Abstract
Brennnesselwurzel (Urtica dioica L.) is recognized for its diverse pharmacological properties. With a range of chemical constituents, such as vitamins, minerals, phenolic compounds, fibers, and amino acids, Brennnesselwurzel (BWE) has a long history of traditional medicinal use in Europe and Asia. The correlation between a plant's metabolite composition and its activity can vary depending on considerations such as geographic location, environmental conditions, and genetic variations. In the present study, we explore the phytochemical profile and biological activity of the 70% acetone extract of the BWE plant. The chemical profile of the BWE extract was explored using several techniques, including amino acid analyzer, HPLC, GC–MS, and other colorimetric analysis. The antioxidant activity of the BWE extract was assessed by evaluating the total antioxidant, free radical scavenging activity (DPPH, ABTS, H2O2), and metal chelating scavenging activity (FRAP, CUPRAC, metal chelating). Furthermore, we assessed the antimicrobial and antiproliferation activities of the BWE extract against 29 microbial strains and 15 cell lines, respectively. Our phytochemical analyzes revealed that the BWE extract has a unique profile of metabolites including amino acids, flavonoids, phenolics, volatile oils, lipids, and vitamins. The BWE extract showed a total antioxidant capacity of 30.94 ± 1.58 mg GAE/g, together with potential free radical scavenging activity towards ABTS (IC50 = 153.51 ± 3.97 µg/ml), DPPH (IC50 = 195.75 ± 5.91 µg/ml), and H2O2 (IC50 = 230.67 ± 5.98 µg/ml). Although the BWE extract showed no significant antifungal activity, our findings revealed that the BWE extract possesses substantial antibacterial activity against Staphylococcus epidermidi, Streptococcus mutants, Enterococcus faecalis, Micrococcus sp., Klebsiella pneumonia and Porphyromonas gingivalis. Furthermore, the BWE extract demonstrated potential antiproliferative activity toward a panel of cancer cell lines with a high selectivity index. Among the cells examined, the BWE extract exhibited significant cytotoxic activity toward HCT-116, A-549, MDA-MB-231 cells with IC50 of 15.11, 15.32, 15.79 µg/mL, respectively, while it possessed no significant cytotoxic activity towards WI-38 cells (IC50 119.62 µg/mL). Taken together, our findings reveal that BWE extract possesses a wide spectrum of biological activities, including antioxidant, antibacterial, and antitumor activities, and could be considered for further research to explore its potential as a natural plant-based supplement for human diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04709-6.
Keywords: Antibacterial activity, Antifungal activity, Antioxidant activity, Antitumor activity, Brennnesselwurzel, GC–MS analysis, HPLC analysis, Medicinal plants, Phytochemical analysis
Introduction
Drug discovery is a complex and challenging process involving the identification and development of new medications for treating various diseases [1, 2]. Meeting regulatory requirements and obtaining approval from health authorities, such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA), is a crucial and complex step in drug discovery [3–5]. Historically, drugs based on natural products have been used in traditional medicine [6–9]. Currently, these drugs play a remarkable role in drug discovery, as they offer a vast source of chemical diversity, with complex structures and unique biological activities [10]. Natural product-based drugs often exhibit remarkable efficacy, high selectivity, good tolerability, and low toxicity [11–15]. Therefore, natural product-based drugs with potential medicinal benefits continue to be an essential area of research. In this context, medicinal plants have become an important source of bioactive compound identification and have played a vital role in human health and well-being since ancient times [16, 17]. With an estimated 85% of the population in Asia and the Middle East relying on traditional medicine for their primary healthcare needs [18], the exploration of medicinal plants holds significant promise for the discovery of novel drugs and therapeutic interventions including inflammation, hypercholesterolemia, atherosclerosis, endothelial dysfunction, asthma, and cancer progression [19]. Most of the medications used in traditional medicine originate from natural products such as plants, and then clinical trials have been conducted to distinguish valuable plants with beneficial impacts [20]. These plants have been used in several contexts as traditional medicines [21], including Ayurveda, traditional Chinese medicine (TCM), and traditional Indian medicine (TIM) [22, 23]. Nowadays, several pharmaceutical drugs are derived from plant sources or are inspired by plant-based compounds. The discovery and development of new drugs often involve investigating the chemical components of plants and their potential therapeutic applications [24–26]. Medicinal plants produce a wide range of bioactive metabolites, including alkaloids, flavonoids, terpenoids, and phenolics, which contribute to their therapeutic effects [27]. These compounds can have various biological effects, such as anti-inflammatory, antimicrobial, and antioxidant activities [28–31].
Among the vast array of medicinal plants, Brennnesselwurzel (the German term for stinging nettle, Urtica dioica L.) has gained significant recognition for its diverse pharmacological properties [32, 33]. According to previous reports, Urtica dioica has a wide range of chemical constituents that contribute to its pharmacological activities, such as vitamins, minerals, phenolic compounds, fibers, and amino acids [32, 34]. In addition, its leaves are rich in carbohydrates, fats, vitamins, chlorophylls, carotenoids, and minerals [32, 35]. Therefore, stinging nettle (Urtica dioica) has been used extensively for several centuries as a traditional medicine, particularly in Europe and Asia [36]. Urtica dioica has been extensively studied for its antioxidant properties, as it contains flavonoids and phenolic chemicals [35]. The antioxidant defense mechanism protects cells from the harmful effects of reactive oxygen species (ROS), preventing lipid peroxidation and DNA damage [37, 38]. In addition to the antioxidant potential of Urtica dioica, the plant also possesses significant antimicrobial activity against both gram-negative and gram-positive bacteria, including Escherichia coli, Bacillus subtilis, Lactobacillus plantarum, and Pseudomonas aeruginosa [39–41]. Urtica dioica extracts have also shown antifungal activity against different fungal species, especially Candida albicans, which is considered a common fungal pathogen responsible for yeast infections. Urtica dioica exhibits various bioactive metabolites, including flavonoids, lectins, and polysaccharides, which may interfere with the integrity of the fungal cell membrane by changing the permeability and fluidity of the cell membrane, which can lead to leakage of intracellular contents [36, 42, 43]. Furthermore, several studies have reported the cytotoxic and antiproliferative activities of Urtica dioica extracts against various cancer cell lines such as prostate and breast cancers, suggesting its potential as a natural anticancer agent. Despite that, the potential anticancer activity of Urtica dioica is an area of ongoing research, and the exact mode of action is not yet fully understood. The reported studies suggested that the antioxidant activity of Urtica dioica extracts, which is attributed to its bioactive compounds such as flavonoids, phenolic compounds, and polysaccharides, may help protect cells from oxidative damage and potentially inhibit tumor growth [44–46].
The correlation between metabolite composition and activity of a plant can vary depending on factors such as geographic location, environmental conditions, and genetic variations. For example, different populations of the same plant species, such as Urtica dioica, found in different regions can exhibit variations in their metabolite profiles and biological activities [36]. These variations can be attributed to various environmental factors, soil composition, climate, and genetic differences among the populations [47]. These differences can potentially lead to variations in the medicinal properties of the plant, including its antimicrobial, antioxidant, or anticancer effects. Based on these facts and our continuous efforts in discovering novel bioactive agents [48–53], the presented research paper aimed to comprehensively explore the phytochemical composition of the 70% acetone extract of the Brennnesselwurzel plant from Germany. In addition, we intended to gain insight into the antioxidant, antimicrobial, and anticancer potency of the extract. The findings of this research will provide valuable information on the therapeutic potential of Brennnesselwurzel extract in the context of antioxidant supplementation and cancer treatment, which will pave the way for further research and development of natural products derived from this medicinal plant.
Materials and methods
Plant material and extraction
The Brennnesselwurzel plant (Urtica dioica) was acquired from Vita Ideal (Germany). The institutional botanist identified the plants and confirmed them by Dr. Ahmed Elbanhawy, Department of Botany, Suez Canal University, Egypt, as Pulicaria Undulata. At least five representative samples have been vouched and deposited at SCUI herbarium under the accession numbers AE_19812023/10. The acquired plant was pulverized using a mixer mill (Retsch MM400, Germany). The powdered Brennnesselwurzel plant was mixed with a solution containing 70% acetone and deionized water (240 ml) and placed at 28° C for 3 days on a rotary shaker for extraction (Clim-O-Shake system Kuhner IRC-1-U). The resulting mixture was double-filtered using filter paper and then dried with a rotary evaporator to obtain the dry lyophilized extract.
Phytochemical characterization
Assessment of the total primary phytochemical metabolites
The evaluation of total primary phytochemical metabolites was carried out using the reported chemical and colorimetric analysis including the Folin-Ciocalteu assay (total phenolic content) [54], Aluminum chloride colorimetric assay (total flavonoid content) [55], Bromocresol green-based colorimetric assay (total alkaloid content) [56], Conventional assay (total lipid content) [57], Bradford assay (total protein content) [58], Modified Vanillin-Sulphuric Acid assay (total saponin content) [59], Phenol–sulphuric acid assay (total carbohydrate content) [60], and acidified-vanillin assay (total tannin content) [61].
GCMS analysis
Analysis using Thermo Scientific's GC–MS-Orbitrap Q Exactive. A vial containing 4 ml of plant leaf extract or 50 µM standard solutions was spun dry at 39° C using an insert. Twenty ml of methoxyamine (20 mg/mL; 90 min at 37°C) and 30 ml of MSTFA were added to the samples automatically using a preparative robot and left for 30 min at 37 °C. To calculate the retention index, each sample received 5 µl of an alkane mix (3 µg.µl−1) containing 14 alkanes ranging from C9-C36 (Connecticut n-hydrocarbon mix, Supelco). The analysis consisted of injecting 1 µl in split mode at 230° C using a Trace 1300 Series GC equipped with a TG-5 SILMS column (30 m × 0.25 mm × 0.25 µm; Thermo Scientific). Helium was continuously flowed at 1 ml/min. First, the GC oven reached 70° C for one minute. It was heated to 325° C at 15°C/minute for four minutes. Full MS scan mode with positive polarity, filament delay 4.12 min, 50–750 m/z mass scan range, 60,000 resolution, AGC target 1E6, and 300° C MS transfer line were used for MS research. Ion source electron impact ionisation at 250° C (70 eV). TraceFinder® (Thermo Scientific) automatically recognized analytes using retention time, a primary characteristic fragment (m / z ion), and a confirmation fragment with a maximum tolerance of 0.00007 Da through focussed screening [62–64].
Analysis of amino acids
Employing a Shimadzu Prominence xR UFLC system in conjunction with a SCIEX hybrid triple quadrupole-linear ion trap MS featuring a Turbo VTM ESI source, we conducted the separation and analysis of all samples. Cysteinic acid was negatively ionized, but all other amino acids were positive. The elution of amino acids was achieved by a 20 mM ammonium formate gradient in water (A) and acetonitrile: water (90:10) with a pH of 3.0, incorporating a concentration that is increasing steadily. A 3-µl sample was introduced into the Infinity Lab 120 Z-HILIC Poroshell column (2.7 µm, 100 × 2.1 mm; Agilent Technologies, Santa Clara, CA, USA). A continuous 0.25 ml/min flow was used for gradient elution of amino acids. After two minutes of 100% to 90% B, six minutes of 50% B and 30 s of 100% B, the column was reequilibrated for 6.5 min. The ESI + and ESI ion spray voltage was 4.5 kV; ion source temperature was 400 °C; and the curtain gas concentrations were 1, 45, 2, 40, and 35. Targeted MRM was utilized to detect and monitor ions. The Quantitation Wizard in Analyst (v. 1.6.2) (SCIEX, Concord, Canada) analyzed the data. To determine the quantities and losses of amino acids, the peak regions were compared directly with isotope-labeled internal standards [65–67].
Analysis of flavonoids and phenolic acids
Flavonoids and phenolic compounds were analyzed using a Waters 2695 Alliance HPLC system (Waters Inc., Milford, CT, USA) and a UV–Vis DAD. Waters SunfireTM C18 reverse phase chromatography column (250 mm length, 4.6 mm width, 5 μm particle size) was used for separation. The equipment received standard phenolic solutions and mixtures via auto-injector. To find an effective standard separation method, gradient, and isocratic mobile phases were investigated at different column temperatures and flow rates. After initial investigations, the gradient method was chosen using acetonitrile (mobile phase A, HPLC grade ≥ 99.9%; Honeywell Seelze, Germany) and phosphoric acid (mobile phase B), prepared by dropwise adding 85% orthophosphoric acid (Sigma-Aldrich, Merck, Darmstadt, Germany) to HPLC grade water (Carlo Erba) until pH = 2. The 60-min run-time of the method adjusted the concentration gradient as follows: A) 5% A and 95% B at first; b) 35% A and 65% B for 15 min; c) 35% A and 65% B for 20 min; d) 40% A and 60% B for 30 min; e) 40% A and 60% B for 35 min; f) 50% A and 50% B for 40 min; g) 70% A and 30% B for 52 min; and h) 0.5 mL/min flow rate and 5° C temperature maintained. This study chose three wavelengths: 210, 280, and 360 nm for the HPLC–DAD analysis after examining the UV–vis spectra of the phenolic standards' UV–Vis spectra [68, 69].
Analysis of total fats and vitamins
Fat methyl esters were evaluated according to Tsiplakou et al. [70]. Overall, a mixture of 15 ml of isopropanol and 8.5 ml of the sample was combined in a separate funnel. Subsequently, hexane (11.25 ml) was added in 3-increments and the mixture was shaken once more. To separate and evaporate the hexane layer (20 mL), (2520 × g at 5° C for 5 min) was centrifuged and 7.5 mL 0.47 M aqueous Na2SO4. 5 ml of hexane was added to 100 mg of lipid. After adding 0.2 ml of 11.2 g of KOH to 100 ml of methanol, the mixture was vortexed and left to react for five minutes. After adding 0.5 g of NaHSO4· × H2O, the mixture was centrifuged at 25° C for 5 min at 5000 × g. Analysis of the supernatant layer was carried out following the method outlined by Gortzi et al. [71], then a fifth of the sample split. For five minutes, the column temperature increased 20° C every minute from 70° C to 160° C for three minutes. At 5° C each minute, the temperature reached 240° C. The compounds were identified using Sigma FAME standards in established amounts. We triple-checked every sample. Following Kondyli et al.. [72], fat-soluble vitamins were detected. A straightforward procedure involved shaking the sample (2 ml) with absolute ethanol containing 0.1% (w/v) ascorbic acid (5 ml), along with 50% KOH (2 ml). The resulting mixture was then allowed to react at 80 degrees Celsius for a duration of 20 min. Subsequently, petroleum ether: diethyl ether (1: 1) (20 ml) with 0.01% butylated hydroxytoluene (BHT) was introduced after cooling the sample. After vortexing and adding 15 ml of water at 2 °C, the mixture was centrifuged at 2000 × g for 15 min. After splitting, the biological layer vanished. Vitamin E analysis was done according to the method of Gortzi et al. (52). Additionally, an l-Porasil column (125, 10 μm, 3.9 × 300 mm; Waters Corp., Waltham, MA, USA) was used. Initially, lipid (1 g) was dissolved in n-hexane (5 ml) and then introduced into the HPLC system. The mobile phase was 2: 97.5: 0.5 2-propanol, n-hexane, and 100% ethanol added at 1 ml / min. Furthermore, vitamin A analysis was determined using Mendoza et al. method [73]. The analysis was carried out using a Shimadzu CBM-20A liquid chromatograph (Shimadzu Europa GmbH, Duisburg, Germany) equipped with a 300° C column oven and SIL-20AC autosampler. The detector was a 325 nm Shimadzu SPDM20A diode array detector. Using a Luna C18 column (Phenomenex, Torrance, CA, USA), 250 × 4.6 mm, 5 μm. The mobile phase was 100% methanol at 1 ml / min. Three times each sample was analyzed.
