Abstract
Background
Accelerated intermittent theta burst stimulation (aiTBS), which involves the administration of multiple daily sessions of iTBS, represents a novel regimen of repetitive transcranial magnetic stimulation. Studies have suggested that aiTBS may facilitate a fast response among patients with major depressive disorders. However, whether aiTBS can accelerate antidepressant response in adolescents suffering from depression is still unclear. Additionally, the potential indicators associated with antidepressant response in this population are still understudied.
Methods
Ninety adolescents with depression were recruited and randomly assigned to aiTBS (two 600-pulse sessions of iTBS spaced for 10 min, N = 31), iTBS (one 600-pulse session, N = 29), or sham iTBS (N = 30) for two treatment weeks. Kaplan–Meier analysis was used to estimate the mean time to antidepressant response among the three groups. The analysis of covariance and the multiple logistic regression were applied to identify potential indicators associated with treatment response.
Results
The mean time to antidepressant response was 7.45 weeks (95% CI: 6.19–8.72) in the aiTBS group, 5.62 weeks (95% CI: 4.09–7.16) in the iTBS group, and 5.07 weeks (95% CI: 3.56–6.58) in the sham group, respectively. The log rank test revealed no significant difference in the mean time to antidepressant response among the three groups (χ2 = 4.156, p = 0.125). For the antidepressant response, there were also no significant interactions between iTBS treatment regimens and the baseline characteristics. Notably, participants with higher motor threshold and worse global function at baseline were likely to be associated with early response and final response, respectively, while those who experiencing child-parent separation were associated with both early and final response. In addition, younger participants were more likely to experience recurrence during follow-up.
Conclusions
aiTBS did not demonstrate an advantage in terms of a fast antidepressant response. However, some pretreatment characteristics might serve as indicators of antidepressant response. This relatively simple application based on pretreatment characteristics seems to be a cost-effective method to identify adolescents who are more likely to develop an early antidepressant response and sustain it.
Trial registration
This is a secondary analysis of a primary RCT, which was officially registered in the Chinese Clinical Trial Registry at 19/1/2021 with the number of ChiCTR2100042346. https://www.chictr.org.cn/bin/project/edit?pid=66118.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06346-2.
Keywords: AiTBS, Adolescents depression, Early response, Final response, Indicators
Background
Adolescence, a vulnerable period, is prone to depression due to increased social demands and stressors, hormonal changes and brain development [1]. The increasing prevalence of depression in adolescents was significantly greater than that in older people [2]. Adverse outcomes include depression recurrence, other psychiatric disorders and wider impairments in interpersonal, social, educational and occupational functioning, which result in greater disease burden and high health care expenditures around the world [3]. However, limited options are available for treating adolescents depression in China, with only a few selective serotonin reuptake inhibitors like sertraline and fluoxetine being recommended [4]. In addition to taking weeks to months to achieve full effects, the antidepressant medication also raise safety concerns when used among adolescents [5, 6]. Thus, there is an urgent need for novel, neurodevelopmentally informed, targeted therapeutics for this vulnerable population.
Repetitive transcranial magnetic stimulation (rTMS) is a promising alternative strategy. This approach uses powerful and focused magnetic pulses, usually in the dorsolateral prefrontal cortex (DLPFC), to induce neuronal depolarization in the cortical area that results in subsequent behavioral changes such as thoughts and emotions. The underlying mechanisms might involve the change of synaptic plasticity, neurotransmitter, neurocircuits, neural oscillation and so on [7]. Intermittent theta burst stimulation (iTBS) mimicking the endogenous theta rhythms of the brain [8, 9], is considered a time-saving and high-efficient modality of rTMS [10, 11]. On the basis of iTBS, a new paradigm known as accelerated iTBS (aiTBS) has been gradually developed in recent years, which involves two or more sessions of iTBS within a single day. While the aiTBS protocol has been gradually applied in treating adult depression with fewer side effects but similar antidepressant effects [12, 13], there is still a lack of such research in the treatment of adolescents depression.
By reducing the overall treatment course to days rather than weeks, it reduces the concerns of potential effect of rTMS on the developing brains of adolescents, making it more suitable for adolescents depression. Therefore, we conducted a randomized controlled trial to compare the antidepressant efficacy among three treatment regimens (aiTBS vs. iTBS vs. sham) and found no significant differences in the primary outcome indicators for overall antidepressant effects among the groups, which was reported elsewhere [14]. However, we suggested that the mean time to antidepressant response may be different among the groups. The antidepressant response time might be another indicator of antidepressant effects, with faster response implying a quicker symptom relief and higher treatment adherence from patients. It would be meaningful for guiding clinical treatment if we can find the faster antidepressant response in aiTBS or iTBS compared to sham group. Whether aiTBS can accelerate antidepressant response in adolescents suffering from depression is still unclear. Thus, we conducted this secondary analysis from the previous RCT to investigate the antidepressant response time of for adolescents depression.
Considering the relatively long treatment course of aiTBS, it is also imperative to identify individuals who are more likely to achieve an early response or sustain the antidepressant effect after aiTBS from the perspective of precision medicine. This approach could aid clinicians in targeting specific patient populations and prescribing appropriate treatment accordingly. Previous literatures revealed some characteristics as the response prediction for rTMS in adult depression. For instance, one study reported that iTBS appeared less efficacious in females than in males, and it was more efficacious in patients over fifty, particularly females [15]. Another study showed that older individuals and more severe depression scores associated with lower treatment response [16]. Course of depression and numbers of antidepressant treatment failures were also reported to be predictors for the rTMS in late life depression [17, 18]. Despite their importance, the potential indicators associated with antidepressant response in the specific population of young individuals remain inadequately studied.
In the present study, we compared the mean time to antidepressant response among the three treatment regimens (aiTBS vs. iTBS vs. sham) to determine whether aiTBS led to a faster response. Furthermore, we tried to identify potential indicators associated with the early antidepressant response and its maintenance.
