Abstract
Background
The psychosocial factors play an important role in the development of depression in adolescents. we used metabolomics techniques to explore the links among childhood trauma, rumination, resilience, and adolescent depression.
Methods
We selected 57 adolescent depression patients and 53 healthy adolescents. The Childhood Trauma Questionnaire (CTQ), Hamilton Depression Scale (HAMD), Resilience Scale (CD-RISC), and Redundant Thinking Response Scale (RRS) were employed for the purpose of psychological assessment. The patients were regrouped according to their scores using the 27% high-low grouping method. Blood specimens were collected from all adolescents and metabolic data were obtained using LC–MS.
Results
We found no statistically significant difference between the groups in terms of age, gender, and body mass index (BMI). HAMD, CTQ, and RRS scores were significantly higher in the adolescent depression group (MDD) than in the adolescent healthy control group (HC), and CD-RISP scores were significantly lower than in the HC group (P < 0.001). There were significant differences between the low childhood trauma group (LCT) and high childhood trauma group (HCT), the low rumination group (LRR) and high rumination group (HRR), and the low resilience group (LPR) and high resilience group (HPR) (P < 0.001). RRS, CTQ and HAMD scores were positively correlated, RRS and CTQ scores were positively correlated, CD-RIS was negatively correlated with HAMD, RRS and CTQ scores (P < 0.01). More importantly, we found that DHEAS and LPA (22:6) were identified as significant differential metabolites in both the depressed and normal groups, as well as in the high and low childhood trauma groups. N-Acetyl-L-aspartic acid and DHEAS were identified as significant differential metabolites in both the depressed and normal groups, as well as in the high and low childhood rumination groups. Pseudouridine and LPA(22:6) were identified as significant differential metabolites in both the depressed and normal groups, as well as in the high and low childhood resilience groups.
Conclusion
Psychological factors (childhood trauma, rumination, resilience) are biologically linked to the development of depression in adolescents. The impact of rumination on adolescent depression may be associated with DHEA. The impact of childhood trauma and resilience on adolescent depression may be associated with LPA (22:6).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06369-9.
Keywords: Adolescent depression, Childhood trauma, Rumination, Resilience, Metabolite
Background
Depression is one of the most common mental disorders that can affect both physical and mental health [1]. The World Health Organization (WHO) has identified depression as the leading cause of disability globally. According to WHO projections, depressive disorders are expected to become the most burdensome disease by 2030. The greatest risk factor for depression in adolescents is family history of depression and the interaction of psychosocial stress and biological factors. [2]. Recent research has shown the prevalence of depression in adolescents to be as high as 25.2% [3]. A high prevalence of depression in the adolescent population often indicates a high risk. Research has demonstrated that adolescents with depression are frequently at elevated risk for suicidal ideation and self-harm [4]. Indeed, suicide has emerged as the second leading cause of death among adolescents. It is imperative that the societal dangers of adolescent depression be acknowledged.
There is a strong link between depression and psychosocial factors, and diathesis-stress model of depression states that the effect of stress on the risk of depression depends on quality or vulnerability, meaning that stress can activate a quality to trigger depression through changes in the external environment [5].A growing body of evidence indicates that adolescents with a lower psychological quality, as evidenced by a tendency to ruminate, are more susceptible to depression when confronted with external stressors [6, 7]. External stressful events often have long-lasting negative effects on the adolescent population, such as Childhood Trauma. Childhood Trauma is a negative experience in one's early life that is significant and beyond the individual's ability to cope with it and brings stress [8]. The available evidence indicates that childhood trauma can influence the trajectory of the brain and may act as a risk factor for the development and maintenance of depression [9, 10].
Rumination is defined as repetitive thoughts focusing on the negative emotion and its causes, effects, and consequences as a way to cope with the negative emotion. Individuals with redundant negative emotions tend to prolong the duration of depression by focusing on their emotional state and repeatedly thinking about the causes of the negative emotion and its consequences [11].Adolescents exhibiting elevated levels of rumination are at an increased risk for developing depressive disorders [12].
