Abstract
Toward the end of spermiogenesis, spermatid nuclei are compacted and the clonally related spermatids individualize to become mature and active sperm. Studies in Drosophila showed that caudal end-directed movement of a microfilament-rich structure, called investment cone, expels the cytoplasmic contents of individual spermatids. F-actin dynamics plays an important role in this process. Here we report that the dynein light chain 1 (DLC1) of Drosophila is involved in two separate cellular processes during sperm individualization. It is enriched around spermatid nuclei during postelongation stages and plays an important role in the dynein-dynactin–dependent rostral retention of the nuclei during this period. In addition, DDLC1 colocalizes with dynamin along investment cones and regulates F-actin assembly at this organelle by retaining dynamin along the cones. Interestingly, we found that this process does not require the other subunits of cytoplasmic dynein-dynactin complex. Altogether, these observations suggest that DLC1 could independently regulate multiple cellular functions and established a novel role of this protein in F-actin assembly in Drosophila.
INTRODUCTION
Sperm elongation and maturation involves complex choreography of a range of different cytoskeletal events leading to a large-scale alteration of cell shape and bulk membrane movement. One of the unique features of sperm differentiation is the clearing of cytoplasmic contents of spermatids after elongation, which also separates them as individual sperm. It is a key step to form mature and active sperm. Morphogenetic changes associated with sperm individualization have been studied in detail in Drosophila (reviewed by Lindsley and Tokuyasu, 1980). It involves compaction of the nuclei, formation of the microfilament rich investment cones around the nucleus, and extrusion of cytoplasmic contents of each spermatid by a caudal end-directed movement of the investment cone. In comparison, the molecular mechanisms underlying these processes are not so well understood.
F-actin and Lamin are the two known cytoskeleton components of investment cone, also known as F-actin cone (Fabrizio et al., 1998; Arama et al., 2003). Pharmacological treatments of isolated cysts established that F-actin dynamics plays a critical role in membrane extraction and F-actin cone movement and that both these processes are microtubule independent (Noguchi and Miller, 2003). F-actin–associated proteins, such as capping protein, cortactin, Arp2/3 complex, and myosin VI, are enriched at the leading edges of F-actin cones, whereas dynamin is localized throughout (Rogat and Miller, 2002), indicating that F-actin assembly would be initiated at the leading edges. Although, a later study showed that the F-actin assembly occurs throughout the cone (Noguchi and Miller, 2003). The F-actin cone bundles appeared disrupted in myosin VI homozygous testes (Hicks et al., 1999) and genetic interaction studies showed that myosin VI (jar1) and dynamin (shits1) could act in parallel pathways to maintain the F-actin levels in these cones (Rogat and Miller, 2002). Because the plasma membrane is not endocytosed at the investment cone, the role of dynamin in this organelle could be limited to F-actin assembly.
Dynamin activity is associated with F-actin dynamics involved in nonendocytic movement of membrane such as the actin comets, the lamellipodia, the podosome, and the membrane ruffles at the leading edges of migrating fibroblast (Schafer, 2002, for review). It is shown to interact with syndapin, cortactin, Abp-1, etc., and initiate F-actin assembly at these sites (McNiven et al., 2000; Kessels et al., 2001; Taunton et al., 2000; Krueger et al., 2003). In spite of all these studies, the mechanism underlying the regulation of dynamin dependent F-actin assembly in these events is still unknown.
The 8-kDa (89 aa) dynein Light Chain 1 (DLC1/LC8) is a conserved protein, which is known to interact with the IC (IC74) subunit of dynein and a large variety of other proteins (Rodriguez-Crespo et al., 2001). Consensus motifs have been identified in many of those proteins for these interactions (Lo et al., 2001). Therefore, it has been suggested that DLC1 could be involved in multiple cellular functions involving not only dynein but also several other proteins. Interestingly, a specific isoform in humans (DLC2) is shown to interact with myosin V but not dynein (Puthalakath et al., 2001) and an independent cell fractionation study with mouse brain extracts revealed that a large fraction of the cellular DLC1/LC8 proteins are not associated with microtubules (Benashski et al., 1997). Furthermore, a column pull-down assay that used the PIN/LC8 homologue revealed that this protein could interact with several isoforms of actin and dynamin-2 in rat brain lysate (Navarro-Lerida et al., 2004). All these suggest that DLC1 could also act independently of dynein, although no in vivo analysis of such functions has been reported so far.
We have recently shown that DLC1 plays an important role in dynein-dynactin–mediated cellular functions involved in spermatid elongation by genetic interaction studies in Drosophila (Ghosh-Roy et al., 2004). Preliminary investigation during this study showed that Drosophila DLC1 (DDLC1) could potentially play a further separate role in sperm individualization. We have investigated this further using a combination of genetic and immunohistochemical techniques to show that DDLC1 plays an important role in two different stages of spermiogenesis. It is involved in the dynein-dynactin–dependent anchoring of nuclei during spermatid elongation and for maintaining the F-actin cone assembly during sperm individualization afterward. The latter function is dynein-independent and requires dynamin. This work is the first to show a role of DLC1 in vivo that is dynein independent.
MATERIALS AND METHODS
Drosophila Stocks
All fly stocks were maintained on standard Drosophila cornmeal agar and sucrose medium at 25°C. Characterization of the ddlc1 alleles and the transgenic stocks used in this study were reported earlier (Ghosh-Roy et al., 2004) and the other stocks were obtained from the fly base (www.flybase.org). The levels of Ddlc1 mRNA was found in a decreasing order in exc39, ins1, and DIIA82 alleles, sw1 is a conditional (temperature sensitive) allele of the Drosophila IC74 gene (cdic; Boylan and Hays, 2002), and shits1 and shits2 are the conditional alleles of Dynamin. The nosGal4-VP16 (nG) was used for exclusive expression of the PUASp-DDLC1 (pDDLC1) and PUAST-MycPIN (mPIN) in male germ line cells for the rescue experiments as described before (Ghosh-Roy et al., 2004). Canton S stock was used as wild-type control for all the experiments.
Immunostaining Techniques and Antibodies Used
The testis dissection and immunostaining procedures were as described by Ghosh-Roy et al. (2004). F-actin and nuclei were stained by incubating the fixed tissue in 0.1 μg/ml RITC:phalloidin and 1 μg/ml DAPI (Sigma Chemical, St. Louis, MO), respectively, in PBST for 30 min, followed by several washes in PBST. The stained specimens were mounted under a coverslip using antifade mounting media (Vectashield, Vector Laboratories, Burlingame, CA). Mouse-anti-P10 (Dick et al., 1996) at 1/500 and rat-anti-DDLC1 at 1/200 (Ghosh-Roy et al., 2004) were used for the immunolocalization of DDLC1. The monoclonal anti-DHC (Sharp et al., 2000) and polyclonal anti-Shibire (Estes et al., 1996) were used at 1/10 and 1/200 dilutions, respectively.