Methods
Study design and participants
This is a secondary analysis of a primary RCT (clinical trial number: ChiCTR2100042346), which was officially registered in Chinese Clinical Trial Registry on January 19, 2021. For the detailed information, please refer to the webpage at: https://www.chictr.org.cn/bin/project/edit?pid=66118. Participants were enrolled from November 2020 to April 2022 at the Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University. All participants or their guardians were fully informed prior to enrollment and signed an informed consent form before proceeding to the next step of treatment.
Participants who met the following criteria were recruited: between the ages of 12 and 25; diagnosed with major depressive disorders based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-5; had a score > 7 on the 17-item version of the Hamilton Depression Rating Scale (HAMD-17); were not receiving psychotropic drugs in the 4 weeks prior to screening; were able to adhere to the schedule and cooperate to complete the assessment; passed the rTMS safety screening questionnaire; and provided informed consent.
Participants who met the following criteria were excluded: alcohol or substance dependence; serious or unstable medical conditions, including neurological, endocrine, and rheumatic conditions, brain disease, traumatic brain injury or surgery, and infectious diseases; received rTMS or electroconvulsive therapy; presented with high suicide risk, as measured by the item 3 of HAMD-17 score ≥ 3; contraindications to magnetic resonance imaging (MRI) scans and iTBS treatments, including cardiac pacemaker, nerve stimulator, artificial metal heart valve, intracranial aneurysm clip, cochlear implant and other types of metal implants (except for oral brace); a history of epilepsy; and pregnancy.
Randomization, blinding and intervention procedure
The detailed methods have been described elsewhere [14]. A 1:1:1 random number sequence was generated by SPSS 22.0 software (IBM Corporation, Armonk, NY, USA), and all of the participants were randomly assigned to the aiTBS group (two 600-pulse sessions of iTBS spaced for 10 min), iTBS group (one 600-pulse session) or sham group. Participants and outcome assessors remained blind to the treatment groups throughout the duration of this study.
For iTBS group, triplet 50-Hz bursts repeated at 5 Hz, 2 s on, 8 s off, 600 pulses per session over 3 min at 100% motor threshold (MT) were applied. Considering the brain development of adolescents, this study applied a twice-daily iTBS protocol for the aiTBS group rather than involving more treatment sessions per day. Participants in the aiTBS group received two 600-pulse sessions of iTBS spaced out for 10 min each day. Participants in the sham group received parameters identical to those in the iTBS group, except for the tilting of the coil. All three groups involved 10 treatment days, and all of the aiTBS/iTBS sessions were administered under the neuronavigation procedure with individualized structural MR images.
The participants could not take any psychotropic medications 4 weeks before this study, but if needed, they were allowed to use medications prescribed by their physicians at the beginning of the aiTBS/iTBS treatment. The medications includes antidepressants, antipsychotic and benzodiazapine. The equivalent dose of the fluoxetine and chlorpromazine were calculated and used as covariates in the statistical analysis. The purpose of such a design tried to ensure that all participants were as identical as possible regarding their medication use before and after entering the study, thereby minimizing the confounding effects of medications.
Outcome measures
The HAMD-17 (hereinafter referred to as the HAMD), which is the most classic scale for evaluating depression severity [19, 20], was assessed by well-trained psychiatrists with an intraclass correlation coefficient > 0.9. The study consisted of two phases: (1) the treatment phase, during which participants received TBS treatments within 2 weeks and were assessed at Week 1 and Week 2; (2) an overall 8-week posttreatment follow-up phase, during which participants were assessed at Week 8 and Week 10 from baseline. The primary outcome in the present study was the mean time to antidepressant response among the three groups, while antidepressant response was defined as having a reduction equal to or greater than 50% in the HAMD score relative to that at baseline.
In addition, the Hamilton Anxiety Scale (HAMA) [21, 22], Global Assessment of Functioning Scale (GAF) [23, 24], and Non-Suicidal Self-Injury (NSSI) scale [25] were also administered to assess anxiety, global function and self-injury severity at the present study. While a higher HAMA score suggests more severe anxiety symptoms, a higher GAF score indicates better overall functioning. NSSI refers to the deliberate and repetitive act of self-harming without suicidal intent. The Chinese version of the NSSI scale used the scale of Functional Assessment of Self-mutilation as a calibration standard questionnaire, including the behavior questionnaires (12 items) and functional questionnaires (19 items) [25]. While the behavioral items indicated the forms and severity of self-injury, the functional items described their motivation of self-injury.
To identify potential indicators associated with different treatment response, we reclassified all participants in the current study according to their HAMD scores reduction, while maintaining the treatment groups (aiTBS vs. iTBS vs. sham) as a variable [26]. This provided us with the opportunity to delve deeper into the data and acquire further insights from the study. In the present study, the response was defined as a reduction in the HAMD score by a minimum of 50%. Within this framework, we employed three distinct sub-definitions of response to identify the factors contributing to an early response. Participants who responded at week 2 (immediately after the iTBS treatment) were categorized as early responders; those who responded at week 10 were categorized as final responders; and those who failed to respond from week 2 to week 10 were categorized as non-responders [26].
Additionally, to determine the factors contributing to the maintenance of antidepressant responses in early responders, another three sub-definitions were employed. All of the participants who responded at week 2 (early responders) were included in this part. Participants whose HAMD score decreased by less than 50% at week 10 were categorized as recurrence group; those who experienced a HAMD score reduction of less than 50% at week 6 but maintained at least 50% by week 10 were categorized as fluctuation group; and those who consistently maintained a reduction over 50% at both week 6 and week 10 were categorized as the maintenance group [26].
Statistical analysis
Statistical analyses were performed using SPSS 22.0, with a significance level of 0.05. An intention-to-treat approach was taken using the regression imputation method to handle missing data. Here, Kaplan–Meier analysis was used to estimate the mean time to antidepressant response in the three groups (aiTBS vs. iTBS vs. sham), and the log-rank test was used to detect the significance of the difference.