Assessment of antioxidant activity
Evaluation of free radical scavenging activity
ABTS method
Two solutions of distilled water were prepared: one with a concentration of 7 mM for ABTS and another with a concentration of 2.45 mM for potassium persulfate. Additionally, a diluted ABTS reagent was used to create a functioning ABTS reagent. Then. Trolox was synthesized as standard solutions with 0.1 mg / ml in a solution of water: ascorbic acid (1: 2) and 1 mg / ml of alpha-tocopherol in hexane. Standard solutions were used to create batch dilutions and then the ABTS working reagent was added. The absorbances were tested after shaking the contents of the tube and letting it sit at room temperature for seven minutes. This approach involved the creation of calibration curves for the reference solutions. The produced samples' hydrophilic and lipophilic extracts underwent identical analyses. At 734 nm, the absorbances were measured [74]. The following formula was used to determine the capacity of extracts to ABTS radical scavenging [75]:
Where, AB is the absorbance of ABTS radical + control and AA is the absorbance of ABTS radical + sample extract.
DPPH method
A 1 mmol/L concentration of DPPH standard solution was produced in solutions of ethanol and methanol. The standard solution of alpha-tocopherol (1 mg / ml) was prepared in hexane. Subsequently, a sequence of dilutions was created, covering a concentration range of 2 to 5 μg/mL. In addition, a standard Trolox solution was formulated, comprising 0.1 mg / ml of the compound dissolved in a solution of water: ascorbic acid (1:2). This solution was diluted several times, with concentrations ranging from 0.4 to 20 μg/mL. Calibration curves were generated for the dilution series of standard alpha-tocopherol and Trolox solutions, after incorporation of the DPPH solution into each prepared solution. Both lipophilic and hydrophilic extracts were prepared, with an equivalent volume of DPPH solution added to each. The material was shaken and exposed to darkness for three hours and thirty minutes. At 517 nm, the absorbances of the samples and standards were determined [74]. The capacity of the extracts to remove DPPH radicals was determined using the formula below [76]:
where: A0 = the control absorbance, A1 = the sample absorbance.
H2O2 method
Following the Ruch et al. [77] method, an aliquot of extracts/ ascorbic acid (0.1 ml, 25–400 μg/mL) was transferred to Eppendorf tubes. The volume was set at 0.4 ml with 50 mM phosphate buffer (pH 7.4) and then a solution of H2O2 (0.6 ml, 2 mM) was added. After the reaction mixture was vortexing and allowed to react for 10 min, the absorbance at 230 nm was measured. The ability to eliminate H2O2 was evaluated using the following formula [78]:
Where: A0 = Absorbance of the control, A1 = Absorbance of a sample.
Evaluation of metal-chelating scavenging activity
FRAP method
FRAP was determined in this study according to Akinmoladun et al. [79] that used a method designed by Benzie and Strain (1996) to quantify the ability to decrease ferric ions. FRAP reagent was made by combining 300 mM sodium acetate buffer (pH 3.6), 10.0 mM TPTZ solution (tripyridyl triazine) and 20.0 mM FeCl3.6H2O solution in a volume ratio of 10: 1: 1. Subsequently, different amounts of leaf extract (2, 3.9, 7.8, 15.6, 31.25, 62.5, 125, 250, 500, and 1000 μg/ml) were added to 3 ml of FRAP reagent and the reaction mixture was incubated at 37 C for 30 min. At 593 nm, an increase in absorbance was measured [80].
Cupric ion reducing antioxidant (CUPRAC) method
Duplicates of all samples and standards were prepared using a mixture consisting of 150 μL each of 7.5 mM neocuproine, 1 M ammonium acetate, and 10 mM copper chloride dihydrate (II). Ascorbic acid (1g) was used to generate the standard curve. The concentrations included a blank (EDTA) and 1000, 500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9, and 2 μg/ml. After adding neocuproine, ammonium acetate, and copper (II) chloride dihydrate to both samples and standards, the test tubes were incubated at room temperature for thirty minutes. Subsequently, the test tubes were examined at 450 nm using a Hitachi U-2001 Spectrophotometer [81].
Metal chelating activity method
Two working solutions were prepared: one for 0.25 mM ferrosine and the other for 0.1 mM ferrous sulfate (II). A 100 mg/L concentration of a standard Na2EDTA solution was obtained. This standard solution was used to create a range of dilutions with concentrations ranging from 1 to 20 mg/L. Reagents that had already been produced were added to the prepared solutions to create the calibration curve. After a ten-minute incubation, the contents of the tubes were shaken and absorbances were measured. The methodology used to generate the EDTA calibration curve was applied similarly to prepare extracts from hydrophilic and lipophilic extracts. At 562 nm, absorbances were measured [74].
Assessment of antimicrobial activity
Microbial strains
A total of 29 pathogenic microbes were used in the study divided into 13 fungal strains, 8 Gram-positive bacteria and 8 Gram-negative bacteria. Fungal strains included Aspergillus fumigatus (RCMB 002008), A. niger (RCMB 002005), Candida albicans RCMB 005003 (1) ATCC 10231, Penicillium aurantiogriseum IMI 89372, Syncephalastrum racemosum RCMB 016001 (1), P. marneffeii (RCMB 001022), Cryptococcus neoformas RCMB 0049001, Candida lipolytica RCMB 005007(1), P. expansum RCMB 001001 (1) IMI 28169, P. italicum RCMB 001018 (1) IMI 193019, Fusarium moniliform (RCMB 008005), Trichophyton rubrum (RCMB 025002), and Geotrichum candidum (RCMB 041001). Gram-positive bacteria included Staphylococcus aureus ATCC 25923, Bacillus subtilis RCMB 015 (1) NRRL B-543, B. cereus RCMB 027 (1), Staphylococcus epidermidis RCMB 009 (2), Micrococcus sp. RCMB 028 (1), Streptococcus mutants RCMB 017 (1) ATCC 25175, Methicillin resistant Staphylococcus aureus (MRSA) ATCC 4330, and Enterococcus faecalis (ATCC 29212). Gram-negative bacteria were Enterobacter cloacae RCMB 001 (1) ATCC 23355, Salmonella typhimurium RCMB 006 (1) ATCC 14028, Escherichia coli ATCC 25922, Klebsiella pneumonia RCMB 003 (1) ATCC 13883, Proteus vulgaris RCMB 004 (1) ATCC 13315, Serratia marcenscens 007001, Pseudomonas aeruginosa ATCC 27853, and Porphyromonas gingivalis RCMB 022001 (1) EMCC 1699.
Antimicrobial activity screening
The diffusion agar technique (bacterial suspension containing 106 CFU/ml of the bacterial test strain disseminated over Muller Hinton agar) was used to verify the antibacterial properties of the plant extracts. Each extract was placed on 5 μg/disc of sterilized filter paper. gentamycin was used as a positive control. These discs were placed on agar plates and left to incubate for 24 h at 37° C. Using a Vernier caliper, the inhibition zones were measured. For antifungal activity, the plant extracts were tested using a well diffusion technique. A 0.2 mL saline inoculum, a fungal strain, was applied to an agar plate. In every plate, five 4 mm ditches were created. 100% ethanolic plant extracts (50 mg/ml) were made and 50 μl of the extracts were added to each well. The positive control in this experiment was ketoconazole (100 µg/ml). Then, 24 h were spent incubating the plates at 37 C. Bacterial and fungal species were acquired from the Regional Center for Mycology and Biotechnology (RCMB) in Egypt.
Assessment of antiproliferation activity
Cell culture
The American Type Culture Collection (ATCC, Rockville, MD) provided the following human cancer cell lines. HepG-2 (human hepatocellular cancer cell line), HCT-116 (human colon cancer cell line), MCF-7 (human breast cancer cell line), HT-29 (human colon cancer cell line), MDA-MB-231 (human breast cancer cell line), PC-3 (human prostate cancer cell line), A-549 (human lung cancer cell line), A-431 (human skin cancer cell line), Panc-1 (human pancreatic cancer cell line), CACO2 (human intestinal ca Furthermore, WI-38, or normal human lung fibroblast cells, were donated by the American Type Culture Collection (ATCC, Rockville, MD). In addition, dimethylsulfoxide (DMSO), MTT, and trypan blue dye were supplied by Sigma (St. Louis, MO, USA). Lonza (Belgium) provided fetal bovine serum, gentamycin, L-glutamine, RPMI-1640, HEPES buffer solution and 0.25% trypsin–EDTA. Cells were grown in RPMI-1640 medium supplemented with gentamycin (50µg/ml) and inactivated foetal calf serum (10%). Cell subculture was performed two or three times a week, and cells were kept at 37° C in a humidified atmosphere with 5% CO2. Cells for WI-38 (Normal Human Cell Lines) were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with L-glutamine (1%), heat-inactivated Foetal Bovine Serum (10%), and HEPES (50µg/ml) [82–84].
Cytotoxicity assay
The MTT cell viability assay was used in 15 different cell lines to assess the activity of the Brennnesselwurzel (nettle root) extract. Cells were distributed in Corning® 96-well tissue culture plates, suspended in media at a concentration of 5 × 104 cells/well, and exposed to varying concentrations of brennnesselwurzel extract (nettle root) extract (0–500 µg/mL). Subsequently, they were incubated in a humidified atmosphere of 5% CO2 at 37° C for 24 h. Following the incubation period, 12 mM MTT stock solution (10 μL, 5 mg MTT/ 1 mL PBS) was added per well, along with fresh RPMI 1640 medium (100 µL) without phenol red (untreated controls). The plate was then shaken and further incubated at 37° C in a humidified environment with 5% CO2 for an additional 4 h. An aliquot of the medium (85 µL) was removed from the wells and DMSO (50 µL) was introduced. Each well was thoroughly mixed using a pipette and the resulting mixture was then incubated at 37° C for 10 min. Subsequently, the reduction of MTT was evaluated by assessing absorbance using a microplate reader (SunRise, TECAN, Inc., USA) at 590 nm. For each concentration, the average of three replicates was calculated [85–87]. The percentage of viability was then computed and expressed as
Subsequently, using GraphPad Prism software (San Diego, CA, USA), the 50% inhibitory concentration (IC50), representing the concentration required to induce detrimental effects in 50% of intact cells, was determined from graphs of the dose–response curve for each concentration [88].
Statistical analysis
Microsoft Excel was used to assess the free radical DPPH, the ABTS radical, H2O2-induced oxidative stress, the ferric reducing antioxidant power (FRAP), CUPRAC, and the metal-chelating scavenging activity. Furthermore, all data were presented as mean values with standard deviations in triplicate. One-way ANOVA was used to assess the mean values. There were significant variations in parameter means (p < 0.05) [89, 90].
Results
Phytochemical characterization
After the plant material was collected cautiously, the extraction process was applied using 70% acetone in a water solution. The Brennnesselwurzel (BWE) plant extract was tested for its primary phytochemical content and secondary metabolites, including amino acids, flavonoids, phenolics, lipids, and vitamins. Furthermore, detailed gas chromatography was employed in association with mass spectrometry to gain more insight into the prominent bioactive metabolites in the BWE extract.
Analysis of total nutrients
In the analysis of total nutrients of the BWE extract, we evaluated the quantitative composition of essential primary metabolites present in the plant. The analysis was carried out using validated spectrophotometric and chromatographic methods, ensuring reliable and accurate results. Our findings revealed that the BWE extract is a valuable source of various bioactive nutrients (Fig. 1). Our results showed that the BWE extract consists of a wide range of primary metabolites, including flavonoids (4.53 mg/g), phenolic acids (81.47 mg/g), alkaloids (0.15 µg/g), lipids (0.12 mg/g), ash (9.31 mg/g), carbohydrates (96.15 mg/g), proteins (92 mg/g), saponin (37.62 mg / g) and tannins (2.62 mg/g). The analysis revealed that the BWE extract has high concentrations of phenolic compounds, carbohydrates, proteins, and saponin. These findings indicate that BWE has significant antioxidant properties due to high levels of phenolic compounds, providing potential health benefits. In addition, significant amounts of carbohydrates and proteins suggest that BWE could serve as a valuable nutritional source. The presence of saponin further implies the chemical diversity of the BWE extract, pointing toward its possible use in therapeutic contexts. On the other hand, the considerable levels of flavonoids and alkaloids suggest the possible antioxidant potential and medicinal properties. Furthermore, the presence of ash and tannins further suggests the mineral content of BWE extract, which is significant both for nutritional and medicinal aspects. These findings provide valuable information on the phytochemical profile of BWE and further indicate its possible potential applications in various contexts.
Fig. 1.
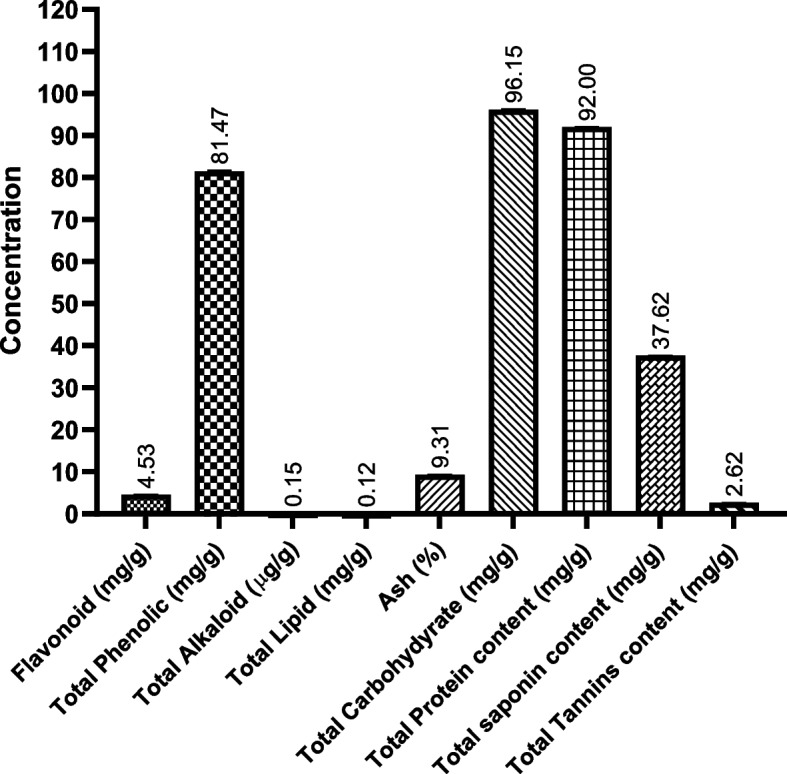
Total primary metabolites detected in BWE extract by phytochemical screening assays. Data were presented as mean values with standard deviations in triplicate
Amino acid analysis
An amino acid analyzer was used to determine the amino acid profile of the BWE extract. Our analysis provided a comprehensive overview of 18 amino acids and related amines, along with their respective retention time, response, and amount. Our results revealed that the BWE extract exhibits a wide range of different amino acids in considerable concentrations, suggesting the BWE extract as a potential source of essential amino acids (Fig. 2, Table S1). Among the amino acids detected, the BWE extract possesses high concentrations of certain amino acids, including glutamic acid (GLU), aspartic acid (ASP), leucine (LEU) and lysine (LYS) (> 20 PPM). The substantial concentration of glutamic acid and aspartic acid in the BWE extract indicates the possible effect of the extract on protein metabolism and synthesis. The existence of leucine and lysine, essential for muscle health and overall physiological functions, further emphasizes the nutritional richness of the BWE extract. These findings underscore the potential significance of BWE extract in supporting protein synthesis and highlight its nutritional value, making it a significant topic in dietary.