Pharmacological Treatment of Isolated Cysts
Testes were dissected from Canton S males in Schneider's Drosophila medium containing l-glutamine (Invitrogen, Carlsbad, CA) and 10% fetal calf serum (Sigma Chemical). Cytochalasin D, 25 μM (Sigma Chemical), or 5 μM vinblastine (Sigma Chemical), or 100 μM colchicine (Sigma Chemical), or 24 μM latrunculin B (Sigma Chemical) was added to the medium before dissection. For controls, dimethyl sulfoxide was added to a final concentration of 0.003% as this was the solvent used to prepare the stocks of different pharmacological agents. Cysts were teased out of the testes during dissection and the preparations were incubated in the same media for 30 min at room temperature before they were fixed and processed for immunostaining as described above.
Quantification of Matured Spermatid Nuclei and IC Organization Index
The F-actin cone and matured nuclear bundle (NB) were labeled with RITC: phalloidin and DAPI, respectively. The NB and IC disruption was assayed using a slight modification of the method described earlier (Fabrizio et al., 1998). We have scored the morphology of the nuclear and F-actin cone bundles within intact testis. An IC was considered intact if the caudal ends of all or most of the constituent cones were organized in register and disrupted if they were out of register. The scoring was performed in a double blind manner by two different observers and the statistical significance between the control and mutants were tested by using the Mann-Whitney two-tailed test of significance.
Temperature Pulse Study
Batches of wild-type and mutant adults were grown at 18°C and 1 d after eclosion they were shifted to 29°C for a specified period. The testes were immediately dissected, fixed, and, then stained with DAPI, RITC:phalloidin, and other antisera, as indicated in the above section.
Quantification of Immunofluorescence Intensity
All images were collected by using a Bio-Rad Radiance 2100 machine (Richmond, CA) with a 60× 1.4 N/A Apochromat (Nikon, Melville, NY) objective. Laser power and the detector settings were kept constant to maintain consistency in the data collection system. Optical sections were scanned at 0.5-μm intervals covering the entire IC. Mean RITC:phalloidin staining intensity was estimated as an indirect measure of F-actin density at the cones. Optical section containing an entire F-actin cone was selected and mean pixel values in each cone was measured by using the ImageJ image analysis software (http://www.rsb.info.nih.gov/ij/). Average density values from several such cones belonging to multiple testes specimens of a particular genotype were plotted together in a histogram. To compare the relative reduction in F-actin levels after heat pulse among different mutant combinations, the intensity values after the heat pulse are presented as percent of the average intensity observed in the same genotypic background grown for the same period at permissive temperature (18°C). For example, the relative F-actin density in ddlc1ins1 after 23-h heat pulse = ([mean pixel value of a cone in ddlc1ins1 testis after the pulse]/[average intensity in the cones from ddlc1ins1 flies grown at 18°C]) × 100. We called this the relative F-actin density. The significance of the data were testes by nonparametric two-tailed Mann-Whitney tests as described above. To minimize fluctuations due to variability in staining, we dissected, fixed, and processed the control and the experimental specimens together.
RESULTS
Morphogenetic analysis of sperm differentiation in Drosophila divided the process into six phases: preelongation, elongation, transition, postelongation, individualization, and coiling (Lindsley and Tokuyasu, 1980). A clone of 64 nearly spherical spermatids is encapsulated by a pair of cyst cells and elongate in synchrony to form an equal number of spermatids of 1.8 mm length. The nuclei remain at the rostral ends of the elongating spermatids and elongate during this stage. It assumes a canoe like shape at the end of the transition period and then compacted into thin needle shaped structures during the postelongation period. A dense complex of microtubules is formed along the fenestrated side of each nucleus as they elongate and get dissolved just before the individualization.
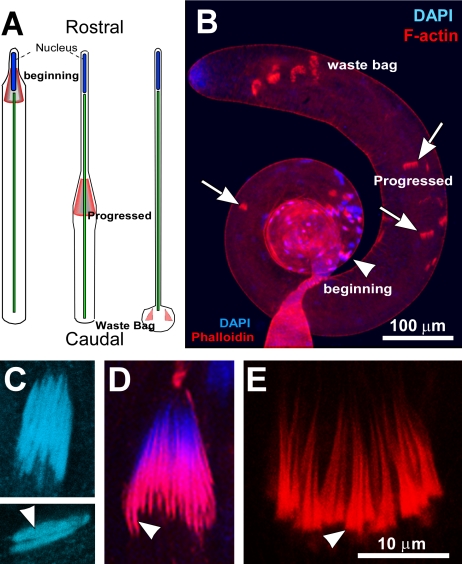
Toward the end of postelongation period a microfilament rich investment cone (length ∼12 μm, dmax ∼2 μm) forms at the caudal end of the nuclei around the basal-body and axoneme (Figure 1A). As it moves toward the tip of the tail, it extrudes all the cytoplasmic content from the spermatid and tightly invests the axoneme-mitochondria complex with a membranous envelope (Figure 1A). All the clonally related spermatids are anchored together at the rostral tips inside the cyst and the complex of 64 investment cones forms and moves in synchrony. Thus, they always remain in register and together the bundle is called an individualization complex (IC). ICs associated with the nuclei are the beginning population (arrowhead, Figure 1B) and they are always present at the basal end of the testis. ICs move toward the apical end of testis, which is also the caudal end of the spermatid, and the ICs are considered progressed (arrows, Figure 1B) if they are at least 10 μm away from the NB. Cytoplasm and extra membrane of each spermatid are collected in a membranous “cystic bulge” ahead of the investment cones, which is finally disposed into a large membranous “waste bag” (Figure 1, A and B) at the end of the spermatid tails and degraded.
Figure 1.
The stages of sperm individualization in wild-type testis. (A) Schematic illustrates relative positions of F-actin cones (marked in red) within a single spermatid cell at different stages of individualization. (B) RITC:phalloidin (red) and DAPI (blue) staining revealed matured nuclear bundles and the beginning stage ICs (arrowhead) at the basal region, progressed ICs (arrows) along the length of the testis and the waste bags near the apical tip. (C) Nuclear bundle (upper panel) and isolated nuclei (arrowhead) from a postelongation stage cyst. (D) Nuclear bundle and freshly formed investment cones (arrowheads) at the early individualization stage. (E) Bundle of progressed investment cones (arrowhead). C, D and E are presented at the same scale as indicated in E.