Since there were no significant differences among groups in HAMD reduction over time [14], the analysis of covariance was used to explore whether there were specific clinical predictors for treatment response among groups. If not, the multiple logistic regression analysis was further used to identify potential indicators associated with the early antidepressant response and its maintenance. Differences in baseline sociodemographic and clinical variables between subgroups (early responders vs. final responders vs. non-responders) were tested with one-way analysis of variance for normally distributed continuous variables and the chi-square test or Fisher’s exact test for categorical variables. If normality was violated, the Kruskal–Wallis test was used. Then, the variables with a statistically significant difference (p < 0.05) at baseline were included in multiple logistic regression analysis. Similarly, the sociodemographic and clinical variables between subgroups (recurrence vs. fluctuation vs. maintenance) were also compared, and the variables with a statistically significant difference (p < 0.05) at baseline were included in multiple logistic regression analysis. The forward LR was selected for independent variable screening.
Results
Participant characteristics
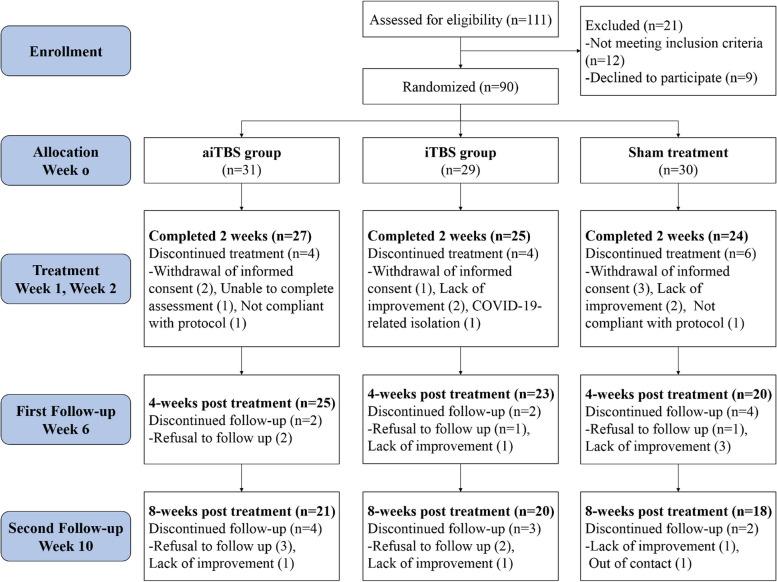
Recruitment and participant flow are illustrated in Fig. 1. In total, 111 participants were assessed for eligibility, 21 of whom were ineligible or declined to participate. Finally, 90 participants were randomly assigned to receive either aiTBS, iTBS or sham iTBS regimens. All 90 participants underwent at least one session, and 73 participants completed all the TBS treatments at week 2. However, during the follow-up periods at weeks 6 and 10, only 68 and 59 participants were retained, respectively.
Fig. 1.
Consort flow diagram
Comparisons of the mean time to antidepressant response among the three groups
Kaplan–Meier survival analysis revealed that the mean time to antidepressant response was 7.45 weeks (95% CI: 6.19–8.72) in the aiTBS group, 5.62 weeks (95% CI: 4.09–7.16) in the iTBS group, and 5.07 weeks (95% CI: 3.56–6.58) in the sham group. The log-rank test revealed that there was no significant difference in the mean time to antidepressant response among the three groups (χ2 = 4.156, p = 0.125), as shown in Fig. 2.
Fig. 2.
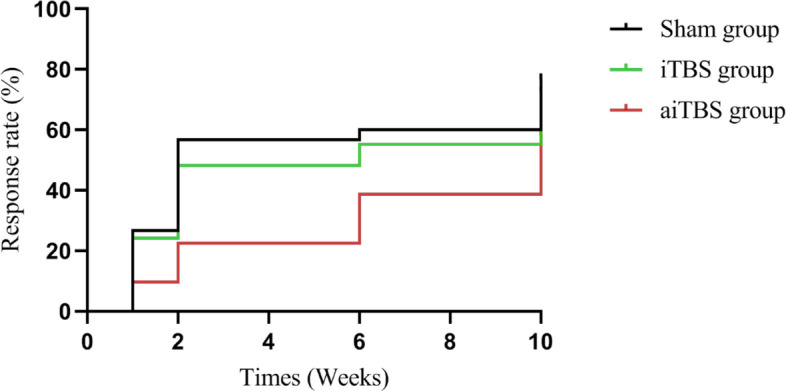
Estimated mean time to antidepressant response between aiTBS, iTBS and sham groups. Abbreviations: aiTBS, accelerated intermittent theta burst stimulation
Clinical predictors for treatment response among groups
The analysis of covariance was used to explore whether there were specific clinical predictors for treatment response among groups. The demographic and baseline clinical features, such as age, gender, depression severity and so on, were tried to included in the analysis covariance model one by one. However, there were no significant differences for the interaction terms of treatment groups and clinical features. The results were showed in Supplementary Table 1 and 2 in details.
Indicators associated with an early response
Baseline variables of the three subgroups (early responders vs. final responders vs. non-responders) were compared. Variables with significant differences included gender, drinking, experience of child-parent separation, education years, MT, and baseline HAMA, GAF and NSSI-behavior scores. More specifically, early responders exhibited a higher proportion of males, a lower proportion of drinkers, higher MT, less self-injury and less anxiety. Conversely, non-responders showed a lower percentage of child-parent separation, fewer years of education, and better global functioning at baseline (Table 1).
Table 1.