Fig. 2.
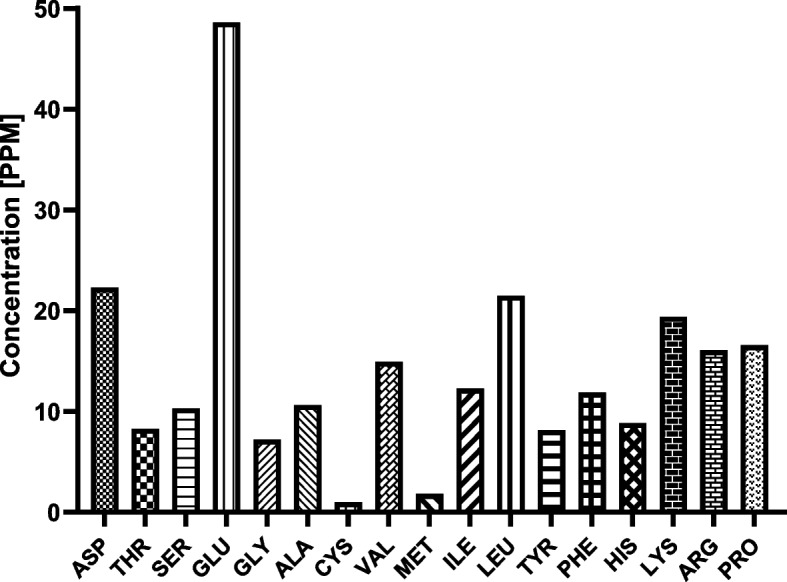
Amino acids detected and their concentrations in the BWE extract by an amino acid analyzer
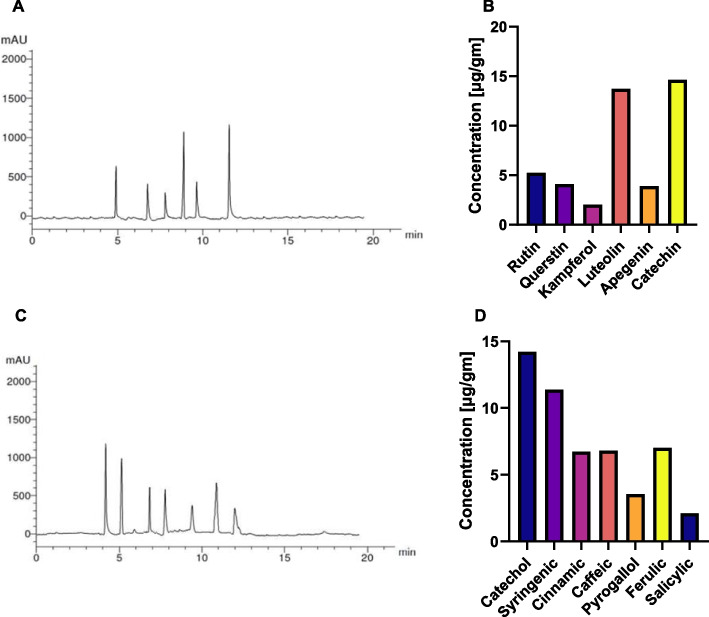
Analysis of phenolic acids and flavonoids
Then, the content of flavonoids and phenolic acids in the BWE extract was assessed using targeted High Performance Liquid Chromatography (HPLC) analysis (Fig. 3). HPLC analysis unraveled the presence of various flavonoid and phenolic metabolites in considerable concentrations. Among the identified flavonoid metabolites, catechin and luteolin exhibit appreciable concentrations (> 10µg/g), further underscoring the possible potential of pf BWE extract as an antioxidant and anti-inflammatory supplement. Additionally, other bioactive flavonoid metabolites were detected at considerable levels, including kampferol, apigenin, quercetin, and rutin (Table S2). On the other hand, the evaluation of phenolic metabolites revealed that catechol and syringic are present in substantial concentrations (14.22 μg/g, 11.38 μg/g, respectively), signifying the possible therapeutic potential of BWE extract as an antitumor and antioxidant supplement. In addition, other phenolic acid metabolites were detected in compounds, such as salicylic, pyrogallol, cinnamic, caffeic and ferulic, which were identified in considerable concentration in the BWE extract, ranging from 2.10 to 7.02 μg/g (Table S3). Taken together, the substantial flavonoids detected in the BWE extract suggest the antioxidant and antitumor characteristics of the BWE extract. The presence of different flavonoid metabolites such as kampferol, apigenin, quercetin, and rutin contributes to the overall chemical profile of the extract. Furthermore, phenolic acids possess several bioactivities including antioxidant, anticancer, and anti-inflammatory effects, suggesting that BWE may provide potential therapeutic benefits.
Fig. 3.
HPLC chromatogram and concentration of the detected flavonoids (A-B) and phenolic acids (C-D) metabolites in the BWE extract
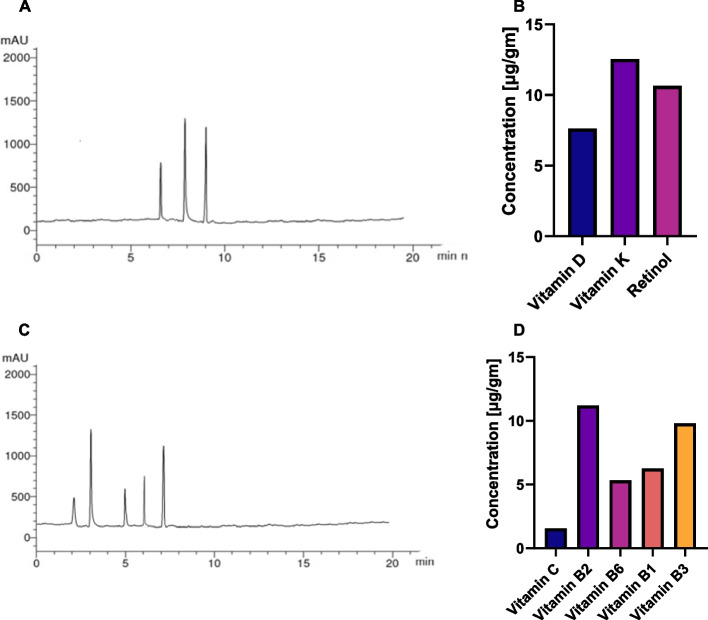
Analysis of total vitamins
Furthermore, we explored the phytochemical profile of the BWE extract employing HPLC analysis to determine the total vitamin profile. The analysis can be divided into two groups according to solubility, namely fat-soluble vitamers (FSV) and water-soluble vitamers (WSV) as shown in Fig. 4 [91]. The results of the HPLC analysis revealed distinctive profiles for both FSV and WSV. Fat-soluble vitamers, including Vitamin D, Vitamin K, and Retinol, demonstrated varying concentrations, with Vitamin K exhibiting the highest concentration at 12.56 μg/gm. These fat-soluble compounds are integral to nutritional and health considerations, and their presence in the BWE extract underscores the potential health benefits associated with the consumption of this botanical extract (Table S4). However, water-soluble vitamers showed a diverse range of concentrations, with Vitamin B2 leading at 11.20 μg/g, followed by Vitamin B3 at 9.80 μg/g. Variable concentrations of water-soluble vitamers, such as Vitamin C, Vitamin B1, and Vitamin B6, point to the diverse nutritional composition of the BWE extract. These water-soluble compounds are essential for physiological functions and are indicative of the potential role of the extract in the health context. Collectively, the HPLC results provide a complete illustration of the vitamin composition of the BWE extract, highlighting its nutritional richness and potential health-promoting properties (Table S5).
Fig. 4.
HPLC chromatogram and concentration of detected (A, B) FSV and (C, D) WSV metabolites in the BWE extract
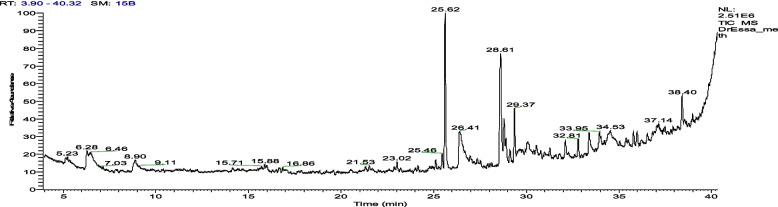
Gas Chromatography (GC) analysis
The identification of secondary metabolites in BWE extract was further achieved by conducting GC–MS analysis with Thermo Scientific's GC–MS-Orbitrap Q Exactive system. In this technique, the plant extracts or standard solutions were dried, then treated with methoxyamine and MSTFA using a preparative robot and subjected to retention index calculation with an alkane mix. The samples were then injected into a Trace 1300 Series GC equipped with a TG-5 SILMS column and the resulting chromatogram was analyzed with a full MS scan mode, offering a mass scan range of 50–750 m/z, a resolution of 60,000, and an AGC target. The ion source operated at 250° C with electron impact ionization (70 eV). Thermo Scientific's TraceFinder® software was employed for automatic analyze recognition based on retention time, a primary characteristic fragment, and a confirmation fragment. The detected metabolites, retention time, peak area, and their molecular formula are summarized in Table 1.
Table 1.
List of the main secondary phytochemical metabolites identified in the acetone extract of BWE
| Number | Compound Name | Chemical Formula | Retention Time (RT) | Area% | R.I |
|---|---|---|---|---|---|
| 1 | cis-13-Octadecenoic acid | C18H34O2 | 5.13 | 0.33 | 2178 |
| 2 | (2-phenyl-1,3-dioxolan-4-yl)methyl ester, cis-9-Octadecenoic acid | C28H44O4 | 5.23 | 1.20 | 3246 |
| 3 | Benzoic acid methyl ester | C8H8O2 | 6.27 | 2.89 | 1084 |
| 4 | Estragole | C10H12O | 8.86 | 0.79 | 1177 |
| 5 | 1-Methoxy-4-(1-propenyl)-benzene | C10H12O | 8.91 | 0.41 | 1264 |
| 6 | Hexadecanoic acid-phenylmethyl ester | C23H38O2 | 15.87 | 0.41 | 2527 |
| 7 | 3-Amino-4-[(1-benzyl-2-methoxy-2-oxoethyl)amino]-4-Oxobutanoic acid | C14H18N2O5 | 15.98 | 0.44 | 2528 |
| 8 | 2H-Indeno[1,2-b]furan-2-one, 3,3a,4,5,6,7,8,8b-octahydro-8,8-dimethyl | C13H18O2 | 16.66 | 1.56 | 1642 |
| 9 | Cholestan -3-ol,2-methylene, (3ß,5α) | C28H48O | 16.84 | 0.26 | 2647 |
| 10 | methyl 8-(2-octylcyclopropyl)octanoate | C20H38O2 | 21.52 | 0.27 | 2276 |
| 11 | 12-Methyl-tetradecanoic acid-methyl ester | C16H32O2 | 23.02 | 0.53 | 959 |
| 12 | 14-Hydroxy-14-methyl-hexadec-15-enoic acid, methyl ester | C18H34O3 | 23.02 | 0.53 | 2060 |
| 13 | , 2-(9-octadecenyloxy)-, (Z)- Ethanol | C20H40O2 | 24.16 | 0.26 | 2640 |
| 14 | 9-Hexadecenoic acid, methyl ester, (Z)- | C17H32O2 | 25.11 | 0.49 | 1885 |
| 15 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester | C15H16O6 | 25.46 | 1.78 | 1329 |
| 16 | Hexadecanoic acid, methyl ester | C17H34O2 | 25.62 | 20.85 | 1908 |
| 17 | 1,25-Dihydroxyvitamin D3, TMS derivative | C30H52O3Si | 26.97 | 0.43 | 3150 |
| 18 | Glycidyl oleate | C21H38O3 | 27.34 | 0.40 | 2374 |
| 19 | Cyclopropanebutanoic acid, 2-[[2-[[2-[(2-pentylcyclopropyl)methyl]cyclopropyl]methyl]cyclopropyl]methyl]-, methyl ester | C25H42O2 | 27.53 | 0.46 | 2528 |
| 20 | 8,11-Octadecadienoic acid methyl ester | C19H34O2 | 28.61 | 15.22 | 2439 |
| 21 | 10-Octadecenoic acid methyl ester | C19H36O2 | 28.80 | 5.86 | 2100 |
| 22 | trans-13-Octadecenoic acid methyl ester | C19H36O2 | 28.80 | 4.86 | 2163 |
| 23 | 16-Octadecenoic acid, methyl ester | C19H36O2 | 28.90 | 1.89 | 2431 |
| 24 | Dasycarpidan-1-methanol, acetate (ester) | C20H26N2O2 | 29.12 | 2.74 | 2453 |
| 25 | Methyl stearate | C19H38O2 | 29.36 | 2.64 | 2133 |
| 26 | Oleic acid | C18H34O2 | 29.63 | 0.26 | 2113 |
| 27 | n-Propyl-11-octadecenoate | C21H40O2 | 30.04 | 0.42 | 2284 |
| 28 | trans-9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester | C28H44O4 | 30.08 | 1.46 | 2175 |
| 29 | Norgestrel trimethylsilyl ether | C24H36O2Si | 30.15 | 0.30 | 2393 |
| 30 | (3ß,24S)-Stigmast-5-en-3-ol | C29H50O | 30.54 | 5.47 | 2657 |
| 31 | 1H-Cyclopropa[3,4]benz[1,2-e]azulene-5,7b,9,9a-tetrol, 1a,1b,4,4a,5,7a,8,9-octahydro-3-(hydroxymethyl)-1,1,6,8-tetramethyl-, 5,9,9a-triacetate | C26H36O8 | 31.27 | 0.44 | 3399 |
| 32 | Champanone A | C19H20O4 | 31.80 | 0.19 | 2470 |
| 33 | 1-Cyclopentyl-4-(3-cyclopentylpropyl)-dodecane | C25H48 | 32.11 | 0.99 | 2785 |
| 34 | Eicosanoic acid methyl ester | C21H42O2 | 32.80 | 4.01 | 2311 |
| 35 | Linoleic acid ethyl ester | C20H36O2 | 33.38 | 5.82 | 2139 |
| 36 | 4-Hexyl-1-(7-methoxycarbonylheptyl)bicyclo[4.4.0]deca-2,5,7-triene | C25H40O2 | 33.95 | 0.83 | 2670 |
| 37 | Dimethyl 2,3-di(8-nonen-1-yl)succinate | C24H42O4 | 34.02 | 0.31 | 2593 |
| 38 | 4-Hydroxy-octadecanoic acid methyl ester | C19H38O3 | 34.41 | 2.86 | 1461 |
| 39 | 3-Ethyl-5-(2-ethylbutyl)-Octadecane, | C26H54 | 34.51 | 1.80 | 2413 |
| 40 | 1,1',2,2'-tetrahydro-1,1'-dimethoxy-.psi,psi.-Carotene | C42H64O2 | 37.93 | 3.78 | 3918 |
| 41 | Icosyl (Z)-octadec-9-enoate | C38H74O2 | 38.40 | 1.86 | 2121 |
The GC–MS chromatogram (Fig. 5 and Table 1) identified a total of 41 secondary metabolites in the BWE acetone extract. The identified metabolites were mainly fatty acids and aromatic metabolites. This categorization showed that BWE extract shows 18 different fatty acid metabolites, indicating the presence of metabolites with long hydrocarbon chains. aqMoreover, various fatty acids and their derivatives play essential roles in energy storage, signaling, and structural components of cells and are associated with lipid metabolism. While, the aromatic class of metabolites exhibited 10 different recognized metabolites, including benzoic acid methyl ester and. psi,.psi-, 1,1',2,2'-tetrahydro-1,1'-dimethoxy- Carotene with the highest peak area % (2.89% and 3.78%, respectively). Aromatic metabolites often contribute to diverse biological activities. Further analysis also revealed the presence of various compounds such as cyclopropaneoctanoic acid, 2-octyl, methyl ester (0.27%), ethanol, 2- (9-octadecenyloxy) -, (Z)—(0.26%) and 2,2,4,4-Tetramethyl-6-(1-oxo-3-phenylprop-2-enyl)-cyclohexane(1aà,1bá,4aá,5á,7aà,7bà,8à,9á,9aà)]- (0.19%), which belong to classes of cyclopropanes, alcohols and terpenes, respectively. These different metabolites possess various biological activities and are often involved in metabolic processes. In particular, the main compounds identified were fatty acid metabolites that included hexadecanoic acid methyl ester and 8,11-octadecadienoic acid methyl ester, with the highest area percentages of 20.85% and 15.22%, respectively (Figure S1).
