Abstract
Aims
More than 220 Mio people live at altitudes above 2000 m, many of whom have pre-existing chronic diseases, including pulmonary vascular diseases (PVDs) such as pulmonary arterial hypertension (PAH) or chronic thromboembolic pulmonary hypertension (CTEPH). We investigated the acute effects of high-dose supplemental oxygen on pulmonary haemodynamics assessed by echocardiography in patients with PVD permanently living at 2850 m.
Methods and results
In a randomized, single-blind, placebo-controlled crossover trial, patients with PVD diagnosed with PAH or CTEPH were allocated to receive 10 L/min supplemental oxygen (FiO2 ≈ 95%) and placebo air administered via a facial mask with reservoir near their living altitude in Quito at 2850 m (FiO20.21, PiO2 ≈ 60% of sea level) in random order with a washout period of >2 h. After >15 min of breathing the respective FiO2, systolic pulmonary artery pressure (sPAP), cardiac output (CO), and other parameters were assessed by echocardiography. Furthermore, radial arterial blood gases were analysed. Twenty-eight patients with PVD (24 females, 26 PAH, age 45 ± 12 years) treated with phosphodiesterase-5 inhibitors (n = 28) and endothelin receptor antagonists (n = 9) were included. With oxygen vs. placebo air, sPAP was 57 ± 23 vs. 68 ± 24 mmHg, mean difference −11 mmHg (−15 to −6 mmHg, P < 0.001), CO was 3.2 ± 0.9 vs. 3.9 ± 1.1 L/min; −0.7 L/min (−0.9 to −0.4 L/min, P < 0.001), while sPAP/CO was unchanged, and the right ventriculo-arterial coupling was increased. PaO2 was 22.5 ± 9.7 vs. 7.6 ± 1.5 kPa; 14.9 kPa (11.4–18.4 kPa, P < 0.001).
Conclusion
High-dose oxygen therapy in prevalent patients with PVD living near 2850 m significantly lowered sPAP but also CO by a reduced heart rate, resulting in an unchanged pulmonary resistance. Whether longer-term oxygen therapy would improve pulmonary vascular resistance requires further investigation.
Registration
NCT06084559 URL: https://clinicaltrials.gov/study/NCT06084559.
Keywords: Pulmonary vascular disease, PVD, High altitude, Oxygen therapy, Pulmonary hypertension
Introduction
In the absence of predominant lung disease, the two major forms of precapillary pulmonary hypertension (PH) are pulmonary arterial hypertension (PAH) and chronic thromboembolic PH (CTEPH), hereafter summarized as pulmonary vascular disease (PVD).1 PVD needs to be diagnosed by right heart catheterization (RHC). In Europe, where the majority of the population lives below 1000 m of altitude, PVD is rare with a reported prevalence that ranges from 26 to 55 cases per million adults.2 However, the systolic pulmonary arterial pressure (sPAP) increases with altitude, even in healthy individuals, due to hypoxic pulmonary vasoconstriction. Typically healthy people rarely have a sPAP >30 mmHg at 2850 m.3 Nevertheless, in subjects with elevated pulmonary arterial pressures living at high altitude, it is important to distinguish between isolated elevated PAP in healthy individuals at high altitude, highlanders with exaggerated PAP (high-altitude PH), and patients with true PVD exhibit pathophysiological changes (e.g. remodelling of the pulmonary vessels with plexiform lesions) identical to those in low-altitude inhabitants.3–5
However, the accurate diagnosis of PVD is difficult, even more in countries with limited medical infrastructure, resources, and is not possible without the access to RHC.6 Furthermore, to date, the diagnosis of PH is based on values assessed at low altitude.1 Many large cities, such as Quito (Ecuador) or Mexico City (Mexico), are located at high altitudes, and it is assumed that the worldwide population permanently living above 2000 m altitude encompasses >220 million people. With such an amount of people living at altitude, it is rather unsurprising to find among them symptomatic patients at living altitudes between 2000 and 3000 m who have severe precapillary PH with a clear PVD phenotype. Previous randomized controlled trials showed that supplemental oxygen therapy, applied in different settings (nocturnal, 5-week domiciliary, during exercise, RHC) improves haemodynamics, exercise capacity, symptoms, and quality of life in patients with PVD living <1000 m.7–9
Therefore, the aim of this study was to investigate the effect of high-dose oxygen therapy on pulmonary haemodynamics in patients with PVD diagnosed by RHC and classified as PAH or distal CTEPH, living in Quito near 2850 m of altitude.
Methods
Study design, randomization, and subjects
This was a randomized, single-blind, placebo-controlled crossover trial conducted between 24 September and 26 September at the Carlos Andrade Marín Hospital in Quito, Ecuador, which is located at 2850 m. Randomization to study sequences (oxygen–placebo/placebo–oxygen) was performed by software-based blocks with random computed block length and allocation concealment. Participants of all sexes with PAH or CTEPH diagnosed with RHC according to the haemodynamic criteria of recent guidelines were recruited from the Pulmonology Department in Quito between January 2023 and September 2023.1 Other forms of PH were excluded. Chronic thromboembolic pulmonary hypertension was diagnosed or excluded by pulmonary angiography. Participants were under stable conditions with unchanged PH-targeted medication for >4 weeks and gave their written informed consent. A resting SpO2 < 80% in Quito, having any inabilities to follow the study protocol, pregnancy, or breast feeding were exclusion criteria. The study complied with the Declaration of Helsinki, was approved by the local ethical authorities, and was registered on clinicaltrials.gov (NCT06084559).