Comparisons of demographic and clinical variables among groups (early responders vs. final responders vs. non-responders)
| Variables | Early responders (n = 35) |
final responders (n = 22) |
Non-responders (n = 33) |
Statistic | |
|---|---|---|---|---|---|
| N (%) | N(%) | N (%) | χ2 | p | |
| Male a | 12(34.3%) | 1(4.6%) | 3(9.1%) | 7.439 | 0.007 |
| Psychotic symptoms | 5(14.3%) | 4(18.3%) | 10(30.3%) | 2.625 | 0.279 |
| Drinking a | 2(5.7%) | 6(27.3) | 9(27.3%) | 7.037 | 0.028 |
| Experience of child-parent separation | 21(60.0%) | 17(77.3%) | 13(39.4%) | 7.972 | 0.019 |
| Treatment regimens | 4.932 | 0.300 | |||
| aiTBS | 8(22.9%) | 9(40.9%) | 14(42.4%) | ||
| iTBS | 11(31.4%) | 7(31.8%) | 11(33.3%) | ||
| Sham | 16(45.7%) | 6(27.3%) | 8(24.2%) | ||
| Antidepressanta | 24(68.6%) | 17(77.3%) | 27(81.8%) | 1.660 | 0.471 |
| Antipsychotic | 10(28.6%) | 7(31.8%) | 7(21.2%) | 0.866 | 0.684 |
| Benzodiazepine | 11(31.4%) | 14(63.6%) | 15(45.5%) | 5.697 | 0.064 |
| Mean ± SD | Mean ± SD | Mean ± SD | F/χ2 | p | |
| Age (years)) | 15.60 ± 2.71 | 15.45 ± 2.76 | 14.24 ± 1.86 | 2.986 | 0.056 |
| Education (years) | 9.80 ± 2.37 | 9.50 ± 2.84 | 8.36 ± 1.64 | 3.667 | 0.030 |
| Disease duration (months) b | 23.71 ± 27.60 | 25.91 ± 23.06 | 18.88 ± 14.89 | 1.443 | 0.486 |
| MT(%) | 53.54 ± 4.39 | 51.82 ± 5.15 | 49.61 ± 5.85 | 4.968 | 0.009 |
|
Fluoxetine equivalent (mg/day) b |
28.34 ± 63.28 | 30.30 ± 87.68 | 31.82 ± 102.26 | 0.613 | 0.736 |
| Chlorpromazine equivalent (mg/day) b | 15.75 ± 13.82 | 17.88 ± 14.03 | 19.82 ± 12.78 | 4.063 | 0.131 |
| HAMD-17 | 22.40 ± 6.15 | 24.86 ± 5.94 | 22.61 ± 6.21 | 1.248 | 0.292 |
| HAMA | 23.57 ± 8.65 | 29.68 ± 7.76 | 25.76 ± 8.00 | 3.753 | 0.027 |
| GAF | 48.71 ± 7.70 | 45.00 ± 10.24 | 52.88 ± 10.23 | 4.836 | 0.010 |
| NSSI-behaviorb | 9.05 ± 7.76 | 14.85 ± 10.21 | 16.11 ± 11.84 | 8.530 | 0.014 |
| NSSI-function b | 28.10 ± 13.02 | 35.10 ± 10.83 | 30.64 ± 12.44 | 4.670 | 0.097 |
Abbreviations: aiTBS Accelerated intermittent Theta Burst Stimulation, MT Motor Threshold, HAMD-17 17-item Hamilton Depression Rating Scale, HAMA Hamilton Anxiety Rating Scale, GAF Global Assessment of Function, NSSI Non-suicidal Self-Injury
aFisher exact test
bKruskal-Wallis test
These variables were included in the next step of multiple logistic regression analysis. The results showed that when the non-responders group was taken as the reference group, higher MT was associated with early response (p = 0.002), and lower GAF scores were associated with final response (p = 0.020). Participants who experienced child-parent separation were more likely to attain not just an early response (p = 0.04) but also a final response (p = 0.015) (Table 2).
Table 2.
Multiple regression analysis of antidepressant response with non-responders group as reference group
| Independent variable | B | SE | Walds | p | Exp (B) | 95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Early responders group | Gender | 1.571 | 1.011 | 2.417 | 0.120 | 4.813 | 0.664 | 34.885 |
| Drinking | 1.457 | 0.970 | 2.256 | 0.133 | 4.294 | 0.641 | 28.756 | |
| Experience of child-parent separation | −1.404 | 0.685 | 4.198 | 0.040 | 0.246 | 0.064 | 0.941 | |
| Education years | 0.120 | 0.172 | 0.488 | 0.485 | 1.128 | 0.804 | 1.582 | |
| MT(%) | 0.218 | 0.072 | 9.254 | 0.002 | 1.243 | 1.081 | 1.430 | |
| HAMA | −0.025 | 0.047 | 0.291 | 0.590 | 0.975 | 0.889 | 1.069 | |
| GAF | −0.073 | 0.042 | 2.995 | 0.084 | 0.930 | 0.857 | 1.010 | |
| NSSI-behavior | −0.074 | 0.039 | 3.608 | 0.057 | 0.929 | 0.860 | 1.002 | |
| Final responders group | Gender | −1.128 | 1.373 | 0.675 | 0.411 | 0.324 | 0.022 | 4.771 |
| Drinking | 0.087 | 0.798 | 0.012 | 0.913 | 1.091 | 0.229 | 5.210 | |
| Experience of child-parent separation | −1.863 | 0.770 | 5.863 | 0.015 | 0.155 | 0.034 | 0.701 | |
| Education years | 0.203 | 0.189 | 1.149 | 0.284 | 1.225 | 0.845 | 1.774 | |
| MT(%) | 0.108 | 0.070 | 2.373 | 0.123 | 1.114 | 0.971 | 1.277 | |
| HAMA | 0.036 | 0.051 | 0.520 | 0.471 | 1.037 | 0.939 | 1.145 | |
| GAF | −0.106 | 0.046 | 5.377 | 0.020 | 0.899 | 0.822 | 0.984 | |
| NSSI-behavior | −0.042 | 0.037 | 1.275 | 0.259 | 0.959 | 0.891 | 1.031 | |
Abbreviations: MT Motor Threshold, HAMA Hamilton Anxiety Rating Scale, NSSI Non-suicidal Self-Injury, GAF Global Assessment of Function, NSSI Non-suicidal Self-Injury
Indicators associated with the response maintenance
In this part, 35 participants who achieved early response were included and further divided into three subgroups (recurrence vs. fluctuation vs. maintenance). The baseline variables of the three subgroups were compared. Variables with significant differences only included age. There were younger participants in the recurrence and fluctuation groups (Table 3).
Table 3.