Fig. 5.
GC–MS chromatogram of the 70% acetone BWE extract showing the detected secondary metabolites
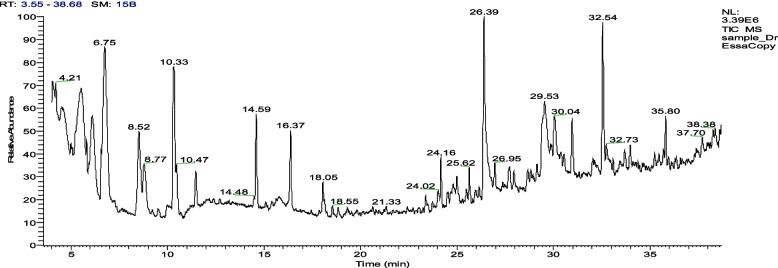
We also used GC–MS analysis with Thermo Scientific's GC–MS-Orbitrap Q Exactive system technique in our study to detect the essential oils and other volatile metabolites in the BWE extract. The GC–MS analysis is effective in separating volatile and semivolatile compounds, as it can separate individual volatile compounds on the basis of their boiling points and chemical properties. The mass spectrometer then identifies these compounds by their unique mass-to-charge ratios, providing an accurate and reliable identification of the components present in the oil. GC–MS analysis of the BWE extract identified a total of 30 essential oils and volatile metabolites (Fig. 6 and Table 2). Among these, fatty acids (FA) were predominant (7 metabolites), including n-hexadecanoic acid (8.81%), 9,12,15-octadecatrienoic acid, (Z,Z,Z) -2,3-dihydroxypropyl ester (6.03%) and 6,9,12-octadecatrienoic acid methyl ester (2.73%) with the highest peak area. These findings suggest a substantial presence of FAs in the BWE extract, which suggests their various roles in biological processes. The most prominent other essential oils include allo-ocimene, cis-4-thujanol, caryophyllene and sabinene with area percentages of 12.61%, 10.56%, 5.66% and 4.74%, respectively, which belong to monoterpenes, oxygenated monoterpenes and sesquiterpenes (Figure S1). The existence of monoterpenes and oxygenated monoterpene metabolites could help in understanding the characteristic of essential oils, as monoterpenes often contribute to the characteristic smell of essential oils, as well as knowing the biological activities of the plant extract, as the additional oxygen atoms in monoterpenes could contribute to the therapeutic properties of the extract. Furthermore, the GC–MS analysis revealed the presence of other bioactive metabolites, highlighting the complexity of the composition of the BWE extract and supporting its potential as a natural source of plant-based supplement (Table 2).
Fig. 6.
GC–MS chromatogram of the 70% acetone BWE extract showing the essential oils and volatile secondary metabolites
Table 2.
List of the main essential oils and volatile secondary metabolites in BWE extract
| Number | Compound Name | Chemical Formula | Retention Time (RT) | Area % | R.I |
|---|---|---|---|---|---|
| 1 | ( +)-3-Carene | C10H16 | 4.21 | 1.13 | 948 |
| 2 | Allo-Ocimene | C10H16 | 5.53 | 12.61 | 993 |
| 3 | p-Mentha-1,4-dien-7-ol | C10H16 | 5.80 | 0.73 | 1240 |
| 4 | Sabinene | C10H16 | 6.09 | 4.74 | 897 |
| 5 | 4- cis-(. ±)-Thujanol | C10H18O | 6.55 | 0.67 | 1311 |
| 6 | 4- cis-Thujanol | C10H18O | 6.74 | 10.56 | 1311 |
| 7 | Terpinen-4-ol | C10H18O | 8.52 | 5.97 | 1137 |
| 8 | (S)-(-)-α-Terpineol | C10H18O | 8.78 | 2.49 | 1158 |
| 9 | 1-methyl-4-(1-methylethylidene)- cyclohexanol | C10H18O | 10.28 | 4.88 | 1346 |
| 10 | cis-Sabinene hydrate acetate | C12H20O2 | 10.33 | 4.41 | 1041 |
| 11 | 1,6-Octadien-3-ol, 3,7-dimethyl-, acetate | C12H20O2 | 10.47 | 0.93 | 1082 |
| 12 | iso-3-Thujyl acetate | C12H20O2 | 11.46 | 1.98 | 1219 |
| 13 | Caryophyllene | C15H24 | 14.59 | 5.66 | 1494 |
| 14 | γ-Elemene | C15H24 | 16.37 | 3.29 | 1431 |
| 15 | (-)-Spathulenol | C15H24O | 18.05 | 1.49 | 1536 |
| 16 | (Z)-2-(9-Octadecenyloxy)-ethanol | C20H40O2 | 24.16 | 2.09 | 2068 |
| 17 | 13-Heptadecyn-1-ol | C17H32O | 24.98 | 0.79 | 1971 |
| 18 | Palmitic acid methyl ester | C17H34O2 | 25.62 | 1.51 | 1878 |
| 19 | n-Hexadecanoic acid | C16H32O2 | 26.38 | 8.81 | 1968 |
| 20 | Hexadecanoic acid ethyl ester | C18H36O2 | 26.95 | 1.25 | 1968 |
| 21 | Dasycarpidan-1-methanol acetate | C20H26N2O2 | 27.72 | 1.66 | 2453 |
| 22 | Oleic Acid | C18H34O2 | 27.94 | 1.15 | 2175 |
| 23 | 6,9,12-Octadecatrienoic acid methyl ester | C19H32O2 | 29.43 | 2.73 | 2447 |
| 24 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester | C21H36O4 | 29.52 | 6.03 | 2586 |
| 25 | Octadecanoic acid, 2,3-dihydroxypropyl ester | C21H42O4 | 30.03 | 2.70 | 2681 |
| 26 | Retinol | C20H30O | 30.96 | 2.82 | 2238 |
| 28 | (3ß,24S)-Stigmast-5-en-3-ol, | C29H50O | 33.96 | 1.20 | 2657 |
| 29 | 1,2-Benzenedicarboxylic acid | C24H38O4 | 35.80 | 2.19 | 2586 |
| 30 | 3-Hydroxyspirost-8-en-11-one | C27H40O4 | 37.69 | 1.16 | 3044 |
Evaluation of antioxidants
Initially, we evaluated the total antioxidant capacity (TAC) of BWE extract, which estimates the total amount of antioxidants in the sample compared to ascorbic acid as a positive control. Our analysis revealed that the BWE extract has a TCA of 30.94 ± 1.58 mg GAE/g, compared to ascorbic acid (72.68 ± 3.74 mg GAE/g). Based on these findings, we encourage you to gain more information about the antioxidant activity of BWE extract by evaluating its ability to remove free radicals and metals. In this regard, various methods were used, including the free radical scavenging activity (ABTS assay, DPPH assay, H2O2 assay) and metal scavenging activity (FRAP scavenging assay, cupric iron-reducing antioxidant (CUPRAC) assay, metal chelating activity). These assays provide quantitative measures of the ability of BWE extracts or metabolites to eliminate free radicals and reduce oxidative stress. In these assessments, ascorbic acid and EDTA were used as standards for the assays carried out.
Evaluation of free radical scavenging activity
ABTS radical scavenging activity
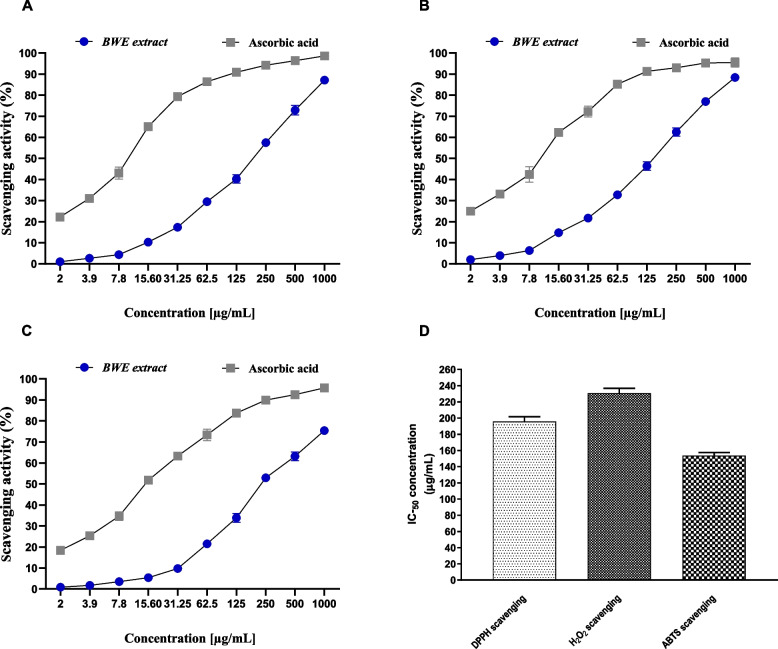
The ability of BWE extract to scavenge ABTS radicals was assessed by using an ABTS (2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) radical scavenging assay. In this assay, the ABTS· + cation radical was produced by the reaction between a 7 mM ABTS solution and a 2.4 mM potassium persulfate (1:1) solution [92]. The antioxidant activity of the BWE extract was evaluated by assessing the ability to eliminate the ABTS radical, compared to ascorbic acid (Fig. 7). Our results revealed that BWE extract exhibits a dose-dependent capability to scavenge ABTS radical with highest scavenging activity at 500 µg/ml and 1000 µg/ml (76.98%, and 88.39%, respectively). The lowest percentage of scavenging activity was observed at 2 µg/ml with 1.98%. Under the experimental conditions given, the BWE extract exhibited antioxidant activity with an IC50 value of 153.51 ± 3.97 µg/ml, as compared to the standard (IC50 = 10.66 ± 0.89 µg/mL). The BWE extract demonstrated an ABTS scavenging activity of 88.39% at 1000 µg/ml, ascorbic acid showed an activity of 95.46% at the same concentration. These results indicate that the BWE extract possesses considerable scavenging activity toward the ABTS radical, as compared to the ascorbic acid standard.
Fig. 7.
The dose-dependent activity of BWE extract to scavenge the radical of DPPH (A), ABTS (B) and H2O2 (C). The radical scavenging activity (IC50) of the BWE extract (D). Data were presented as mean values with standard deviations in triplicate
DPPH radical scavenging activity
The DPPH (2,2-Diphenyl-1-picrylhydrazyl) scavenging assay is a robust assay to evaluate antioxidant activity by eliminating the DPPH radical. The DPPH free radical interacts with an odd electron to give a noticeable absorbance at a specific wavelength (517 nm). As shown in Fig. 7, the BWE extract demonstrated considerable and dose-dependent scavenging activity toward the DPPH radical, with the highest activity of 87.13% at 1000 µg/ml. On the contrary, the lowest percentage of inhibition is observed at a concentration of 2 µg/ml, with a value of 0.97%. In this assay, the BWE extract exhibited antioxidant activity with an IC50 of 195.75 ± 5.91 µg/ml, as compared to ascorbic acid with an IC50 of 10.21 ± 0.77 µg/ml. Our findings indicated that the BWE extract has DPPH scavenging activity compared to that of ascorbic acid, as indicated by the activity at 1000 µg/ml (87.13% and 98.65%, respectively). Furthermore, at lower concentration (3.9 µg/ml), the BWE extract showed a percentage of DPPH scavenging of 2.65%, while ascorbic acid showed 1.04% activity. These findings indicate that the BWE extract exhibits considerable antioxidant activity by scavenging the DPPH radical.
H2O2radical scavenging activity
The H2O2 scavenging assay was also applied to test the antioxidant activity of the BWE extract at different concentrations (2, 3.9, 7.8, 31.25, 62.5, 250, 500, and 1000 μg/ml) by neutralizing hydrogen peroxide, a reactive oxygen species that can cause oxidative damage. As indicated in Fig. 7, our analysis indicated that the BWE extract shows a moderate and dose-dependent radical scavenging activity against H2O2 radicals. At high concentrations (1000 µg/ml, 500 µg/ml, and 250 µg/ml), BWE extract possessed considerably high percentages of scavenging H2O2 with 75.42%, 63.14%, and 52.95%, respectively. However, at low concentration (2 µg/ml), the percentage of H2O2 scavenging decreased significantly to 0.85%. IC50 assessment revealed that BWE exhibits an IC50 of 230.67 ± 5.98 µg/ml, while ascorbic acid showed an IC50 of 14.77 ± 0.69 µg/ml, at similar conditions. Compared to ascorbic acid, the BWE extract showed moderate H2O2 scavenging activity, as indicated by the activity at 1000 µg/ml (75.42%, compared to 95.73% for ascorbic acid). These findings further support the antioxidant potential of BWE extract by scavenging for radicals.
