Abstract
Background
Less than one-third of sub-Saharan Africans have access to improved water sources. In US, Indian, and African studies, Bacterial vaginosis (BV) is increased among women with poor water, sanitation, and hygiene (WASH). We examined water source, sanitation (latrine type), and rainfall in relation to the vaginal microbiome (VMB).
Methods
In a cluster randomized controlled trial of menstrual cups and cash transfer, we measured the impact of cups on VMB via 16S rRNA gene amplicon sequencing in a subset of 436 adolescent girls. We analyzed how self-reported water source and latrine type at home related to VMB over 18-months, examining community state type I (CST-I, L. crispatus dominant) vs. other CST; alpha diversity; targeted taxa (coliform and other water-related pathogens); and non-targeted taxa via machine learning approaches. Mixed effects multivariable longitudinal models were adjusted for intervention arm, age, socioeconomic status, sexual activity, and cluster-level school WASH and rainfall (in millimeters).
Results
Adjusting for all covariates in all models: (1) the odds of CST-I were increased among participants with piped water (vs. pond), and decreased with traditional pit latrine vs. flush toilet. (2) Alpha diversity varied by water source and latrine type without consistent trends. (3) Coliform bacteria relative abundance (RA) was higher among participants with traditional pit or ventilated improved pit latrines vs. flush toilet, and higher among participants relying on stream vs. pond water. Streptococcus agalactiae RA was higher among participants with non-flush toilets, while Bacteroides fragilis RA was lower with non-flush toilets. (4) Key taxa from non-targeted analyses associated with water source and latrine type included typical vaginal bacteria, opportunistic pathogens, and urinary tract pathobionts. (6) Increased rainfall was associated with decreased odds of CST-I.
Trial registration
ClinicalTrials.gov NCT03051789, February 14, 2017.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10313-3.
Keywords: Vaginal microbiome, Water sanitation and hygiene, WASH, Climate change, Water, Latrine, Menstrual hygiene management, Bacterial vaginosis
Introduction
Access to clean water and sanitation is essential for health and wellness, and is particularly challenging in sub-Saharan Africa (SSA) where 32% of people lack access to improved water sources [1]. Bacterial contamination of water sources is a known risk factor for numerous human diseases [2, 3], but its link to reproductive health is less well established.
Bacterial vaginosis (BV) is the most common gynecologic infection [4], affecting approximately 20% of women worldwide and 20–50% of women living in SSA [5, 6]. In addition to causing distressing symptoms, BV increases the risk of acquiring HIV and other STIs [7] and experiencing adverse pregnancy outcomes [8]. In meta-analyses, consistent risk factors for BV include sexual exposures [9] and intravaginal washing [10]. Changes in the vaginal microbiome (VMB) from a Lactobacillus crispatus predominant state to one with a diverse range of bacteria is associated with development of BV [11]. The VMB composition has been characterized by community state types (CST), with CST-I representing a Lactobacillus crispatus dominant composition, CST-II representing a L. gasseri dominated composition, CST-III representing a L. iners dominated composition, and CST-IV representing more diverse compositions [12]. CST-I is considered optimal and is associated with lower rates of BV and STIs, while CST-IV is considered non-optimal and associated with greater rates of BV, vaginal mucosal inflammation and increased risk of HIV acquisition [13].
Non-optimal CST-IV has been associated with greater sexual exposure, having an uncircumcised male sexual partner, vaginal hygiene practices such as douching, and menstrual hygiene (e.g. type of material used) [14, 15]. While water, sanitation, and hygiene (WASH) conditions may contribute to BV risk, studies of water source in relation to VMB composition are lacking. In one study among United States military women, poorer WASH conditions were associated with higher rates of genitourinary infections including BV, urinary tract infections, and vulvovaginal candidiasis [16]. Both availability and water quality are likely to impact genitourinary health. One study in India found that body washing more than twice per day was associated with 1.5-fold increased odds of BV [17]; while not addressed in the study, more frequent washings may lead to an increased exposure of the VMB to waterborne organisms. Interestingly, this finding differed from a prior study by the same authors in a similar setting which found that increased frequency of bathing was associated with decreased rates of BV, though the prior study used the less sensitive Amsel criteria for BV diagnosis, rather than Nugent’s criteria used in the later study [18]. In a longitudinal study of women and girls aged 14–39 years in Rakai, Uganda, those using unprotected water (rainwater, pond, lake, or stream) for bathing had a greater risk of BV compared to those collecting water from a protected water (well, tap, or borehole) supply, adjusted for sexual practices, use of soap and water for vaginal washing, age, socioeconomic status, and HIV status [19]. While diarrheal and other waterborne diseases are known to fluctuate with seasonal rainfall [20], the effect of precipitation in relation to VMB is unknown and will become particularly important as the changing climate causes more extreme rainfall patterns [21].
Given the lack of data regarding the impact of water source and latrine type on the VMB, and scale of the potential exposure based on the high percent of people worldwide living with unimproved water source and limited sanitation facilities, we sought to examine water source, latrine type, and rainfall in relation to VMB in a cohort of adolescent schoolgirls in rural western Kenya. Better understanding the factors which affect the VMB among adolescents is critically important to targeting interventions which may have a lifelong impact on health including, but not limited to, risks of HIV/STI acquisition and maternal/fetal health. Intersecting with this, women in low resource settings are disproportionately adversely impacted by poor WASH. Generating understanding on these reproductive tract impacts can inform WASH that is tailored to women’s needs, opportunities for integrated health programming, and reducing gender inequalities [22].
Methods
This study was approved by the Institutional Review Boards of the Kenya Medical Research Institutes (#3215), Liverpool School of Tropical Medicine (#15 − 005), and University of Illinois at Chicago (#2017 − 1301).
Study setting
This study used data and specimens from Cups and Community Health (CaCHe), a prospective cohort study of adolescent secondary school girls in Siaya County, Kenya. The CaCHe study was nested in the Cups or Cash for Girls (CCG) Trial, a large cluster randomized controlled trial assessing the impact of menstrual cup and cash transfer interventions on a composite outcome of school dropout, HIV and HSV-2 [23] (ClinicalTrials.gov NCT03051789, February 14, 2017).
Siaya County is a largely rural area positioned 400 km west of Nairobi, adjacent to Lake Victoria. From the 2022 Kenya Demographic and Health Survey, 48.5% of the Siaya County population reported having access to basic services for drinking water, defined as having an improved water source within their residence or within a 30-minute round trip collection time (versus 98.6% in Nairobi and 67.9% nationally); 54.7% reported household excreta connected to sewers or was safely disposed of on-site or removed off-site (versus 91.6% in Nairobi and 65.9% nationally) [24]. According to 2017 data, the prevalence of HIV among adult men and women in Siaya County is the highest in the country at 21.0% (22.4% among women and girls and 19.4% among men and boys), compared to 4.9% in Kenya nationally [24].
Data collection
Participants self-completed a tablet-based survey in their language of choice (English or Dholuo) using Open Data Kit (v2.0.5) to obtain socio-demographic information and to assess sexual and menstrual hygiene management practices. Socio-demographic data included age and assessment of household amenities for socioeconomic status (SES) estimation following health and demographic surveillance system (HDSS) household survey questions. Construction of the SES score followed an absolute index method [25], following details published in [26]. Quintiles of SES were dichotomized as poorer (quintiles 1 to 2) vs. wealthier (quintiles 3 to 5). School-level WASH score was assessed and analyzed as previously described [26], dichotomized as better school WASH conditions vs. worse conditions. At each study visit, ever having sexual intercourse was assessed via two questions capturing coerced or forced sex and willing sexual activity, and transactional intercourse was assessed via a series of questions regarding sex in exchange for money, goods, or favors.