Morphological analysis indicated that the bundling of nuclei marks the beginning of the IC formation and movement in wild-type testes (Figure 1D). The entire individualization process is estimated to take nearly 24 h at 25°C inside the testis (reviewed in Lindsley and Tokuyasu, 1980) and the ICs take 10 h to complete the 1.8-mm journey under specific culture conditions in vitro (Noguchi and Miller, 2003).
DDLC1 Localizations in Postelongated Spermatids
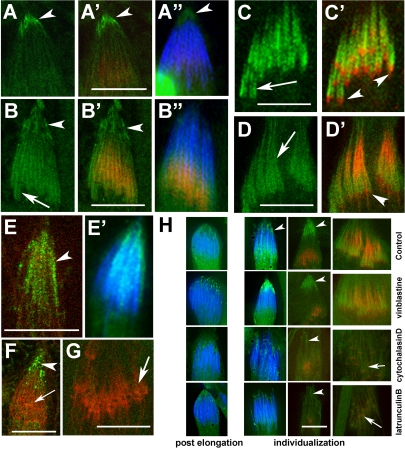
To determine the role of DDLC1 in the later stages of spermiogenesis, we studied its subcellular localization pattern during postelongation and individualization stages. DDLC1 staining was enriched in the rostral tips of the cells at the end of postelongation stage (arrowhead, Figure 2A). Afterward, as the F-actin cones are formed, the staining was found along the newly formed F-actin cones (arrow, Figure 2B). The staining was also found along the F-actin assembly in progressed investment cones (arrows, Figure 2, C and D), but it was always excluded from the leading edges of the cones (arrowheads, Figure 2, C and D). Identical staining along the progressed cones was observed with another DDLC1 specific antisera as well (Dick et al., 1996), and both the antisera recognized an approximately 10-kDa band in the adult head and abdomen extracts (unpublished data). Such enrichments along the F-actin cones were not visible in ddlc1ins1 hemizygous testis, which contains significantly lower levels of Ddlc1 mRNA (Ghosh-Roy et al., 2004). Hence, we concluded that the staining pattern is specific to DDLC1 and the diversity in spatiotemporal localization suggested that it could be involved in multiple different functions during postelongation and individualization stages.
Figure 2.
Immunolocalization of DDLC1 in maturing spermatids. (A–D) Squashed testes preparations stained with anti-DDLC1 (green), RITC:phalloidin (red), and DAPI (blue). (A–A″) Arrowheads indicate perinuclear localization of DDLC1 at the rostral end of a postelongation stage cyst. (B–B″) DDLC1 is enriched at both the perinuclear (arrowheads) as well as along the F-actin cones (arrows) during the early individualization stage. (C and C′) DDLC1 localization at the F-actin cones excludes the leading edges (arrowheads). (D and D′) DDLC1 localization in progressed investment cones (arrow). Arrowhead indicates the leading edge. (E–G) Anti-DHC (green), RITC:phalloidin and DAPI (blue) staining at the early (E and F) and progressed (G) stages of individualization. (E and E′) Arrowhead indicates DHC localization around the nuclei in a postelongation stage spermatid. (F) DHC (green) is condensed to perinuclear region (arrowhead) during early individualization stage, but it was absent from the F-actin cone (arrow). (G) DHC was also absent from the progressed F-actin cones (arrow). (H) DAPI (blue), DDLC1 (green), and RITC:phalloidin (red) staining in isolated cysts treated with different pharmacological agents as indicated at the right margins of each row. Pictures of equivalent stages are organized in a column. The first two columns from the left show epifluorescence images of the rostral ends of the cysts form postelongation and early individualization stages. Third and the fourth column contain confocal images of the rostral regions from early individualization stage cysts and the regions containing progressed ICs, respectively. Arrowheads indicate the perinuclear region in each figure, and arrows indicates the expected position of progressed investment cones in cytochalasin D– and latrunculin B–treated specimens. Scale bars in all figures indicate 10 μm.
DLC1 homologues are known to associate with cytoplasmic dynein complex in vivo. We observed the DHC staining around the nuclei of postelongation stage spermatids (arrowhead, Figure 2, E and E′) and later at the rostral region during early individualization stage (Figure 2F). This matched the pattern of DDLC1 staining during these stages. However, DHC was not found along the F-actin assembly at investment cones both at the early (arrow, Figure 2F) and at the later stages (arrow, Figure 2G). These observations suggested that cytoplasmic dynein may play a role at the early stages of individualization, but it is unlikely to be involved in investment cone assembly and movement afterward.
To learn more about the role of cytoskeleton in DDLC1 localization, we treated isolated cysts with different pharmacological agents. Treatments with microtubule-depolymerizing agents such as vinblastine and colchicine did not alter the rostral enrichment of DDLC1 (arrowheads, Figure 2H). In contrast, similar treatments with F-actin depolymerizing agents such as latrunculin B and cytochalasin D eliminated the localization (arrowheads, Figure 2H). This showed that F-actin and not microtubules play an important role in DDLC1 localizations at the rostral regions and along the investment cone. In addition, F-actin disruptions were also found to disrupt the nuclear organization at the early individualization stage (Figure 2H). This defect was less frequent in vinblastine-treated samples and did not occur after the colchicine treatment. Thus, DDLC1 appears to mediate an interaction between the spermatid nuclei and cortical actin cytoskeleton for the rostral retention of the nuclei at the beginning of individualization. This could be a dynein-mediated process. DDLC1 localization at the F-actin cones at a later stage were also found to be F-actin–dependent because it was eliminated by both cytochalasin D and latrunculin B treatments (arrows, Figure 2H). Altogether, these data indicate that DDLC1 primarily interacts with F-actin, which is surprising because it is known as a subunit of the Dynein complex.
Loss of ddlc1 Disrupts the Nuclear and F-actin Cone Bundles
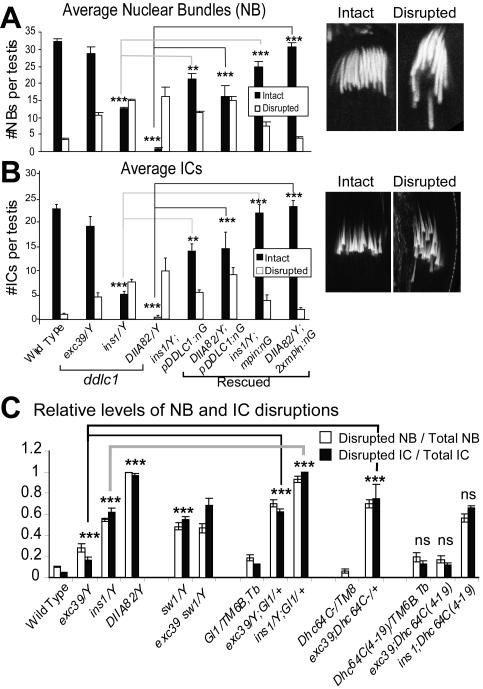
A preliminary observation showed that organizations of both the NBs and the F-actin cones in ICs were indeed disrupted in ddlc1ins1 testis. To ascertain whether ddlc1 would be involved in these processes, the NB and IC disruptions were quantified in different hypomorphic alleles. This was done to rule out nonspecific influences from the background and to establish a genetic correlation between the loss of ddlc1 expression and the phenotype. On an average 32 ± 5 intact NBs and 23 ± 4 intact ICs were found in wild-type testis. These numbers were significantly reduced in all three ddlc1 alleles (solid bars, Figure 3, A and B) and a large number of the NBs and ICs appeared disrupted (open bars, Figure 3, A and B). The defect was most severe in DIIA82 allele where almost all the NBs and ICs were disrupted (Figure 3, A and B).