Intervention
According to the crossover design, patients received both interventions in a randomized order with a washout period of >2 h in between. The experimental intervention was calm breathing of 10 L/min supplemental oxygen (FiO2 ≈ 95%). The control intervention was calm breathing of placebo air (FiO20.21, PiO2 ≈ 60% of sea level); both interventions were supplied via a facial mask with reservoir in supine or lateral position. The setting of both interventions was identical, and participants were blinded to the interventions. Patients who were treated with nocturnal oxygen therapy were asked to pause the therapy for the study.
Assessments
Echocardiography was performed after 15 min of rest while breathing oxygen or placebo air, respectively. Echocardiographic recordings were conducted using a real-time sector scanner (CX 50, Philips, Philips Respironics, Zofingen, Switzerland) with an integrated colour, continuous-wave (CW), and pulsed-wave Doppler system. Recordings and measurements were performed according to guidelines of the American Society of Echocardiography.10 To estimate sPAP, the maximal pressure gradient of the tricuspid regurgitation was calculated from the maximal tricuspid regurgitation velocity (TRV) determined with CW Doppler using the modified Bernoulli equation: ΔPressure = 4 × TRVmax2 with adding the right atrial pressure. Four experienced and well-trained investigators performed the echocardiographic imaging according to standard operating procedures. Repeated investigations of each patient with both conditions (air and oxygen therapy) were performed by the same investigator to minimize inter-observer variance. The analysis of the echocardiographic data was conducted by the same investigator who performed the imaging and was supervised by the principal investigator to ensure uniformity in measurements. Heart rate (HR) was derived continuously from a 3-lead ECG, and blood pressure was measured using an arm cuff. After the echocardiography while still breathing oxygen or placebo air, respectively, arterial blood samples were taken from the radial artery for immediate analysis of arterial blood gases (epoc Blood Analysis System, Siemens, Berlin, Germany). Baseline characteristics were collected from medical records from the Carlos Andrade Marín Hospital in Quito.
Statistical analysis and sample size
Based on the primary outcome sPAP, a sample size of 24 was calculated, assuming an minimal clinically important difference of 5 mmHg, a standard deviation of 5 mmHg applying a significance level alpha of 0.05 and a power 0.9. To account for dropouts, we aimed to include 28 participants.
Primary analysis
To compare the main outcome between oxygen and placebo air after 15 min of calm breathing, physiological data were summarized as means ± standard deviation. The distribution of the data was checked by visual inspection of histograms and Shapiro–Wilk test. Furthermore, test assumptions for linear mixed models such as homogeneity and normality of the residuals were checked visually with Tukey–Anscombe plots and Q–Q plots. A linear mixed model was fitted to the data using intervention (oxygen vs. placebo air), period, and intervention–period interaction as fixed effects and subject (id) as random intercept, so that carry-over effects (treatment-period interaction) and period effects were controlled according to the standards of crossover trials. We tested whether the intervention–period interaction could be removed from the model.
The analysis of the secondary outcomes followed the same procedure as described above, with the addition of baseline characteristics as covariates to control for bias and confounding. In cases of missing data or incomplete data records, no imputation was performed, and an intention-to-treat analysis was used.11
Prediction analysis
To further investigate whether a special set or combination of baseline characteristics may determine if a patient will respond to oxygen therapy or not, a post hoc analysis was performed. A responder was defined by a reduction in sPAP ≥10 mmHg when breathing oxygen compared with placebo air. A logistic regression analysis was performed to determine how useful certain baseline values might be in predicting a responder. For continuous variables, the optimal cut-off for the identified variables was estimated using receiver operating characteristic (ROC) curves.
In all analyses, a 95% confidence interval (CI) that excluded the null effect was considered as evidence of statistical significance. Analyses were performed using R-Studio software version 2023.12.1+402.
Results
Model assumptions of homogeneity and normality of the residuals and random effects were met, and there were neither carry-over nor period effects.