Resilience is understood to be a protective factor that enables an individual to withstand adverse events, thereby facilitating positive developmental outcomes. It is a constructive response to stressful circumstances that enables individuals to withstand the negative effects of stressors. Resilience can serve to mitigate the negative emotional impact of external factors, thereby reducing the likelihood of developing [13]. The literature indicates that resilience can assist the hippocampus, prefrontal cortex, and reward system in maintaining optimal integrity and functionality during periods of stress, thereby preventing the progression of mental illness [14]. Concurrently, a robust immune system and an optimal gut microbiota can enhance resilience. Resilience plasticity can be intervened with antidepressants, anti-inflammatory drugs, and dietary control [14, 15].
Childhood trauma, rumination, and resilience as influences on depression may be contributing to the development of mental illness by affecting individual biology. Childhood Trauma has been found to have lasting effects on the structure and functioning of the brain, leading to mental illness, And researchers believe that childhood trauma can lead to poor development of executive function areas, reward areas [16, 17]. There may be a genetics link to Rumination as a chronic coping modality [18]. The severity of rumination in individuals with depression is associated with functional connectivity between the posterior cingulate cortex and the right temporoparietal junction [19]. A retrospective study suggests that resilience may be associated with metabolic disorders [20]. Resilience acts as a moderator of mental illness through the hippocampus, prefrontal lobes, and reward system [14, 15].
Metabolomics is a systematic analysis of all metabolites of an organism. Metabolomics reflects biological events that have occurred, and the effects of disease on a population can be reflected in metabolites. Metabolomic analysis is now a valuable tool for understanding the pathogenesis of depression in adolescents. It can be employed to identify potential biomarkers and associated signaling pathways that are linked to depression [21–23]. Recent studies have demonstrated a correlation between childhood trauma and levels of isocitric acid, fatty acids, acylcarnitines, and 4-hydroxyproline [24]. It is hypothesized that resilience may be associated with lactate and N-acetylaspartate levels [25, 26]. A current search did not yield any articles that explored the potential link between rumination and metabolites. In conclusion, there is a dearth of articles that examine the effects of psychological traits on the biology of depression through metabolomics techniques.
The novelty of this study lies in its use of metabolomics to elucidate the biological links between psychological factors and adolescent depression. The objective of this study was to investigate the biological correlates of childhood trauma, resilience, rumination, and adolescent depression. The significance of this study is that it provides a new perspective for the study of the interaction between psychosocial and biological factors in adolescent depression.
Methods
Objects of research
Informed consent was obtained from all participants for this study. In the case of participants under the age of 16, informed consent was obtained from both the participants themselves and their legal guardians. The study was reviewed and approved by the Medical Ethics Committee of The Affiliated Hospital of Guizhou Medical University(2021 Lunar Review No. 688) and was in accordance with the Declaration of Helsinki. Adolescent depression inpatients in the psychiatry department of Guizhou Medical University Hospital and healthy individuals were selected, and individuals and their families were required to have no history of psychiatric disorders, no history of psychoactive substance abuse, and no history of serious organic diseases. A diagnosis of depression is based on at least two of the following three core symptoms over the past two weeks: (1) depressed mood; (2) loss of interest and enjoyment; (3) decreased energy leading to increased exertion and decreased activity; and seven additional symptoms:(1) Decreased attention span; (2) Decreased self-evaluation and self-confidence; (3) Concepts of self-guilt and feelings of worthlessness; (4) Perception of a bleak and pessimistic future; (5) Self-injurious or suicidal concepts or behaviors; (6) Sleep disturbances; and (7) Decreased appetite.articipants will first be divided into two groups as follows.