We obtained rainfall data from IMERG (Integrated Multi-satellitE Retrievals for GPM) [27] and CHIRPS (Climate Hazards group Infrared Precipitation with Stations) [28]. Data were extracted via application programming interface (API) calls based on date of study visit and the latitude and longitude of participants’ school, as we did not collect any home addresses. IMERG estimates precipitation via active and passive microwave satellite observations via Global Precipitation Measurement (GPM). CHIRPS data is obtained based on satellite-derived rainfall combined with rain-gauge data. IMERG has performed well over Lake Victoria in relation to capturing diurnal cycle magnitude and temporal variation in precipitation [27] CHIRPS also has accurate performance for spatial and temporal coverage of precipitation for East Africa [28]. Therefore, we assessed both rainfall variables in analyses.
Characterization of the vaginal microbiome
Specimen collection has been detailed elsewhere [26]. At each study visit, participants provided self-collected vaginal swabs for assessment of VMB. Swabs for amplicon sequencing (collected using OMNIgene vaginal (OMR-130, DNA Genotek, Ontario, Canada), were transported to the UNIM Research and Training Centre laboratory in Kisumu and placed at − 80 °C until shipment to Chicago for processing.
We sequenced the V3-V4 region of the microbial 16 S rRNA genes by employing a two-stage PCR protocol and primers 341 F and 806R to generate amplicon libraries, followed by sequencing on an Illumina MiSeq instrument, implementing V3 chemistry (600 cycles), as described elsewhere [29]. DNA extraction, library preparation, and sequencing were performed by the Genome Research Core at the University of Illinois Chicago. Annotation and community state types (CSTs) were conducted by University of Maryland Institute for Genomic Science [30], generating a biological observation matrix at the lowest taxonomic level identifiable. Vaginal CSTs were identified in a reference dataset using nearest centroid classification [31]. Among 1,655 observations, the median read count was 28,564; n = 23 (1.4%) observations had < 5,000 total sequence reads and were excluded from analyses for potentially poor read depth.
Following removal of putative contaminants via decontam, data were filtered to retain taxa that contributed at least 100 total sequence reads, resulting in the retention of 580 vaginal taxa (from 1,345 taxa in the initial observation matrix). Next, we filtered taxa based on having sufficient non-zero observations, and retained those which had at most 98% zeroes, resulting in 219 taxa for analyses. Raw sequence data (FASTQ files) were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), under BioProject identifier PRJNA746243.
Targeted taxa analysis
Specific taxa were selected based on known associations with water source contamination, colonization of the gastrointestinal or genital tract, and/or relation to Lake Victoria. PubMed Title and Abstract fields were searched for manuscripts related to water source contamination, vaginal microbiome or microbiota, and Kenya or Lake Victoria. Additional searches were performed as needed to clarify species and clinical significance of broader taxa. Primarily, the studies were concerned with identifying putative pathogens and those with impact on human health, and are summarized in Supplemental Table 1. Results of the literature review were used to group 181 taxa into 20 variables for analysis (Supplemental Table 2). The mean RA of targeted taxa was 3.4% and median 1.0% (Supplemental Table 3), with 90% of observations having less than 10% of total RA of the VMB attributed to these targeted taxa.
Statistical analyses
We examined the association of main water source and latrine type at home as reported at each visit with: (1) CST, (2) alpha diversity metrics, (3) targeted taxa of hypothesized importance, and (4) non-targeted taxa. This analysis makes use of data collected from baseline (May 2 – July 6, 2018) through 18-month (October 3–24, 2019) study visits. The 24-month visit (planned April 2020) was missed due to the COVID-19 pandemic. Thus, data from study visits subsequent to 18-months were excluded from analysis to avoid the potential confounding introduced by the pandemic.
Multivariable modeling
Modeling of CST, alpha diversity, and specific taxa relative abundances employed longitudinal mixed effects modeling, with random effects for cluster (school) and participant, and with robust variance estimation. CST was dichotomized as CST-I vs. other CSTs, and modeled with binomial distribution and logit link. Alpha diversity was analyzed using Shannon diversity index and richness, with Gaussian distribution. The relative abundance (RA) of targeted taxa and selected non-targeted taxa were modeled with Poisson distribution and log link with robust variance estimation to estimate the prevalence rate ratio [32]. Taxa distributions were truncated at the 99.5th percentile to exclude observations with overly high influence and obtain robust results.
Multivariable models were adjusted for: assigned intervention status (menstrual cup arm or control arm), baseline SES, baseline school WASH score, and time-varying age, sexual activity, having transactional sex, having a boyfriend, cluster-level rainfall, and study visit. Because having a boyfriend, sexual activity, and transactional sex were highly correlated, selection among these variables was according to minimized Akaike’s information criterion (AIC) and p-value. A similar approach was used to select between the two available values for rainfall (i.e., CHIRPS or IMERG). These mixed effects models were conducted in Stata/SE v17 using meglm for modeling CST, alpha diversity, and relative abundances of taxa.
Selection of non-targeted taxa via stability selection
Among the 219 taxa, we applied a machine learning approach called L0L2 regression within L0Learn R package [33], and Lasso regression within the stabs [34] package in R. L0Learn is advantaged to parsimoniously select predictors in low signal-to-noise settings, and was applied to 250 bootstrapped subsets of the data. Each subset underwent L0L2 regression, resulting in six logistic models corresponding to seven water categories (bore/lake/pipe/rain/river/stream vs. pond); for latrine type, there were 3 models corresponding to four categories (bush/traditional pit/ventilated improved pit vs. flush toilet). Taxa were selected using a cutoff p-value < 0.10, and occurrence in at least one multinomial model in more than 75% of the subsets. We then applied Lasso regression within stability selection, using 250 bootstrapped subsets of taxa and water source or latrine type, using a selection probability ≥ 0.85 for each taxon. We report the taxa common to both selection approaches to improve confidence in robustness of findings.
Results
Characteristics of the study sample
CaCHe enrolled a total of 436 participants, with 223 (51.1%) in the control arm and 213 (48.9%) receiving menstrual cups (Table 1). Reporting piped water in the home was uncommon (5.3%), with a plurality reporting lake (19.5%), rainwater (19.3%), river (18.6%), borehole (16.0%), pond (14.2%) or stream (7.0%) as the main water source. Among latrine types at home, the most common was traditional pit (45.7%) followed by ventilated improved pit (39.7%), flush toilet (11.6%), and bush/field (2.1%). Across 1,653 observations, unspecified responses for latrine type (n = 4) or water source (n = 6) were excluded from subsequent analyses.
Table 1.