Assessment of metal scavenging activity
FRAP scavenging assay
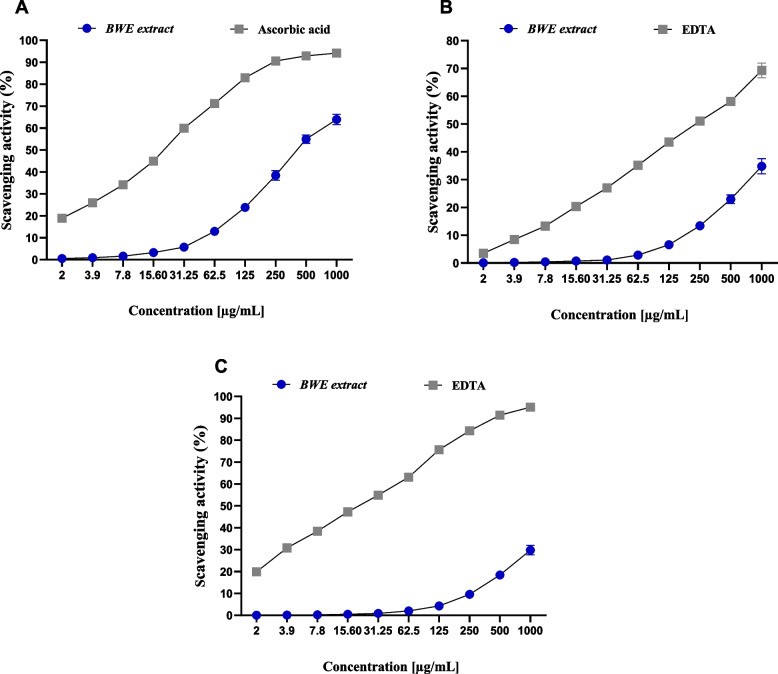
The FRAP (Ferric reducing antioxidant power) scavenging assay was performed to test the antioxidant activity of BWE extract. As depicted in Fig. 8, the BWE extract exhibited considerable FRAP scavenging activity, with the highest activity recorded at 1000 µg/ml and 500 µg/ml (63.89% and 54.93%, respectively). On the other hand, the lowest activity was observed at 2 µg/ml, with a minimal scavenging activity of 0.51%. These results underscore the concentration-dependent nature of the scavenging activity of the plant extract. Under the experimental conditions specified, the BWE extract showed antioxidant activity with an IC50 of 425.43 ± 9.61 µg/ml, as compared to ascorbic acid (IC50 = 20.89 ± 1.25 µg/ml). These results were supported by comparing activity at high concentration (1000 µg/ml). While the BWE extract showed a FRAP scavenging percentage of 63.89%, ascorbic acid showed 94.19% activity. These results indicate that the BWE extract has moderate metal scavenging activity.
Fig. 8.
The dose-dependent activity of BWE extract to chelate the metal of FRAP (A), CUPRAC (B), and metal chelating activity (C). Data were presented as mean values with standard deviations in triplicate
Cupric Iron Reducing Antioxidant (CUPRAC) assay
Next, we evaluated the metal scavenging activity of the BWE extract by conducting the CUPRAC assay. As shown in Fig. 8, the CUPRAC assay revealed that the BWE extract exhibits the highest scavenging activity (34.81%) observed at a concentration of 1000 µg/ml, while the lowest percentage of scavenging activity (0.09%) was observed at 2 µg/ml. These results indicate a comparatively weak metal chelating activity of BWE extract under the given experimental conditions. On the contrary, EDTA (positive control) demonstrated significant metal chelating activity with an IC50 of 232.28 ± 4.73 µg/ml, under the same experimental conditions. At a concentration of 1000 µg/ml, EDTA showed a chelating activity of 69.32%, while the BWE extract showed 34.81% at the same concentration. These findings provide a comparative perspective on the moderate ability of the BWE extract to scavenge metals.
Metal chelating activity assay
Finally, the evaluation of the antioxidant activity of the BWE extract was conducted by applying the metal chelating activity assay.
As shown in Fig. 8, the metal chelating activity assay revealed the nonsignificant activity of the BWE extract. Although the BWE extract exhibited dose-dependent activity, the highest activity observed was 29.76% at 1000 µg/ml concentration. These findings further suggest the relatively low metal chelating activity of the BWE extract, under the specified experimental conditions. On the contrary, EDTA showed dose-dependent and potential metal chelating activity with an IC50 of 21.16 ± 2.08 µg/ml. Furthermore, at a concentration of 1000 µg/ml, EDTA showed a metal chelating activity of 95.13%, while at a concentration of 2 µg/ml, 19.87% was observed. Taken together, the evaluation of antioxidant activity by several assays reveals that the BWE extract possesses potential antioxidant activity, which could be mainly attributed to its ability to scavenge free radicals.
Antimicrobial assessment
Despite the increasing availability of antimicrobials, the rate at which microorganisms develop drug resistance is concerning. Therefore, more research is needed to identify alternative treatments that are both more efficient and safer than the drugs currently available (30). Toward this, we assessed the antimicrobial activity of BWE extract against 29 microbial strains mainly (13 fungal strains, 8 Gram positive bacterial strains, and 8 Gram negative bacterial strains).
Evaluation of antifungal activity
Assessment of antifungal activity was performed using the well-established diffusion agar technique. The antifungal activity of the BWE extract was evaluated against the growth of 13 different fungal strains at a concentration of 10 mg / ml. In our assays, ketoconazole was used as a positive antifungal drug at 100 μg/mL concentration. As shown in Table 3, the BWE extract displayed a non-significant antifungal effect against the examined strains at the applied concentrations. These findings indicate that the BWE extract has no considerable activity toward the microorganisms tested for fungi.
Table 3.
The antifungal activity of BWE extract at 10mg / ml against the growth of 13 fungal stains
| Fungal strain | Inhibition Zone (mm) | |
|---|---|---|
| BWE extract | Ketoconazole | |
| Aspergillus fumigatus | NA | 17 |
| Aspergillus niger | NA | 15 |
| Candida albicans | NA | 20 |
| Penicillium aurantiogriseum | NA | 25 |
| Syncephalastrum racemosum | NA | 15 |
| Penicillium marneffeii | NA | 13 |
| Cryptococcus neoformas | NA | 25 |
| Candida lipolytica | NA | 18 |
| Penicillium expansum | NA | 17 |
| Penicillium italicum | NA | 18 |
| Fusarium moniliform | NA | 21 |
| Trichophyton rubrum | NA | 22 |
| Geotrichum candidum | NA | 14 |
Evaluation of antibacterial activity
The diffusion agar technique was also utilized to evaluate the antibacterial activity of the BWE extract. In this regard, the BWE sample was tested at 10 mg/ml concentration, while gentamycin was used as a positive control at 4μg/ml concentration. As shown in Table 4, the BWE extract exhibited considerable antibacterial activity against a set of Gram-positive bacteria, including Enterococcus faecalis, Streptococcus mutants, Staphylococcus epidermidis, and Micrococcus sp. strains. However, BWE extract did not show significant antibacterial activity against the strains of Staphylococcus aureus, Bacillus subtilis, Bacillus cereus and MRSA. These results indicate that BWE extract could be a natural medicinal extract available for the treatment of infections caused by susceptible bacterial strains, particularly Enterococcus faecalis, Streptococcus mutants, Staphylococcus epidermidis, and Micrococcus sp..
Table 4.
Antibacterial activity of BWE extract against a panel of Gram-positive bacterial strains
| Gram Positive Bacteria | Inhibition Zone (mm) | |
|---|---|---|
| BWE extract | Gentamycin | |
| Staphylococcus aureus | NA | 24 |
| Bacillus subtilis | NA | 26 |
| Bacillus cereus | NA | 25 |
| Staphylococcus epidermidis | 9 | 28 |
| Micrococcus sp. | 8 | 21 |
| Streptococcus mutants | 9 | 22 |
| Methicillin-Resistant Staphylococcus aureus (MRSA) | NA | 15 |
| Enterococcus faecalis | 9 | 26 |
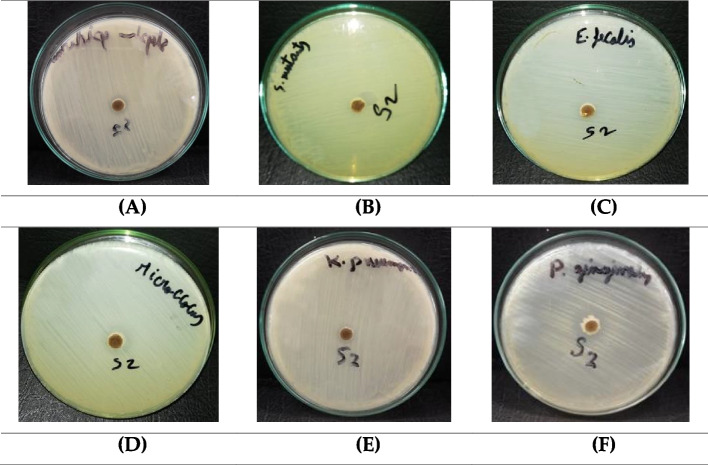
Furthermore, the BWE extract showed notable antibacterial efficacy against the growth of Gram-negative bacteria, including Klebsiella pneumonia and Porphyromonas gingivalis (Table 5). In particular, BWE demonstrates a substantial capacity to prevent the growth of Porphyromonas gingivalis bacteria, surpassing the effectiveness of the antibiotic Gentamycin. Porphyromonas gingivalis is recognized as a causative agent of periodontal diseases. Our findings underscore the considerable antibacterial potential of the BWE extract against the Porphyromonas gingivalis strain (Fig. 9).
Table 5.
The antibacterial activity of BWE extract against a panel of Gram-negative bacterial strains
| Gram Negative Bacteria | Inhibition Zone (mm) | |
|---|---|---|
| BWE extract | Gentamycin | |
| Enterobacter cloacae | NA | 30 |
| Salmonella typhimurium | NA | 17 |
| Escherichia coli | NA | 30 |
| Klebsiella pneumonia | 8 | 21 |
| Proteus vulgaris | NA | 25 |
| Serratia marcenscens | NA | 25 |
| Pseudomonas aeruginosa | NA | 27 |
| Porphyromonas gingivalis | 17 | 18 |
Fig. 9.
The growth inhibition zones of Staphylococcus epidermidi. (A), Streptococcus mutants (B), Enterococcus faecalis (C), Micrococcus sp. (D) Klebsiella pneumonia (E) and Porphyromonas gingivalis (F) after treatment with BWE extract
Evaluation of antitumor activity
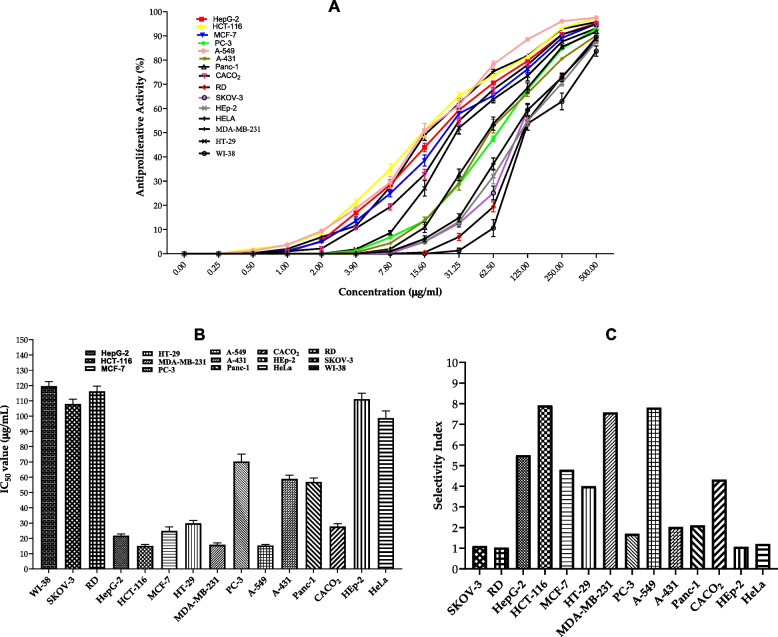
Finally, we assessed the antitumor activity of the BWE extract by screening its antiproliferation activity against a panel of 15 cell lines. The activity of the BWE extract was evaluated in triplicate at different concentrations, including 0.25, 0.5, 1, 2, 3.9, 780, 15.60, 31.25, 62.50, 125, 250, and 500 µg/mL. As shown in Fig. 10, the BWE extract exhibited considerable and dose-dependent antiproliferative activity toward the panel of texted cancer cell lines. The BWE extract demonstrated moderate cytotoxic activity towards SKOV, RD, HeLa and Hep2 cells with IC50 > 100µg/mL, while showing considerable activity toward PC-3, A-431 and Panc-1 cells with an IC50 of 55-70µg/mL. The evaluation of the selectivity index indicated that the extract displays a selectivity index of 1 towards SKOV, RD, HeLa, and Hep2 cancer cells, compared to WI-38 cells, indicating that the BWE extract does not possess significant selectivity toward these cell lines. On the other hand, BWE extract displayed selectivity index > 2 toward PC-3, A-431, and Panc-1 cells. Furthermore, the BWE extract demonstrated substantial antiproliferative activity toward the growth of HCT-116, A-549, MDA-MB-231, HepG-2, MCF-7, CACO2 and HT-29 cell lines with IC50 ranging from 15–30 µg/mL, and selectivity index > 3, as compared to WI-38 cells. Among the cells examined, the BWE extract exhibited the most cytotoxic activity toward HCT-116, A-549, MDA-MB-231cells with an IC50 of 15.11, 15.32, 15.79 µg/mL, respectively, and a selectivity index ~ 8. Furthermore, the evaluation of the cytotoxicity of the BWE extract on WI-38 cells (normal cells) indicated that the BWE extract possesses a non-significant cytotoxic activity on WI-38 cells with IC50 119.62 µg/mL. Collectively, these findings indicate that the BWE extract possesses potential antitumor activity and could be considered as a natural plant-based antitumor supplement.
Fig. 10.
A Evaluation of the antiproliferative activity of BWE extract against a panel of 14 cancer cell lines and normal human lung fibroblast WI-38 cells. B IC50 values of the antiproliferative activity of the BWE extract against examined cell lines. C The selectivity index of the BWE extract toward cancer cells compared to WI-38 cells. Data were presented as mean values with standard deviations in triplicate
Discussion
Various global markets have used various medicinal plants in the treatment of health conditions such as hepatotoxicity, cardiovascular disease, neurotoxicity, gastrointestinal diseases, and even cancer [93]. The various effects of the therapeutic effectiveness of most medicinal plants are believed to be caused by the complex chemical composition of the active phytochemical components [94, 95]. The presented study investigated the phytochemical profile of the acetone extract of BWE plant from the plant of Germany. We extensively explored the bioactivity of the BWE extract by assessing antioxidant, antimicrobial, and anticancer activity. In agreement with Khan et al., the GC–MS analysis of BWE extract demonstrated a set of metabolites, including: (-)-Spathulenol, Cholestan-3-ol, 2-methylene-, (3á,5à)-, Hexadecanoic acid, ethyl ester, Ethanol, 2-(9-octadecenyloxy)-, (Z)-, Hexadecanoic acid, methyl ester and Linoleic acid ethyl ester [96]. On the other hand, Khan et al. study detected other unique metabolites, including carophyllen oxide, β-Himachalenoxide, 3, 7, 11, 15-Tetramethyl-2-hexadecen-1-ol and 9, 12-Octadecadienoic acid, ethyl ester, despite sharing the same chemical formula as our compounds. Differences in the metabolites identified through gas chromatography (GC) analysis can stem from various factors, even when substances with identical chemical formulas are examined. These factors include column temperature, mobile phase flow rate, column length, and polarity of the compounds [97]. In addition, GC–MS analysis revealed a wide spectrum of metabolites within the BWE extract, each of which possesses different chemical structures. In particular, the presence of metabolites such as eicosanoic acid, methyl ester (also known as methyl eicosanoate), oleic acid, linoleic acid ethyl ester, and caryophyllene suggests the potential occurrence of bioactive molecules with known anticancer activities. For cis-13-octadecenoic acid, 9-octadecenoic acid (cis and trans isomers), hexadecanoic acid, methyl ester, methyl stearate, and terpinen-4-ol have been reported to possess antimicrobial properties. Furthermore, metabolites such as (-)-alpha-terpineol, (-)-spathulenol, and certain cyclopropane derivatives have been investigated for their antioxidant properties. It is essential to recognize that the biological activities of these metabolites are context-dependent and influenced by factors such as concentration, formulation, and the specific biological model used for the assessment.