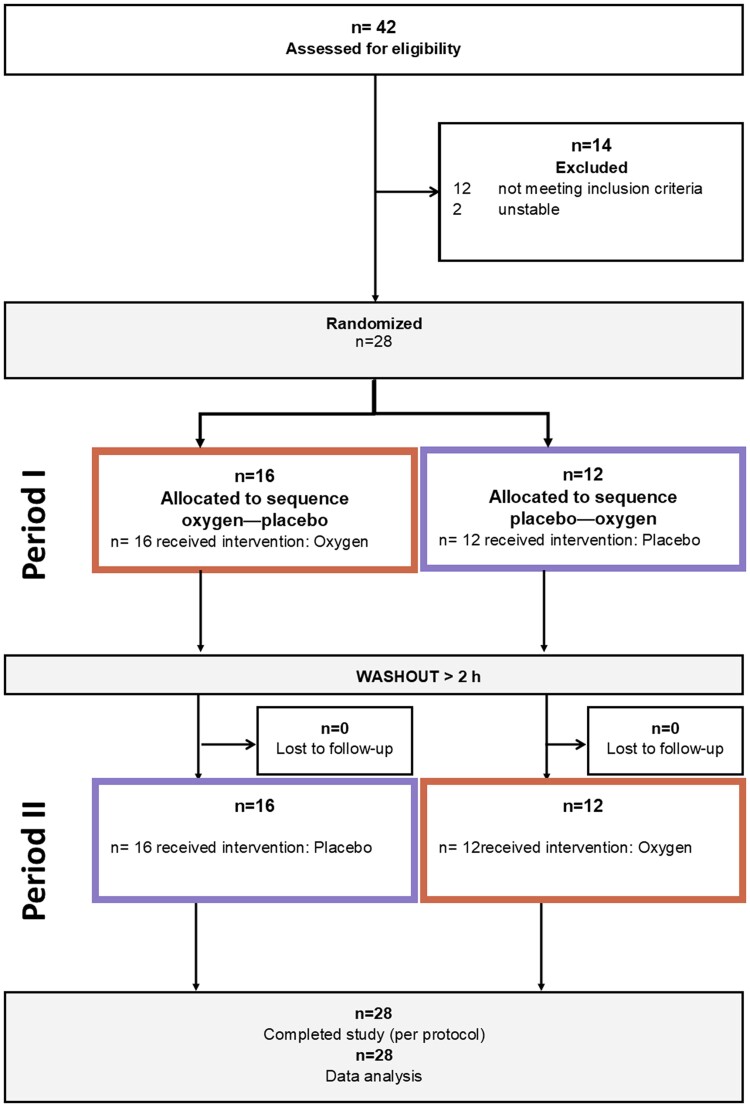
A total of 28 participants (26 PAH and 2 CTEPH, 24 females, age 45 ± 12 years) were included and completed the trial per protocol. According to the classical PAH phenotype, patients were predominantly female, were relatively young, and had severely impaired haemodynamics. The characteristics of the participants are listed in Table 1. The flow chart of the study is shown in Figure 1. All participants completed the study per protocol, and as the participants were predominantly younger to middle-aged females, the echocardiographic quality was good. A sensitivity analysis excluding the two patients with CTEPH was conducted to reduce the heterogeneity of the sample. Since the results were unchanged, we decided to report the data from all 28 patients.
Table 1.
Baseline characteristics
| All | Responder | Non-responder | |
|---|---|---|---|
| Number of participants | 28 | 13 (46%) | 15 (54%) |
| Female/male | 24 (86%):4 (14%) | 10 (77%):3 (23%) | 14 (93):1 (7%) |
| Age (years) | 45 ± 12 | 47 ± 14 | 43 ± 12 |
| Pulmonary arterial hypertension | 26 (93%) | 13 (100%) | 13 (87%) |
| Idiopathic | 21 (75%) | 12 (92%) | 9 (60%) |
| Associated with: | |||
| Congenital heart disease | 4 (14%) | 1 (8%) | 3 (20%) |
| Connective tissue disease | 1 (4%) | 0(0%) | 1 (7%) |
| Chronic thromboembolic pulmonary hypertension | 2 (7%) | 0 (0%) | 2 (13%) |
| WHO functional class (I–IV) | II: 10 (36%), III: 18 (64%) | II: 3 (23%), III: 10 (77%) | II: 8 (53%), III: 7 (57%) |
| Body mass index (kg/m2) | 25.6 ± 3.8 | 26.3 ± 3.9 | 25.1 ± 3.6 |
| Current smoker/ex-smoker | 0 (0%)/1 (4%) | 0 (0%)/0 (0%) | 0 (0%)/1 (7%) |
| SpO2 at rest (%) | 87 ± 5 | 84 ± 6 | 89 ± 3 |
| NT-proBNP (ng/L) | 531 ± 844 | 404 ± 860 | 684 ± 908 |
| Six minute walk distance (m) | 499 ± 94 | 452 ± 104 | 470 ± 100 |
| Diagnostic right heart catheter data | |||
| Mean pulmonary artery pressure (mmHg) | 52 ± 16 | 53 ± 14 | 52 ± 16 |
| Pulmonary artery wedge pressure (mmHg) | 11 ± 3 | 11 ± 3 | 10 ± 3 |
| Pulmonary vascular resistance (WU) | 16.3 ± 8.6 | 14.5 ± 9.2 | 17.7 ± 8.2 |
| Cardiac output (L/min) | 2.9 ± 0.9 | 2.7 ± 0.9 | 3.2 ± 1.0 |
| Arterial blood gases at time of diagnosis | |||
| Arterial partial pressure for oxygen (kPa) | 8.5 ± 1.5 | 8.4 ± 1.2 | 8.6 ± 1.9 |
| Arterial partial pressure for carbon dioxide ([kPa) | 3.9 ± 0.5 | 4.0 ± 0.5 | 3.8 ± 0.6 |
| Arterial oxygen saturation (%) | 91 ± 5 | 91 ± 6 | 91 ± 5 |
| Echocardiography data | |||
| Left ventricular ejection fraction (%) | 63 ± 5 | 63 ± 5 | 63 ± 5 |
| Left atrial volume index (mL/m2) | 23 ± 12 | 25 ± 12 | 20 ± 11 |
| Right atrium area (cm2) | 25.4 ± 12.4 | 26.7 ± 12.5 | 24.0 ± 12.7 |
| Right atrium dilated | 24 (86%) | 10 (77%) | 14 (93%) |
| Pericardial effusion | 6 (21%) | 3 (23%) | 3 (20%) |
| Pulmonary hypertension-targeted medication | |||
| Endothelin receptor antagonist | 9 (32%) | 6 (46%) | 3 (20%) |
| Phosphodiesterase-5 inhibitor | 28 (100%) | 13 (100%) | 15 (100%) |
| Calcium channel blocker | 13 (46%) | 6 (46%) | 7 (47%) |
| Anticoagulation | 19 (68%) | 9 (69%) | 10 (67%) |
| Diuretics | 17(61%) | 9 (69%) | 8 (53%) |
| Nocturnal oxygen therapy | 15 (54%) | 7 (54%) | 8 (53%) |
| 24-h oxygen therapy | 1 (4%) | 1 (8%) | 0 (0) |
Data are presented as mean ± standard deviation, absolute numbers, or proportions.