Distribution of participant characteristics by study time point
| Variablesa | Baseline, N = 436 n (%) |
6 Monthsb, N = 424 n (%) |
12 Months, N = 395 n (%) |
18 Months, N = 398 n (%) |
|---|---|---|---|---|
| Intervention status | ||||
| Control arm | 223 (51.2) | 219 (51.6) | 202 (51.1) | 207 (52.0) |
| Menstrual cup arm | 213 (48.8) | 205 (48.4) | 193 (48.9) | 191 (48.0) |
| Median age in years (SD) | 17.0 (1.31) | 17.4 (1.35) | 18.0 (1.28) | 18.3 (1.24) |
| Baseline socioeconomic status, lower quintiles | 127 (29.1) | 123 (29.0) | 118 (29.9) | 116 (29.2) |
| Main source of water at home | ||||
| Pond | 61 (14.2) | 78 (20.7) | 61 (15.6) | 76 (19.3) |
| Borehole | 69 (16.0) | 42 (11.1) | 66 (16.8) | 65 (16.5) |
| Lake | 84 (19.4) | 88 (23.3) | 82 (20.9) | 85 (21.6) |
| Pipe in house | 23 (5.3) | 12 (3.2) | 20 (5.1) | 14 (3.5) |
| Rainwater | 83 (19.3) | 58 (15.4) | 65 (16.6) | 78 (19.8) |
| River | 80 (18.6) | 67 (17.8) | 75 (19.1) | 62 (15.7) |
| Stream | 30 (7.0) | 20 (5.3) | 22 (5.6) | 13 (3.3) |
| Other | 1 (0.2) | 12 (3.2) | 1 (0.3) | 1 (0.3) |
| Latrine at home | ||||
| Flush toilet | 50 (11.6) | 35 (9.3) | 47 (12.0) | 46 (11.7) |
| Bush/Field | 9 (2.1) | 10 (2.7) | 8 (2.0) | 11 (2.8) |
| Traditional Pit | 197 (45.7) | 237 (62.9) | 245 (62.5) | 228 (57.9) |
| VIP | 171 (39.7) | 95 (25.2) | 91 (23.2) | 109 (27.7) |
| Other | 4 (0.9) | 0 (0.0) | 1 (0.3) | 0 (0.00) |
|
IMERG rainfall: Median, mm (IQR) |
3.7 (1.2–13.4) | 2.0 (0–4.6) | 0.8 (0.1–13.8) | 5.2 (2.1–11.7) |
|
CHIRPS rainfall: Median, mm (IQR) |
7.0 (0–8.3) | 4.4 (0–10.2) | 4.5 (0–6.7) | 4.7 (2.3–5.1) |
| Baseline School-Level Water, Sanitation, and Hygiene (WASH) score (higher) | 262 (60.1) | 253 (59.7) | 233 (59.0) | 235 (59.1) |
| Has a boyfriend | ||||
| No | 410 (94.3) | 409 (96.5) | 302 (77.0) | 290 (73.6) |
| Yes | 25 (5.7) | 15 (3.5) | 90 (23.0) | 104 (26.4) |
| Reports being sexually active | ||||
| No | 287 (66.6) | 311 (82.5) | 155 (39.5) | 158 (40.1) |
| Yes | 144 (33.4) | 66 (17.5) | 237 (60.5) | 236 (59.9) |
| Reports engaging in transactional sex | ||||
| No | 379 (88.1) | 360 (95.5) | 346 (88.3) | 358 (90.9) |
| Yes | 51 (11.9) | 17 (4.5) | 46 (11.7) | 36 (9.1) |
| Bacterial vaginosis | ||||
| Negative (Nugent score 0–6) | 387 (88.8) | 385 (90.8) | 338 (85.6) | 342 (85.9) |
| Positive (Nugent score 7–10) | 49 (11.2) | 39 (9.2) | 57 (14.4) | 56 (14.1) |
| Vaginal Community State Type (CST) | ||||
| CST-I (L. crispatus dominant) | 187 (43.3) | 179 (45.5) | 146 (38.1) | 154 (39.1) |
| CST-II (L. gasseri dominant) | 12 (2.7) | 9 (2.1) | 9 (2.3) | 6 (1.5) |
| CST-III (L. iners dominant) | 147 (34.0) | 149 (35.4) | 146 (38.1) | 140 (35.5) |
| CST-IV (G. vaginalis, mixed) | 78 (18.2) | 78 (18.5) | 79 (20.6) | 86 (21.8) |
| CST-V (L. jensenii dominant) | 8 (1.8) | 6 (1.4) | 3 (0.78) | 8 (2.0) |
| Alpha diversity, mean (SD) | ||||
| Shannon diversity index | 1.36 (0.88) | 1.35 (0.89) | 1.46 (0.85) | 1.32 (0.87) |
| Richness | 33.3 (21.4) | 31.0 (19.8) | 29.1 (19.4) | 30.6 (21.2) |
aNot all cells sum to N due to missing data
bAt the 6-month study visit, n = 45 surveys were lost due to tablet failure
Association of main water source and latrine type with community state type
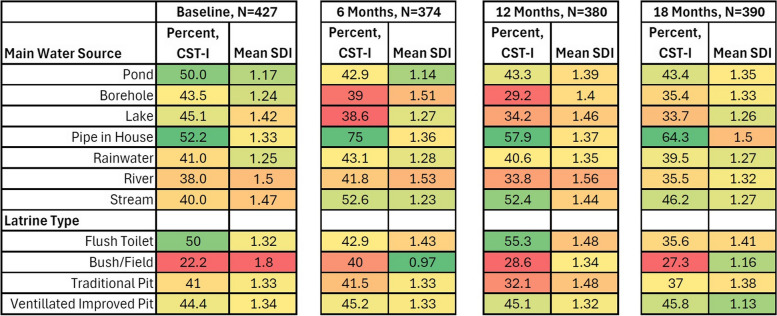
Participants with piped water had the highest proportion of L. crispatus dominated CST-I (52.2–75%) across all visits (Fig. 1). Among latrine types, flush toilet or ventilated improved pit were associated with higher percent of observations with CST-I (35.6–55.3% and 44.4–45.8%, respectively), and use of bush/field consistently had the lowest number of participants with CST-I (22.2–40%, with three of the four visits < 30%). Participants using traditional pit latrines showed an intermediate percent of observations with CST-I (32.1–41.5%). In regression (Table 2), participants reporting piped water (vs. pond water) were more likely to have CST-I (OR = 2.94, 95% CI: 1.08–3.82), though this attenuated when adjusted for all covariates (aOR = 1.61; 95% CI: 0.92–1.80). Compared to participants with a flush toilet, those with all other latrine types were 30–50% less likely to have CST-I, though after adjustment this was significant only for those with traditional pit latrines (aOR = 0.51; 95% CI: 0.26–0.99). Among the other factors analyzed, CST-I was higher among participants in the menstrual cup arm (aOR = 1.42; 95% CI: 1.13–1.79), and those attending schools with better WASH scores (aOR = 1.46, 95% CI: 1.00–2.11).
Fig. 1.
Distribution of vaginal community state type and shannon diversity index by water source and latrine type over time. Legend: Color shading and intensity represent proportions of optimal community state type-I (CST-I; L. crispatus dominant) and Shannon diversity index (SDI) by category of water source and latrine type. A lower proportion of observations CST-I and higher Shannon diversity index are represented with red shades, and a higher proportion of CST-I and lower Shannon diversity index are represented with green shades, with medium values transitioning through yellow. Thus, for example, participants with piped water in the home consistently have the highest proportion of observations with CST-I, while those relying on bush/field for latrine type consistently have the lowest proportion of observations with CST-I. Alpha diversity, as represented here by Shannon diversity index, is less consistent over visits
Table 2.
