Abstract
Purpose
To investigate the prognostic value of human epididymis protein 4 (HE4) in patients with acute myocardial infarction (AMI).
Patients and Methods
A total of 212 consecutive patients diagnosed with AMI in the Department of Cardiovascular Medicine of Hunan Provincial People’s Hospital from June 2020 to May 2021 were enrolled. We determined plasma HE4 levels at baseline. The patients were followed up regularly and the occurrence of major adverse cardiac events (MACE) was recorded after discharge.
Results
After a mean follow-up period of 242 (159–427) days, 67 patients had MACE. Multivariate Cox regression analysis showed that HE4 was an independent predictor of MACE in patients with AMI [HR = 1.004 (1.002–1.007), P = 0.002]. Kaplan-Meier survival curves showed that patients with HE4 levels > 532.9 pmol/L had higher MACE compared with patients with ≤ 532.9 pmol/L HE4 levels (HR=4.044, 95% CI 2.373–6.890, P <0.001). Receiver operating characteristic (ROC) curve analysis showed that the area under the curve (AUC) of HE4 for predicting MACE was 0.734 (95% CI: 0.669–0.792, P < 0.001).
Conclusion
Human epididymis protein 4 (HE4) might be a novel biomarker for predicting the prognosis of patients with AMI.
Keywords: acute myocardial infarction, biomarkers, human epididymis protein
Introduction
Acute myocardial infarction(AMI) is an acute heart disease with a high disability rate and mortality. Although current reperfusion therapy has improved the short-term prognosis of AMI to some extent, some survivors are repeatedly readmitted for heart failure (HF) after myocardial infarction due to necrotic cardiomyocytes fail to regenerate. The 1-year mortality rate of patients with AMI remains as high as 9% after discharge, which places a heavy burden on families and public health.1,2 Cardiac biomarkers can reflect the pathophysiological characteristics of AMI from different perspectives such as myocardial ischemia injury, ventricular remodeling, and oxidative stress, and have good advantages in the prognostic evaluation of AMI.3 The traditional biomarker N-Terminal B-type natriuretic peptide (NT-proBNP) can predict the prognosis of patients with AMI to some extent.4 However, its evaluation value has certain limitations. Its half-time is relatively short, and it is affected by factors such as Body Mass Index (BMI), renal function, infection, cirrhosis, and β-blockers.5,6 Therefore, searching for novel biomarkers for precise risk stratification and prognostic evaluation of AMI patients as well as the early intensive intervention of patients according to risk stratification is important to improve the prognosis of AMI patients.
Human epididymis protein 4 (HE4) was first discovered as a secreted protein in the human epididymis and is currently mainly used in the diagnosis and treatment of gynecological neoplastic diseases.7 Several studies reported that HE4 was associated with the severity of HF and can predict the prognosis of patients with HF, which was independent of NT-proBNP.8,9 Our previous study showed that HE4 could predict the prognosis of idiopathic pulmonary arterial hypertension (IPAH) patients with right HF and ischemic cardiomyopathy (ICM) patients. The area under the curve values of HE4 were higher than that of NT-proBNP in these studies.10,11 A recent study showed that in acute ST-segment elevation myocardial infarction (STEMI) patients undergoing revascularization, HE4 levels were significantly increased in patients with low left ventricular ejection fraction (LVEF).12 The decrease in LVEF was closely related to the occurrence of adverse cardiovascular events after AMI,13,14 suggesting that HE4 may be a novel biomarker of poor prognosis in AMI. Therefore, this study was intended to detect HE4 levels at admission in patients with AMI and to investigate its value in predicting the prognosis of AMI.