Criteria for enrollment in the adolescent depression group: (1) meeting the diagnostic criteria for depression in the World Health Organization Classification of Mental and Behavioral Disorders (ICD-10); (2) age 13–19; (3) no history of antibiotic and antidepressant use in the last 2 weeks; (4) no history of mental or somatic disorder. Enrollment in the healthy adolescents group Criteria: (1) no history of mental or somatic disorder; (2) age 13–19 years old; (3) no history of antibiotic use in the past 2 weeks;
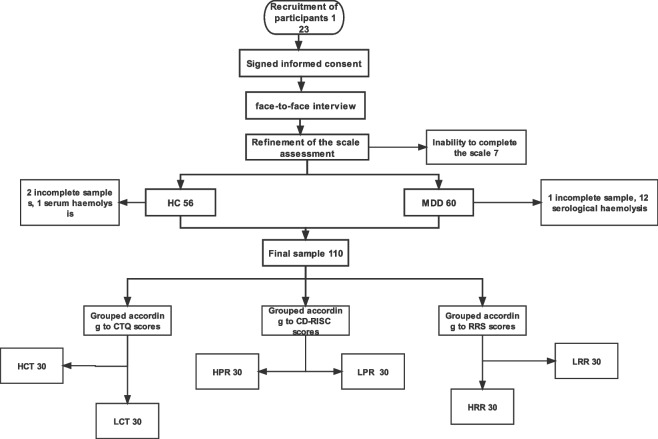
A total of 123 subjects were initially enrolled in the study; 13 subjects withdrew during the course of the experiment, resulting in a final sample size of 110. These subjects had no history of colds, viral infections, or antibiotic use in the past two weeks. The primary source of sample wastage is the result of errors made by the subjects during the centrifugation, separation, preservation, and transportation of the serum specimen after collection. The individual samples were a consequence of subjects' inability to complete the scale or serum haemolysis. The adolescent healthy control group (HC) comprised 53 participants, while the adolescent depression group (MDD) consisted of 57 individuals. Furthermore, the Childhood trauma questionnaire (CTQ), The Connor- Davidson resilience scale (CD-RISC), and The Ruminative Responses Scale (RRS) scale scores were ranked in ascending order and subsequently classified according to the critical ratio method, as proposed by Kelley and based on a 27% high-low grouping [27]. 30 cases each were categorized into low childhood trauma group (LCT) and high childhood trauma group (HCT) according to CTQ scores; 30 cases each were categorized into low resilience group (LPR) and high resilience group (HPR) according to CD-RISC scores; and 30 cases each were categorized into low redundancy group (LRR) and high redundancy group (HRR) according to RRS scores (Fig. 1). Participants are placed on a one-to-one basis in a quiet and comfortable room in order to facilitate their relaxation and optimal participation in the assessment. Prior to the commencement of the assessment, participants were provided with standardised instructions, which were subsequently verified at the conclusion of the assessment.
Fig. 1.
Experimental flow chart
Screening tools
Socio-demographic and clinical form: A self-administered case report recording form was developed under the supervision of a psychiatrist and based on reference to a large body of literature, which collected information on the patient's gender, age, height, weight, education, family situation, medication history, treatment history of the disease, and the scale. Scale evaluation was completed by a specialized psychiatrist, and scale consistency was evaluated at the beginning of the study.
Hamilton depression scale(HAMD): created by Hamilton in 1960 [28], It is widely used by scholars in China to study depression [29]. In this study, the Cronbach's alpha coefficient for this scale was 0.98, indicating a high level of internal consistency.
Childhood trauma questionnaire (CTQ): the CTQ is a widely utilized tool to evaluate early traumatic experiences [30]. The Chinese version was initially translated by Zhao et al. [31]. In the present study study, it demonstrated excellent reliability and high internal consistency, with a Cronbach's alpha of 0.886. The CTQ comprises 28 entries, with five subscales and three validity entries, covering five types of childhood trauma. The total scores on the CTQ range from 25—125, with higher scores indicating more early life trauma experienced before the age of 16.
The Connor- Davidson resilience scale (CD-RISC): The CD-RISC assesses mental toughness [32]. Translated into Chinese by Yu et al. [33]. With a total of 10 entries, each scored from 0–4 and the overall score ranges from 0–40, indicating higher mental resilience with higher scores. The Cronbach's alpha coefficient for the CD-RISC in this study was 0.946.