Crude and multivariable random effects analysis: factors associated with Community State Type I vs. other CST
| Covariates | Crude Odds Ratio (95% CI) |
Adjusted Odds Ratioa (95% CI) |
|---|---|---|
| Main Water Source at Home | ||
| Pond | Reference | Reference |
| Borehole | 0.71 (0.37, 1.36) | 0.64 (0.35, 1.16) |
| Lake | 0.73 (0.33, 1.63) | 0.64 (0.31, 1.32) |
| Pipe in House | 2.04 (1.08, 3.82) | 1.61 (0.92, 2.80)+ |
| Rainwater | 1.07 (0.71, 1.63) | 0.89 (0.64, 1.25) |
| River | 0.78 (0.37, 1.67) | 0.70 (0.33, 1.45) |
| Stream | 1.06 (0.67, 1.68) | 1.00 (0.60, 1.66) |
| Latrine Type at Home | ||
| Flush Toilet | Reference | Reference |
| Bush/Field | 0.52 (0.20, 1.34) | 0.54 (0.19, 1.52) |
| Traditional Pit | 0.51 (0.24, 1.07) | 0.51 (0.26, 0.99)^ |
| Ventilated Improved Pit | 0.82 (0.51, 1.30) | 0.74 (0.51, 1.07) |
| Intervention Arm (vs. Control) | 1.37 (1.12, 1.66) | 1.42 (1.13, 1.79)* |
| Age (in Years) | 0.74 (0.66, 0.83) | 0.77 (0.65, 0.92)* |
| Baseline Socioeconomic Status (lower vs. higher) | 0.66 (0.34, 1.27) | 0.77 (0.46, 1.30) |
| Baseline School-Level Water, Sanitation, and Hygiene (higher vs. lower) | 1.12 (0.76, 1.65) | 1.46 (1.00, 2.11)^ |
| Sexually Active | 0.66 (0.42, 1.02) | 0.73 (0.46, 1.18) |
| IMERG Rainfall Estimate (per 10 mm) | 0.98 (0.92, 1.06) | 0.95 (0.91, 1.00)* |
| Study Visit | ||
| Baseline | Reference | Reference |
| 6 month visit | 0.87 (0.67, 1.14) | 0.97 (0.70, 1.34) |
| 12 month visit | 0.66 (0.51, 0.85) | 0.96 (0.71, 1.30) |
| 18 month visit | 0.70 (0.44, 1.13) | 1.15 (0.68, 1.93) |
aSimultaneously adjusted for all variables presented. Final model includes N = 433 participants and N = 1,494 observations
*p < 0.01; ^ p < 0.05; +p < 0.10
Association of main water source and latrine type with alpha diversity
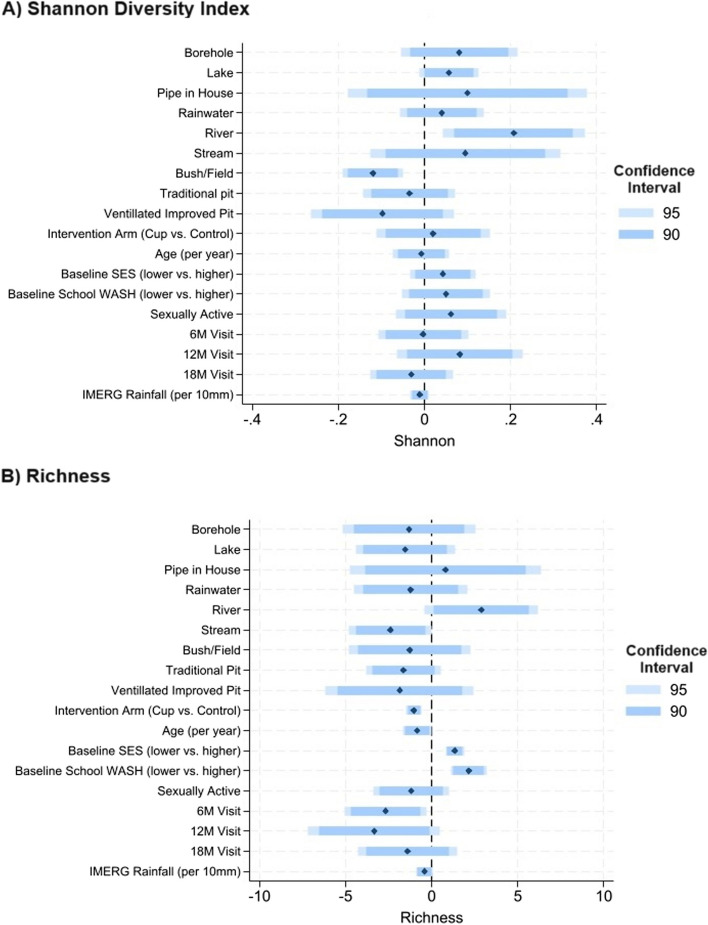
Compared to participants reporting pond as their main water source at home, Shannon diversity index was higher for participants with all other water sources, and significant in the adjusted analyses for those relying on lake or river water (Fig. 2). Compared to those with flush toilets, Shannon diversity index was lower for those with all other latrine types, reaching significance (p < 0.05) for those relying on bush/field. Shannon diversity index also decreased with increasing rainfall. There was not a consistent direction of association between water source and richness (number of taxa present), although richness was significantly elevated for those relying on rivers. No associations between latrine type and richness reached significance at the p < 0.05 level. In contrast to findings with Shannon diversity index, richness was significantly decreased among participants in the intervention arm.
Fig. 2.
Results of multivariable alpha diversity analyses. Legend: The results of multivariable mixed effects linear regression are summarized in the plots above for alpha diversity measures: (A) Shannon diversity index and (B) Richness. Coefficients are represented with black diamonds, and 95% and 90% Confidence Intervals (CI) in blue bars, as shown in the legend. Models are simultaneously adjusted for all variables presented. For water sources, the reference is “pond” and for latrine type the reference is “flush toilet”
Results of targeted taxa analysis
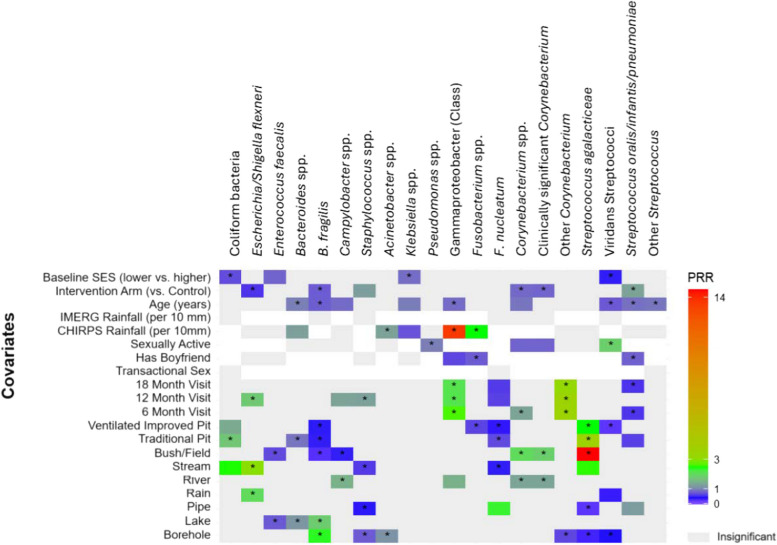
In multivariable analyses, the relative abundance (RA) of coliform bacteria was higher in the VMB of participants with stream compared to pond as main water source, and participants using a traditional pit or ventilated improved pit compared to those reporting a flush toilet at home (Fig. 3, Supplemental Fig. 1). Relying on rainwater and stream water was associated with a higher RA of Escherichia/ Shigella flexneri compared to pond water (p < 0.05, all). The RA of Escherichia/Shigella in the VMB did not vary by latrine type. Notably, among other factors examined, participants randomized to the menstrual cup arm had substantially lower RA of these species. The RA of Enterococcus faecalis was lower in participants using lake as a primary water source compared to pond and those using bush/field compared to those with a flush toilet in the home (p < 0.05, both). The RA of Staphylococcus spp. was lower in participants using borehole, piped water, or stream as compared to those using pond as main water source, and did not vary by latrine type. There was a greater RA of Campylobacter spp. in participants relying on river water compared to pond, and a lower RA in those who relied on bush/field compared to flush toilet.
Fig. 3.
Results of mixed effects multivariable poisson regression: targeted taxa association with water source and latrine type. Legend: Heatmap summarizing prevalence rate ratios (PRRs) for the associations between water source, latrine type, and other covariates in relation to the targeted taxa. The heatmap represents the direction and magnitude of the PRR for the covariates. PRRs < 1 (i.e., inverse relationship) are shaded in blue, and positive PRRs (> 1) are shaded in green to red, with deeper intensity representing the magnitude of the association. Covariate associations with p-value < 0.10 are shown in color, and those with p-value < 0.05 are indicated with an asterisk (*). Covariates associations that are not significant but maintained in the model for fit are shown in light grey. White space indicates the variable is not included in the model. For water sources, the reference is “pond” and for latrine type the reference is “flush toilet”
The RA of Klebsiella spp. did not significantly differ by water source or latrine type, but was inversely associated with rainfall, SES, and age. The RA of Acinetobacter spp. was higher among participants reporting a borehole as main water source compared to a pond, but did not vary by any other water sources or latrine type. Among other variables, only higher rainfall was associated with increased RA of Acinetobacter. The vaginal RA of Pseudomonas spp. did not vary by water source or latrine type, and was inversely associated with sexual activity. Participants who used a borehole or lake as their main water source had higher RA of Bacteroides fragilis compared to those who relied on pond water. There was a lower RA of B. fragilis among participants who used anything other than flush toilet at home, and among participants randomized to menstrual cups. A similar pattern was observed for Bacteroides spp.