Figure 3.
NB and IC organization defects in ddlc1 testes. (A and B) Average intact (solid bars) and disrupted (open bars) nuclear (A) and F-actin cone (B) bundles in wild-type and mutant testes. exc39, ins1, and DIIA82 represent weak, moderate, and strong alleles of ddlc1. pDDLC1:nG and mPIN:nG indicates the presence of UAS-DDLC1/+; nosGal4-VP16/+ and UAS-Myc-PIN/+; nosGal4-VP16/+, respectively, in the backgrounds. N = 8 or more for each set of plots; error bars, ±SEM. Statistical significance is tested with respect to the Canton S values as control for the ddlc1 mutants and appropriate ddlc1 allele for the rescue data as indicated by link lines between the bars. Extremely significant (p < 0.0001), very significant (p < 0.001) and significant (p < 0.01) differences are indicated by ***, **, and *, respectively, at the top of the bar. Typical examples of an intact and a disrupted NBs (A) and ICs (B) are shown at the right margins of the respective plots. (C) Relative levels of NB and IC disruptions in various genetic combinations with ddlc1 alleles are calculated by the ratio of disrupted to total population for each testis and plotted. N equals 8 or more for each data point and the error bars indicates ±SEM. For double mutant combinations, significance values were calculated with respect to each of the single mutant values (see Supplementary Table 1 for details).
Germ line–specific expression of either the UAS-DDLC1 (pDDLC1), or, the UAS-mycPIN (mPIN) transgenes by the nosGal4-VP16 (nG) driver in ddlc1 backgrounds (Figure 3, A and B) rescued the NB and IC disruptions. Because nosGal4-VP16 is an ectopic promoter, we studied the rescue with different copies of the transgenes. The disruptions were completely rescued with two copies of the transgenes in the ins1 and DIIA82 backgrounds. These results established that the loss of DDLC1 caused the phenotypes and further confirmed the requirement of a conserved DLC1 function in NB and IC organization process. exc39, ins1, and DIIA82 are the weak, moderate, and strong alleles, respectively, of ddlc1 according to the decreasing levels of Ddlc1 mRNA present in the tissue extracts from these alleles (Ghosh-Roy et al., 2004). Our results showed that the extent of the disruptions was proportional to the relative reduction of Ddlc1 levels in these alleles, suggesting a direct involvement of the protein in the NB and IC organization processes.
Cdic, P150dynactin, and Dhc64C Are Also Involved in ddlc1-dependent Nuclear and F-actin Cone Organization
To further test the involvement of dynein and dynactin in these processes, we studied the NB and IC disruptions in mutants of different dynein subunits and compared their interactions with ddlc1 alleles. sw1 is a conditional allele of the cdic, which codes for the IC74 subunit of the dynein-dynactin complex, and loss of the IC74 was shown to disrupt dynein-dynactin functions in Drosophila and other organisms (Bolyan et al., 2002). The ddlc1exc39 sw1 double mutants are viable at 25°C, but almost all ddlc1ins1 sw1 and ddlc1DIIA82 sw1 hemizygous males failed to emerge even at 18°C. About 60% of the NBs and ICs were disrupted in the sw1 hemizygous testes at 25°C (Figure 3D) and the level of IC disruption was marginally increased in ddlc1exc39 sw1 (Figure 3D) double mutant background. All these indicated that ddlc1 interacts with cdic, which is also involved in the NB and IC organization.
The levels of NB and IC disruptions were also dominantly enhanced by the presence of Glued1/+ (dominant negative allele of P150dynactin) and Df(3L)GN24/+ (deletion uncovering cytoplasmic Dhc64C gene; Gepner et al., 1996) in ddlc1 backgrounds (Figure 3D), whereas the Dhc64C4–19, which is known to suppress the ddlc1 defects in earlier stages of spermatogenesis (Ghosh-Roy et al., 2004), had no dominant effect on these phenotypes (Figure 3D). These suggested that along with the ddlc1 and cdic, the Glued and perhaps the Dhc64C gene products are also required to maintain the nuclear positions at the beginning of sperm individualization. In addition, we observed a tight correlation between the NB and IC disruptions, which indicates a role of dynein-dynactin complex in positioning the F-actin cones around nuclei. Alternatively, this could happen if the nuclear membrane act as a scaffold for the F-actin cones assembly. Similar linked disruptions were also observed in several other mutants before (Castrillon et al., 1993; Fabrizio et al., 1998).
DDLC1 Is Independently Required for the F-actin Assembly at the Investment Cones
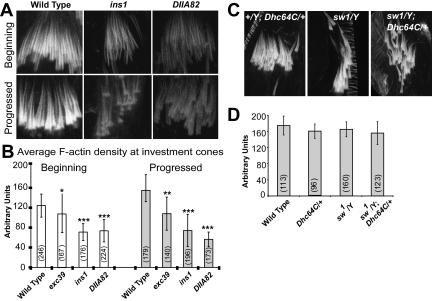
Nuclear bundling and F-actin cone assembly occurs in successive stages during postelongation and early individualization stages of spermiogenesis. The continuing presence of DDLC1 at the investment cones even during the later stages of individualization suggested a separate role of this protein during individualization. We found that the RITC:phalloidin staining intensity, which indicates F-actin levels, were visibly reduced at investment cones in ddlc1 hemizygous testes (Figure 4A). In addition, both the beginning and the progressed cones appeared thinner. Further measurement of average staining intensity at the cones revealed that indeed loss of ddlc1 reduced the F-actin levels in these organelles (Figure 4B). Once again, the levels of reductions were correlated directly to the reduction in the dose of ddlc1 in respective alleles. This evidence, together with the DDLC1 localization along the F-actin cones suggested that the protein is also required for F-actin assembly at investment cones. Interestingly, though the IC bundles appeared highly disorganized in sw1 and sw1, Dhc64C6–10/+ testes (Figure 4C), the F-actin density in the cones was not significantly different from that of the wild-type control (Figure 4D). This suggested that cytoplasmic dynein is unlikely to be involved in F-actin assembly at the cones, and, further underlined that IC disruption and the reduction in F-actin density are mutually independent phenotypes.