Materials and Methods
Study Population
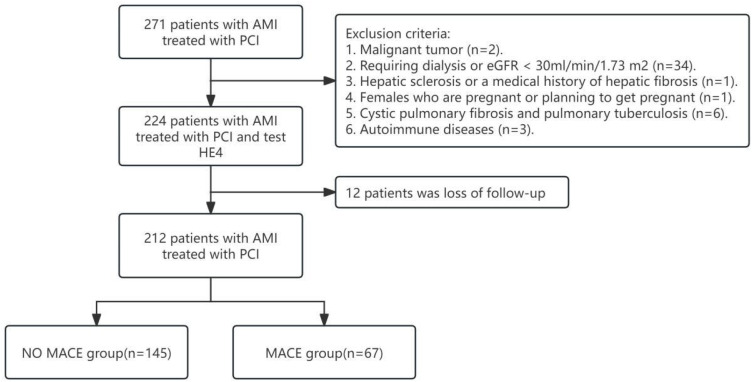
We enrolled 212 patients who were hospitalized in the Department of Cardiovascular Medicine of Hunan Provincial People’s Hospital from June 2020 to May 2021 and diagnosed with AMI and underwent percutaneous coronary intervention (PCI), and the diagnostic criteria for AMI were based on clinical guidelines15 (Figure 1). AMI was diagnosed if a patient had a cardiac troponin I level exceeding the 99th percentile of a normal reference population with ≥1 of the following: ①ischemic chest pain symptoms, chest pain lasting >20 min; ②new ischemic electrocardiogram changes: new ST-segment and T-wave changes; ③new pathologic Q waves; ④imaging evidence of new loss of myocardial activity or new local wall motion abnormalities; ⑤coronary angiography confirmed the presence of coronary thrombosis. As tumor and renal failure can obviously affect the expression of HE4, patients with a medical history of malignant tumor and end-stage renal disease (requiring dialysis or eGFR < 30mL/min/1.73 m2) were excluded from this study. Female patients who were preparing to become or had become pregnant were also excluded from our study in consideration of ethics. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) simplified formula. eGFR(mL/min/1.73m2) = 30,849 × [Scr(μmol/L)] −1.154 × (age)−0.203 × (0.742 if patient is female). Patients’ baseline data and clinical data were collected through the electronic medical records (EMR) system.
Figure 1.
Flowchart: Patients’ flow-chart in our study.
HE4 Measurement
Blood samples for measurement of NT-proBNP were collected from patients with AMI on the day following admission. All blood samples were allowed to stand in a freezer at 4 °C for half an hour and then centrifuged at 3000 rpm for 10 minutes to obtain plasma, followed by storage in a −80 °C freezer. After all samples collection, HE4 levels were uniformly detected using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, Minnesota). Processing of all blood samples and measurement of the biomarker HE4 were performed at a central laboratory.
Enzyme-linked immunosorbent assay (ELISA) brief procedure: (1) Add 100ul of Assay Diluent RD1W to dilute into small holes coated with anti-HE4 antibody microplate strips. (2) Add 20ul of standard plasma sample and incubate at room temperature for 2 hours. (3) Wash the strips with washing buffer salt water and repeat washing 4 times. (4) Add 200ul Human HE4/WFDC2 Conjugate and incubate for 2 hours and wash 3 times. (5) Add 200ul substrate solution and incubate at room temperature without light for 30 minutes. (6) Observe the reaction by adding a stop solution and determine the optical density at 450 nm in the spectrometer. All blood samples were multiwell tested.
Endpoint and Follow-Up
The endpoint was the composite of major adverse cardiovascular events (MACE), which were defined as readmission for unstable angina and revascularization, readmission for HF, nonfatal myocardial infarction, and all-cause mortality. Readmission for unstable angina and revascularization was defined as hospital readmission for which unstable angina and revascularization was the primary reason. Readmission for HF was defined as an unplanned admission for worsening HF. Patients had to have typical symptoms and signs of HF. Nonfatal myocardial infarction was diagnosed following established criteria as described.15 Follow-up data were obtained by regular telephone and outpatient follow-up by healthcare professionals at 1 month, 3 months, 6 months, 12 months, and 18 months after discharge in combination with the review of medical records of the hospital. The follow-up contents mainly included the treatment effect on the patient’s disease, medication, and the occurrence of endpoint events. The follow-up time was from discharge to the time of the endpoint event or until December 05, 2021.
Statistical Analysis
Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and MedCalc software 18.11.3 (MedCalc Software, Mariakerke, Belgium). Results were presented as mean ± standard deviation for continuous variables that met normal distribution, as the median or interquartile range for continuous variables that were not normally distributed, and as frequency (percentage) for categorical and ordinal variables. For comparisons between groups, independent sample t-tests were used for normally distributed variables and U-tests were used for non-normally distributed variables. The Chi-square test was used for categorical variables. Correlations between the two variables were investigated using Spearman correlation coefficients. The variables predictive of MACE by univariate analysis (P<0.05) were then entered into a multivariable Cox regression analysis to determine independent predictors of MACE. Receiver operating characteristic curves (ROC) were plotted to calculate the area under the ROC curve (AUC) and cut-off points, and Kaplan-Meier curve analysis was performed to group the cut-off points calculated according to ROC analysis and identify the differences in events between the two groups using the Log rank test. Univariate and multivariate Cox proportional hazards regression models were used for survival analysis. Two-sided P < 0.05 was defined as statistically significant.