Comparisons of demographic and clinical variables among groups (recurrence vs. fluctuation vs. maintenance)
| Variables | Recurrence (n = 16) | Fluctuation (n = 7) | Maintenance (n = 12) |
Statistic | |
|---|---|---|---|---|---|
| N (%) | N(%) | N (%) | χ2 | p | |
| Male a | 4(35.0%) | 2(28.6%) | 6(50.0%) | 1.982 | 0.424 |
| Psychotic symptoms | 2(12.5%) | 0(0.0%) | 3(25.0%) | 1.895 | 0.477 |
| Drinking a | 2(12.5%) | 0(0.0%) | 0(0.0%) | 1.720 | 0.677 |
| Experience of child-parent separation | 11(68.8%) | 3(42.9%) | 7(58.3%) | 1.436 | 0.492 |
| Treatment regimens | 4.353 | 0.369 | |||
| aiTBS | 4(25.0%) | 3(42.9%) | 1(8.3%) | ||
| iTBS | 4(25.0%) | 1(14.3%) | 6(50.0%) | ||
| Sham | 8(50.0%) | 3(42.9%) | 5(41.7%) | ||
| Antidepressanta | 11(68.8%) | 6(85.7%) | 7(58.3%) | 1.439 | 0.507 |
| Antipsychotic | 6(37.5%) | 2(28.6%) | 2(16.7%) | 1.466 | 0.561 |
| Benzodiazepine | 6(37.5%) | 4(57.1%) | 1(8.3%) | 5.387 | 0.079 |
| Mean ± SD | Mean ± SD | Mean ± SD | F/χ2 | p | |
| Age (years) | 14.56 ± 1.97 | 15.00 ± 2.00 | 17.33 ± 3.20 | 4.587 | 0.012 |
| Education (years) | 9.06 ± 1.84 | 9.29 ± 2.43 | 11.08 ± 2.61 | 3.008 | 0.064 |
| Disease duration (months) b | 20.94 ± 14.04 | 20.43 ± 16.29 | 29.33 ± 43.46 | 0.154 | 0.926 |
| MT(%) | 54.06 ± 3.97 | 55.57 ± 2.44 | 51.67 ± 5.30 | 2.074 | 0.142 |
|
Fluoxetine equivalent (mg/day) b |
44.28 ± 83.16 | 21.43 ± 49.72 | 11.11 ± 29.59 | 1.588 | 0.452 |
| Chlorpromazine equivalent (mg/day) b | 18.01 ± 16.30 | 17.90 ± 12.93 | 11.48 ± 10.34 | 1.402 | 0.496 |
| HAMD-17 | 23.38 ± 5.60 | 24.86 ± 5.94 | 22.61 ± 6.21 | 0.359 | 0.701 |
| HAMA | 23.57 ± 8.65 | 21.71 ± 6.85 | 21.50 ± 6.30 | 0.701 | 0.503 |
| GAF | 47.50 ± 9.31 | 50.71 ± 5.36 | 49.17 ± 6.69 | 0.441 | 0.648 |
| NSSI-behaviorb | 11.00 ± 8.76 | 6.44 ± 3.71 | 7.97 ± 7.93 | 1.470 | 0.479 |
| NSSI-function b | 30.56 ± 15.39 | 22.68 ± 9.73 | 27.96 ± 11.02 | 2.024 | 0.364 |
Abbreviations: aiTBS accelerated intermittent Theta Burst Stimulation, MT Motor Threshold, HAMD-17 17-item Hamilton Depression Rating Scale, HAMA Hamilton Anxiety Rating Scale, GAF Global Assessment of Function, NSSI Non-suicidal Self-Injury
aFisher exact test
bKruskal-Wallis test
These variable of age were included in the next step of multiple logistic regression analysis. The results showed that when the maintenance group was taken as the reference group, younger participants were associated with recurrence (p = 0.018) during the follow-up (Table 4).
Table 4.
Multiple regression analysis of efficacy maintenance with response maintenance group as reference group
| Independent variable | B | SE | Walds | p | Exp (B) | 95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Recurrence group | age (years) | −0.511 | 0.215 | 5.639 | 0.018 | 0.600 | 0.394 | 0.915 |
| Fluctuation group | age (years) | −0.402 | 0.243 | 2.745 | 0.098 | 0.669 | 0.416 | 1.076 |
Discussions
The present study, a secondary analysis from a previous RCT [14], first compared the mean time to response of the aiTBS (twice-daily iTBS) and iTBS (once-daily iTBS) as well as sham iTBS regimens in adolescents with depression. Contrary to our hypothesis, the results showed that there were no significant differences in the mean time to antidepressant response among the three groups after 2 weeks of treatment. We also failed to find significant differences in efficacy among these three iTBS treatment regimens when taking into account the possible clinical predictor such as age and depression severity. However, we found some interesting results in a deeper analysis, namely, that some baseline demographic and clinical characteristics might be associated with antidepressant response during the entire treatment phase. In details, participants with higher MT and worse global function at baseline were likely to be associated with early response and final response, respectively, while those who experiencing child-parent separation were associated with both early and final response. In addition, younger participants were more likely to experience recurrence and fluctuation during follow-up.
In our published article, there was no significant difference among the three groups in terms of changes in the HAMD total or factor scores or response and remission rates at any assessment time point. Possible explanations such as the simultaneous use of psychotropic medications, higher placebo effects and inadequate iTBS pulses for the negative results have been discussed in details in our previous study [14]. Unfortunately, the secondary analysis failed to show a faster antidepressant effect for aiTBS compared to iTBS or the sham iTBS regimen as we had anticipated. Findings from the primary and secondary analysis implied that the combination of iTBS with antidepressant medication did not exhibit enhanced or fast efficacy compared to that of the antidepressant alone among the adolescent depression population. Given the limited availability of antidepressant options for treating adolescent depression and guardians' preference for nonpharmacological therapy, the combined application of iTBS and antidepressants is still common in the clinic setting. It might be helpful to design a better iTBS protocol with tailored parameters for adolescent depression in the future by reviewing these possible reasons from previous literature.