Figure 4.
F-actin levels at the investment cones in wild-type and ddlc1 alleles. (A) RITC-phalloidin staining at the ICs in wild type and mutants. (B) Average intensity of the staining at the investment cones as measured in arbitrary data units (ADU) and plotted in a scale of 0–255 Gy levels. N values are indicated under parenthesis on each bar; error bars, ±SD. Significance levels are measured with respect to the wild-type value and indicated on top of each bar as before. (C) Investment cone bundles from the +/Y; Dhc64C6–10/+ (control), sw1/Y, and sw1/Y; Dhc64C6–10/+ hemizygous testes. (D) F-actin levels at the progressed cones from the same set of alleles were measured and plotted as above. Dhc64C6–10 is referred as Dhc64C in C and D.
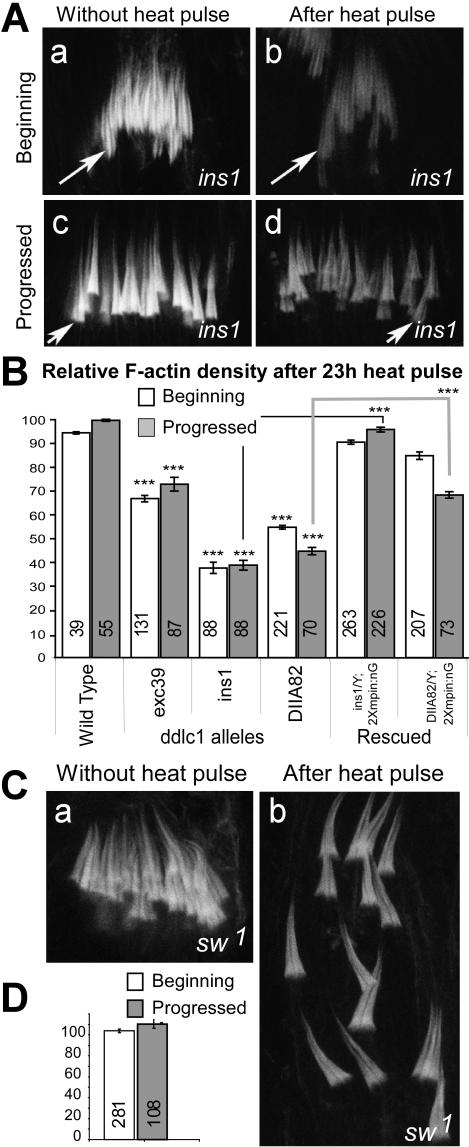
The recessive lethality of ddlc1 alleles were enhanced if they were grown at 29°C (Ghosh-Roy et al., 2004) and studies in Aspergillus showed that hypomorphic mutations in nudG (DLC1/LC8) could cause temperature-sensitive growth defects (Liu et al., 2003). Although, little is known about the temperature-dependent activity of DLC1/LC8 in vitro, these results indicate that the threshold of DLC1 activity could be lowered at higher temperature and this could cause conditional phenotypes in hypomorphic alleles. We utilized this conditional trait to establish further the role of DDLC1 in F-actin assembly at the investment cones. A systematic analysis showed that shifting 2-d-old mutant adults to 29°C for 23 h could visibly reduce the F-actin density at the cones in all three ddlc1 alleles (Figure 5A). Although the ICs appeared disrupted, their average number per testis remained unchanged. The controls were the same mutants grown constantly at 18°C. Further measurements revealed that the F-actin levels were significantly reduced in both the ins1 and DIIA82 backgrounds and both the beginning (open bars, Figure 5B) and the progressed populations (gray bars, Figure 5B) were affected. The level of reduction was proportional to the decrease in the Ddlc1 transcript levels in the respective alleles and it was rescued by the ectopic expression of mycPIN in ddlc1 backgrounds. Because individualization lasts for 24 h, a sizable fraction of the cysts containing progressed cones would have been formed before the heat pulse. Hence, these results suggest that DDLC1 would separately act at investment cones to maintain the F-actin assembly.
Figure 5.
Analysis of ddlc1 function during F-actin cone assembly. (A) RITC:phalloidin staining at the ICs from ddlc1ins1 testes grown continually at 18°C and after a 23-h pulse at 29°C. (B) Relative F-actin density at the wild-type and ddlc1 hemizygous cones after a 23-h pulse, calculated with respect to the average control values as per the descriptions provided in Materials and Methods. N-values are indicated on each bar; error bars, ±SEM. Significance of the mutant values are tested with respect to the wild-type and for the rescued data it was compared with the corresponding mutants as indicated by the link lines. (C) RITC:phalloidin staining in the ICs from sw1 testes before and after heat pulse. ICs were disrupted after the heat pulse but the staining levels remained unaltered. (D) Relative F-actin density after heat pulse in the sw1 alleles as described above.
In addition, we found that the F-actin cone bundles were nicely organized in ICs in sw1 hemizygous testis when the flies were grown at 18°C and they were severely disorganization after a 23-h heat pulse (Figure 5C), but the relative F-actin density in these cones remained unchanged even after the pulse (Figure 5D). This, together with the previous results, clearly established that the F-actin assembly at investment cones is dynein independent and highlighted that the role of DDLC1 in the F-actin assembly would be a novel one.
DDLC1 Regulates Dynamin Activity Required for the F-actin Assembly at Investment Cones
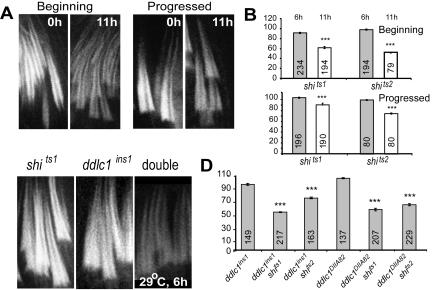
shibire is the Drosophila homologue of dynamin and certain mutations in the gene cause temperature-sensitive paralysis at nonpermissive temperatures (van der Bliek and Meyerowitz, 1991). Dynamin is present along the F-actin cones and it was proposed to play a role in F-actin assembly at the cones (Rogat and Miller, 2002). Therefore, we decided to investigate this further by shifting 2-d-old shits adults to 29°C for defined periods. F-actin density at the cones was significantly (p < 0.0001) reduced in the shits1 and shits2 hemizygous testes after 11 h of heat pulse (Figure 6, A and B) and the F-actin cones were not detectable in shits2 testes after a 16-h pulse (unpublished data). This indicated that dynamin activity is required for F-actin assembly at the ICs. Interestingly, there was no significant reduction in the relative F-actin levels after a 6-h pulse (Figure 6B), which perhaps indicate that the threshold of dynamin activity required to maintain F-actin levels at the cones is not sufficiently affected after this period.
