WC, Sedaghat AR, Yadav P, Fay A, Metson R. Orbital Decompression in the Endoscopic Age: The Modified Inferomedial Orbital Strut. Otolaryngol Head Neck Surg. 2016;154(5):963–9. [DOI] [PubMed]
- 105.Ye Y, Hu F, Ji Y, Wang R, Zhu K, Kong Q. The outcomes of endoscopic orbital decompression combined with fat decompression for thyroid-associated ophthalmopathy. BMC Ophthalmol. 2023;23(1):217. [DOI] [PMC free article] [PubMed]
- 106.Yeo JH, Park SJ, Chun YS, Kim JT, Moon NJ, Lee JK. The effect of orbital decompression surgery on interpupillary distance and angle kappa in patients with thyroid-associated orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):825–30. [DOI] [PubMed]
- 107.Yinghong Z, Jichao Z, Zhidi Z, Chiyu X, Haipeng Z, Yanrong R, et al. Combined endonasal and orbital approach for annulus of Zinn area decompression in dysthyroid optic neuropathy. Am J Otolaryngol. 2023;44(2):103692. [DOI] [PubMed]
- 108.Zhang S, Li Y, Wang Y, Zhong S, Liu X, Huang Y, et al. Comparison of rim-sparing versus rim-removal techniques in deep lateral wall orbital decompression for Graves' orbitopathy. Int J Oral Maxillofac Surg. 2019;48(4):461–7. [DOI] [PubMed]
- 109.Parrilla C, Mele DA, Gelli S, Zelano L, Bussu F, Rigante M, et al. Multidisciplinary approach to orbital decompression. A review. Acta Otorhinolaryngologica Italica: Organo Ufficiale Della Soc Italiana di Otorinolaringologia e Chirurgia cervico-facciale. 2021;41(Suppl 1):S90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boboridis KG, Bunce C. Surgical orbital decompression for thyroid eye disease. Cochrane Database Syst Rev. 2011;12:Cd007630. [DOI] [PubMed] [Google Scholar]
- 111.Mourits MP, Bijl H, Altea MA, Baldeschi L, Boboridis K, Currò N, et al. Outcome of orbital decompression for disfiguring proptosis in patients with Graves’ orbitopathy using various surgical procedures. Br J Ophthalmol. 2009;93(11):1518–23. [DOI] [PubMed] [Google Scholar]
- 112.Willaert R, Maly T, Ninclaus V, Huvenne W, Vermeersch H, Brusselaers N. Efficacy and complications of orbital fat decompression in Graves’ orbitopathy: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2020;49(4):496–504. [DOI] [PubMed] [Google Scholar]
- 113.Gioacchini FM, Kaleci S, Cassandro E, Scarpa A, Tulli M, Cassandro C, et al. Orbital wall decompression in the management of Graves’ orbitopathy: a systematic review with meta-analysis. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German society for Oto-Rhino-Laryngology. Head Neck Surg. 2021;278(11):4135–45. [DOI] [PubMed] [Google Scholar]
- 114.Bailey KL, Tower RN, Dailey RA. Customized, single-incision, three-wall orbital decompression. Ophthal Plast Reconstr Surg. 2005;21(1):1–9 discussion – 10. [DOI] [PubMed] [Google Scholar]
- 115.Bleier BS, Lefebvre DR, Freitag SK. Endoscopic orbital floor decompression with preservation of the inferomedial strut. Int Forum Allergy Rhinology. 2014;4(1):82–4. [DOI] [PubMed] [Google Scholar]
- 116.Kim JH, Lee IG, Lee JS, Oh DY, Jun YJ, Rhie JW, et al. Restoration of the inferomedial orbital strut using a standardized three-dimensional printing implant. J Anat. 2020;236(5):923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim JW, Goldberg RA, Shorr N. The inferomedial orbital strut: an anatomic and radiographic study. Ophthal Plast Reconstr Surg. 2002;18(5):355–64. [DOI] [PubMed] [Google Scholar]
- 118.Tu Y, Mao B, Li J, Liu W, Xu M, Chen Q, et al. Relationship between the 24-h variability of blood pressure, ocular perfusion pressure, intraocular pressure, and visual field defect in thyroid associated orbitopathy. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2020;258(9):2007–12. [DOI] [PubMed] [Google Scholar]
- 119.Al-Qadi M, Hussain A. Influence of orbital decompression on upper eyelid retraction in Graves' orbitopathy: a systematic review and meta-analysis. Orbit. 2024;43(4):549–54. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.