The Ruminative Responses Scale (RRS): The RRS has been used to measure repetitive thought patterns [34]. Han and other scholars translated it into Chinese [35]. Comprising 22 items, the scores from all items are combined to yield a total redundancy score. Higher score son this scale indicate more pronounced repetitive thinking tendencies. In this study, the Cronbach's alpha coefficient for the RRS was 0.947.
Sample collection and handling
Blood samples were collected from adolescents with depression and healthy adolescents, samples were collected by medical professionals. Fasting blood samples were used to minimise the effects of biorhythms. Blood processing procedure: (1) 5 ml of fasting peripheral elbow vein blood was taken from adolescents and immediately turned up and down 7–8 times, followed by 15 min of static coagulation. (2) The serum was isolated through centrifugation at 1500 r/min for 10 min at 4 °C. Afterwards, the serum was drawn using a pipette, transferred into EP tubes, and sealed and then stored in a refrigerator at −80 °C. (3) The extract containing theisotope-labeled internal standard was prepared by mixing acetonitrile and methanol in the same proportion. Then 100ul of sample and 300ul of extract were placed in 1.5 ml EP tubes, vortexed for 30 s and sonicated for 10 min. the supernatant was collected by centrifugation at 12,000 r/min for 15 min at 4 °C and then analyzed by LC–MS. Quality control samples were prepared by pooling aliquots of supernatants from all samples.
LC–MS analysis
Fasting blood samples were used to minimise the effects of biorhythms.All solvents and chemicals were of chromatographic grade, methanol and Acetonitrile were from CNW Technologies, Ammonium acetate was from SIGMA-ALDRICH and Ethanoic acid was from Fisher Chemical.The samples were processed as follows.100 μL of sample was transferred to an EP tube. After the addition of 300 μL ofextract solution (methanol, containing isotopically-labelled internal standard mixture), the samples were vortexed for 30 s, sonicated for 10 min in ice-water bath, and incubated for 1 h at −40℃ to precipitate proteins. Then the sample was centrifuged at 12,000 rpm(RCF = 13,800(× g),R = 8.6 cm) for 15 min at 4℃. The resulting supernatant was transferred to a fresh glass vial for analysis. The quality control (QC) sample was prepared by mixing an equal aliquot of the supernatants from all of the samples.
Utilizing a UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 µm), connected to an Orbitrap Exploris 120 mass spectrometer (Orbitrap MS, Thermo). The mobile phases consisted of 5 mmol/L ammonium acetate and 5 mmol/L acetic acid, water (A) and acetonitrile (B). The autosampling temperature was set at 4 °C and each sample injected had a volume of 2 μL.
Full-scan MS spectra were continuously evaluated in information-dependent acquisition (IDA) mode by using an Orbitrap Exploris 120 mass spectrometer and acquisition software (Xcalibur, Thermo). The ESI source specifications were set as follows:(1) The sheath gas flow and Aux gas flow were 50 Arb and 15 Arb, respectively.(2) The capillary temperature was set to 320 °C.(3) The full mass spectrometry resolution MS/MS resolution was 60,000 and 15,000, respectively.(4) Collision energies in NCE mode were 10/30/60.(5) The injection voltages were 3.8 kV (positive) or −3.4 kV (negative).
Data processing and bioinformatic analysis
Metabolite data in its raw form were converted into mzML format using the ProteoWizard converter and peak detection, extraction, comparison and integration were carried out using an custom program (developed using R and based on XCMS). The RTs were linearly shifted throughout the metabolite analysis using internal standard normalization. Peak annotation was then accomplished using an in-house MS/MS database. The resulting data matrix was subsequently transferred into SIMCA-P V16.0.2 software (Umetrics, Umea, Sweden) for complex statistical analysis involving multiple variables. Then, orthogonal projection latent structure discriminant analysis (OPLS-DA) model was employed to acquire variable importance (VIP) values from each variable whithin the OPLS-DA model, which were validated through a sevenfold cross validation and 200 permutation tests. Since the metabolic data are non-normally, metabolite analysis involved employing a nonparametric Mann—Whitney U test, which was followed by the application of benjaminii—hochberg correction for controlling multiple testing in order to evaluate statistical significance. Metabolites with VIP values of > 1.0, P value < 0.05 were regarded as statistically significant and variables with insignificant changes were ignored. The recognized metabolites were matched based on the similarity of their MS/MS spectral.