Results
Baseline Characteristics
A total of 224 patients with AMI treated with PCI were included in this study, 7 patients (4.8%) in the high HE4 group (> 532.9 pmol/L) and 5 patients (6.5%) in the low HE4 group (≤532.9 pmol/L) were not included in the study due to loss of follow-up. 212 patients were finally included in the analysis, including 144 patients with acute ST-segment elevation myocardial infarction (STEMI) and 68 patients with acute non-ST-segment elevation myocardial infarction (NSTEMI). Table 1 describes the baseline data and clinical characteristics of the patients. The median age of all subjects was 63 years (interquartile range 54–71), and the proportion of males was 70.8%. Compared with the no MACE group, the patients in the MACE group were likely to be older and have hypertension and diabetes. In addition, total cholesterol, HE4, NT-proBNP, Killip class, and triple vessel lesion proportions were higher in the MACE group, and eGFR, high-density lipoprotein cholesterol, hemoglobin levels, and LVEF were lower. There was no significant difference in gender as well as smoking between the two groups.
Table 1.
Baseline Characteristics of the Study Population by MACE
| Variable | Overall (N=212) | NO MACE (N=145) | MACE (N=67) | P-value |
|---|---|---|---|---|
| Age (yr) | 63.0 [53.8, 71.0] | 61.0 [51.0, 69.0] | 66.0 [58.0, 73.0] | 0.008 |
| Sex (male) | 150 (70.8) | 103 (71.0) | 47 (70.1) | >0.99 |
| BMI (kg/m2) | 23.8 [22.0, 25.7] | 23.9 [21.9, 25.7] | 23.4 [22.0, 25.7] | 0.773 |
| Killip class III/IV | 31 (14.6) | 13 (9.0) | 18 (26.9) | 0.001 |
| STEMI (%) | 144 (67.9) | 98 (67. 6) | 46 (68.7) | >0.99 |
| Smoking (%) | 132 (62.3) | 88 (60.7) | 44 (65.7) | 0.587 |
| Hypertension (%) | 132 (62.3) | 83 (57.2) | 49 (73.1) | 0.039 |
| Diabetes mellitus (%) | 67 (31.6) | 36 (24.8) | 31 (46.3) | 0.003 |
| Atrial fibrillation (%) | 9 (4.2) | 4 (2.8) | 5 (7.5) | 0.225 |
| Previous PCI history (%) | 8 (3.8) | 3 (2.1) | 5 (7.5) | >0.99 |
| CABG (%) | 1 (0.5) | 1 (0.7) | 0 (0.0) | >0.99 |
| Main epicardial coronary arteries ≥70% stenosis | 0.042 | |||
| 1 | 44 (20.8) | 34 (23.4) | 10 (14.9) | 0.155 |
| 2 | 62 (29.2) | 47 (32.5) | 15 (22.4) | 0.136 |
| 3 | 106 (50.0) | 64 (44.1) | 42 (62.7) | 0.012 |
| Left main ≥ 50% stenosis | 22 (10.4) | 15 (10.3) | 7 (10.4) | >0.99 |
| WBC (×109/l) | 8.8 [7.0, 11.0] | 8.8 [7.1, 10.4] | 8.8 [6.8, 11.8] | 0.471 |
| PLT (×109/l) | 215.5 [169.3, 260.0] | 216.0 [171.0, 260.0] | 206.0 [166.5, 261.0] | 0.812 |
| Hemoglobin (g/dl) | 12.8 [11.5, 14.0] | 13.0 [11.7, 14.1] | 12.4 [10.8, 13.7] | 0.048 |
| eGFR (mL/min/1.73 m2) | 87.9 [68.0, 100.1] | 90.2 [76.1, 102.7] | 71.6 [53.7, 95.8] | <0.001 |
| Total cholesterol (mg/dL) | 170.9 [142.6, 190.6] | 174.0 [149.7, 193.0] | 161.3 [133.8, 181.9] | 0.078 |
| Triglycerides (mg/dL) | 143.4 [98.7, 188.6] | 130.2 [93.2, 180.4] | 170.0 [107.6, 209.4] | 0.042 |
| HDL cholesterol (mg/dL) | 37.9 [33.6, 45.6] | 39.4 [34.4, 46.8] | 36.7 [32.1, 42.5] | 0.021 |