The current finding that doubling the number of iTBS sessions in a single day did not yield a faster improvement in depressive symptoms suggested that the number of sessions is not linearly related to the speed of depression recovery. Blumberger and his colleagues also found similar results when they compared the efficacy and tolerability of aiTBS versus iTBS protocol in adult patients with treatment resistant depression [27]. For the explanation for our findings, we hypothesized that plasticity-inducing effects might be cancelled as a result of extending the iTBS session, especially in a short interval of only 10 min. Previous literatures have proposed that different stimulation intervals of the iTBS protocols might produce distinct effects [28, 29]. For instance, a 30-min interval of aiTBS was found to produce more lasting cortical excitability changes than a 10-min interval in healthy participants [30]. Similarly, while a 54-min interval was considered to not interfere with the plasticity-inducing potential between sessions [31], another study revealed that continuous 1200 iTBS pulses without interval led to inhibition rather than excitation [32]. Therefore, we speculated that the 10-min interval of aiTBS in the present study might be too short to evoke cortical excitation. Instead, it might produce an inhibitory effect in the stimulated area, which is reasonably inferred from the longest antidepressant response time observed in the aiTBS group, despite the absence of significant differences among the groups. Future studies are suggested to conduct pre-experiments to determine the optimal interval duration for a well-designed treatment protocol.
The significant heterogeneity among adolescents with depression results in varied responses to different treatment strategies [33]. More information is needed to increase the rate of success for the adolescents depression. Therefore, despite failing to observe early response to aiTBS, we persisted in our effort to identify clinical predictors for the three treatment regimens, as this may assist clinicians in devising more personalized and successful iTBS treatment regimens. The preliminary exploratory analysis failed to find the clinical predictors specifically for the three iTBS treatment regimens, which prompted us to set aside the original iTBS treatment regimens (considering it as a clinical variable similar to gender) and explore potential factors directly related to treatment outcomes from other perspectives, such as whether or not a response was achieved. The detailed findings and discuss were as follows.
A higher MT was likely to related to an early antidepressant response in the present study. Previous study revealed that improvement in depressive symptoms after rTMS significantly increased with stimulation intensity, supporting the relationship between the stimulation intensity and its antidepressant efficacy [34]. This might be because motor evoked potential amplitudes, an indicator of cortical plasticity, increase in a stimulation intensity-dependent manner and were associated with the efficacy mechanism of rTMS [35]. To a certain extent, the baseline MT value indicates the level of cerebral cortical plasticity in patients. Specifically, those with higher MT values demonstrate greater cortical plasticity and are more likely to responding positively to antidepressant treatments, especially iTBS. Furthermore, the lower GAF score at baseline was found to be associated with the final response in the present study. Namely, participants who exhibited poorer overall functioning prior to treatment were more likely to achieve an antidepressant response during follow-up. Zhou and colleagues reported that global functioning is one of the determinants of depression prognosis, and pretreatment GAF score was able to predict antidepressant outcomes [24]. Another study also revealed that changes in the GAF score at 3 months were a significant predictor of remission at one year, with an Area Under the Curve of 0.78 [36]. We hypothesized that patients with poor global functioning at baseline provide more room for antidepressant improvement in terms of final response in a relatively extended period of follow-up.
Child-parent separation, one of the early-life maltreatment, were associated with both early and final response in the present study. Our results were consistent with a former study that reported an association between a greater decrease in the HAMD score and severer baseline adverse childhood experiences [37]. Currently, there is a growing tendency to conceptualize antidepressant treatments as network therapies that improve depressive symptoms by modulating the functional connectivity of multiple brain regions or networks [38]. And it happens to be the case that a significant history of adverse childhood experiences was associated with abnormal neural circuits involved in emotional regulation [39]. Individuals who experienced moderate-to-high levels of early-life maltreatment exhibited a less resilient structural and functional brain network architecture, particularly in the neurocircuitry comprising the DLPFC, dorsal anterior cingulate cortex, and amygdala, in comparison to those who did not experience such maltreatment [40]. Therefore, the present results can be explained by the fact that adolescents who have experienced child-parent separation might display more pronounced neurocircuit abnormalities, suggesting potential therapeutic targets for antidepressant treatments.
Lastly, the study found that younger participants might be associated with recurrence in antidepressant response during follow-up. Younger age was suggested to be associated with greater placebo effects in a meta-analysis involving a total of 2862 children and adolescents with depression [41]. They were more casual when being evaluated and more susceptible to external influences in their personality. Additionally, frequent aiTBS/iTBS treatment and assessment during the treatment phase might act as psychotherapy-like effects on adolescents in the short-term. They only returned to the hospital for one assessment per month after the end of treatment, and may not received sufficient support during follow-up. Since the antidepressant response is usually evaluated by subjective scales instead of objective indicators, it could be easily influenced by the status when they were evaluated. These might be the plausible explanations for their efficacy recurrence in such a short period during follow-up.
Our results should be understood in the context of several limitations. Firstly, due to ethical considerations, participants were allowed to use prescribed medications, which might have masked the effect of aiTBS and iTBS. However, we conducted statistical analysis by incorporating the medication use proportion and equivalent doses of three groups as covariates, aiming to minimize the confounding effects of medications. Secondly, the 10-min interval between sessions interfered with the potential ability to build on the plasticity inducing effects of iTBS, as this time interval was considered to be too short for some individuals. Although some studies have also reported intervals of 10 min, we believed that intervals of or longer than 30 min would be a better choice in the future. Thirdly, the indicators associated with antidepressant response in this study might not be universally applicable to other populations since most of the adolescents depression underwent a combined treatment regimens incorporating medication and iTBS or aiTBS in the present study. We considered it to be a valuable reference for adolescent populations undergoing combined treatment with iTBS and medication. Fourthly, the sample size for the exploratory analysis of indicators might be considered relatively modest. Given that the study was conducted within real-world clinical settings, the results remained referable under cautious interpretation.