There was a higher RA of Corynebacterium spp. in participants with river as the primary water source compared to pond. Corynebacterium spp. RA was also increased among those using bush/field or ventilated improved pit, and was decreased among participants reporting being sexually active, and those in the menstrual cup arm. Of the clinically relevant Corynebacterium species, a similar trend was observed in relation to water source and latrine type (Fig. 2). Among Corynebacterium species without known clinical relevance, there was a decrease in RA among those using a borehole as the primary source compared to pond and no association with latrine type. Fusobacterium spp. was not associated with water source, but there was a lower RA for those relying on ventilated improved pit latrines compared to a flush toilet, and increasing RA with increasing rainfall. There was greater RA of F. nucleatum in the VMB of those with piped water and lower RA among those relying on streams. F. nucleatum RA was decreased among users of traditional pit or ventilated improved pit compared to flush toilet.
Streptococcus agalactiae RA was decreased among participants who relied on boreholes or piped water compared pond water, and was increased for those relying on bush/field, traditional pit, and ventilated improved pit compared to flush toilet. Viridans group Streptococcus species were detected in lower RA when borehole or rainwater was the primary water source compared to pond, and when ventilated improved pit was the primary latrine type compared to flush toilet. The RA of viridans Streptococcus species was increased for participants reporting sexual activity, and decreased for those with higher SES and increasing age. The RA of Streptococcus pneumoniae (analyzed by the ASV including Streptococcus oralis and Streptococcus infantarius) was increased in participants reporting piped water compared to those relying on pond water, and was decreased for users of traditional pit compared to flush toilet and lower among participants reporting having a boyfriend, and greater among participants who received menstrual cups.
Results of non-targeted analyses
The union of L0Learn and Lasso applied within stability selection identified the following taxa differed by water source: Porphromonas somerae, Staphylococcus homonis/hamolyticus/simulans, Facklamia hominis, Peptoniphilus coxii, and family Prevotellacceae (Supplemental Fig. 2, Supplemental Table 3). The VMB RA of Porphyromonas somerae was increased for users of borehole, pipe, rainwater, river, and stream, and was decreased among those with flush toilet. Staphylococcus hominis/haemolyticus/simulans had lower RA in participants who reported relying on river water, traditional pit and ventilated improved pit. Facklamia hominis RA was increased among participants who reported lake as main water source, and was not associated with latrine type. Despite being identified through stability selection, Peptoniphlus coxii was not associated with water source or latrine type in adjusted models. The RA of Prevotellaceae was increased among participants relying on borehole for water source, and decreased for those relying on lake water.
The intersection of stability selection using L0Norm and stability selection using Lasso identified two taxa differing by latrine types: Sneathia sp. and Corynebacterium urealyticum. In multivariable adjusted analyses, the RA of Sneathia sp. was reduced among participants reporting piped or stream as home water source, and increased with all latrine types compared to flush toilet, though most so for participants relying on bush/field. Corynebacterium urealyticum RA was reduced for participants reporting using bush/field at home, and did not vary by water source.
Discussion
Vaginal microbiome composition was found to vary by water source, latrine type, and rainfall independent of age, socioeconomic status, sexual activity, school WASH score, and menstrual hygiene management. In non-targeted analyses via stability selection, several known vaginal commensals as well as Porphyromonas somerae (typical bovine rumen), opportunistic pathogens (Peptoniphilus coxii, Facklamia hominis) were identified to vary by water source and latrine type.
The likelihood of having CST-I, considered optimal for VMB, was highest amongst participants with pipe water in their house. This is consistent with prior studies showing lower rates of BV among women with a protected water source in their home [18, 19]. This is potentially due to contamination of unprotected water sources with bacteria from human and animal waste (as summarized in Supplemental Table 1). Our study of water quality in five sub-locations in Ahero County, another county in western Kenya approximately 90 km from Siaya County, measured home water sources: 42.9 − 87.5% had unsafe drinking levels of coliform bacteria (≥ 1 coliform/100 mL E. coli) in both open defecation free (ODF) and non-ODF locations [35]. Thus, if water sources in the study locations in Siaya are similarly contaminated, this could explain the recovery of these bacteria from VMB in relation to water source.
Associations of richness and Shannon diversity index in relation to water source and latrine type were sometimes divergent. For example, surface water sources including river and lake were associated with increased alpha diversity, although stream was associated with lower diversity, and Shannon diversity index was lower for participants reporting bush/field as their primary latrine type. These results were somewhat unexpected as more diverse VMB has been commonly associated with CST-IV [13], and participants reporting piped water and flush toilets were more likely to have with CST-I. Additional work is needed to evaluate the relative influence of water source and latrine type on VMB diversity vs. specific pathobionts and CST.
Consistent with CST analysis results, compared to pond water, improved water sources including borehole and piped water were associated with lower RA of potentially pathogenic bacteria overall including Streptococcus agalactieae (“group B strep”), and Staphylococcal species. Interestingly, borehole but not piped water was associated with a lower RA of both viridans group streptococcal species and Enterococcus faecalis. While it is not clear why this difference would be present for the borehole water source but not piped water, it may be related to the small number of participants with pipe as the primary water source, reducing validity and reliability of the observed association. Borehole was also associated with increased RA of Acinetobacter spp., Bacteroides fragilis, and Fusobacterium nucleatum. As Acinetobacter baumannii is a well-known colonizer of hospital plumbing [36], it may also colonize borehole associated pipes and faucets. While pipe colonization is not well-described for Bacteroides fragilis and Fusobacterium nucleatum, both of these organisms are known to form biofilms [37, 38], which may similarly permit colonization of water sources. Further study of colonization of pipes and faucets used to extract water across these sources is needed.
Use of river as the primary water source was associated with increased RA of Corynebacterium spp., class Gammaproteobacteria, and Campylobacter spp., and relying on stream water was associated with increased RA of coliform bacteria and Escherichia/Shigella, possibly from groundwater contamination or direct anthropogenic contamination of surface water, with identification of these taxa in several studies of groundwater near Kisumu and Lake Victoria [39, 40]. Participants reporting rainwater as their main source of water had notably higher RA of Escherichia/Shigella flexneri in the VMB. This is consistent with prior studies showing high rates of point of use contamination of rainwater [41].
As water source and SES are known to be associated [42], and given epidemiologic associations between water conditions in relation to BV [16, 17, 19], it is possible that water source and associated microbial contamination, has a direct contribution to non-optimal VMB. Conversely, as these organisms are commensals of the gastrointestinal tract, detection in the VMB could be a result of bacterial transfer from the rectum/gastrointestinal tract rather than direct exposure to water. In a cohort of pregnant women in Japan, BV-associated bacterial species in the vagina and rectum were correlated, and authors posited that the rectum served as an extragenital reservoir for these bacteria [43]. In a case control study of women infected with C. trachomatis comparing VMB among those with concomitant rectal infection to those with cervical infection only, approximately 60% of taxa presence was similar between rectal and vaginal microbiome [44]. Because the main water source used at home likely serves multiple purposes (e.g., drinking, bathing, washing, cooking), future studies need to assess these uses, practices of water treatment (e.g., filtration, boiling, chlorination), and correlation with gut/rectal microbiome.
In non-targeted analyses, several taxa varied by water source and latrine type. Notably, non-targeted analyses did not identify typically dominant vaginal taxa, such as Lactobacilli species or Gardnerella vaginalis. This suggests that while water source and latrine type may contribute to VMB composition, they are unlikely to be primary drivers of dominant VMB composition. As noted, the mean RA of targeted taxa was just 3.4% and even less for the non-targeted taxa (Supplemental Table 3). However, in approximately 5% of observations, targeted taxa accounted for more than 15% of the overall RA, and often yielded high RAs of enteric bacteria, S. agalactiae, and Staphylococcus. These bacteria are associated with aerobic vaginitis (AV) [45], a condition which is distinct from BV but similarly associated with increased inflammation [46] and a range of clinical implications, including preterm birth, low birth weight, and puerperal sepsis [47]. Future analyses will examine the association between these taxa and adverse birth outcomes in this cohort.