| LDL cholesterol (mg/dL) | 100.5 [81.8, 124.3] | 102.5 [81.2, 126.5] | 97.5 [82.4, 119.1] | 0.681 |
| Troponin I (ng/mL) | 4.0 [0.7, 15.3] | 3.6 [0.7, 13.0] | 4.2 [0.6, 22.3] | 0.644 |
| HE4 (pmol/L) | 462.9 [343.9, 565.9] | 399.3 [306.3, 518.3] | 562.0 [443.4, 603.5] | <0.001 |
| NT-proBNP (pg/mL) | 1215.0 [388.3, 2897.0] | 1090.0 [364.0, 2060.0] | 1820.0 [481.0, 6720.0] | 0.004 |
| HbA1c (%) | 6.0 [5.7, 7.1] | 5.9 [5.6, 6.6] | 6.7 [6.0, 8.1] | 0.001 |
| LVDd (mm) | 48.0 [44.0, 52.0] | 48.0 [44.0, 52.0] | 48.0 [45.0, 54.0] | 0.349 |
| LVEF (%) | 52.7 (12.2) | 54.2 (11) | 49.5 (14.0) | 0.009 |
| Aspirin (%) | 199 (93.9) | 139 (95.9) | 60 (89.6) | 0.141 |
| P2Y12 inhibitor (%) | 185 (87.3) | 126 (86.9) | 59 (88.1) | 0.988 |
| β-blocker (%) | 191 (90.1) | 131 (90.3) | 60 (89.6) | >0.99 |
| ACEI/ARB (%) | 178 (84.0) | 125 (86.2) | 53 (79.1) | 0.267 |
| Diuretic (%) | 31 (14.6) | 13 (9.0) | 18 (26.9) | 0.001 |
| Anticoagulant (%) | 9 (4.2) | 7 (4.8) | 2 (3.0) | 0.801 |
| Statin (%) | 203 (95.8) | 142 (97.9) | 61 (91.0) | 0.052 |
Note: Continuous data are mean ± standard deviation or median (interquartile range), and categorical variables are n (%).
Abbreviations: ACEI/ARB, Angiotensin-converting enzyme inhibit or angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; PCI, Percutaneous coronary intervention; eGFR, Estimated glomerular filtration rate; HbA1c, hemoglobinA1c; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; HE-4, Human epididymis factor-4; LV, left ventricular; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation myocardial infarction; NT-proBNP, N-terminal pro-brain natriuretic peptide; PLT, platelet; STEMI, ST-segment elevation myocardial infarction; WBC, White blood cell.
The median follow-up time was 241(159–427) days, and 67 patients developed MACE during the follow-up period, including 28 patients with unstable angina pectoris, 28 patients readmitted due to HF, 6 patients with nonfatal myocardial infarction, and 9 patients with all-cause mortality (Table 1).
Correlation Analysis
With the increase in Killip class, the levels of HE4 were increased [423.1 pmol/L (Killip classI/II) vs 565.5 pmol/L (Killip class III/IV), P < 0.001]. Correlation analysis showed that plasma HE4 levels were positively correlated with NT-proBNP (r = 0.362, P < 0.001) and age (r = 0.375, P < 0.001), and negatively correlated with eGFR (r = −5.30, P < 0.001) and LVEF (r = −0.218, P = 0.001).
HE4 and Clinical Outcomes
Univariate Cox regression analysis showed that HE4, NT-proBNP, Age, Hypertension, Diabetes, Killip class, eGFR, White blood cell, LVEF, and Main epicardial coronary arteries ≥70% stenosis were all significant predictors of MACE during follow-up in patients with AMI (all P<0.05). The results of multivariate Cox analysis showed that HE4 [HR = 1.004 (1.002 −1.007), P =0.002] were independent predictors of MACE (Table 2).