Results
General demographic survey
A total of 110 samples were recovered, of which 46 were male, contistuting 41.8%, and 64 were female, representing 58.2% of which 57 were in the depressed group (MDD) and 53 were in the healthy group (HC). According to Kelley's [27] 27% high/low grouping basis, using the critical ratio method, the CTQ scores were divided into a low childhood trauma group (CTQ total score ≤ 32) and a high childhood trauma group (CTQ total score ≥ 54), each with 30 cases; the RRS scores were divided into a low rumination group (RRS total score ≤ 32) and a high rumination group (RRS total score ≥ 58), each with 30 cases; the CDS scores were divided into a low redundancy group (RRS total score ≤ 32) and a high redundancy group (RRS total score ≥ 58), each with 30 cases. There were 30 cases each in the low resilience group (total CD-RISC score ≤ 14) and the high resilience group (total CD-RISC score ≥ 30) according to the CD-RISC score.
The results of this study indicated that there were no statistically significant variations in gender, age, and BMI when comparing the healthy and depressed groups, as well as the low and high childhood trauma groups, low and high rumination, and low and high resilience groups (p > 0.05). See Table 1.
Table 1.
Comparison of socio-demographic and clinical form
| Gender | Age | BMI (kg/m2) | ||
|---|---|---|---|---|
| Male | Famale | P50(P25,P75) | ||
| HC | 27 | 26 | 16.26(15.50,16.94) | 19.03(18.32,21.91) |
| MDD | 19 | 38 | 15.96(14.88,16.92) | 20.31(18.00,22.77) |
| χ2/Z | 3.5 | −1.441 | −0.73 | |
| LCT | 13 | 17 | 15.96(14.88,16.92) | 15.96(14.88,16.92) |
| HCT | 11 | 19 | 16(14.75,17) | 20.3(17.85,22.3) |
| χ2/Z | 0.278 | −1.145 | −0.015 | |
| LRR | 14 | 16 | 16(16,17) | 19.26(18.48,22.42) |
| HRR | 7 | 23 | 16(15,17) | 19.53(17.96,22.86) |
| χ2/Z | 3.59 | −1.578 | 0.631 | |
| LPR | 11 | 19 | 16(15,17) | 18.66(17.76,22.77) |
| HPR | 18 | 12 | 16(15.75,17) | 19.69(18.59,23.53) |
| χ2/Z | 3.27 | −1.312 | −1.153 | |
BMI body mass index, HC adolescent healthy control group, MDD adolescent depression group, HCT high childhood trauma group, LPR low resilience group, HPR high resilience group, LRR low redundancy group, HRR high redundancy group; the same below
*stands for P less than 0.05
**stands for P less than 0.01
***stands for P less than 0.001
Comparison of scale scores between groups
Due to the non-normal distribution of scale scores, the Mann—Whitney U test was employed to examine score differences between the depressed group and the healthy group. It was found that the depression group exhibited significantly higher scores in HAMD, CTQ, and RRS compared to the healthy group, and the CD-RISP score was significantly lower than that of the healthy group compared with the healthy group (P < 0.001), as what was summarized in Table 2. This shows that psychological resilience, rumination, and childhood trauma in depressed individuals and healthy individuals are significant.
Table 2.
Comparison of scale scores for group between HC and MDD
| HAMD | CTQ | RRS | CD-RISP | |
|---|---|---|---|---|
| P50(P25,P75) | ||||
| HC | 0(0,2) | 32(29,35) | 31(27.5,37) | 31(26.5,34) |
| MDD | 43(33,48) | 54(42,62) | 59(52,66) | 12(7,16) |
| Z | −9.127*** | −7.593*** | −8.651*** | 8.408*** |
HAMD stands for Hamilton Depression Scale score, CTQ stands for Childhood Trauma Questionnaire, CD-RIS is the Resilience Score, and RRS is the rumination Response Scale score, below
***stands for P less than 0.001
The Mann—Whitney U test was employed to assess the diversity in HAMD between high and low subgroups. The results showed that HAMD scores were significantly different in the high versus low childhood trauma group, high versus low rumination group, and high versus low resilience (p < 0.001), as shown in Table 3. This suggests that individuals who are differently affected by childhood trauma, psychological resilience, and rumination are also significantly different in their scores on the depression assessment.