Increased rainfall was associated with increased RA of Acinetobacter spp., Bacteroides spp., class Gammaproteobacteria, and Fusobacterium spp. (excluding F. nucleatum) as well as decreased odds of CST-I. This is consistent with prior studies showing increased microbial contamination of water following increased rainfall [48] as well as decreased use of improved water sources during periods of heavy rainfall [49]. Unexpectedly, while CST-I and rates of specific contaminants increased, alpha diversity decreased; the meaning of this is unclear. It is likely other factors modify the association between rainfall and vaginal microbiome, operating through household- specific water source and WASH impacts.
Among other factors evaluated, randomization to menstrual cups was associated with increased odds of CST-I, in keeping with previous findings [26]. Sexual activity was associated with reduced RA of Corynebacterium spp., Fusobacterium spp. excluding Fusobacterium nucleatum, Streptococcus pneumoniae and an increase in the RA of Streptococcus viridans; reduction in these mostly enteric pathogens with onset of sexual activity may be related to competition from introduction of new bacteria as sexual activity is known to increase diversity of the VMB [9, 14, 50].
We found that latrine type varied over time in 34.9% of reports, with 55.3% stemming from shifts between traditional pit latrine and VIP latrines: 22.0% from traditional to VIP; 33.3% from VIP to traditional. The Kenyan government has been scaling community led total sanitation (CLTS) since 2011 [51]. Often more than one round of CLTS is needed: latrines periodically collapse and are in need of rebuilding [52], or inevitably fill and need emptying [53, 54]. More well-resourced households are more likely to transition from traditional pit latrine to improved pit latrine [55]. Future study of the impact of latrine type on VMB should include direct observation of latrine types, including their structural properties (e.g., duration of use, type of slabs), and extent of sharing. Water source varied over time in 45.5% of reports. Highlighting the precariousness of this resource, frequent variability in water source is in keeping with previous studies in Kenya, and stems from use/availability ratio, climate variability, and resource degradation, among several other factors [56]. Future study will need attention to definitions of the water service ladder (e.g., protected, unprotected, collection times, safe management) for comparability to Sustainable Development Goals, and for more refined understanding of water source contribution to VMB.
Future study of the impact of water source on VMB requires contemporaneous and repeated water and VMB assessment to differentiate transient presence from habitation of GI-related bacteria. Such studies must also incorporate functional analyses to determine whether vaginally atypical bacteria have altered function, and whether differential mucosal immune responses are elicited. Metagenomic studies of streams and rivers have demonstrated a variety of microorganisms, including fecal bacteria and antibiotic resistant bacteria and pathogens, and different functional dynamics of the water microbial communities. The taxonomic and functional composition of these natural water sources changes with effluent and stormflow events, and the impact on human health is unknown. For example, the abundance of gene content related to pathogenic processes and relative abundance of antibiotic resistance genes in urban rivers have been shown to differ before and after rain falls [57]; if rainfall leads to increases in pathogenesis, exposure to these water sources could plausibly contribute to vaginal dysbiosis. Therefore, prospective studies with user location-matched and contemporaneous water sampling in relation to VMB are necessary to verify the potential direct transfer of bacteria, complemented with behavioral assessments to understand mechanisms of exposure. A systematic sampling design at the watershed scale and during different weather regimes to survey the different water sources for their microbiome will enable a robust estimation of the factors and sources contributing to the assembly of these aquatic microbial communities, and the potential public health risks involved [40, 58, 59]. Shotgun metagenomics will help in linking the microbial phylogenetic information with the functional gene content of these microbial communities [60].
Improved WASH is a public health priority [22], and the impact on reproductive tract health is of growing importance due to climate change and increasing frequency of extreme weather events and water scarcity. While the effects of climate change on HIV epidemiology have been evaluated, the conceptual framework has focused on broader structural factors including degradation of infrastructure, increased migration, food insecurity, and increased sexual risk behaviors including sexual violence and transactional sex [61, 62]. To our knowledge, no studies have been published on the direct effect of climate change on the biology of STI/HIV transmission, as BV and a non-optimal VMB are significant risk factors for transmission of HIV, STIs, and adverse pregnancy outcomes.
Limitations
This study capitalizes on reported sources of water and latrine type in the home and examines them in relation to VMB in a large sample of adolescent girls and young women, measured prospectively for 18 months. We did not collect water samples to verify the presence of the bacteria identified by 16 S rRNA gene amplicon sequencing, nor do we have direct observations of water treatment (e.g., boiling, chlorination, filtering) or latrine conditions to verify type and other WASH factors, such as availability of water, soap, and shared use (e.g., private household latrine vs. community or shared latrine). While we observed that VMB composition varied by reported main water source and latrine type at home, there were inconsistencies across associations, and this may be due to misclassification or differences from one home to the next: e.g., the microbial composition of water from different points along Lake Victoria have been shown to vary [39]; water from a borehole serving a particular location could have different conditions and microbial composition than water from a borehole a few kilometers away. Despite these limitations, our adjusted analyses demonstrate a potentially impactful role for water source, latrine type, and rainfall in VMB health that provides rationale for intentionally designed prospective study.
Conclusions
This study observed associations between water source, latrine type, and rainfall on the VMB composition, through alterations in CST and detection of pertinent taxa. If water source, latrine type, and rainfall contribute to non-optimal vaginal microbiome composition, then current and planned therapeutics (e.g., antibiotics, live biotherapeutics) may be less effective or have reduced durability in populations whose VMB is continually exposed through the environment. We recommend prospective studies of water source, latrine type, climate, and other WASH factors in relation to VMB, to better inform structural and individual interventions to optimize VMB and reproductive tract health.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- AIC
Akaike’s Information Criterion
- BV
Bacterial vaginosis
- CaCHe
Cups and Community Health
- CCG
Cups or Cash for Girls
- COVID
Corona Virus Disease
- CHIRPS
Climate Hazards Group InfraRed Precipitation with Station data
- CST
Community State Type
- HIV
Human Immunodeficiency Virus
- IMERG
Integrated Multi-satellitE Retrievals for GPM
- NCBI
National Center for Biotechnology Information
- ODF
Open Defecation Free
- OR
Odds Ratio
- RA
Relative Abundance
- RNA
Ribonucleic acid
- SES
Socioeconomic Status
- SRA
Sequence Read Archive
- SSA
Sub Saharan Africa
- STI
Sexually Transmitted Infection
- UNIM
University of Nairobi, Illinois, and Manitoba
- VMB
Vaginal Microbiome
- WASH
Water, sanitation, and hygiene
Authors’ contributions
Conceptualization, S.D.M. and A.C.; Methodology, S.D.M., S.P., W.A., R.B., S.J.G., P.A.P.-H.; Formal analysis, S.P., S.D.M.; Investigation, S.D.M., W.A., G.Z., E.N., S.J.G., A.M.v.E., F.O.O. and P.A.P.-H.; Resources, E.N. and F.O.O.; Data curation, G.Z., S.J.G., A.M.v.E. and F.O.O.; Writing—original draft, A.C.; Writing—review & editing, S.P., W.A., G.Z., E.N., R.B., S.J.G., A.M.v.E., F.O.O., P.A.P.-H.; Supervision, S.D.M., E.N., F.O.O. and P.A.P.-H.; Project administration, S.D.M., E.N., F.O.O. and P.A.P.-H.; Funding acquisition, S.D.M. and P.A.P.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Institutes of Health Eunice Shriver National Institute of Child Health and Human Development (R01-HD093780 to SDM), and the Joint Global Health Trials Initiative (UK-Medical Research Council/ Department for International Development/ Wellcome Trust/Department of Health and Social Care; MR/N006046/1 to PPH). The funders had no role in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
Data availability
Raw sequence data (FASTQ files) were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), under BioProject identifier PRJNA746243. This study was conducted with approval from the Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU), which requires that data be released from any KEMRI-based Kenyan studies (including de-identified data) only after their written approval for additional analyses. In accordance, data for this study will be available upon request, after obtaining written approval for the proposed analysis from the KEMRI SERU. Their application forms and guidelines can be accessed at https://www.kemri.go.ke/scientific-ethics-review-unit-seru/. To request these data, please contact the KEMRI SERU at seru@kemri.org.