Figure 6.
F-actin density at the investment cones is synergistically reduced in the shi ddlc1 double mutants after a 6-h heat pulse. (A) RITC:phalloidin staining at the F-actin cones from shits1 hemizygous testis, before and after an 11-h heat pulse. (B) Gray and open bars indicate relative F-actin levels at the investment cones after a 6- and an 11-h heat pulse, respectively, from shits1 and shits2 hemizygous testes. N values are indicated on each bar; error bars, ±SEM. (C) F-actin cones from shits1 ddlc1ins1 and ddlc1ins1 shits1 double mutant testes after a 6-h heat pulse. (D) Relative F-actin levels after a 6-h heat pulse in different mutant combinations. The data are presented for the progressed cones to highlight the synergistic level of the interaction.
Although there was no report of DLC1-dynamin interaction in vivo, the rat homologue (PIN/LC8) of this protein pulled down dynamin-2 from rat brain lysate (Navarro-Lerida et al., 2004). In addition, the pattern of dynamin localization along the F-actin cone indicated that such an interaction could also occur in Drosophila. We found that though a 6-h heat pulse causes no significant change in F-actin levels at the investment cones in ddlc1 alleles (Figure 6, C and D), the F-actin density was significantly (p < 0.0001) decreased in the ddlc1 shi double mutants after the same duration of pulse (Figure 6, C and D). Such synergistic interaction established that DDLC1 and dynamin interact with each other, or, act in parallel pathways leading to F-actin assembly at investment cones.
DDLC1 Is Required to Maintain Dynamin along the Investment Cones and Vice Versa
To determine further the mechanism underlying DDLC1 and dynamin actions during F-actin assembly at the ICs, we analyzed immunolocalization profiles of dynamin (Shibire) at the cones under different background conditions. In wild-type specimens, it was localized along the F-actin cones in both the beginning and the progressed populations (arrows, Figure 7, A and B). Like DDLC1, the staining excluded the leading edges (arrowheads, Figure 7, A and B) and overlapped with that of DDLC1 (arrows, Figure 7C). Dynamin staining at the cones disappeared after latrunculin B treatments (Figure 7D), indicating that the localization is F-actin dependent.
Figure 7.
Dynamin localization at the investment cones is reduced in ddlc1 testes. (A and B) Anti-dynamin (green) and RITC:phalloidin (red) staining of the wild-type ICs. Dynamin is found along the cone (arrow) excluding the leading edges (arrowheads). (C) Dynamin (green) is colocalized with DDLC1 (magenta) at the F-actin cones (arrows). (D) Dynamin (green) and RITC:phalloidin (red) staining at the cones (arrows) after only buffer (control), colchicine and latrunculin B treatments. (E and F) Gray levels of RITC:phalloidin and dynamin stainings at the ICs in specimens from ddlc1ins1 testes before (E) and after an 11-h (F) heat pulse are displayed in a false color scale (as depicted in F). (F) Dynamin staining is considerably reduced at the cones (arrows) after the heat pulse. (G) A typical IC from a shits2 hemizygous testis after a 16-h pulse shows loss of F-actin staining from the cones and diffused DDLC1 staining in the cytoplasm. Dynamin staining remained along the cones (arrow), but it was less compact than those found in the control (shits2 without heat pulse). Scale bar in A, 10 μm.
In ddlc1ins1 testes, dynamin was present at the cones when the flies were grown at 18°C (Figure 7E), but it was considerably reduced after an 11-h growth at 29°C (arrow in Figure 7F) and substantially reduced in the mutants grown constantly at 25°C (Supplementary Figure 1). We observed a similar reduction in ddlc1exc39 testes after a 16-h heat pulse (unpublished data). These results indicated that DDLC1 activity is required to maintain dynamin at investment cones. Thus, it could regulate dynamin dependent F-actin assembly at the cones.
Furthermore, because DDLC1 localization at the cones is F-actin dependent, the dynamin activity could in turn maintain DDLC1 at the ICs. This was confirmed when we found that a 16-h heat pulse could eliminate the RITC:phalloidin staining at the ICs and delocalize DDLC1 from the cones (Figure 7G) in shits2 hemizygous testes. However, dynamin staining at the cones remained unaltered even after the heat pulse (arrow, Figure 7G). Dynamin associates with the particulate fraction at nonpermissive temperature in shi mutants (Chen et al., 2002). Therefore, even though it dissolved F-actin at the cones, a local precipitation of the antigen during heat pulse could cause the retention of dynamin staining in this region in shits2 background. Altogether, these results confirmed that a dynamin → F-actin → DDLC1 feedback loop plays a critical role in maintaining F-actin dynamics at investment cones.
DISCUSSION
The DLC1/LC8 is an integral component of the dynein complex and it is expected to regulate dynein-dynactin interaction in vivo. However, its role in dynein-independent pathways was unknown. This study elucidated that DLC1 regulates two independent cellular functions within the same cell during spermatid differentiation in Drosophila: 1) dynein-dynactin–dependent nuclear positioning at the early stages of sperm individualization, and, 2) dynamin-mediated F-actin assembly during the later stages. The role of DLC1 in F-actin assembly at the ICs is a novel finding of this study. It provided an interesting clue about the possible regulation of dynamin dependent F-actin assembly in vivo and may have a greater impact on understanding the mechanism of F-actin assembly in actin comets, membrane ruffles, and in other similar processes.
DLC1 Regulates Dynein-Dynactin Function Involved in Nuclear Positioning in Sperm Heads
How nuclei remain anchored to the rostral ends of the spermatids during elongation is still an open question. Independent studies have shown that cytoplasmic dynein (Yoshida et al., 1994) and components of the dynactin (Fouquet et al., 2000) associate with the nuclear membrane, and they are present at the interface of the manchette microtubules and nuclear envelope in mammalian spermatids. The manchette is proposed to play a role in nuclear compaction during sperm maturation and the nuclei are anchored to acrosome though acroplaxome, which is an F-actin and keratin rich structure (Kierszenbaum et al., 2004). A dense complex of microtubules persists around sperm nuclei in Drosophila until the early individualization stage (Lindsley and Tokuyasu, 1980 for review), which may play a role equivalent to that of the manchette.