Table 3.
Comparison of HAMD scores between groups
| Groups | HAMD | Z |
|---|---|---|
| P50(P25,P75) | ||
| LCT | 0.5(0,2) | −5.716*** |
| HCT | 43(33.75,46.75) | |
| LRR | 0(0,1.25) | −6.751*** |
| HRR | 45.5(34.25,49.25) | |
| LPR | 44(34,49) | 6.693*** |
| HPR | 0(0,2) |
***stands for P less than 0.001
Correlation analysis between variables
Correlation analysis was performed on all subjects, and Spearman correlation analysis was used because the scores of the variables did not conform to normality. The results showed that RRS, CTQ and HAMD scores were positively correlated, RRS and CTQ scores were positively correlated, and CD-RIS was negatively correlated with the scores of HAMD, RRS and CTQ (P < 0.01), as shown in Table 4, which indicates that the correlations between RRS, CTQ and HAMD were all significantly positive. regardless of whether the score of one of the two was higher, the scores of the remaining two were significantly higher; CD-RIS correlated with the scores of any of RRS, CTQ and HAMD; CD-RIS correlated with the scores of RRS, CTQ and HAMD. CD-RIS was significantly negatively related to any of the RRS, CTQ and HAMD scores.. the higher the CD-RIS score, the lower the RRS, CTQ and HAMD scores. From this we know that HAMD is positively correlated with RRS and CTQ scores two by two, and all three are negatively correlated with CD-RIS scores.
Table 4.
Correlation analysis of psychosocial factors affecting depression (N = 110)
| HAMD | CTQ | CD-RIS | RRS | |
|---|---|---|---|---|
| HAMD | 1 | |||
| CTQ | 0.609** | 1 | ||
| CD-RIS | −0.734** | −0.667** | 1 | |
| RRS | 0.767** | 0.646** | −0.686** | 1 |
**stands for P less than 0.01
OPLS-DA
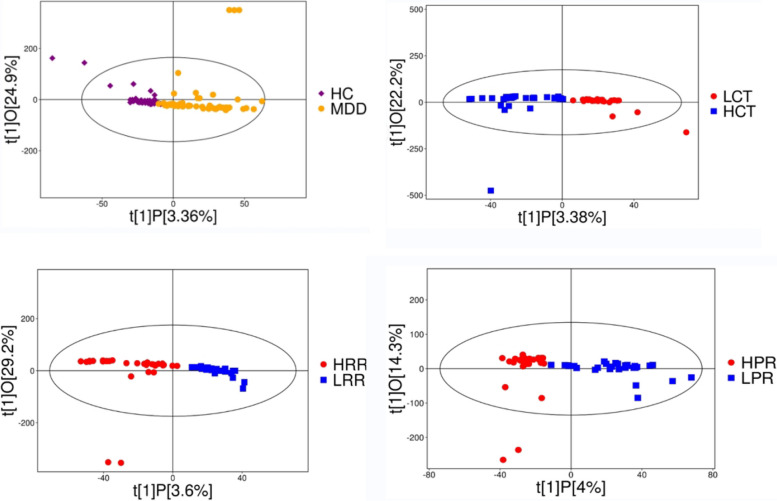
The OPLS-DA analysis provides better access to information about differences between groups, effectively filtering out information unrelated to the classification information, thus making the model simple and easy to understand, and improving the parsing ability and effectiveness of the model. The results of the serum metabolome were assessed using the LC–MS/MS metabolomics platform. After data normalization, the OPLS-DA model showed plots of scores for the healthy control (HC) versus depressed (MDD), low childhood trauma (LCT) versus high childhood trauma (HCT), low rumination response (LRR) versus high rumination response (HRR), and low resilience (LPR) versus high resilience (HPR) groups (Fig. 2). As can be seen from the figures, the corresponding two groups are basically on the positive and negative sides of the first principal component, with little overlap. From the OPLS-DA score graph we can conclude that there is a significant difference between the sample groups.