WHO, World Health Organization; SpO2, oxygen saturation by pulse oximetry; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Figure 1.
The study flow chart for crossover trials (adapted from CONSORT12). All participants who were randomized completed the study per protocol and were included into the analysis.
Oxygen vs. placebo air
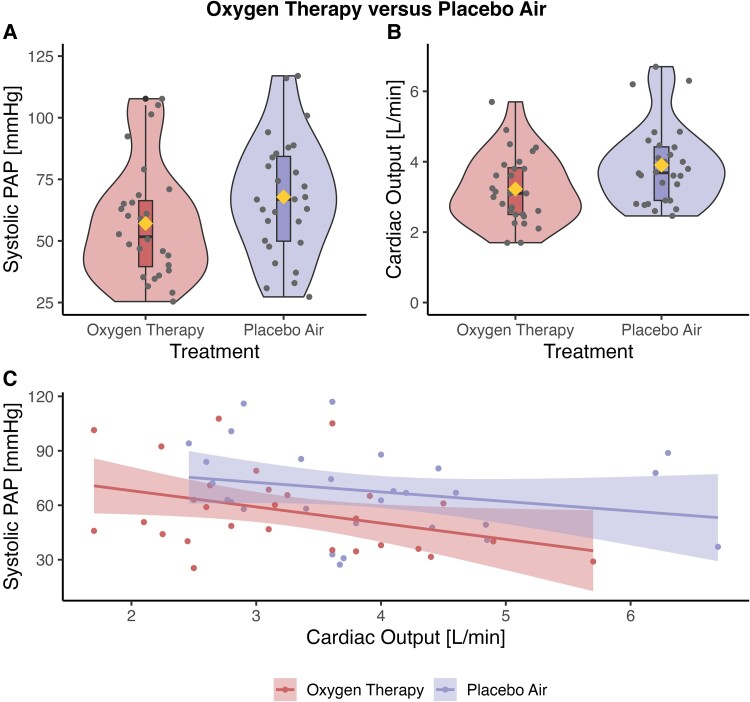
After 15 min of breathing oxygen, the sPAP was −11 mmHg (95% CI: −15 to −6 mmHg, P < 0.001) lower than after breathing 15 min of placebo air (Figure 2). Individual changes in sPAP are shown in Supplementary material online, Figure S1.
Figure 2.
Echocardiographic parameters assessed after breathing 15 min of oxygen therapy vs. 15 min of placebo air. (A) The systolic pulmonary arterial pressure. (B) The cardiac output. The boxplots show the median, the inter-quartile ranges, the minimum, the maximum, and outliers. The violin plots represent the distribution of the data, while the width represents the frequency. The mean is displayed as diamonds, and the dots represent the individual data. (C) The pulmonary resistance. The individual patient data are represented by dots, and the lines show the regression lines with standard errors. There is a left shift with oxygen therapy in the lower left corner compared with placebo air, but the resistance remains unchanged as both systolic pulmonary arterial pressure and cardiac output decreased with oxygen therapy. The cardiac output decreases by a reduction in heart rate, while the stroke volume remains constant.
Heart rate and consequently CO were lower by 9 b.p.m. (95% CI: −14 to −5 b.p.m., P < 0.001) and −0.7 L/min (95% CI: −0.9 to −0.5 L/min, P < 0.001) with oxygen. After breathing 15 min of oxygen compared with placebo air, tricuspid annular plane systolic excursion (TAPSE)/sPAP was higher by 0.05 mm/mmHg (95% CI: 0.01–0.09 mm/mmHg, P = 0.024). None of the other echocardiographic parameters differed statistically significantly between oxygen and placebo air. The shunt fraction with oxygen (Qs/Qt) was 14 ± 4%. All outcomes are presented in Table 2.
Table 2.