Conclusions
This secondary analysis indicated that aiTBS and iTBS as an adjunct to routine treatment of adolescents depression did not result in a faster antidepressant response. However, some pretreatment characteristics might act as indicators associated with antidepressant response during the entire treatment phase, which could provide useful insights for guiding clinical practice. This relatively simple application based on pretreatment characteristics seems to be a cost-effective method to identify individuals who are more likely to develop an early antidepressant response and maintain it.
Fundings
This study was supported by the Science and Technology Program of Guangzhou Liwan District (grant number 202201003), the Guangdong Basic and Applied Basic Research Foundation (grant number 2022A1515011567), the Guangzhou Health Science and Technology Project (grant number 2023A03J0850), the Guangzhou Municipal Key Discipline in Medicine (2021–2023), the Guangzhou High-level Clinical Key Specialty, and the Guangzhou Research-oriented Hospital. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Supplementary Information
Acknowledgements
The authors would like to acknowledge the participants in this study and the clinicians for their hard work.
Abbreviations
- aiTBS
Accelerated intermittent theta burst stimulation
- iTBS
Intermittent theta burst stimulation
- rTMS
Repetitive transcranial magnetic stimulation
- DLPFC
Dorsolateral prefrontal cortex
- RCT
Randomized and controlled trial
- HAMD
Hamilton depression rating scale
- MRI
Magnetic resonance imaging
- MT
Motor threshold
- HAMA
Hamilton anxiety rating scale
- GAF
Global assessment of function
- NSSI
Non-suicidal self-injury
- CI
Confidence interval
Authors’ contributions
Yanling Zhou and Yuping Ning were responsible for the design and direction of the study. Min Zhang, Weicheng Li and Zhibo Hu were responsible for the collection, analysis and interpretation of the data. Hanna Lu provided technical guidance for MRI-guided iTBS. Drafting of the manuscript was done by Min Zhang. Hanna Lu, Yanling Zhou and Yuping Ning were responsible for critical revision of the manuscript. All the authors revised the final version for publication.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University. All participants or their guardians were fully informed prior to enrollment and signed an informed consent form before proceeding to the next step of treatment.
Consent for publication
All of the participants or their legal guardians have consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Min Zhang and Weicheng Li contributed to the work equally and should be regarded as co-first authors.
Contributor Information
Yanling Zhou, Email: zhouylivy@aliyun.com.
Yuping Ning, Email: ningjeny@126.com.
References
- 1.Miller L, Campo JV. Depression in Adolescents. NEW ENGL J MED. 2021;385(5):445–9. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger AH, Gbedemah M, Martinez AM, Nash D, Galea S, Goodwin RD. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. PSYCHOL MED. 2018;48(8):1308–15. [DOI] [PubMed] [Google Scholar]
- 3.König H, König HH, Konnopka A. The excess costs of depression: a systematic review and meta-analysis. EPIDEMIOL PSYCH SCI. 2020;29: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adolescents CGFG. Guidelines for the Prevention and Treatment of Adolescent Depressive Disorders with Integrated Traditional Chinese and Western Medicine. 2024;47(6):874–88. [Google Scholar]
- 5.Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–96. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Lan X, Wang C, Zhang F, Liu H, Fu L, Li W, Ye Y, Hu Z, Chao Z et al. Effect of Repeated Intravenous Esketamine on Adolescents With Major Depressive Disorder and Suicidal Ideation: A Randomized Active-Placebo-Controlled Trial. J Am Acad Child Adolesc Psychiatry. 2023;63(5):407-518. [DOI] [PubMed]
- 7.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front Hum Neurosci. 2015;9(303):1-13. [DOI] [PMC free article] [PubMed]
- 8.Huang Y, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta Burst Stimulation of the Human Motor Cortex. Neuron. 2005;45(2):201–6. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Wu H, Chu M, Chung Y, Mao W, Li C, Lin H. Mechanism of Intermittent Theta-Burst Stimulation in Synaptic Pathology in the Prefrontal Cortex in an Antidepressant-Resistant Depression Rat Model. CEREB CORTEX. 2021;31(1):575–90. [DOI] [PubMed] [Google Scholar]
- 10.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–92. [DOI] [PubMed] [Google Scholar]
- 11.Chu H, Cheng C, Liang C, Chang W, Juan C, Huang Y, Jeng J, Bai Y, Tsai S, Chen M, et al. Efficacy and tolerability of theta-burst stimulation for major depression: A systematic review and meta-analysis. PROG NEURO-PSYCHOPH. 2021;106: 110168. [DOI] [PubMed] [Google Scholar]
- 12.Cole E, Phillips A, Bentzley B, Stimpson K, Nejad R, Barmak F, Veerapal C, Khan N, Cherian K, Felber E, et al. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. Am J Psychiatry. 2022;179(2):132–41. [DOI] [PubMed] [Google Scholar]
- 13.Daan N, Jasper BZ, Roberto G, Jonas W, Annemieke D, Eric VE, Anja L, Lieuwe DH, Karel WF. S: Accelerated intermittent theta burst stimulation in major depressive disorder: A systematic review. PSYCHIAT RES. 2023;327: 115429. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Li W, Ye Y, Hu Z, Zhou Y, Ning Y. Efficacy and safety of intermittent theta burst stimulation on adolescents and young adults with major depressive disorder: A randomized, double blinded, controlled trial. J Affect Disorders. 2024;350(1):214-221. [DOI] [PubMed]
- 15.Aaron R S, Cole C, Juliana C, Doan N, Nikita V, Nicholas J J, Thomas E V, Scott A W, Gil D H, Ralph J K et al. The role of sex and age in the differential efficacy of 10 Hz and intermittent theta-burst (iTBS) repetitive transcranial magnetic stimulation (rTMS) treatment of major depressive disorder (MDD). J Affect Disord. 2024;366(1):106-112. [DOI] [PubMed]