Table 2.
Cox Analysis of Proportional Risks for MACE
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| HE4 | 1.006 | 1.004–1.008 | <0.001 | 1.004 | 1.002–1.007 | 0.002 |
| Log 10 NT-proBNP | 2.202 | 1.448–3.35 | <0.001 | 1.041 | 0.611–1.772 | 0.883 |
| Age | 1.034 | 1.011–1.058 | 0.003 | 1.004 | 0.978–1.032 | 0.748 |
| Hypertension | 1.79 | 1.042–3.075 | 0.035 | 1.418 | 0.798–2.522 | 0.234 |
| Diabetes | 2.073 | 1.281–3.354 | 0.003 | 1.432 | 0.858--2.390 | 0.169 |
| Killip class II/IV | 2.990 | 1.738–5.143 | <0.001 | 1.148 | 0.565–2.330 | 0.703 |
| eGFR | 0.977 | 0.967–0.987 | <0.001 | 0.955 | 0.981–1.008 | 0.448 |
| WBC(×109/L) | 1.065 | 1.007–1.127 | 0.027 | 1.012 | 0.945–1.083 | 0.736 |
| LVEF | 0.969 | 0.949–0.988 | 0.002 | 0.991 | 0.965–1.017 | 0.478 |
| Main epicardial coronary arteries ≥70% stenosis | 1.511 | 1.079–2.117 | 0.016 | 1.129 | 0.790–1.611 | 0.506 |
Abbreviations: CI, confidence interval; eGFR, Estimated glomerular filtration rate; HE4, Human epididymis factor-4; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; WBC, White blood cell.
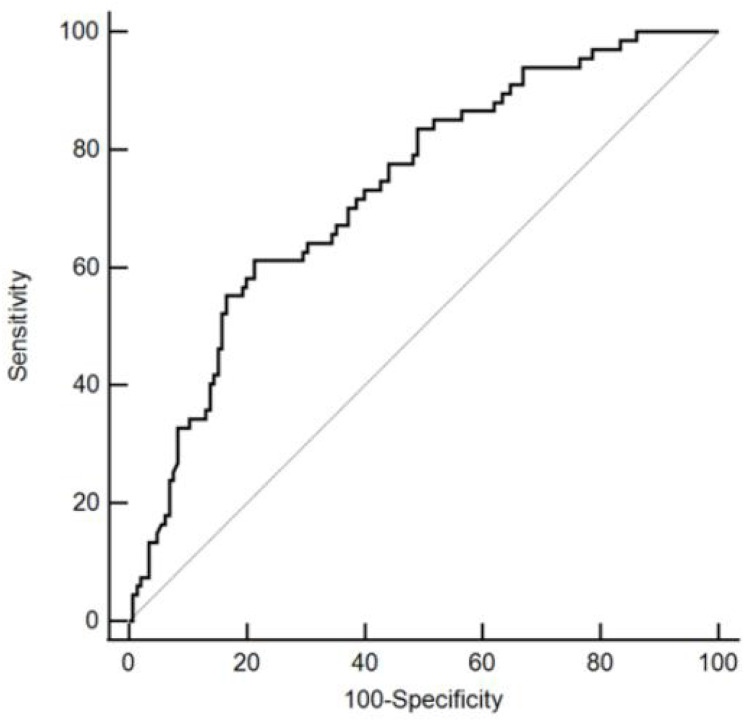
Receiver operating characteristic (ROC) curve analysis discriminated MACE showed that HE4 had the largest area under the curve (AUC), with an AUC value of 0.734 (95% CI: 0.669–0.792, P < 0.001). The cut-off point was 532.9 pmol/L and a specificity of 78.62% and a sensitivity of 61.19%. (Figure 2).
Figure 2.
Receiver operating characteristic curve showing the sensitivity and specificity of HE4.
Abbreviation: HE4, human epididymis protein 4.