Echocardiographic data placebo air vs. oxygen therapy in Quito at 2850 m (PiO2 ≈ 60% of sea level). Values in bold indicate statistical significance.
| Parameter | Placebo air (FiO2 ≈ 0.21) | Oxygen (FiO2 ≈ 0.95) | Mean difference (95% CI) | P-value |
|---|---|---|---|---|
| Tricuspid regurgitation pressure gradient (mmHg) | 62 ± 22 | 52 ± 23 | −10 (−14 to −5) | <0.001 |
| Right atrial pressure (mmHg) | 6 ± 4 | 5 ± 4 | −1 (−1.6 to 0.3) | 0.212 |
| Systolic pulmonary arterial pressure (mmHg) | 68 ± 24 | 57 ± 23 | −11 (−15 to −6) | <0.001 |
| Heart rate (beats/min) | 74 ± 14 | 65 ± 10 | −9 (−14 to −5) | <0.001 |
| Velocity time integral (cm) | 20.5 ± 4.7 | 20.3 ± 5.4 | −0.2 (−1.5 to 1.6) | 0.888 |
| Stroke volume ((mL) | 53 ± 13 | 53 ± 17 | 0 (−6 to 5) | 0.929 |
| Cardiac output (L/min) | 3.9 ± 1.1 | 3.2 ± 1.0 | −0.7 (−0.9 to −0.5) | <0.001 |
| sPAP/CO (mmHg/L/min) | 19 ± 10 | 20 ± 12 | 1 (−1 to 3) | 0.380 |
| Tissue Doppler index S′ | 10.8 ± 2.2 | 10.6 ± 2.3 | −0.2 (−0.9 to 0.5) | 0.575 |
| TAPSE (mm) | 15.9 ± 4.1 | 15.9 ± 3.9 | 0 (−0.8 to 0.9) | 0.912 |
| TAPSE/sPAP (mm/mmHg) | 0.28 ± 0.16 | 0.33 ± 0.17 | 0.05 (0.01–0.09) | 0.024 |
| Fractional area change (%) | 33 ± 7 | 32 ± 6 | −1 (−3 to 2) | 0.661 |
| Right atrium area (cm2) | 25.4 ± 12.4 | 24.5 ± 11.0 | −0.9 (−2.6 to 1.4) | 0.559 |
| Right ventricle/left ventricle | 1.4 ± 0.6 | 1.3 ± 0.4 | −0.1 (−0.3 to 0.1) | 0.232 |
| Systolic blood pressure (mmHg) | 108 ± 13 | 111 ± 12 | 3 (−3 to 6) | 0.493 |
| Diastolic blood pressure (mmHg) | 66 ± 14 | 64 ± 13 | −2 (−7 to 1) | 0.076 |
| Pulse oximetry oxygen saturation (%) | 87 ± 5 | 97 ± 3 | 10 (9–12) | <0.001 |
| Arterial partial pressure for oxygen (kPa) | 7.6 ± 1.5 | 22.5 ± 9.7 | 14.9 (11.4–18.4) | <0.001 |
| Arterial partial pressure for carbon dioxide (kPA) | 4.0 ± 0.5 | 4.1 ± 0.5 | 0.1 (−0.2 to 0.0) | 0.132 |
| Shunt fraction (Qs/Qt) (%) | NA | 14 ± 4 | NA | NA |
| Arterial partial pressure for oxygen (kPa) | ΔPaO2 in responder 13.9 ± 10.8 |
ΔPaO2 in non-responder 16.3 ± 7.7 |
2.4 (−5.4 to 10.2) | 0.530 |
Data are presented as mean ± standard deviation.
TAPSE, tricuspid annular plane systolic excursion; CO, cardiac output; sPAP, systolic pulmonary arterial pressure.
Responders to high-dose oxygen therapy
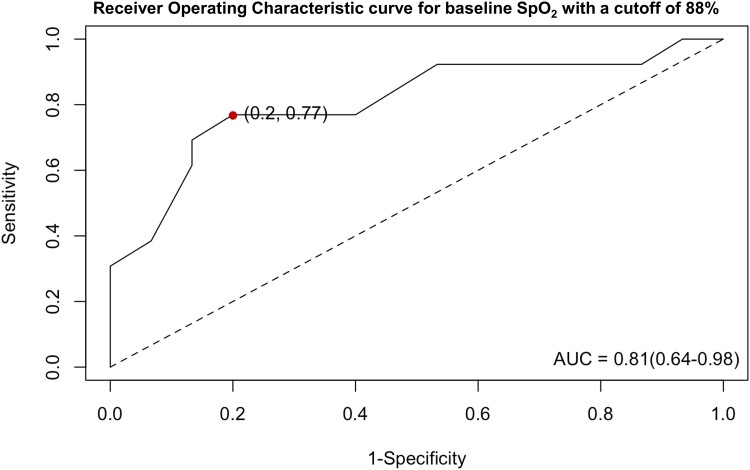
A total of 13 participants were identified as responders (sPAP reduction by ≥10 mmHg with oxygen). Logistic regression analysis showed that neither baseline RHC nor echocardiographic data or PH-targeted medication were useful in predicting whether a patient might be a responder to oxygen therapy. The best predictor was baseline SpO2, with a cut-off point of 88% identified by ROC curve analysis [area under the curve (AUC) = 0.81, sensitivity = 0.77, specificity = 0.80, see Figure 3]. The odds ratio of being a responder was 21.7 (95% CI: 3.0–155.4, P = 0.002) for patients with a baseline SpO2 ≤ 88% compared with those with a baseline SpO2 > 88%.
Figure 3.
The receiver operating characteristic curve for baseline SpO2 to predict whether a patient will be a responder to oxygen therapy. The optimal cut-off was identified at 88%, with a sensitivity of 77% and a specificity of 80% (dot). AUC, area under the curve.