Furthermore, phytochemical screening investigations have been carried out to gain more information on the categories of metabolites that are present in BWE, such as amino acids, flavonoids and phenolic acids, vitamins, and total nutrients. According to our results, the BWE extract displayed significant concentrations of the following metabolites: amino acids (especially glutamic acid (GLU) and aspartic acid (ASP)), flavonoids (such as catechin and luteolin), phenolic acids (such as catechol and syringenic), vitamins, fatty acids, saponins, tannins, carbohydrates, and protein. Joshi et al. 2014 showed that flavonoids, tannins, volatile chemicals, fatty acids, polysaccharides, isolectins, sterols, terpenes, protein, vitamins, and minerals are among the primary chemical components of Urtica dioica [98]. Furthermore, Kasouni et al. 2021 showed that the phytochemical analysis of the BWE extract demonstrated the presence of the following metabolites: coumarins, ketoses, carbohydrates, flavonoids, saponins, and resins. On the other hand, the plant extract under scrutiny revealed the absence of several metabolites, including steroids, proteins, tannins, alkaloids, and terpenoids [99]. Minor variations in the composition of the phytochemical profile may depend on the specific part of the plant subjected to extraction and the geographical location (demography) where the plant was harvested.
Our phytochemical analyses suggest potential biological activities of the BWE extract, including antioxidant, antimicrobial, and anticancer activities. The assessment of TAC revealed that the BWE extract possesses considerable antioxidant activity. Accordingly, we extended our investigation to explore the free radical scavenging activity and the metal chelating activity of BWE extract. The BWE extract showed significant scavenging activity for the free radicals ABTS, DPPH, and H2O2, compared to the ascorbic acid standard. Khan et al. 2023 [96] and Yousuf et al. 2022 [100] reported similar activity for the DPPH scavenging assay. Furthermore, Yousuf et al. 2022 (78) demonstrated strong scavenging activity for the ABTS assay. Also, Carvalho et al. [101] and Kukrić et al. 2012 [102] showed that Urtica dioica has strong antioxidant activity using ABTS, DPPH, and FRAP assays. The effective antioxidant activity of the BWE extract could be correlated with the inclusion of phenolic metabolites and their analogues [98, 103–105]. According to previous reports, the strong antioxidant activity of plant extract could also be associated with the presence of ascorbic acid, chlorophylls, carotenoids, and other metabolites [106–108]. In addition, the variation in ROS scavenging activity observed in different research studies may be ascribed to differences in pre- and post-harvest conditions. Variables such as soil types, organic manure types, weather conditions, and genetic differences can markedly affect variability in nutritional accumulation, consequently influencing biological activity [106]. For example, various agroecological conditions led to the identification of fluctuating amounts of nutrients in nearly identical species of Urtica dioica [103]. Although the BWE extract exhibited lower percentages compared to the reference EDTA in the CUPRAC and metal chelating activity assays, these values are not considered insignificant for a natural product [109]. Beyond mitigating oxidative stress by inhibiting the Fenton reaction, the chelating activity of plant extracts serves as a preventive measure against bacterial infections. The chelating properties of the extract induce bacteriostasis by extracting iron from the wound site since iron is essential for bacterial growth. The lactoferrin protein shows a comparable mode of action demonstrated by the lactoferrin protein [110].
Recently, microorganisms have begun to exhibit resistance to existing antimicrobials. Consequently, additional studies are imperative to identify alternative medicines that are not only more effective but also secure [111, 112]. Our results showed that the BWE extract does not have a significant antifungal impact against the 13 fungal microorganisms tested, compared to the reference Ketoconazole drug. On the other hand, Taheri et al. 2022 [36] and Gulçin et al. 2004 [113] showed that Urtica dioica extract has considerable antifungal activity against Candida albicans. The Brennnesselwurzel plant extract has also shown antibacterial activity against Gram-positive and Gram-negative bacteria. Our results showed that the BWE extract exhibits significant antibacterial activity against Staphylococcus epidermidi, Streptococcus mutants, Enterococcus faecalis, Micrococcus sp., Klebsiella pneumonia and Porphyromonas gingivalis. Contrary to our findings, the study reported by Zeković et al. 2017 [114] confirmed that Urtica dioica extract has considerable antibacterial activity with a MIC of 9.76 µg/ml against the methicillin-resistant staphylococcus aureus (MRSA) strain. Zenão et al. 2017 [115] revealed that these observed effects were related to their high amount of flavonoids (quercetin) and hydroxycinnamic acids/phenolic acids (chlorogenic, caffeic and rosmarinic acids). Furthermore, Ghaima et al. 2013 [116] also evaluated the antibacterial potential of Urtica dioica extract against various bacteria strains, including Salmonella typhi, Escherichia coli, Staphylococcus aureus, and Bacillus cereus, using cephalothin as standard drug. Their findings showed that the zone of inhibition of the plant extract against Salmonella typhi, Escherichia coli, Staphylococcus aureus, and Bacillus cereus was found to be 22, 10, 20, and 24 mm, respectively. On the contrary, the results of our plant extract did not demonstrate a significant impact against the same bacterial strains. Additionally, Modarresi-Chahardehi et al. 2012 [40] investigated the antibacterial activity of Urtica dioica against Methicillin-resistant Staphylococcus aureus (MRSA) and Bacillus cereus and showed that BWE exhibits MIC values of 16.33 and 8.33 mg/mL, respectively. On the other hand, our findings showed an insignificant impact against both strains. Further, Gulçin et al. 2004 [113] demonstrated that the BWE extract exhibits antibacterial effects against Escherichia coli, Staphylococcus epidermidis and Micrococcus luteus. The disparity in results could be attributed to the preparation of the Urtica dioica extract using different solvents, namely butanol, chloroform, diethyl ether, ethyl acetate, hexane, and methanol. Khan et al. 2023 [96] also tested the antibacterial activity of Urtica dioica against different bacterial strains, such as Escherichia coli, Bacillus subtilis, Staphylococcus aureus and Salmonella typhimurium. Their results showed that BWE has respectable antibacterial activity (1000 g/mL) against the tested bacterial strains when compared to ofloxacin (0.25 µg/mL). These findings are consistent with Jyoti et al. 2016 [117] and Zeroual et al. 2021 [118], both of whom reported similar results against the selected pathogens. Motamedi et al. 2012 [41] elucidated that the resistance of Gram-negative bacteria, particularly Escherichia coli and Salmonella typhimurium, can be attributed to their distinctive bacterial structure. They possess an outer membrane envelope that functions as a barrier that limits the entry of certain substances. Moreover, their porins control the type and size of molecules allowed to enter their cytoplasm. Some of these resilient species can also produce polysaccharide capsules, which hinder the entry of specific chemicals into the cytoplasm. These findings also implied that Urtica dioica extracts show significant antibacterial activity against Gram-positive species. This suggests the potential use of the plant as a selective treatment for infections, particularly the methicillin-resistant strain of S. aureus (MRSA), which poses a significant threat to human health. Furthermore, Dogruoz et al. 2008 [119] reported that the efficacy of bacteria inhibition or eradication can be affected by the specific plant extract, the solvent used, and the organisms under examination.
In addition to antioxidant assays, we also performed a cytotoxicity assay on 15 different cell lines to test the antitumor activity of BWE extract. The dose-dependent curve for the different cell lines showed that the BWE extract significantly reduces the viability percentage of the cancerous cell lines. In particular, the BWE extract showed substantial cytotoxic activity against HCT-116 (colorectal cancer), A-549 (lung cancer), MDA-MB-231 (breast cancer), HepG-2 (liver cancer), MCF-7 (breast cancer), CACO2 (colorectal cancer) and HT-29 (colorectal cancer) human cell lines, as indicated by IC50 values (15.11, 15.32, 15.79, 21.73, 24.9, 27.68, and 29.89 µg/ml, respectively). Interestingly, the BWE extract demonstrated a high selectivity index for cancer cell lines compared to WI-38 cells. In line with our findings, Mansoori et al. 2017 [120] showed that the Urtica dioica extract significantly suppressed the cancer cell line with an IC50 value of 31.37 mg/ml against the MCF-7 cell line and 38.14 mg/ml mg/ml against the MDA-MB-231 cell line. They concluded that according to QRT-PCR, Urtica dioica extracts reduced cell migration by upregulating the expression of E-cadherin and downregulating the expression of miR-21, matrix metalloproteinase (MMP) 1, MMP9, and MMP13. Moreover, Yousuf et al. 2022 [100] reported that BWE extract exhibits an IC50 of 114 µg/ml against the HepG-2 cell line. However, our results showed an IC50 of 21.73 µg/ml against the same cell line, suggesting that the current BWE extract possesses a unique set of secondary metabolites with antitumor characteristics. The difference in anticancer activity could be due to the different solvents that have been utilized in the extraction methodology. The extraction solvent may affect the phytochemical profile of the BWE extract, including flavonoids and tannin [121]. Ghasemi et al. 2016 [122] showed that the BWE extract has antiproliferation activity against human colon cancer HT-29 with an IC50 value of 24.7 μg/ml. Furthermore, Mohammadi et al. 2016 [123] revealed that the BWE extract exhibits cytotoxic activity against PC-3 with an IC50 value of 15.54 μg/ml. In alignment with these studies, our findings revealed that the BWE extract has IC50 of 29.89 μg/ml against HT-29 cells and IC50 of 70.24 μg/ml against PC-3 cells. Variation in antitumor activity of the BWE extract could be attributed to differences in demography and duration of treatment. In the Ghasemi et al. study, HT-29 cells were treated for 72 h, while in the study by Mohammadi et al. the PC-3 cells were treated for 48 h. Furthermore, Karakol et al. 2022 [46] detected cytotoxic activity of BWE extract against MCF-7 and MDA-MB-231 cells, as the IC50 of 18 and 14 μg/ml, respectively. Our results indicated a significant impact against MCF-7 and MDA-MB-231 cells with IC50 of 24.9 and 15.79 μg/ml, respectively. Cytotoxic activity may be due to polyphenolic chemicals, such as flavonoids, which may have anticancer effects through a variety of mechanisms, including antioxidant activity, activation of apoptosis, reduction of cell growth, and cell migration (42). Taking into account our results presented, the acetone BWE extract seems to exhibit considerable antioxidant, antibacterial, and antitumor activities. These activities could be associated with a wide variety of metabolites detected, including amino acids, flavonoids, phenolic acids, vitamins, fatty acids, tannins, carbohydrates, and proteins. More research is needed to explore the therapeutic potential of BWE extract in the animal disease model and to gain more insight into the possible applicability as a medicinal plant supplement against human diseases.
Conclusions
In summary, our study delved into the complete exploration of the 70% acetone extract of Brennnesselwurzel (Urtica dioica L.), revealing its diverse pharmacological potential. The phytochemical analysis showed a complex profile encompassing amino acids, flavonoids, phenolics, volatile oils, lipids, and vitamins. This intricate composition likely contributes to the observed biological activities of the extract. It should be noted that among its attributes, the BWE extract demonstrated robust antioxidant capabilities, as reflected in its substantial total antioxidant capacity and effective free radical scavenging against ABTS, DPPH, and H2O2. The potent antioxidant properties suggest a potential role in mitigating oxidative stress-related conditions. Furthermore, the extract demonstrated pronounced antibacterial efficacy against a variety of microbial strains, including Staphylococcus epidermidi, Streptococcus mutants, Enterococcus faecalis, Micrococcus sp., Klebsiella pneumonia and Porphyromonas gingivalis. This broad-spectrum antibacterial activity positions the BWE extract as a promising candidate for further exploration in the realm of antimicrobial agents. Furthermore, the study revealed compelling antiproliferative effects of the BWE extract against various cancer cell lines. The significant cytotoxic activity of HCT-116, A-549, and MDA-MB-231 cells was particularly striking, emphasizing its potential as an antitumor agent. Importantly, the extract showed a favorable selectivity index, indicating its ability to target cancer cells while sparing normal cells, as evidenced by its nonsignificant cytotoxicity towards WI-38 cells. Collectively, these findings underscore the multifaceted biological activities of BWE extract, which span antioxidant, antibacterial, and antitumor properties. The observed effects provide a strong reason for further investigations, including in vivo studies, to elucidate the full therapeutic potential of BWE as a natural plant-based supplement for human diseases.
Supplementary Information
Supplementary Material 1: Figure S1: Chromatogram of amino acids detected in the BWE extract by amino acid analyzer; Figure S2: Representative metabolites predominantly detected in the BWE extract by GC-MS analysis; Table S1: List of amino acid detected in BWE extract by Amino Acid Analyzer; Table S2: List of flavonoids detected by HPLC analysis in BWE extract; Table S3: List of phenolics detected by HPLC analysis in BWE extract; Table S4: List of fat-soluble vitamins detected by HPLC analysis in BWE extract; Table S5: List of water-soluble vitamins detected by HPLC analysis in BWE extract.
Acknowledgements
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RP23064).
Authors’ contributions
Amr Elkelish, Essa M. Saied, Sulaiman Lakoh, Mai Labib conceptualization, Supervision, Writing - Original Draft, Writing - Review & Editing, Data Curation, software and resourses. Abdelghafar M. Abu-Elsaoud, Alaa M. Alqahtani,:Norah Al harthi, Najwa Al harthi,:, Methodology, Investigation, Validation, Data Curation, Writing - Review & Editing,; Methodology.; Mohammad El-Nablaway: Project administration; Funding, Project, Review & Editing, Data Curation. All authors have read and agreed to the published version of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amr Elkelish and Mohammad El-Nablaway contributed equally to this work.