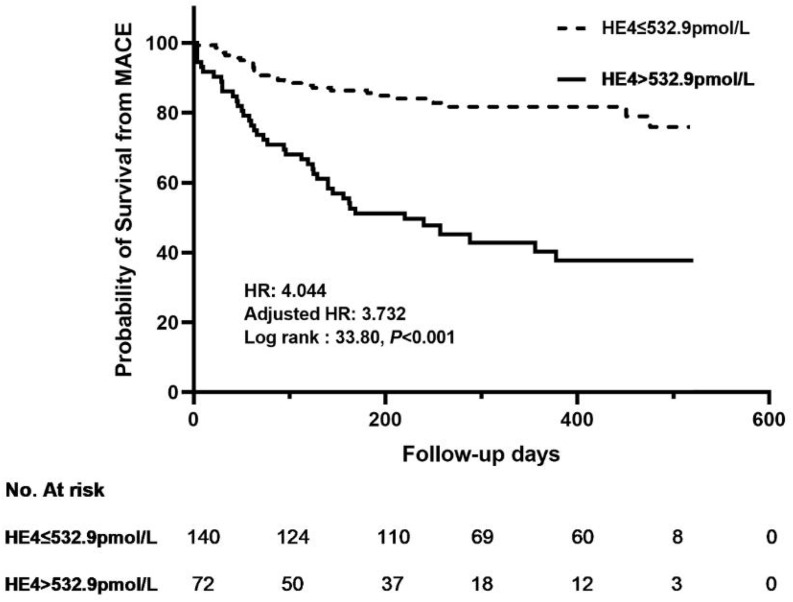
Kaplan-Meier survival analysis was performed to identify the survival difference between the two groups after grouping patients at the best cut-off point. The study results showed patients in the high HE4 level group (HE4>532.9 pmol/L) had a higher risk of major adverse cardiovascular events (MACE) (log-rank:χ2=33.8, HR=4.044, 95% CI 2.373–6.890, P <0.001) (Figure 3). After adjusting for age and sex, the HR was 3.732(95% CI:2.197–6.339, P<0.001).
Figure 3.
Kaplan-Meier analysis of human epididymis protein (HE4) for MACEs. Adjusted HR indicates hazard ratio (HR) adjusted for age and gender.
Discussion
In this study, we evaluated the predictive value of HE4 in patients with AMI. We found that HE4 levels were closely associated with the severity of HF in patients with AMI, and further analysis indicated that HE4 was an independent predictor of MACE in patients with AMI.
Human epididymis protein 4, also known as whey acidic protein 4-disulfide core domain 2 (WFDC2), was a novel cross-class protease inhibitor, which applied to the diagnosis and prognosis evaluation of ovarian and endometrial cancer currently.16 In normal tissues, it is mainly expressed in the glandular epithelium of the female genital tract, ovarian surface epithelium, epididymis and vas deferens, respiratory epithelium, distal renal tubules, and salivary glands.7,17 In recent years, it has been found that blood HE4 levels are closely associated with renal function. Its levels are significantly elevated in patients with chronic renal function and renal fibrosis and are closely associated with renal function,18,19 which may be mediated through the reduced degradation of type I collagen via the HE4/ mitogen-activated protein kinase/(Matrix metalloproteinases)MMPs pathway and the reduced MMP2 activity by upregulation of tissue metalloproteinase inhibitor 1 in the HIF-1α/HE4/NF-κB signaling pathway to inhibit the chances of extracellular matrix degradation leading to renal fibrosis.20,21 These results suggest that HE4 plays an important role in the process of renal fibrosis.
Our study showed a significant negative correlation between HE4 and renal function, suggesting that HE4 levels increase as renal function deteriorates.de Boer RA et al found elevated HE4 levels in the blood of patients with chronic HF and acute HF could reflect the severity of HF and predict the prognosis of patients. In addition, HE4 levels in these HF patients were significantly positively correlated with GAL3,8,9 an indicator of fibrosis, indicating that HE4 may be closely related to myocardial fibrosis. Our previous study found that HE4 could reflect the severity of HF and predict patients’ prognosis in a population with ischemic cardiomyopathy, further suggesting that HE4 may be involved in myocardial fibrosis. After AMI, activation of neuroendocrine systems (renin-angiotensin-aldosterone system, sympathetic nervous system, and natriuretic peptide system) and inflammatory immune response lead to myocardial fibrosis and ventricular remodeling.22–24 Myocardial fibrosis and ventricular remodeling play a key role in cardiac events and long-term prognosis in patients with AMI.25 In our study, we found that HE4 could reflect the severity of HF in AMI, and HE4 could predict the prognosis of AMI, which was independent of other established risk factors, including age, gender, NT-proBNP level, renal function and LVEF. This result was consistent with previous findings. The reason may be attributed to HE4 involved in myocardial fibrosis and ventricular remodeling after AMI. However, the exact molecular mechanistic pathways of HE4 elevation post-acute myocardial infarction is unknown, further studies are needed to explored it.