Discussion
In this randomized, single-blind, placebo-controlled crossover trial, we found that high-dose oxygen therapy significantly reduced sPAP compared with placebo air, in 28 patients with PVD permanently residing at an altitude of 2850 m. Furthermore, the CO was lower with oxygen therapy, mainly due to a reduction in heart rate, but pulmonary resistance was unchanged. TAPSE/sPAP was significantly higher under oxygen, indicating improved coupling between the right ventricle and the pulmonary arteries. Forty-six per cent of the patients were responders to oxygen therapy, defined as a decrease in sPAP by ≥10 mmHg with oxygen therapy, and it was found that SpO2 on ambient air at 2850 m using a cut-off of 88% is useful in predicting whether a patient will be a responder.
The severity of PVD at baseline and the reduction in PAP with PH-targeted medication are of prognostic importance for survival in patients with PVD living at low altitude.13,14 In a previous study, it was shown that hyperoxia (FiO2, 1.0) applied at 470 m of altitude via a facial mask reduced sPAP from 54 to 48 mmHg (11%) in patients with PVD.15 Therefore, the magnitude of sPAP reduction with oxygen observed in this trial (from 68 to 57 mmHg, 16%) in patients with PVD permanently living at 2850 m of altitude was higher.
Elevated sPAP represents right ventricular overload, which impairs right ventricular function.1 However, not solely sPAP was lower with oxygen. TAPSE/sPAP as an indicator of right ventricular–arterial coupling and thus right ventricular contractile response to increased afterload was higher with oxygen compared with placebo air.16,17 This suggests that oxygen improved right ventricular contractile function compared with placebo air.
Of interest, CO was also lower with oxygen compared with placebo air, leading to an unchanged total pulmonary resistance. Lower CO with oxygen can be explained by a 25% increase in systemic vascular resistance.18 The reduction in CO was driven by a decrease in heart rate of 9 b.p.m. with oxygen. Since stroke volume was unchanged, the decrease in heart rate might be a sign of less sympathetic over excitation.19 This was also observed in PVD at low altitude, healthy volunteers, and coronary artery disease, while in patients with heart failure CO decreased during oxygen therapy through a reduction in stroke voloume.18 The 12% decrease in heart rate, with preserved stroke volume, indicates that right ventricular load is reduced with oxygen therapy, which is an important observation for patients with PVD. Similar heart rate reduction was also found when patients with PVD exercised with high-dose oxygen vs. placebo.19 Recently, sotatercept, which is a novel pharmacological treatment for PAH, has been shown in a Phase-3 study to improve the 6-min walk distance, time to clinical worsening, and haemodynamics in patients with PAH.20 Interestingly, Sotatercept also improved haemodynamics by reducing PAP, while CO was unchanged.21
When total pulmonary resistance remains unchanged while sPAP declines during oxygen therapy, the lower CO must be considered as main factor to the decrease in pressure. When the oxygen content of the arterial blood is increased, as was here the case with oxygen therapy, even with lower CO, the oxygen delivery remains similar. In other words, a decrease in CO even in patients with PVD should not be problematic as long as the oxygen delivery remains similar due to the increased blood oxygen content.18 A lower CO due to the lower HR with unchanged SV might even be considered as relieve for the cardiopulmonary system. The opposite physiological reaction can be observed when patients with PVD are acutely breathing hypoxic air, which leads to an increased PAP and increased CO.22
Vasoreactivity testing during RHC is not yet available in Ecuador. Although oxygen is a vasodilator in the pulmonary vessels, it cannot replace nitric oxides for vasoreactivity testing. Nevertheless, for a positive vasoreactivity test, a decrease in mPAP of ≥10 mmHg, to below 40 mmHg with constant CO, must be achieved, which is not approximately the case here.1,23 In addition, as the availability of PAH-targeted medication in Ecuador is still limited (see Table 1), around 50% of the patients with PVD included in this study empirically received calcium channel blockers. However, considering the severely compromised haemodynamics in the present PVD cohort with a mean sPAP of 68 mmHg with therapy, the vast majority of the presently investigated patients with PVD are very unlikely to be vasoreactive.
We identified 13 (46%) responders to oxygen therapy defined by a decrease in sPAP ≥10 mmHg. None of the demographic, RHC, or echocardiographic data at baseline differed between responders and non-responders. Furthermore, neither medical therapy nor nocturnal oxygen therapy did influence the responsiveness to oxygen therapy. Only SpO2 while breathing ambient air at 2850 m was shown to be a useful predictor for sPAP responsiveness to oxygen. An SpO2 of 88% with ambient air was identified as an optimal cut-off for the highest diagnostic accuracy to identify a responder (Figure 3). When a patient had a baseline SpO2 of <88%, the chance to be a responder to oxygen therapy was around 22 times higher compared with patients with a baseline SpO2 ≥ 88%, in 95% of the times. With an AUC of 0.81, SpO2 represents a quite accurate, simple, and cheap test that might help decision-making, especially in countries with limited medical infrastructure. However, whether improving haemodynamics by supplemental oxygen in these patients with PVD would translate into improved patient-reported outcomes, quality of life, exercise capacity, and survival remains to be investigated. In addition, the oxygen dose of 10 L/min was high, so it is unknown whether a lower dose, as usually given in every day practice, would have similar effects.