Fig. 2.
Scatter plot of OPLS-DA model obtained for group. HC vs MDD;LCT vs HCT; LRR vs HRR; LPR vs HPR. Note: The closer longitudinal distance indicates better intra-group reproducibility
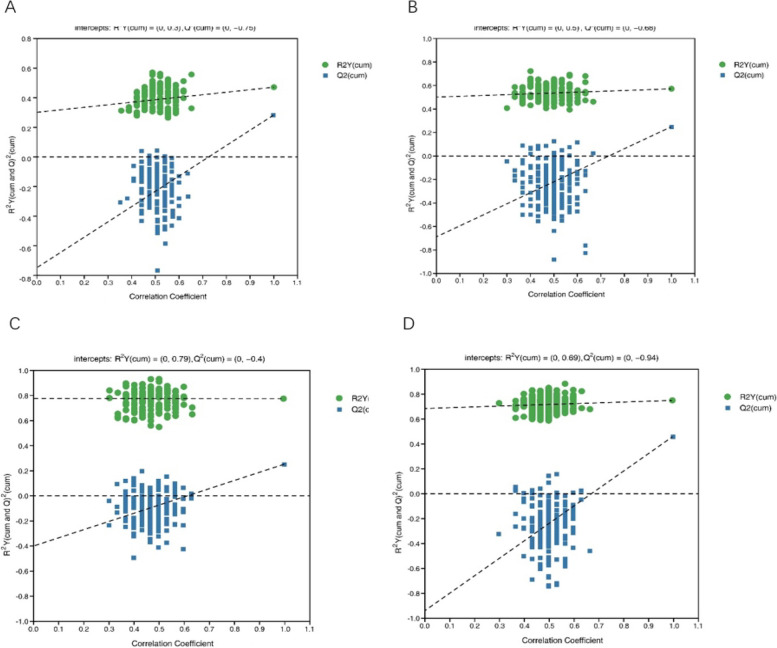
In addition, in order to verify the accuracy of the model and to prevent the test model from being excessively fitted, the permutation experiments were performed several times (number n = 200) using the permutation test Fig. 3). The Q2 value is a measure of the predictive power of the model.
Fig. 3.
Plot of the permutation test results of the OPLS-DA model group. A HC vs MDD; B LCT vs HCT; C LRR vs HRR; D LPR vs HPR. Note: The replacement test stochastic model exhibits lower Q2 (predictability of the model) values compared to the original model; the intercept point between the regression line and the vertical axis of Q2 is negative; additionally, as replacement retention decreases, the replaced Y proportion increases, leading to a gradual decrease in Q2, indicating a well-fitted model
Differential metabolite screening
To identify the differential metabolites that have a significant impact in the depressed group (MDD) versus healthy control (HC), high childhood trauma (HCT) versus low childhood trauma (LCT), low rumination response(LRR) versus rumination response (HRR), and low resilience (LPR) versus high resilience (HPR), we chose to screen for metabolites that have a VIP of the primary component of the OPLS-DA model VIP > 1 and a P-value < 0.05 metabolites. (Table 5).
Table 5.