The cortical localization of dynein-dynactin in mammalian cells, which plays an important role in spindle positioning, requires F-actin (Busson et al., 1998; Dujardin and Vallee, 2002). It is involved in attaching spindle body to the cortical actin through dynactin, which facilitates nuclear movement along the microtubule into the daughter cell in S. cerevisiae and in several other fungi (Yamamoto and Hiraoka, 2003; for review). In addition, cytoplasmic dynein is shown to move isolated nuclei along the astral microtubules in a cell free system (Reinsch and Karsenti, 1997) and facilitate nuclear fusion after fertilization in vivo (Payne et al., 2003). Although DLC1 is an integral component of the cytoplasmic dynein complex and its homologue plays a key role in both dynein localization and nuclear migration processes in Aspergillus (Beckwith et al., 1998), its role in nuclear positioning process was unknown in higher eukaryotes.
Here we have shown that F-actin plays a key role in the rostral anchoring of nuclei in postelongation stage spermatids and DDLC1 along with the dynein-dynactin complex plays a key role in this process. In addition, both DDLC1 and DHC are enriched at the perinuclear region during this period. Interestingly, F-actin plays a role in the DDLC1 localization as well. All these suggest that the dynein-dynactin would retain the nuclei by a direct interaction between the cortical F-actin and nuclear membrane at the rostral end and DLC1 would play a critical role in it. In addition, these data also suggest that DLC1 could directly localize the Dynein complex to the cortex.
DLC1 Regulates Dynamin-mediated F-actin Assembly at the Investment Cones during Sperm Individualization
The DDLC1 localization along the investment cones and its involvement in the F-actin cone assembly at the cones were both unexpected and novel findings. Cytoplasmic DHC was not enriched along the investment cones and genetic studies showed that the cdic locus, which codes for the IC74 subunit of the dynein-dynactin complex, as well as the Dhc64C, was not involved in F-actin cone assembly. These results established that DDLC1 functions at ICs would be dynein independent. Furthermore, the synergistic reduction of F-actin density at ICs in the ddlc1 shi double mutant backgrounds and loss of dynamin localization at the ICs in ddlc1 alleles suggested an essential role of this protein in the dynamin mediated F-actin assembly process.
The characteristics of F-actin dynamics at the cones suggest that they are similar to the leading edges of lamellipodia (Noguchi and Miller, 2003) and they are compared with the actin comets found in mammalian cells (Bazinet and Rollins, 2003). Actin comets are dynamic actin-based structures that form on the endocytic vesicles and power their movement inside the cell. Bacteria, e.g., Listeria, Rickettsiae, etc., (Gouin et al., 1999) and some viruses (Gilbert et al., 2003) utilize this mechanism to invade and move inside the host cells. Dynamin is an integral component of F-actin comets (Lee and DeCamilli, 2002; Orth et al., 2002; Schafer et al., 2002); and it is found to organize F-actin assembly in lamellipodia (Schafer, 2002, for review). Dynamin interacts with proteins like syndapin, cortactin, Abp-1, etc., to initiate F-actin assembly at different intracellular sties (McNiven et al., 2000; Kessels et al., 2001; Taunton et al., 2000;; Krueger et al., 2003) and this regulates the membrane remodeling in lamellipodia, podosomes, and membrane ruffles.
What would be the role of such DLC1 at the F-actin cones? We propose that it could harness dynamin at the investment cone and thus regulate the nucleation of F-actin assembly on the axonemal sheath. This would result a rapid F-actin assembly, which in turn would propel the membrane toward the caudal end of spermatids. The DLC1 action in dynamin pathway could be limited to two main activities: 1) harnessing the protein along the F-actin cones through some membrane-anchored complex, and/or 2) regulate its interactions with different actin-binding proteins. Both these functions are equally likely. DLC1 is known to associate with transmembrane receptor complexes as well as various actin isoforms and dynamin (Navarro-Lerida et al., 2004), and we find that its localization at the cone requires F-actin. Furthermore, analysis of various dynamin sequences from the human, C. elegans, and Drosophila revealed a probable DLC1-binding motif in the GTPase effector domain of dynamin, which lies at the N-terminus of the proline-rich domain (A. Ghosh-Roy, unpublished results). Abp-1, syndapin, cortactin, and Arp2/3 interact with dynamin through the PRD domain. Hence, there is an interesting possibility that DLC1 could regulate these interactions.
Supplementary Material
Acknowledgments
We thank R. B. Vallee, Columbia University, New York, and K. S. Krishnan, Tata Institute of Fundamental Research, Mumbai, for their kind assistance and support, J. S. Scholey for generous supply of antibodies, and M. Peckham for a careful reading of the manuscript. A.G.R. and B.D. were supported by Kanwal Rekhi Career Development scholarship. Research described in this article was supported by an intramural grant from TIFR, India to K.R.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–02–0103) on April 13, 2005.
Abbreviations used: DDLC1, Drosophila dynein light chain 1; NB, nuclear bundle; IC, individualization complex.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Arama, E., Agapite, J., and Steller, H. (2003). Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell. 4, 687–697. [DOI] [PubMed] [Google Scholar]
- Bazinet, C., and Rollins, J. E. (2003). Rickettsia-like mitochondrial motility in Drosophila spermiogenesis. Evol. Dev. 5, 379–385. [DOI] [PubMed] [Google Scholar]
- Beckwith, S. M., Roghi, C. H., Liu, B., and Ronald-Morris, N. (1998). The “8-kD”cytoplasmic dynein light chain is required for nuclear migration and for dynein heavy chain localization in Aspergillus nidulans. J. Cell Biol. 143, 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benashski, S. E., Harrison, A., Patel-King, R. S., and King, S. M. (1997). Dimerization of the highly conserved light chain shared by dynein and myosin V. J. Biol. Chem. 272, 20929–20935. [DOI] [PubMed] [Google Scholar]
- Boylan, K. L., and Hays, T. S. (2002). The gene for the intermediate chain subunit of cytoplasmic dynein is essential in Drosophila. Genetics 162, 1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson, S., Dujardin, D., Moreau, A., Dompierre, J., and De Mey, J. R. (1998). Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8, 541–544. [DOI] [PubMed] [Google Scholar]
- Castrillon, D. H., Gonczy, P., Alexander, S., Rawson, R., Eberhart, C. G., Viswanathan, S., DiNardo, S., and Wasserman, S. A. (1993). Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics 135, 489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. L. et al. (2002). Unique biochemical and behavioral alterations in Drosophila shibire(ts1) mutants imply a conformational state affecting dynamin subcellular distribution and synaptic vesicle cycling. J. Neurobiol. 53, 319–329. [DOI] [PubMed] [Google Scholar]