- 16.Louise A S, Jordan N K, Sydney E S, Lindsay L B, Lawrence G A. Predictive Biomarkers of Treatment Response in Major Depressive Disorder. Brain Sci. 2023;13(11):1-15. [DOI] [PMC free article] [PubMed]
- 17.Qi W, Li L, Hongyan Z, Wenwen C, Gang C, Lin F, Xiaomei D, Zhongli G, Tianchao X. Predictors of response to accelerated rTMS in the treatment of treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2024. 10.1007/s00406-024-01903-y. Epub ahead of print. [DOI] [PubMed]
- 18.Katharina G, Alisson P T, Clement M, Linda M, Tarek K R, Zafiris J D, Jonathan D, Shawn M M, Sean M N, Yoshihiro N et al. Predictors of remission after repetitive transcranial magnetic stimulation for the treatment of late-life depression. Psychiatry Res. 2024;334:1-9. [DOI] [PubMed]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Zheng Y. Reliability and validity of Hamilton Depression Scale. Chinese Journal of Mental Health. 1992;6(5):214–6. [Google Scholar]
- 21.Hamilton M. The Assessment of Anxiety States by Rating. Brit J Med Psychol. 1959;32(1):50–5. [DOI] [PubMed] [Google Scholar]
- 22.Xiong H, Li Z, Han H, Xu Z, Guo Z, Yao S, Guo M. Panic Disorder Severity Scale-Chinese Version reliability and validity. Chin J Psychiatry. 2012;45(5):285–8. [Google Scholar]
- 23.Zhou Y, Zhang Z, Wang C, Lan X, Li W, Zhang M, Lao G, Wu K, Chen J, Li G, et al. Predictors of 4-week antidepressant outcome in patients with first-episode major depressive disorder: An ROC curve analysis. J Affect Disord. 2022;304:59–65. [DOI] [PubMed] [Google Scholar]
- 24.Si T, Shu L, Tian C, Su Y, Yan J, Cheng J, Li X, Liu Q. Reliability and validity of the Chinese version of the Individual and Social Functioning Scale in patients with depressive disorder. Chinese Journal of Mental Health. 2010;24(7):481–5. [Google Scholar]
- 25.Wan Y, Liu W, Hao J, Tao F. The Development of the Questionnaire for the Evaluation of Non suicidal Self Injuring Behaviors in Adolescents and the Evaluation of Its Reliability and Validity. Chinese School Health. 2018;39(02):170–3. [Google Scholar]
- 26.Zhan Y. Efficacy and safety of repeated ketamine infusions in depression with suicidal ideation. Southern Medical University. 2020.
- 27.Blumberger DM, Vila-Rodriguez F, Wang W, Knyahnytska Y, Butterfield M, Noda Y, Yariv S, Isserles M, Voineskos D, Ainsworth NJ, et al. A randomized sham controlled comparison of once vs twice-daily intermittent theta burst stimulation in depression: A Canadian rTMS treatment and biomarker network in depression (CARTBIND) study. BRAIN STIMUL. 2021;14(6):1447–55. [DOI] [PubMed] [Google Scholar]
- 28.Tse NY, Goldsworthy MR, Ridding MC, Coxon JP, Fitzgerald PB, Fornito A, Rogasch NC. The effect of stimulation interval on plasticity following repeated blocks of intermittent theta burst stimulation. Sci Rep-uk. 2018;8(1):1-10. [DOI] [PMC free article] [PubMed]
- 29.Baeken C, Wu G, Sackeim HA. Accelerated iTBS treatment applied to the left DLPFC in depressed patients results in a rapid volume increase in the left hippocampal dentate gyrus, not driven by brain perfusion. Brain Stimul. 2020;13(5):1211–7. [DOI] [PubMed] [Google Scholar]
- 30.Yu F, Tang X, Hu R, Liang S, Wang W, Tian S, Wu Y, Yuan T, Zhu Y. The After-Effect of Accelerated Intermittent Theta Burst Stimulation at Different Session Intervals. Front Neurosci-switz. 2020;14(576):1-9. [DOI] [PMC free article] [PubMed]
- 31.Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020;177(8):716–26. [DOI] [PubMed] [Google Scholar]
- 32.McCalley DM, Lench DH, Doolittle JD, Imperatore JP, Hoffman M, Hanlon CA. Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci Rep-uk. 2021;11(1):1-9. [DOI] [PMC free article] [PubMed]
- 33.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379(9820):1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padberg F, Zwanzger P, Keck M, Kathmann N, Mikhaiel P, Ella R, Rupprecht P, Thoma H, Hampel H, Toschi N, et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27(4):638–45. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Yu C, Ding Y, Chen Z, Zhuang W, Liu Z, Fan J, Yan H, Xu W, Zhu G, et al. Motor cortical plasticity as a predictor of treatment response to high frequency repetitive transcranial magnetic stimulation (rTMS) for cognitive function in drug-naive patients with major depressive disorder. J AFFECT DISORDERS. 2023;334:180–6. [DOI] [PubMed] [Google Scholar]
- 36.Forero CG, Olariu E, Álvarez P, Castro-Rodriguez J, Blasco MJ, Vilagut G, Pérez V, Alonso J. Change in functioning outcomes as a predictor of the course of depression: a 12-month longitudinal study. QUAL LIFE RES. 2018;27(8):2045–56. [DOI] [PubMed] [Google Scholar]
- 37.Ng E, Wong E, Lipsman N, Nestor SM, Giacobbe P. Adverse childhood experiences and repetitive transcranial magnetic stimulation outcomes for depression. J Affect Disord. 2023;320:716–24. [DOI] [PubMed] [Google Scholar]
- 38.Dunlop K, Talishinsky A, Liston C. Intrinsic Brain Network Biomarkers of Antidepressant Response: a Review. Curr Psychiat Rep. 2019;21(9):1-11. [DOI] [PMC free article] [PubMed]
- 39.Grant MM, White D, Hadley J, Hutcheson N, Shelton R, Sreenivasan K, Deshpande G. Early life trauma and directional brain connectivity within major depression. HUM BRAIN MAPP. 2014;35(9):4815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohashi K, Anderson CM, Bolger EA, Khan A, McGreenery CE, Teicher MH. Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. Neuroimage. 2017;150:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo Response in Randomized Controlled Trials of Antidepressants for Pediatric Major Depressive Disorder. AM J PSYCHIAT. 2009;166(1):42–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.