References
- 1.Abd Elbar OH, Elkelish A, Niedbała G, Farag R, Wojciechowski T, Mukherjee S, et al. Protective effect of γ-aminobutyric acid against chilling stress during reproductive stage in tomato plants through modulation of sugar metabolism, chloroplast integrity, and antioxidative defense systems. Front Plant Sci. 2021;12:663750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahan MS, Hasan MM, Alotaibi FS, Alabdallah NM, Alharbi BM, Ramadan KMA, et al. Exogenous putrescine increases heat tolerance in tomato seedlings by regulating chlorophyll metabolism and enhancing antioxidant defense efficiency. Plants. 2022;11:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moridani M, Harirforoosh S. Drug development and discovery: challenges and opportunities. Drug Discov Today. 2014;19:1679–81. [DOI] [PubMed] [Google Scholar]
- 4.Disorders F on N and NS, Policy B on HS, Medicine I of. Drug Development Challenges. In: Improving and Accelerating Therapeutic Development for Nervous System Disorders: Workshop Summary. National Academies Press (US); 2014. [PubMed]
- 5.El-Mogy MM, Atia MAM, Dhawi F, Fouad AS, Bendary ESA, Khojah E, et al. Towards better grafting: SCoT and CDDP analyses for prediction of the tomato rootstocks performance under drought stress. Agronomy. 2022;12:153. [Google Scholar]
- 6.El-Nashar HAS, Mostafa NM, El-Shazly M, Eldahshan OA. The role of plant-derived compounds in managing diabetes mellitus: a review of literature from 2014 To 2019. Curr Med Chem. 2021;28:4694–730. [DOI] [PubMed] [Google Scholar]
- 7.Bhuia MDS, Chowdhury R, Sonia FA, Kamli H, Shaikh A, El-Nashar HAS, et al. Anticancer potential of the plant-derived saponin gracillin: a comprehensive review of mechanistic approaches. Chem Biodivers. 2023;20:e202300847. [DOI] [PubMed] [Google Scholar]
- 8.Gaber A, Alsanie WF, Kumar DN, Refat MS, Saied EM. Novel papaverine metal complexes with potential anticancer activities. Molecules. 2020;25:5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saied EM, El-Maradny YA, Osman AA, Darwish AMG, Abo Nahas HH, Niedbała G, et al. A comprehensive review about the molecular structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics. 2021;13:1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskar YB, Mazumder PB. Insight into the molecular evidence supporting the remarkable chemotherapeutic potential of Hibiscus sabdariffa L. Biomed Pharmacother Biomed Pharmacother. 2020;127:110153. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Jiang J, Wu P, Zou J, Le J, Lin J, et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm Sin B. 2021;11:246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashad Y, Aseel D, Hammad S, Elkelish A. Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules. 2020;10:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Nashar HAS, Abbas H, Zewail M, Noureldin MH, Ali MM, Shamaa MM, et al. Neuroprotective effect of artichoke-based nanoformulation in sporadic Alzheimer’s disease mouse model: focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceuticals. 2022;15:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elshaer SE, Hamad GM, Sobhy SE, Darwish AMG, Baghdadi HH, H. Abo Nahas H, et al. Supplementation of Saussurea costus root alleviates sodium nitrite-induced hepatorenal toxicity by modulating metabolic profile, inflammation, and apoptosis. Front Pharmacol. 2024;15. [DOI] [PMC free article] [PubMed]
- 16.Phillipson JD. Phytochemistry and medicinal plants. Phytochemistry. 2001;56:237–43. [DOI] [PubMed] [Google Scholar]
- 17.Mushtaq S, Abbasi BH, Uzair B, Abbasi R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018;17:420–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devkota HP, Paudel KR, Khanal S, Baral A, Panth N, Adhikari-Devkota A, et al. Stinging Nettle (Urtica dioica L.): nutritional composition, bioactive compounds, and food functional properties. Mol Basel Switz. 2022;27:5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy A, Khan A, Ahmad I, Alghamdi S, Rajab BS, Babalghith AO, et al. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res Int. 2022;2022:5445291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Mol Basel Switz. 2016;21:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F. Worldwide research trends on medicinal plants. Int J Environ Res Public Health. 2020;17:3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid-Based Complement Altern Med ECAM. 2005;2:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doosti F, Dashti S, Tabatabai SM, Hosseinzadeh H. Traditional Chinese and Indian medicine in the treatment of opioid-dependence: a review. Avicenna J Phytomedicine. 2013;3:205–15. [PMC free article] [PubMed] [Google Scholar]
- 24.Romano B, Lucariello G, Capasso R. Topical collection “pharmacology of medicinal plants.” Biomolecules. 2021;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azab A, Nassar A, Azab AN. Anti-inflammatory activity of natural products. Mol Basel Switz. 2016;21:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nugraha AS, Agustina RP, Mirza S, Rani DM, Winarto NB, Triatmoko B, et al. Phytochemistry and pharmacology of medicinal plants used by the Tenggerese society in Java Island of Indonesia. Mol Basel Switz. 2022;27:7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeleye OA, Bamiro OA, Bakre LG, Odeleye FO, Adebowale MN, Okunye OL, et al. Medicinal plants with potential inhibitory bioactive compounds against coronaviruses. Adv Pharm Bull. 2022;12:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Zhao Y, Liu X, Li J, Zhang J, Liu D. Chemical constituents and pharmacological activities of medicinal plants from Rosa genus. Chin Herb Med. 2022;14:187–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D-P, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci. 2017;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su C, Li N, Ren R, Wang Y, Su X, Lu F, et al. Progress in the medicinal value, bioactive compounds, and pharmacological activities of gynostemma pentaphyllum. Mol Basel Switz. 2021;26:6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helmy YA, Taha-Abdelaziz K, Hawwas HAEH, Ghosh S, AlKafaas SS, Moawad MMM, et al. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics. 2023;12:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhusal KK, Magar SK, Thapa R, Lamsal A, Bhandari S, Maharjan R, et al. Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): a review. Heliyon. 2022;8:e09717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Große Brennnessel. Wikipedia. 2023.
- 34.Kregiel D, Pawlikowska E, Antolak H. Urtica spp.: ordinary plants with extraordinary properties. Mol Basel Switz. 2018;23:1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulauskienė A, Tarasevičienė Ž, Laukagalis V. Influence of harvesting time on the chemical composition of wild stinging nettle (Urtica dioica L.). Plants Basel Switz. 2021;10:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taheri Y, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Ezzat SM, Merghany RM, et al. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid-Based Complement Altern Med ECAM. 2022;2022:4024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Güder A, Korkmaz H. Evaluation of in-vitro antioxidant properties of hydroalcoholic solution extracts urtica dioica L., Malva neglecta Wallr. and Their Mixture. Iran J Pharm Res IJPR. 2012;11:913–23. [PMC free article] [PubMed] [Google Scholar]
- 38.Komes D, Belščak-Cvitanović A, Horžić D, Rusak G, Likić S, Berendika M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem Anal PCA. 2011;22:172–80. [DOI] [PubMed] [Google Scholar]
- 39.Kukrić: Characterization of antioxidant and antimicrobia... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Acta+Period.+Technol.&title=Characterization+of+antioxidant+and+antimicrobial+activities+of+nettle+leaves+(Urtica+dioica+L.)&author=Z.Z.+Kukri%C4%87&author=L.N.+Topali%C4%87-Trivunovi%C4%87&author=B.M.+Kukavica&author=S.B.+Mato%C5%A1&author=S.S.+Pavi%C4%8Di%C4%87&publication_year=2012&pages=257-272&doi=10.2298/APT1243257K&. Accessed 8 Jun 2023.
- 40.Modarresi-Chahardehi A, Ibrahim D, Fariza-Sulaiman S, Mousavi L. Screening antimicrobial activity of various extracts of Urtica dioica. Rev Biol Trop. 2012;60:1567–76. [DOI] [PubMed] [Google Scholar]
- 41.Motamedi H, Seyyednejad SM, Bakhtiari A, Vafaei M. Introducing Urtica dioica, A Native Plant of Khuzestan, as an antibacterial medicinal plant. Jundishapur J Nat Pharm Prod. 2014;9:e15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peralta MA, da Silva MA, Ortega MG, Cabrera JL, Paraje MG. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine Int J Phytother Phytopharm. 2015;22:975–80. [DOI] [PubMed] [Google Scholar]
- 43.Nematollahi: Antifungal activity of nettle (Urtica... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Int.+J.+Mol.+Clin.+Microbiol.&title=Antifungal+activity+of+nettle+(Urticadioica+L.)+and+European+pennyroyal+(Menthapulegium+L.)+extracts+on+Alternaria+alternata&author=S+Nematollahi&author=M+Sayidi&volume=7&publication_year=2017&pages=869-874&. Accessed 8 Jun 2023.
- 44.Saponaro M, Giacomini I, Morandin G, Cocetta V, Ragazzi E, Orso G, et al. Serenoa repens and urtica dioica fixed combination: in-vitro validation of a therapy for Benign Prostatic Hyperplasia (BPH). Int J Mol Sci. 2020;21:9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esposito S, Bianco A, Russo R, Di Maro A, Isernia C, Pedone PV. Therapeutic perspectives of molecules from urtica dioica extracts for cancer treatment. Mol Basel Switz. 2019;24:2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karakol P, Saraydin SU, Bozkurt M, Hepokur C, Inan ZDS, Turan M. Anticancer Effects of urtica dioica in breast cancer. Asian Pac J Cancer Prev APJCP. 2022;23:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrew RL, Wallis IR, Harwood CE, Foley WJ. Genetic and environmental contributions to variation and population divergence in a broad-spectrum foliar defence of Eucalyptus tricarpa. Ann Bot. 2010;105:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohamed DI, Alaa El-Din Aly El-Waseef D, Nabih ES, El-Kharashi OA, Abd El-Kareem HF, Abo Nahas HH, et al. Acetylsalicylic acid suppresses alcoholism-induced cognitive impairment associated with atorvastatin intake by targeting cerebral miRNA155 and NLRP3: in vivo, and in silico study. Pharmaceutics. 2022;14:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samaha D, Hamdo HH, Cong X, Schumacher F, Banhart S, Aglar Ö, et al. Liposomal fret assay identifies potent drug-like inhibitors of the Ceramide Transport Protein (CERT). Chem – Eur J. 2020;26:16616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khirallah SM, Ramadan HMM, Shawky A, Qahl SH, Baty RS, Alqadri N, et al. Development of novel 1,3-Disubstituted-2-Thiohydantoin analogues with potent anti-inflammatory activity; in vitro and in silico assessments. Molecules. 2022;27:6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salem MG, El-Maaty DMA, El-Deen YIM, Elesawy BH, Askary AE, Saleh A, et al. Novel 1,3-Thiazole Analogues with potent activity against breast cancer: a design, synthesis, in vitro, and in silico study. Molecules. 2022;27:4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohamed DI, Ezzat SF, Elayat WM, El-Kharashi OA, El-Kareem HFA, Nahas HHA, et al. Hepatoprotective role of carvedilol against ischemic hepatitis associated with acute heart failure via targeting miRNA-17 and mitochondrial dynamics-related proteins: an in vivo and in silico study. Pharmaceuticals. 2022;15:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdel-Wahab BA, F. Abd El-Kareem H, Alzamami A, A. Fahmy C, H. Elesawy B, Mostafa Mahmoud M, et al. Novel Exopolysaccharide from marine bacillus subtilis with broad potential biological activities: insights into antioxidant, anti-inflammatory, cytotoxicity, and Anti-Alzheimer activity. Metabolites. 2022;12:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 55.Sembiring E, Elya B, Sauriasari R, Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn J. 2018;10:123–7. [Google Scholar]
- 56.Shamsa F, Monsef H, Ghamooshi R, Verdian-rizi M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J Pharm Sci. 2008;32:17–20. [Google Scholar]
- 57.Rymbai H, Verma VK, Talang H, Assumi SR, Devi MB, Vanlalruati, et al. Biochemical and antioxidant activity of wild edible fruits of the eastern Himalaya, India. Front Nutr. 2023;10. [DOI] [PMC free article] [PubMed]
- 58.Kruger NJ. The Bradford Method For Protein Quantitation. In: Walker JM, editor. The Protein Protocols Handbook. Totowa, NJ: Humana Press; 2009. p. 17–24. [Google Scholar]
- 59.V. Le A, E. Parks S, H. Nguyen M, D. Roach P. Improving the Vanillin-Sulphuric Acid Method for Quantifying Total Saponins. Technologies. 2018;6:84.
- 60.Total Carbohydrate by Phenol-Sulfuric Acid Method. springerprofessional.de. https://www.springerprofessional.de/en/total-carbohydrate-by-phenol-sulfuric-acid-method/12344818. Accessed 14 Jan 2024.
- 61.Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Sci Food Agric. 1978;29:788–94. [Google Scholar]
- 62.Abadie C, Lalande J, Tcherkez G. Exact mass GC-MS analysis: protocol, database, advantages and application to plant metabolic profiling. Plant Cell Environ. 2022;45:3171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth - PubMed. https://pubmed.ncbi.nlm.nih.gov/32973295/. Accessed 22 Dec 2023. [DOI] [PMC free article] [PubMed]
- 64.Anzano A, de Falco B, Ammar M, Ricciardelli A, Grauso L, Sabbah M, et al. Chemical analysis and antimicrobial activity of moringa oleifera Lam. Leaves and Seeds Mol Basel Switz. 2022;27:8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kambhampati S, Li J, Evans BS, Allen DK. Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry. Plant Methods. 2019;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kahsay BN, Ziegler J, Imming P, Gebre-Mariam T, Neubert RHH, Moeller L. Free amino acid contents of selected Ethiopian plant and fungi species: a search for alternative natural free amino acid sources for cosmeceutical applications. Amino Acids. 2021;53:1105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharar M, Saied EM, Rodriguez MC, Arenz C, Montes-Bayón M, Linscheid MW. Elemental labelling and mass spectrometry for the specific detection of sulfenic acid groups in model peptides: a proof of concept. Anal Bioanal Chem. 2017;409:2015–27. [DOI] [PubMed] [Google Scholar]
- 68.Mizzi L, Chatzitzika C, Gatt R, Valdramidis V. HPLC analysis of phenolic compounds and flavonoids with overlapping peaks. Food Technol Biotechnol. 2020;58:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kebal L, Pokajewicz K, Djebli N, Mostefa N, Poliwoda A, Wieczorek PP. HPLC-DAD profile of phenolic compounds and In vitro antioxidant activity of Ficus carica L. fruits from two Algerian varieties. Biomed Pharmacother Biomedecine Pharmacother. 2022;155:113738. [DOI] [PubMed]
- 70.Tsiplakou: Sheep and goats differences in CLA and... - Google Scholar. https://scholar.google.com/scholar_lookup?title=Sheep%20and%20goats%20differences%20in%20CLA%20and%20fatty%20acids%20milk%20fat%20content%20in%20relation%20with%20mRNA%20stearoyl-CoA%20desaturase%20and%20lipogenic%20genes%20expression%20in%20their%20mammary%20gland&publication_year=2009&author=E.%20Tsiplakou&author=E.%20Flemetakis&author=C.%20Kalloniati&author=G.%20Papadomichelakis&author=P.%20Katinakis&author=G.%20Zervas. Accessed 22 Dec 2023.
- 71.Applied Sciences | Free Full-Text | Comparative Analysis of Fatty Acid Profile and Fat-Soluble Vitamin Content in Sheep and Goat Milk of Organic and Conventional Origin. https://www.mdpi.com/2076-3417/12/6/2809. Accessed 22 Dec 2023.