- Dick, T., Ray, K., Salz, H. K., and Chia, W. (1996). Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. Mol. Cell. Biol. 16, 1966–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin, D. L., and Vallee, R. B. (2002). Dynein at the cortex. Curr. Opin. Cell Biol. 14, 44–49. [DOI] [PubMed] [Google Scholar]
- Estes, S. P., Roos, J., Van der Bliek, A., Regis, B. K., Krishnan, K., and Ramaswami, M. (1996). Traffic of dynamin within single Drosophila synaptic boutons relative to compartment specific presynaptic markers. J. Neurosci. 16, 5443–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio, J. J., Hime, G., Lemmon, S. K., and Bazinet, C. (1998). Genetic dissection of sperm individualization in Drosophila melanogaster. Development 125, 1833–1843. [DOI] [PubMed] [Google Scholar]
- Fouquet, J., Kann, M., Soues, S., and Melki, R. (2000). ARP1 in Golgi organisation and attachment of manchette microtubules to the nucleus during mammalian spermatogenesis. J. Cell Sci. 113, 877–886. [DOI] [PubMed] [Google Scholar]
- Gepner, J., Li, M., Ludmann, S., Kortas, C., Boylan, K., Iyadurai, S. J., McGrail, M., and Hays, T. S. (1996). Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics 142, 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Roy, A., Kulkarni, M., Kumar, V., Shirolikar, S., and Ray, K. (2004). Cytoplasmic dynein-dynactin complex is required for spermatid growth but not axoneme assembly in Drosophila. Mol. Biol. Cell 15, 2470–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, J. M., and Goldberg, I. G., and Benjamin, T. L. (2003). Cell penetration and trafficking of polyomavirus. J. Virol. 77, 2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin, E., Gantelet, H., Egile, C., Lasa, I., Ohayon, H., Villiers, V., Gounon, P., Sansonetti, P. J., and Cossart, P. (1999). A comparative study of the actinbased motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J. Cell Sci. 112, 1697–1708. [DOI] [PubMed] [Google Scholar]
- Hicks, J. L., Deng, W. M., Rogat, A. D., and Miller, K. G., and Bownes, M. (1999). Class VI unconventional myosin is required for spermatogenesis in Drosophila. Mol. Biol. Cell 10, 4341–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels, M. M., Engqvist-Goldstein, A.E.Y., Drubin, D. G., and Qualmann, B. (2001). Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J. Cell Biol. 153, 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum, A. L., Rivkin, E., and Tres, L. L. (2003). Acroplaxome, an F-actin-keratin-containing plate, anchors the acrosome to the nucleus during shaping of the spermatid head. Mol. Biol. Cell 14, 4628–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, E. W., Orth, J. D., Cao, H., and McNiven, M. A. (2003). A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14, 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, E., and DeCamilli, P. (2002). Dynamin at actin tails. Proc. Natl. Acad. Sci. USA 99, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and Tokuyasu, K. T. (1980). Spermatogenesis. In: The Genetics and Biology of Drosophila, Chapter 32, Vol. 2d, ed. M. Ashburner and T.R.F. Write, Oxford: Academic Press, 226–287. [Google Scholar]
- Liu, B., Xiang, X., and Lee, Y. R. (2003). The requirement of the LC8 dynein light chain for nuclear migration and septum positioning is temperature dependent in Aspergillus nidulans. Mol. Microbiol. 47, 291–301. [DOI] [PubMed] [Google Scholar]
- Lo, K. W., Naisbitt, S., Fan, J. S., Sheng, M., and Zhang, M. (2001). The 8-kDa dynein light chain binds to its targets via a conserved (K/R)XTQT motif. J. Biol. Chem. 276, 14059–14066. [DOI] [PubMed] [Google Scholar]
- McNiven, M. A., Kim, L., Krueger, E. W., Orth, J. D., Cao, H., and Wong, T. W. (2000). Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape J. Cell Biol. 151, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Lerida, I., Martinez Moreno, M., Roncal, F., Gavilanes, F., Albar, J. P., and Rodriguez-Crespo, I. (2004). Proteomic identification of brain proteins that interact with dynein light chain LC8. Proteomics 4, 339–346. [DOI] [PubMed] [Google Scholar]
- Noguchi, T., and Miller, K. G. (2003). A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development 130, 1805–1816. [DOI] [PubMed] [Google Scholar]
- Orth, J. D., Krueger, E. W., Cao, H., and McNiven, M. A. (2002). The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl. Acad. Sci. USA 99, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, C., Rawe, V., Ramalho-Santos, J., Simerly, C., and Schatten, G. (2003). Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. J. Cell Sci. 116, 4727–4738. [DOI] [PubMed] [Google Scholar]
- Puthalakath, H., Villunger, A., O'Reilly, L. A., Beaumont, J. G., Coultas, L., Cheney, R. E., Huang, D. C., and Strasser, A. (2001). Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293, 1829–1832. [DOI] [PubMed] [Google Scholar]
- Reinsch, S., and Karsenti, E. (1997). Movement of nuclei along microtubules in Xenopus egg extracts. Curr. Biol. 7, 211–214. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Crespo, I., Yelamos, B., Roncal, F., Albar, J. P., Ortiz de Montellano, P. R., and Gavilanes, F. (2001). Identification of novel cellular proteins that bind to the LC8 dynein light chain using a pepscan technique. FEBS Lett. 503, 135–141. [DOI] [PubMed] [Google Scholar]
- Rogat, A. D., and Miller, K. G. (2002). A role for myosin VI in actin dynamics at sites of membrane remodeling during Drosophila spermatogenesis. J. Cell Sci. 115, 4855–4865. [DOI] [PubMed] [Google Scholar]
- Schafer, D. A. (2002). Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol. 14, 76–81. [DOI] [PubMed] [Google Scholar]
- Schafer, D. A., Weed, S. A., Binns, D., Karginov, A. V., Parsons, J. T., and Cooper, J. A. (2002). Dynamin2 and cortactin regulate actin assembly and filament organization. Curr. Biol. 12, 1852–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. J., Brown, H. M., Kwon, M., Rogers, G. C., Holland, G., and Scholey, J. M. (2000). Functional coordination of three mitotic motors in Drosophila embryos. Mol. Biol. Cell 11, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton, J., Rowning, B. A., Coughlin, M. L., Wu, M., Moon, R. T., Mitchison, T. J., and Larabell, C. A. (2000). Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 148, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek, A. M., and Meyerowitz, E. M. (1991). Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351, 411–414. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A., and Hiraoka, Y. (2003). Cytoplasmic dynein in fungi: insights from nuclear migration. J. Cell Sci. 116, 4501–4512. [DOI] [PubMed] [Google Scholar]
- Yoshida, T., Ioshii, S. O., Imanaka-Yoshida, K., and Izutsu, K. (1994). Association of cytoplasmic dynein with manchette microtubules and spermatid nuclear envelope during spermiogenesis in rats. J. Cell Sci. 107, 625–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.