Recently, Hakan Kilci et al found that HE4 levels were significantly increased in patients with low left ventricular ejection fraction (the fourth day after admission) in STEMI patients undergoing revascularization, and the authors hypothesized that HE4 levels might be an evaluation of reduced left ventricular systolic function in STEMI patients undergoing revascularization.12 Patients with STEMI and NSTEMI were included in this study, and all patients underwent cardiac ultrasound and HE4 testing the next day, and our results also showed that HE4 levels were high in patients with low left ventricular ejection fraction. In addition, HE4 levels increased with the elevation of Killip class and HE4 levels were positively correlated with NT-proBNP. Therefore, HE4 is very useful in the risk stratification of AMI patients.
According to the current guidelines, aspirin, P2Y12 inhibitors and ACEI/ARB are recommended for all patients with acute myocardial infarction unless they has contraindications.26 Compared with the no MACE group, the rates of hypertension and diabetes, NT-proBNP, Killip class, and triple vessel lesion proportions were higher, eGFR, LVEF were lower in the MACE group, and the patients in the MACE group were older. However, all these factors are not the contraindications for the use of aspirin, P2Y12 inhibitors and ACEI/ARB. This could be the reason that there was no statistically significant difference in the history of Aspirin, P2Y12 inhibitor and ACEI/ARB administration between the two group.
Our study is limited as follows. First, it is a single-center study with a small sample size and short follow-up duration. Second, we did not perform serial measurements of plasma HE4 levels after the onset of AMI. Larger clinical studies are still needed to further verify the prognostic predictive value of HE4 for AMI and to further perform basic experiments to explore its mechanism.
Conclusion
Human epididymis protein 4 independently predicts the occurrence of adverse cardiovascular events in AMI and might be a novel biomarker for prognostic evaluation in patients with AMI.
Acknowledgment
We thank all subjects and colleagues for participating in the study.
Funding Statement
This work was supported by grants from the National Clinical Key Specialty Construction Project Cardiovascular Department (Cardiopulmonary Function Rehabilitation Direction) (Z2023123), Renshu Fund project of Hunan Provincial People’s Hospital (RS2022A12), Key Project of Hunan provincial science and technology innovation (2020SK1013) and Hunan Provincial Health Commission Fund (202214013647).
Data Sharing Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Statement of Ethics
The study complied with the Declaration of Helsinki. This study protocol was reviewed and approved by the Ethics Committee of Hunan Provincial People’s Hospital, approval number (2021-103). All patients gave their written informed consent to participate.
Author Contributions
Yi Tang, Wen-Yu Zhu, Si-ling Peng and Shuai Huang contributed equally to this work and share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Kostis WJ, Deng Y, Pantazopoulos JS, Moreyra AE, Kostis JB. Myocardial infarction data acquisition system study G. Trends in mortality of acute myocardial infarction after discharge from the hospital. Circ Cardiovasc Qual Outcomes. 2010;3(6):581–589. doi: 10.1161/CIRCOUTCOMES.110.957803 [DOI] [PubMed] [Google Scholar]
- 3.Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med. 2010;8(1):34. doi: 10.1186/1741-7015-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leistner DM, Klotsche J, Pieper L, et al. Prognostic value of NT-pro-BNP and hs-CRP for risk stratification in primary care: results from the population-based DETECT study. Clin Res Cardiol. 2013;102(4):259–268. doi: 10.1007/s00392-012-0530-5 [DOI] [PubMed] [Google Scholar]
- 5.Kuwahara K. The natriuretic peptide system in heart failure: diagnostic and therapeutic implications. Pharmacol Ther. 2021;227:107863. doi: 10.1016/j.pharmthera.2021.107863 [DOI] [PubMed] [Google Scholar]
- 6.Piek A, Du W, de Boer RA, Sillje HHW. Novel heart failure biomarkers: why do we fail to exploit their potential? Crit Rev Clin Lab Sci. 2018;55(4):246–263. doi: 10.1080/10408363.2018.1460576 [DOI] [PubMed] [Google Scholar]