Declarations
Ethics approval and consent to participate
Ethics committees approved this study by the following names of the Institutional Review Boards (reference number): Kenya Medical Research Institutes (KEMRI, SERU #3215), Liverpool School of Tropical Medicine (LSTM, #15-005), and University of Illinois at Chicago (UIC, #2017-1301). Written informed parental consent and written informed assent from minors was obtained for all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fils LRN. Linking Water Access and Education in the Sustainable Development Goals in Sub-saharan Africa. Asian J Empir Res. 2021;11:50–8. 10.18488/journal.1007.2021.116.50.58. [Google Scholar]
- 2.Lin L, Yang H, Xu X. Effects of Water Pollution on Human Health and Disease Heterogeneity: a review. Front Environ Sci. 2022; 10:880246. 10.3389/fenvs.2022.880246.
- 3.Wolf J, Johnston RB, Ambelu A, et al. Burden of disease attributable to unsafe drinking water, sanitation, and hygiene in domestic settings: a global analysis for selected adverse health outcomes. Lancet. 2023;401:2060–71. 10.1016/S0140-6736(23)00458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khedkar R, Pajai S. Bacterial vaginosis: a Comprehensive Narrative on the etiology, clinical features, and Management Approach. Cureus. 2022. 10.7759/cureus.31314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peebles K, Velloza J, Balkus JE, et al. High global burden and costs of bacterial vaginosis: a systematic review and Meta-analysis. Sex Transm Dis. 2019;46:304–11. 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 6.Torrone EA, Morrison CS, Chen PL, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. 2018;15(2):e1002511. 10.1371/journal.pmed.1002511. [DOI] [PMC free article] [PubMed]
- 7.Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–501. 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenfack-Zanguim J, Kenmoe S, Bowo-Ngandji A, et al. Systematic review and meta-analysis of maternal and fetal outcomes among pregnant women with bacterial vaginosis. Eur J Obstet Gynecol Reproductive Biology. 2023;289:9–18. [DOI] [PubMed] [Google Scholar]
- 9.Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47:1426–35. [DOI] [PubMed] [Google Scholar]
- 10.Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8(2):e1000416. 10.1371/journal.pmed.1000416. [DOI] [PMC free article] [PubMed]
- 11.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N Engl J Med 2005;353(18):1899–911. 10.1056/NEJMoa043802. [DOI] [PubMed]
- 12.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108:4680–7. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinnon LR, Achilles SL, Bradshaw CS, et al. The evolving facets of bacterial vaginosis: implications for HIV Transmission. AIDS Res Hum Retroviruses. 2019;35:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta SD, Zulaika G, Otieno FO, et al. High prevalence of Lactobacillus crispatus dominated vaginal microbiome among Kenyan secondary school girls: negative effects of Poor Quality Menstrual Hygiene Management and sexual activity. Front Cell Infect Microbiol. 2021;11:716537. 10.3389/fcimb.2021.716537. [DOI] [PMC free article] [PubMed]
- 15.Tuddenham S, Ravel J, Marrazzo JM. Protection and risk: male and female genital microbiota and sexually transmitted infections. J Infect Dis. 2021;223:S222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kostas-Polston EA, Buechel JJ, Ryan-Wenger NA, Remesz-Guerrette J. U.S. active-duty service women’s urogenital health and operational readiness through the lens of the IBM-WASH model: a systematic integrative review. Appl Nurs Res. 2022;67:151620. 10.1016/j.apnr.2022.151620. [DOI] [PubMed]
- 17.Das P, Lisnek D, Sahoo KC, et al. Identifying risk factors for lower reproductive tract infections among women using reusable absorbents in Odisha, India. Int J Environ Res Public Health. 2021;18:4778. 10.3390/ijerph18094778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das P, Baker KK, Dutta A, et al. Menstrual hygiene practices, WASH access and the risk of urogenital infection in women from Odisha, India. PLoS ONE. 2015;10(6):e0130777. 10.1371/journal.pone.0130777. [DOI] [PMC free article] [PubMed]
- 19.Thoma ME, Brotman RM, Gray RH, et al. Risk and protective factors associated with BV chronicity among women in Rakai, Uganda. Sex Transm Infect. 2020;96:380–6. 10.1136/sextrans-2019-054145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraay ANM, Man O, Levy MC, et al. Understanding the impact of rainfall on diarrhea: testing the concentration-dilution hypothesis using a systematic review and meta-analysis. Environ Health Perspect. 2020;128:126001–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kogo BK, Kumar L, Koech R. Climate change and variability in Kenya: a review of impacts on agriculture and food security. Environ Dev Sustain. 2021;23:23–43. [Google Scholar]
- 22.Scahs JD, Lafortune G, Fuller G, Drumm E. Sustainable development report 2023. Implementing the SDG stimulus; 2023. [Google Scholar]
- 23.Zulaika G, Kwaro D, Nyothach E, et al. Menstrual cups and cash transfer to reduce sexual and reproductive harm and school dropout in adolescent schoolgirls: study protocol of a cluster-randomised controlled trial in western Kenya. BMC Public Health. 2019;19(1):1317. 10.1186/s12889-019-7594-3. [DOI] [PMC free article] [PubMed]
- 24.Kenya National Bureau of Statistics, Ministry of Health, The DHS Program ICF. Kenya Demographic and Health Survey 2022: Volume 1. Nairobi, Kenya, and Rockville, Maryland, USA: KNBS and ICF; 2023.
- 25.Kabudula CW, Houle B, Collinson MA, et al. Assessing changes in Household Socioeconomic Status in Rural South Africa, 2001–2013: a distributional analysis using Household Asset indicators. Soc Indic Res. 2017;133:1047–73. 10.1007/s11205-016-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta SD, Zulaika G, Agingu W, et al. Analysis of bacterial vaginosis, the vaginal microbiome, and sexually transmitted infections following the provision of menstrual cups in Kenyan schools: results of a nested study within a cluster randomized controlled trial. PLoS Med. 2023;20(7):e1004258. 10.1371/journal.pmed.1004258. [DOI] [PMC free article] [PubMed]
- 27.Dezfuli AK, Ichoku CM, Huffman GJ, et al. Validation of IMERG Precipitation in Africa. J Hydrometeorol. 2017;18:2817–25. 10.1175/JHM-D-17-0139.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funk C, Peterson P, Landsfeld M, et al. The climate hazards infrared precipitation with stations - a new environmental record for monitoring extremes. Sci Data. 2015. 10.1038/sdata.2015.66. 2:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naqib A, Poggi S, Wang W, et al. Making and sequencing heavily multiplexed, high-throughput 16S ribosomal RNA gene amplicon libraries using a flexible, two-stage PCR protocol. Method Mol Biol. 2018;1783:149–69. 10.1007/978-1-4939-7834-2_7. [DOI] [PubMed]
- 30.Holm JB, Humphrys MS, Robinson CK, et al. Ultrahigh-Throughput Multiplexing and sequencing of > 500-Base-pair amplicon regions on the Illumina HiSeq 2500 platform. mSystems. 2019;4(1):e00029–19. 10.1128/msystems.00029-19 [DOI] [PMC free article] [PubMed]
- 31.France MT, Ma B, Gajer P, et al. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome. 2020;8(1):166. 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed]
- 32.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 200;3:21. 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed]
- 33.Hazimeh H, Mazumder R. Fast best subset selection: coordinate descent and local combinatorial optimization algorithms. Oper Res. 2020;68:1517–37. 10.1287/opre.2019.1919. [Google Scholar]
- 34.Meinshausen N, Bühlmann P. Stability selection. J R Stat Soc B, Stat Methodology. 2010;72:417–73. 10.1111/j.1467-9868.2010.00740.x.