The increase in PaO2 (ΔPaO2) with oxygen was similar between responders and non-responders. Furthermore, the calculated shunt fraction (Qs/Qt) with 100% oxygen was 14%, which is identical to that found in patients with PVD at 470 m of altitude.15 Thus, undetected cardiac or pulmonary shunts that might confound the responsiveness to oxygen therapy are unlikely.
Recent European guidelines recommend oxygen therapy only for hypoxaemic patients with PVD at rest.1 In the cohort of patients with PVD who live at high altitude, we found that the haemodynamic response to oxygen is dependent on the SpO2 with ambient air.
High-dose oxygen therapy was previously shown to increase exercise capacity in patients with PVD.8,19 However, whether lower doses of oxygen as applied via nasal cannula in everyday practice with up to 4 L/min would be similarly beneficial to improve exercise performance remains to be elucidated. Nocturnal or domiciliary oxygen therapy, which is comparatively easy to apply, has been shown to improve daytime performance even when patients are breathing ambient air during the day.7,9 Nevertheless, long-term effects or potential benefits for patients with PVD have not been investigated so far.
Limitations
The dose of oxygen chosen for this study on haemodynamics (10 L/min) via rebreathing facial mask was high, and thus, we do not know the haemodynamic effects of lower oxygen doses as used at home, during nights, or ambulatory in hypoxaemic patients with PVD. There were 15 patients on nocturnal and one patient on 24-h oxygen therapy, which might have influenced the chemosensitivity to high-dose oxygen therapy. However, oxygen therapy at baseline had no statistically significant effect on the responsiveness to oxygen therapy within the logistic regression analysis. The sample size of our trial was rather small; however, in a crossover trial, it is possible to reduce the sample size by 50–75% without losing power. Therefore, the sample size of 28 patients was considerable, given the relatively rare collective with predominantly typical phenotype of patients with PVD, and we did not have a carry-over or period effect. This phenotype is characterized by low age, female predominance, and severe haemodynamic compromise, all of whom live at high altitude. Whether these results are applicable to different populations living at varying altitudes is uncertain and requires further investigation. The investigators were not blinded to the study intervention.
Conclusions
High-dose oxygen therapy in prevalent patients with PVD, predominantly PAH, who permanently live at high altitude near 2850 m, significantly lowered sPAP and reduced CO, resulting in an unchanged pulmonary resistance. This indicates oxygen-induced pulmonary vasodilatation, and along with the improved right ventricular–arterial coupling, it suggests a reduced load on the right heart with supplemental oxygen. Forty-six per cent of patients responded to oxygen therapy (sPAP at least −10 mmHg), and a SpO2 cut-off of 88% was an accurate, simple, and inexpensive test to predict responders to high-dose oxygen therapy. Whether longer-term oxygen therapy given to patients with PVD living at higher altitude would decrease pulmonary vascular resistance remains to be studied.
Supplementary Material
Acknowledgements
All authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors contributed to the production of the final manuscript with revision for important intellectual content.
Contributor Information
Julian Müller, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Faculty of Medicine, University of Zurich, Pestalozzistrasse 3, 8032 Zurich, Switzerland.
Mona Lichtblau, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Faculty of Medicine, University of Zurich, Pestalozzistrasse 3, 8032 Zurich, Switzerland.
Stéphanie Saxer, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Department of Health, Eastern Swiss University of Applied Sciences, Rosenbergstrasse 59, 9000 St. Gallen, Switzerland.
Mirjam Schmucki, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Faculty of Medicine, University of Zurich, Pestalozzistrasse 3, 8032 Zurich, Switzerland.
Michael Furian, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Simon R Schneider, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Joël J Herzig, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Meret Bauer, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Diego Saragoni, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Faculty of Medicine, University of Zurich, Pestalozzistrasse 3, 8032 Zurich, Switzerland.
Esther I Schwarz, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Faculty of Medicine, University of Zurich, Pestalozzistrasse 3, 8032 Zurich, Switzerland.
Elizabeth Cajamarca, Pneumology Unit, Carlos Andrade Marín Hospital, Av. Universitaria, 170103 Quito, Ecuador.
Rodrigo Hoyos, Pneumology Unit, Carlos Andrade Marín Hospital, Av. Universitaria, 170103 Quito, Ecuador.
Silvia Ulrich, Department of Pulmonology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Faculty of Medicine, University of Zurich, Pestalozzistrasse 3, 8032 Zurich, Switzerland.
Data availability
The data from this study will be available from the corresponding author upon reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
The trial was publicly funded by the Swiss National Science Foundation (SNSF, grant number 219199).