- 72.Kondyli: Chemical composition and microbiological... - Google Scholar. https://scholar.google.com/scholar_lookup?title=Chemical+composition+and+microbiological+quality+of+ewe+and+goat+milk+of+native+Greek+breeds&author=Kondyli,+E.&author=Svarnas,+C.&author=Samelis,+J.&author=Katsiari,+M.C.&publication_year=2012&journal=Small+Rumin.+Res.&volume=103&pages=194%E2%80%93199&doi=10.1016/j.smallrumres.2011.09.043. Accessed 22 Dec 2023.
- 73.Mendoza-Castillo: Impact of Mucuna bean (Mucuna spp.)... - Google Scholar. https://scholar.google.com/scholar_lookup?title=Impact+of+Mucuna+bean+(Mucuna+spp.)+supplementation+on+milk+production+of+goats&author=Mendoza-Castillo,+H.&author=Castillo-Caamal,+J.B.&author=Ayala-Burgos,+A.&publication_year=2003&journal=Trop.+Subtrop.+Agroecosyst.&volume=1&pages=93%E2%80%9396. Accessed 22 Dec 2023.
- 74.Imamović B, Ivazović I, Alispahić A, Bečić E, Dedić M, Dacić A. Assessment of the suitability of methods for testing the antioxidant activity of anti-aging creams. Appl Sci. 2021;11:1358. [Google Scholar]
- 75.Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci. 2011;73:146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of ficus religiosa. Mol Basel Switz. 2022;27:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41. [DOI] [PubMed] [Google Scholar]
- 78.Bhatti MZ, Ali A, Ahmad A, Saeed A, Malik SA. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res Notes. 2015;8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akinmoladun VI, Owotade FJ, Olusanya AA. Trace metals and total antioxidant potential in head and neck cancer patients. Ann Afr Med. 2013;12:131–4. [DOI] [PubMed] [Google Scholar]
- 80.Selvam R, Muruganantham K, Subramanian S. Phytochemical Screening and Evaluation of In Vitro Antioxidant Efficacy of Enicostemma littorale Blume Leaves Extract.
- 81.Callaghan CM, Leggett RE, Levin RM. A Comparison of total antioxidant capacities of concord, purple, red, and green grapes using the CUPRAC assay. Antioxid Basel Switz. 2013;2:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
- 83.Riyadh S, Gomha S, Mahmmoud E, Elaasser M. ChemInform abstract: synthesis and anticancer activities of thiazoles, 1,3-Thiazines, and thiazolidine using chitosan-Grafted-Poly(vinylpyridine) as basic catalyst. Heterocycles. 2015;91:1227. [Google Scholar]
- 84.Saied EM, Banhart S, Bürkle SE, Heuer D, Arenz C. A series of ceramide analogs modified at the 1-position with potent activity against the intracellular growth of Chlamydia trachomatis. Future Med Chem. 2015;7:1971–80. [DOI] [PubMed] [Google Scholar]
- 85.Alzamami A, Radwan EM, Abo-Elabass E, Behery ME, Alshwyeh HA, Al-Olayan E, et al. Novel 8-Methoxycoumarin-3-Carboxamides with potent anticancer activity against liver cancer via targeting caspase-3/7 and β-tubulin polymerization. BMC Chem. 2023;17:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Azab IH, Saied EM, Osman AA, Mehana AE, Saad HA, Elkanzi NA. Novel N-bridged pyrazole-1-carbothioamides with potential antiproliferative activity: design, synthesis, in vitro and in silico studies. Future Med Chem. 2021;13:1743–66. [DOI] [PubMed] [Google Scholar]
- 87.G. Salem M, El-ata SAA, H. Elsayed E, N. Mali S, Abdullah Alshwyeh H, Almaimani G, et al. Novel 2-substituted-quinoxaline analogs with potential antiproliferative activity against breast cancer: insights into cell cycle arrest, topoisomerase II, and EGFR activity. RSC Adv. 2023;13:33080–95. [DOI] [PMC free article] [PubMed]
- 88.Radwan EM, Abo-Elabass E, Abd El-Baky AE, Alshwyeh HA, Almaimani RA, Almaimani G, et al. Unveiling the antitumor potential of novel N-(substituted-phenyl)-8-methoxycoumarin-3-carboxamides as dual inhibitors of VEGFR2 kinase and cytochrome P450 for targeted treatment of hepatocellular carcinoma. Front Chem. 2023;11:1231030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohamed DI, Abou-Bakr DA, Ezzat SF, El-Kareem HFA, Nahas HHA, Saad HA, et al. Vitamin D3 prevents the deleterious effects of testicular torsion on testis by targeting miRNA-145 and ADAM17: in silico and in vivo study. Pharmaceuticals. 2021;14:1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohamed DI, Abo Nahas HH, Elshaer AM, El-Waseef DAE-DA, El-Kharashi OA, Mohamed SMY, et al. Unveiling the interplay between NSAID-induced dysbiosis and autoimmune liver disease in children: insights into the hidden gateway to autism spectrum disorders. Evidence from ex vivo, in vivo, and clinical studies. Front Cell Neurosci. 2023;17. [DOI] [PMC free article] [PubMed]
- 91.Arachchige GRP, Thorstensen EB, Coe M, McKenzie EJ, O’Sullivan JM, Pook CJ. LC-MS/MS quantification of fat soluble vitamers - a systematic review. Anal Biochem. 2021;613:113980. [DOI] [PubMed] [Google Scholar]
- 92.Arnao: The hydrophilic and lipophilic contribution... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Food+Chem&title=The+hydrophilic+and+lipophilic+contribution+to+total+antioxidant+activity&author=MB+Arnao&author=A+Cano&author=M+Acosta&volume=73&publication_year=2001&pages=239-44&. Accessed 22 Jun 2023.
- 93.Nirmala C, Bisht MS, Bajwa HK, Santosh O. Bamboo: a rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci Technol. 2018;77:91–9. [Google Scholar]
- 94.Hoang HT, Moon J-Y, Lee Y-C. Natural antioxidants from plant extracts in skincare cosmetics: recent applications challenges and perspectives. Cosmetics. 2021;8:106. [Google Scholar]
- 95.Danciu C, Zupko I, Bor A, Schwiebs A, Radeke H, Hancianu M, et al. Botanical therapeutics: phytochemical screening and biological assessment of chamomile, parsley and celery extracts against a375 human melanoma and dendritic cells. Int J Mol Sci. 2018;19:3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khan MZ, Azad AK, Jan S, Safdar M, Bibi S, Majid AMSA, et al. An Experimental and computational analysis of plant compounds from whole Urtica dioica L. Plant’s essential oil for antioxidant and antibacterial activities. Metabolites. 2023;13:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gas Chromatography | SpringerLink. https://link.springer.com/chapter/10.1007/978-981-15-1547-7_15. Accessed 22 Dec 2023.
- 98.Joshi: Pharmacognostical review of Urtica dioica L. - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Int.+J.+Green+Pharm.&title=Pharmacognostical+review+of+Urtica+dioica+L&author=B.+C.+Joshi&author=M.+Mukhija&author=A.+N.+Kalia&volume=8&publication_year=2014&pages=201-209&doi=10.4103/0973-8258.142669&. Accessed 22 Dec 2023.
- 99.The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8540079/. Accessed 22 Dec 2023. [DOI] [PMC free article] [PubMed]
- 100.Appraisal of the Antioxidant Activity, Polyphenolic Content, and Characterization of Selected Himalayan Herbs: Anti-Proliferative Potential in HepG2 Cells - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9735473/. Accessed 22 Dec 2023. [DOI] [PMC free article] [PubMed]
- 101.Carvalho AR, Costa G, Figueirinha A, Liberal J, Prior JAV, Lopes MC, et al. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res Int Ott Ont. 2017;99(Pt 1):485–94. [DOI] [PubMed] [Google Scholar]
- 102.Serbia - Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.) - Kukrić, Zoran Z.; Topalić-Trivunović, Ljiljana N.; Kukavica, Biljana M.; Matoš, Snježana B.; Pavičić, Svetlana S.; Boroja, Mirela M.; Savić, Aleksandar V. https://serbia.nb.rs/Article.aspx?ID=1450-71881243257K. Accessed 22 Dec 2023.
- 103.Otles S, Yalcin B. Phenolic compounds analysis of root, stalk, and leaves of nettle. ScientificWorldJournal. 2012;2012:564367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grauso L, de Falco B, Lanzotti V, Motti R. Stinging nettle, Urtica dioica L.: botanical, phytochemical and pharmacological overview. Phytochem Rev. 2020;19:1341–77. [Google Scholar]
- 105.Kőszegi: Changes in total polyphenol content and... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Period.+Polytech.+Chem.+Eng.&title=Changes+in+Total+Polyphenol+Content+and+Antioxidant+Capasity+of+Stinging+Nettle+(Urtica+dioica+L.)+from+Spring+to+Autumn&author=K.+K%C5%91szegi&author=E.+B%C3%A9k%C3%A1ssy-Moln%C3%A1r&author=N.+Koczka&author=T.+Kerner&author=%C3%89.+Stefanovits-B%C3%A1nyai&volume=64&publication_year=2020&pages=548-554&doi=10.3311/PPch.14338&. Accessed 22 Dec 2023.
- 106.Shonte TT, Duodu KG, de Kock HL. Effect of drying methods on chemical composition and antioxidant activity of underutilized stinging nettle leaves. Heliyon. 2020;6:e03938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drincovich MF, Voll LM, Maurino VG. Editorial: on the diversity of roles of organic acids. Front Plant Sci. 2016;7:1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jan KN, Zarafshan K, Singh S. Stinging nettle (Urtica dioica L.): a reservoir of nutrition and bioactive components with great functional potential. J Food Meas Charact. 2017;11:423–33. [Google Scholar]
- 109.Gioti EM, Fiamegos YC, Skalkos DC, Stalikas CD. Antioxidant activity and bioactive components of the aerial parts of Hypericum perforatum L. from Epirus, Greece. Food Chem. 2009;117:398–404. [Google Scholar]
- 110.Irshad M, Zafaryab M, Singh M, Rizvi MMA. Comparative analysis of the antioxidant activity of cassia fistula extracts. Int J Med Chem. 2012;2012:157125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taheri Y, Joković N, Vitorović J, Grundmann O, Maroyi A, Calina D. The burden of the serious and difficult-to-treat infections and a new antibiotic available: cefiderocol. Front Pharmacol. 2020;11:578823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zlatian O, Balasoiu AT, Balasoiu M, Cristea O, Docea AO, Mitrut R, et al. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp Ther Med. 2018;16:4499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gülçin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004;90:205–15. [DOI] [PubMed] [Google Scholar]
- 114.Zeković: Chemical and biological screening of stinging... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Industrial+Crops+and+Products&title=Chemical+and+biological+screening+of+stinging+nettle+leaves+extracts+obtained+by+modern+extraction+techniques&author=Z.+Zekovi%C4%87&author=A.+Cvetanovi%C4%87&author=J.+%C5%A0varc-Gaji%C4%87&volume=108&publication_year=2017&pages=423-430&. Accessed 22 Dec 2023.
- 115.Zenão: Antibacterial potential of Urtica dioica... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Journal+of+Herbal+Medicine&title=Antibacterial+potential+of+Urtica+dioica+and+Lavandula+angustifolia+extracts+against+methicillin+resistant+staphylococcus+aureus+isolated+from+diabetic+foot+ulcers&author=S.+Zen%C3%A3o&author=A.+Aires&author=C.+Dias&author=M.+Saavedra&author=C.+Fernandes&volume=10&publication_year=2017&pages=53-58&. Accessed 22 Dec 2023.
- 116.Ghaima: Antibacterial and antioxidant activities... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=J.+Appl.+Pharm.+Sci.&title=Antibacterial+and+Antioxidant+Activities+of+Ethyl+Acetate+Extract+of+Nettle+(Urtica+dioica)+and+Dandelion+(Taraxacum+Officinale)&author=K.K.+Ghaima&author=N.M.+Hashim&author=S.A.+Ali&volume=3&publication_year=2013&pages=96-99&doi=10.7324/JAPS.2013.3518&. Accessed 22 Dec 2023.
- 117.Jyoti: Characterization of silver nanoparticles synthesiz... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=J.+Radiat.+Res.+Appl.+Sci.&title=Characterization+of+silver+nanoparticles+synthesized+using+Urtica+dioica+Linn.+leaves+and+their+synergistic+effects+with+antibiotics&author=K.+Jyoti&author=M.+Baunthiyal&author=A.+Singh&volume=9&publication_year=2016&pages=217-227&doi=10.1016/j.jrras.2015.10.002&. Accessed 22 Dec 2023.
- 118.Zeroual: Wild chamomile [Cladanthus mixtus (L.) chevall.]... - Google Scholar. https://scholar.google.com/scholar_lookup?journal=Biointerface+Res.+Appl.+Chem.&title=Wild+chamomile+%5bCladanthus+mixtus+(L.)+chevall.%5d+collected+from+central-northern+Morocco:+Phytochemical+profiling,+antioxidant,+and+antimicrobial+activities&author=A.+Zeroual&author=E.H.+Sakar&author=N.+Eloutassi&author=F.+Mahjoubi&author=M.+Chaouch&volume=11&publication_year=2021&pages=11440-11457&. Accessed 22 Dec 2023.
- 119.ZEYBEK: Antibacterial activity of some plant extracts - Google Scholar. https://scholar.google.com/scholar_lookup?journal=IUFS+J+Biol.&title=Antibacterial+activity+of+some+plant+extracts.&author=N+Dogruoz&author=Z+Zeybek&author=A+Karagoz&volume=67&issue=1&publication_year=2008&pages=17-21&. Accessed 22 Dec 2023.
- 120.Mansoori B, Mohammadi A, Hashemzadeh S, Shirjang S, Baradaran A, Asadi M, et al. Urtica dioica extract suppresses miR-21 and metastasis-related genes in breast cancer. Biomed Pharmacother Biomedecine Pharmacother. 2017;93:95–102. [DOI] [PubMed] [Google Scholar]
- 121.Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol Adv. 2020;38:107382. [DOI] [PubMed] [Google Scholar]
- 122.Cytotoxic effects of Urtica dioica radix on human colon (HT29) and gastric (MKN45) cancer cells mediated through oxidative and apoptotic mechanisms - PubMed. https://pubmed.ncbi.nlm.nih.gov/27755943/. Accessed 22 Dec 2023. [PubMed]
- 123.Mohammadi A, Mansoori B, Aghapour M, Baradaran B. Urtica dioica dichloromethane extract induce apoptosis from intrinsic pathway on human prostate cancer cells (PC3). Cell Mol Biol Noisy--Gd Fr. 2016;62:78–83. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Figure S1: Chromatogram of amino acids detected in the BWE extract by amino acid analyzer; Figure S2: Representative metabolites predominantly detected in the BWE extract by GC-MS analysis; Table S1: List of amino acid detected in BWE extract by Amino Acid Analyzer; Table S2: List of flavonoids detected by HPLC analysis in BWE extract; Table S3: List of phenolics detected by HPLC analysis in BWE extract; Table S4: List of fat-soluble vitamins detected by HPLC analysis in BWE extract; Table S5: List of water-soluble vitamins detected by HPLC analysis in BWE extract.
Data Availability Statement
No datasets were generated or analysed during the current study.