- 7.Hellstrom I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 8.de Boer RA, Cao Q, Postmus D, et al. The WAP four-disulfide core domain protein HE4: a novel biomarker for heart failure. JACC Heart Fail. 2013;1(2):164–169. doi: 10.1016/j.jchf.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Piek A, Meijers WC, Schroten NF, Gansevoort RT, de Boer RA, Sillje HH. HE4 serum levels are associated with heart failure severity in patients with chronic heart failure. J Card Fail. 2017;23(1):12–19. doi: 10.1016/j.cardfail.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Jin Q, Tang Y, Liu Z, et al. Serum human epididymis protein 4 level as a predictor of clinical worsening in idiopathic pulmonary arterial hypertension: a pilot study. BMC cardiovasc disord. 2020;20(1):175. doi: 10.1186/s12872-020-01461-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Wang Y, Xu X, et al. Human epididymis protein 4: a novel predictor of ischemic cardiomyopathy. BMC cardiovasc disord. 2021;21(1):511. doi: 10.1186/s12872-021-02319-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilci H, Altinbilek E, Cetinkal G, et al. Relation of a novel fibrosis marker and post-myocardial infarction left ventricular ejection fraction in revascularized patients. Biomarker Med. 2021;15(17):1651–1658. doi: 10.2217/bmm-2021-0003 [DOI] [PubMed] [Google Scholar]
- 13.Agra Bermejo R, Cordero A, Garcia-Acuna JM, et al. Determinants and prognostic impact of heart failure and left ventricular ejection fraction in acute coronary syndrome settings. Rev Esp Cardiologia. 2018;71(10):820–828. doi: 10.1016/j.recesp.2017.10.047 [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Hu S, Cao M, et al. Evaluation of acute myocardial infarction patients with mid-range ejection fraction after emergency percutaneous coronary intervention. Postgrad Med J. 2019;95(1125):355–360. doi: 10.1136/postgradmedj-2018-136334 [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618–e51. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 16.Chhikara N, Saraswat M, Tomar AK, Dey S, Singh S, Yadav S. Human epididymis protein-4 (HE-4): a novel cross-class protease inhibitor. PLoS One. 2012;7(11):e47672. doi: 10.1371/journal.pone.0047672 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Ferraro S, Panteghini M. Making new biomarkers a reality: the case of serum human epididymis protein 4. Clin Chem Lab Med. 2019;57(9):1284–1294. doi: 10.1515/cclm-2018-1111 [DOI] [PubMed] [Google Scholar]
- 18.Wan J, Wang Y, Cai G, et al. Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease. Oncotarget. 2016;7(42):67748–67759. doi: 10.18632/oncotarget.11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen P, Yang Q, Li X, Qin Y. Potential association between elevated serum human epididymis protein 4 and renal fibrosis: a systemic review and meta-analysis. Medicine. 2017;96(36):e7824. doi: 10.1097/MD.0000000000007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBleu VS, Teng Y, O’Connell JT, et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med. 2013;19(2):227–231. doi: 10.1038/nm.2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Zhao H, Xu F, Zhang P, Zheng Y, Jia N. Human epididymis protein 4 (HE4) protects against cystic pulmonary fibrosis associated-inflammation through inhibition of NF-kappaB and MAPK singnaling. Genes Genomics. 2019;41(9):1045–1053. doi: 10.1007/s13258-019-00836-4 [DOI] [PubMed] [Google Scholar]
- 22.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122(25):2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anzai T. Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J. 2013;77(3):580–587. doi: 10.1253/circj.CJ-13-0013 [DOI] [PubMed] [Google Scholar]
- 24.Frangogiannis NG. The extracellular matrix in ischemic and nonischemic heart failure. Circ Res. 2019;125(1):117–146. doi: 10.1161/CIRCRESAHA.119.311148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St John Sutton M, Ferrari VA. Prevention of left ventricular remodeling after myocardial infarction. Curr Treat Options Cardiovasc Med. 2002;4(2):97–108. doi: 10.1007/s11936-002-0030-4 [DOI] [PubMed] [Google Scholar]
- 26.Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–3826. doi: 10.1093/eurheartj/ehad191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.