References
- 1. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S; ESC/ERS Scientific Document Group . 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–3731. [DOI] [PubMed] [Google Scholar]
- 2. Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ 2021;11:2045894020977300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lichtblau M, Saxer S, Furian M, Mayer L, Bader PR, Scheiwiller PM, Mademilov M, Sheraliev U, Tanner FC, Sooronbaev TM, Bloch KE, Ulrich S. Cardiac function and pulmonary hypertension in Central Asian highlanders at 3250 m. Eur Respir J 2020;56:1902474. [DOI] [PubMed] [Google Scholar]
- 4. Lichtblau M, Bader PR, Carta AF, Furian M, Muralt L, Saxer S, Hartmann SE, Rawling JM, Poulin MJ, Bloch KE, Ulrich S. Extravascular lung water and cardiac function assessed by echocardiography in healthy lowlanders during repeated very high-altitude exposure. Int J Cardiol 2021;332:166–174. [DOI] [PubMed] [Google Scholar]
- 5. Soria R, Egger M, Scherrer U, Bender N, Rimoldi SF. Pulmonary artery pressure and arterial oxygen saturation in people living at high or low altitude: systematic review and meta-analysis. J Appl Physiol (1985) 2016;121:1151–1159. [DOI] [PubMed] [Google Scholar]
- 6. Hasan B, Hansmann G, Budts W, Heath A, Hoodbhoy Z, Jing Z-C, Koestenberger M, Meinel K, Mocumbi AO, Radchenko GD, Sallmon H, Sliwa K, Kumar RK. Challenges and special aspects of pulmonary hypertension in middle- to low-income regions: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:2463–2477. [DOI] [PubMed] [Google Scholar]
- 7. Ulrich S, Saxer S, Hasler ED, Schwarz EI, Schneider SR, Furian M, Bader PR, Lichtblau M, Bloch KE. Effect of domiciliary oxygen therapy on exercise capacity and quality of life in patients with pulmonary arterial or chronic thromboembolic pulmonary hypertension: a randomised, placebo-controlled trial. Eur Respir J 2019;54:1900276. [DOI] [PubMed] [Google Scholar]
- 8. Müller J, Lichtblau M, Saxer S, Schneider SR, Appenzeller P, Bauer M, Hasler ED, Schwarz EI, Bloch KE, Ulrich S. Hyperoxia improves exercise capacity in cardiopulmonary disease: a series of randomised controlled trials. ERJ Open Res 2023;9:00563–02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ulrich S, Keusch S, Hildenbrand FF, Lo Cascio C, Huber LC, Tanner FC, Speich R, Bloch KE. Effect of nocturnal oxygen and acetazolamide on exercise performance in patients with pre-capillary pulmonary hypertension and sleep-disturbed breathing: randomized, double-blind, cross-over trial. Eur Heart J 2015;36:615–623. [DOI] [PubMed] [Google Scholar]
- 10. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 11. Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health 2001;25:464–469. [PubMed] [Google Scholar]
- 12. Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ 2019:l4378. 10.1136/bmj.l4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, Rainisio M, Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002;40:780–788. [DOI] [PubMed] [Google Scholar]
- 14. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension. Circulation 2010;122:164–172. [DOI] [PubMed] [Google Scholar]
- 15. Carta AF, Lichtblau M, Berlier C, , Saxer S, Schneider SR, Schwarz EI, Furian M, Bloch KE, Ulrich S. The impact of breathing hypoxic gas and oxygen on pulmonary hemodynamics in patients with pulmonary hypertension. Front Med (Lausanne) 2022;9:791423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Mohajerani E, Seeger W, Herberg U, Sommer N, Gall H, Richter MJ. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging 2019;12:e009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naeije R, Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev 2014;23:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smit B, Smulders YM, van der Wouden JC, Oudemans-van Straaten HM, Spoelstra-de Man AME. Hemodynamic effects of acute hyperoxia: systematic review and meta-analysis. Critical Care 2018;22:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ulrich S, Hasler ED, Saxer S, Furian M, Müller-Mottet S, Keusch S, Bloch KE. Effect of breathing oxygen-enriched air on exercise performance in patients with precapillary pulmonary hypertension: randomized, sham-controlled cross-over trial. Eur Heart J 2017;38:1159–1168. [DOI] [PubMed] [Google Scholar]
- 20. Hoeper MM, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M, McLaughlin VV, Preston IR, Souza R, Waxman AB, Grünig E, Kopeć G, Meyer G, Olsson KM, Rosenkranz S, Xu Y, Miller B, Fowler M, Butler J, Koglin J, de Oliveira Pena J, Humbert M. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med 2023;388:1478–1490. [DOI] [PubMed] [Google Scholar]
- 21. Souza R, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M, McLaughlin VV, Preston IR, Waxman AB, Grünig E, Kopeć G, Meyer G, Olsson KM, Rosenkranz S, Lin J, Johnson-Levonas AO, de Oliveira Pena J, Humbert M, Hoeper MM. Effects of sotatercept on haemodynamics and right heart function: analysis of the STELLAR trial. Eur Respir J 2023;62:2301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schneider SR, Mayer LC, Lichtblau M, Berlier C, Schwarz EI, Saxer S, Furian M, Bloch KE, Ulrich S. Effect of normobaric hypoxia on exercise performance in pulmonary hypertension: randomized trial. Chest 2021;159:757–771. [DOI] [PubMed] [Google Scholar]
- 23. Gerhardt F, Fiessler E, Olsson KM, Kayser MZ, Kovacs G, Gall H, Ghofrani HA, Badr Eslam R, Lang IM, Benjamin N, Grünig E, Halank M, Lange TJ, Ulrich S, Leuchte H, Held M, Klose H, Ewert R, Wilkens H, Pizarro C, Skowasch D, Wissmüller M, Hellmich M, Olschewski H, Hoeper MM, Rosenkranz S. Positive vasoreactivity testing in pulmonary arterial hypertension: therapeutic consequences, treatment patterns, and outcomes in the modern management era. Circulation 2024;149:1549–1564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study will be available from the corresponding author upon reasonable request.