- 35.Babb C, Makotsi N, Heimler I, et al. Evaluation of the effectiveness of a latrine intervention in the reduction of childhood diarrhoeal health in Nyando District, Kisumu County, Kenya. Epidemiol Infect. 2018;146:1079–88. 10.1017/S0950268818000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cullom A, Spencer MS, Williams MD, et al. Influence of pipe materials on in-building disinfection of P. Aeruginosa and A. Baumannii in simulated hot water plumbing. Water Res X. 2023;21:100189. 10.1016/j.wroa.2023.100189. [DOI] [PMC free article] [PubMed]
- 37.Chen Y, Huang Z, Tang Z, et al. More than just a Periodontal Pathogen –the Research Progress on Fusobacterium nucleatum. Front Cell Infect Microbiol. 2022;12:815318. 10.3389/fcimb.2022.815318. [DOI] [PMC free article] [PubMed]
- 38.Reis ACM, Silva JO, et al. Virulence factors and biofilm production by isolates of Bacteroides fragilis recovered from dog intestinal tracts. Braz J Microbiol. 2014;45(2):64–50. 10.1590/s1517-83822014000200037. [DOI] [PMC free article] [PubMed]
- 39.Khatiebi S, Kiprotich K, Onyando Z, et al. Shotgun metagenomic analyses of Microbial assemblages in the aquatic ecosystem of Winam Gulf of Lake Victoria, Kenya reveals Multiclass Pollution. Biomed Res Int. 2023;2023(1):3724531. 10.1155/2023/3724531. [DOI] [PMC free article] [PubMed]
- 40.Odhiambo KA, Ogola HJO, Onyango B, et al. Contribution of pollution gradient to the sediment microbiome and potential pathogens in urban streams draining into Lake Victoria (Kenya). Environ Sci Pollut Res. 2023;30:36450–71. 10.1007/s11356-022-24517-0. [DOI] [PubMed] [Google Scholar]
- 41.da Silva DT, Ebdon J, Okotto-Okotto J, et al. A longitudinal study of the association between domestic contact with livestock and contamination of household point-of-use stored drinking water in rural Siaya County (Kenya). Int J Hyg Environ Health. 2020;230:113602. 10.1016/j.ijheh.2020.113602. [DOI] [PMC free article] [PubMed]
- 42.Ocholla G, Letema S, Mireri C. Socioeconomic determinants of water delivery satisfaction in a medium sub-saharan Africa city: a case of Kisumu, Kenya. Water Supply. 2022;22:8682–97. 10.2166/ws.2022.388. [Google Scholar]
- 43.Fudaba M, Kamiya T, Tachibana D, et al. Bioinformatics analysis of oral, vaginal, and rectal microbial profiles during pregnancy: a pilot study on the bacterial co-residence in pregnant women. Microorganisms. 2021;9(5):1027. 10.3390/microorganisms9051027. [DOI] [PMC free article] [PubMed]
- 44.Raimondi S, Candeliere F, Amaretti A, et al. Vaginal and anal Microbiome during Chlamydia trachomatis infections. Pathogens. 2021;10:1347. 10.3390/pathogens10101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donders GGG, Bellen G, Grinceviciene S, et al. Aerobic vaginitis: no longer a stranger. Res Microbiol. 2017;168:845–58. 10.1016/j.resmic.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Han C, Wu W, Fan A, et al. Diagnostic and therapeutic advancements for aerobic vaginitis. Arch Gynecol Obstet. 2015;291:251–7. 10.1007/s00404-014-3525-9. [DOI] [PubMed] [Google Scholar]
- 47.Kaambo E, Africa C, Chambuso R, et al. Vaginal microbiomes Associated with Aerobic Vaginitis and bacterial vaginosis. Front Public Health. 2018;6:78. 10.3389/fpubh.2018.00078. [DOI] [PMC free article] [PubMed]
- 48.Powers JE, Mureithi M, Mboya J, et al. Effects of high temperature and Heavy Precipitation on Drinking Water Quality and child hand contamination levels in Rural Kenya. Environ Sci Technol. 2023;57:6975–88. 10.1021/acs.est.2c07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson P, Bradley D, Katilu A, et al. Rainfall and groundwater use in rural Kenya. Sci Total Environ. 2019;649:722–30. 10.1016/j.scitotenv.2018.08.330. [DOI] [PubMed] [Google Scholar]
- 50.Wessels JM, Lajoie J, Vitali D, et al. Association of high-risk sexual behaviour with diversity of the vaginal microbiota and abundance of Lactobacillus. PLoS ONE. 2017;12(11):e0187612. 10.1371/journal.pone.0187612. [DOI] [PMC free article] [PubMed]
- 51.Wasonga J, Miyamichi K, Hitachi M, et al. Effects of Community-Led Total Sanitation (CLTS) Boosting and Household factors on latrine ownership in Siaya County, Kenya. Int J Environ Res Public Health. 2023;20:6781. 10.3390/ijerph20186781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peletz R, Macleod C, Kones J, et al. When pits fill up: supply and demand for safe pit-emptying services in Kisumu, Kenya. PLoS ONE. 2020;15:e0238003. 10.1371/journal.pone/02348003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gudda FO, Moturi WN, Oduor OS, et al. Pit latrine fill-up rates: variation determinants and public health implications in informal settlements, Nakuru-Kenya. BMC Public Health. 2019;19:68. 10.1186/s12889-019-6403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinton RGK, Macleod CJA, Troldborg M, et al. The status of sanitation in Malawi: is SDG6.2 achievable? Int J Environ Res Public Health. 2023;20:6528. 10.3990/ijerph20156528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Njuguna J. Progress in sanitation among poor households in Kenya: evidence from demographic and health surveys. BMC Public Health. 2019;19:135. 10.1186/s12889-019-6459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mogaka H. Climate variability and water resources degradation in Kenya: improving water resources development and management. Volume 69. World Bank; 2006. [Google Scholar]
- 57.Chaudhary A, Kauser I, Ray A, et al. Taxon-Driven Functional shifts Associated with Storm Flow in an urban Stream Microbial Community. mSphere. 2018;3(4):10–128. 10.1128/msphere.00194-18. [DOI] [PMC free article] [PubMed]
- 58.Zhang SY, Tsementzi D, Hatt JK, et al. Intensive allochthonous inputs along the Ganges River and their effect on microbial community composition and dynamics. Environ Microbiol. 2019;21:182–96. 10.1111/1462-2920.14439. [DOI] [PubMed] [Google Scholar]
- 59.Baral D, Speicher A, Dvorak B, et al. Quantifying the relative contributions of environmental sources to the microbial community in an urban stream under dry and wet weather conditions. Appl Environ Microbiol. 2018;84. 10.1128/AEM.00896-18. [DOI] [PMC free article] [PubMed]
- 60.Meziti A, Tsementzi D, Ar. Kormas K, et al. Anthropogenic effects on bacterial diversity and function along a river-to-estuary gradient in Northwest Greece revealed by metagenomics. Environ Microbiol. 2016;18:4640–52. 10.1111/1462-2920.13303. [DOI] [PubMed] [Google Scholar]
- 61.Lieber M, Chin-Hong P, Whittle HJ, et al. The synergistic relationship between Climate Change and the HIV/AIDS epidemic: a conceptual Framework. AIDS Behav. 2021;25:2266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guinto RR, Cahatol JJF, Lazaro KYMS, et al. Pathways linking climate change and HIV/AIDS: an updated conceptual framework and implications for the Philippines. J Clim Change Health. 2022;6:100106. 10.1016/j.joclim.2021.100106. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence data (FASTQ files) were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), under BioProject identifier PRJNA746243. This study was conducted with approval from the Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU), which requires that data be released from any KEMRI-based Kenyan studies (including de-identified data) only after their written approval for additional analyses. In accordance, data for this study will be available upon request, after obtaining written approval for the proposed analysis from the KEMRI SERU. Their application forms and guidelines can be accessed at https://www.kemri.go.ke/scientific-ethics-review-unit-seru/. To request these data, please contact the KEMRI SERU at seru@kemri.org.