Significantly different metabolites for group
| group | NO | metabolites | rt | mz | VIP | P-VALUE | FOLD CHANGE |
|---|---|---|---|---|---|---|---|
| HC-MDD | 1 | DHEAS | 468.42 | 367.16 | 1.60 | 0.003593019 | 1.29 |
| HC-MDD | 2 | N-Acetyl-L-aspartic acid | 38.73 | 174.04 | 1.85 | 0.022292785 | 1.15 |
| HC-MDD | 3 | Pseudouridine | 76.34 | 243.06 | 2.11 | 0.000254541 | 1.24 |
| HC-MDD | 4 | Bilirubin | 539.49 | 585.27 | 1.01 | 0.029616405 | 1.39 |
| HC-MDD | 5 | LPA(22:6) (DHA) | 596.04 | 481.23 | 1.83 | 0.004864482 | 1.28 |
| HC-MDD | 6 | Creatinine | 66.37 | 112.05 | 1.98 | 0.039443612 | 0.90 |
| HC-MDD | 7 | Taurocholic acid | 594.28 | 516.31 | 1.02 | 0.001697389 | 1.24 |
| LCT-HCT | 1 | Taurine | 42.67 | 124.01 | 3.04 | 0.031237121 | 1.38 |
| LCT-HCT | 2 | DHEAS | 468.42 | 367.16 | 1.33 | 0.047713942 | 1.30 |
| LCT-HCT | 3 | L-Acetylcarnitine | 48.56 | 204.12 | 1.87 | 0.00811418 | 1.28 |
| LCT-HCT | 4 | Dodecanoylcarnitine | 537.09 | 344.28 | 1.05 | 0.000862536 | 1.77 |
| LCT-HCT | 5 | LPA(18:2) | 595.99 | 433.23 | 2.84 | 0.010703892 | 1.22 |
| LCT-HCT | 6 | LPA(22:6) (DHA) | 596.04 | 481.23 | 2.37 | 0.017396497 | 1.34 |
| LRR-HRR | 1 | N-Acetyl-L-aspartic acid | 38.73 | 174.04 | 1.72 | 0.049262933 | 1.17 |
| LRR-HRR | 2 | Linoleic acid | 572.81 | 281.25 | 1.08 | 0.044686889 | 1.20 |
| LRR-HRR | 3 | Taurine | 42.67 | 124.01 | 3.31 | 0.025334133 | 1.39 |
| LRR-HRR | 4 | Nicotinamide | 154.44 | 123.06 | 2.90 | 0.019866477 | 1.45 |
| LRR-HRR | 5 | Cholesterol sulfate | 700.33 | 465.30 | 2.30 | 0.011606822 | 1.27 |
| LRR-HRR | 6 | DHEAS | 468.42 | 367.16 | 2.15 | 0.009710871 | 1.37 |
| LRR-HRR | 7 | Dodecanoylcarnitine | 537.09 | 344.28 | 1.51 | 0.000826136 | 1.59 |
| LRR-HRR | 8 | alpha-Linolenic acid | 714.88 | 277.22 | 3.61 | 0.000161289 | 1.55 |
| LPR-HPR | 1 | LPA(22:6) (DHA) | 596.04 | 481.23 | 2.80 | 0.001295733 | 0.67 |
| LPR-HPR | 2 | Pseudouridine | 76.34 | 243.06 | 2.20 | 0.000726917 | 0.74 |
RT is the chromatographic retention time of the metabolite, MZ is the mass-to-charge ratio of the distinctive ion associated with the metabolite
The findings revealed (1) a total of 78 significantly different metabolites between HC and MDD, of which 13 metabolites were relatively evaluated and 65 metabolites were relatively reduced in the HC group compared to MDD group; additionally, 7 distictively different metabolites were retrieved as significantly associated with depression, of which 1 metabolite was evaluated and 6 metabolites were reduced in MDD group compared to HC group.
There were a total of 69 significantly different metabolites between the groups with high and low childhood trauma, with 17 metabolites relatively up-regulated and 52 metabolites relatively down-regulated in HCT group compared to LCT group; a total of 6 significantly different metabolites were retrieved as significantly associated with depression, with 6 of these metabolites being down-regulated compared to the low childhood trauma group.
A total of 83 metabolites exhibited significant differences when comparing LRR group and HRR group, 58 metabolites were upregulated in LRR, 25 metabolites were downregulated, and 8 metabolites were retrieved that had a significant association with depression, of which all 8 metabolites were upregulated.
A total of 50 metabolites exhibited significant differences when comparing the LPR and HPR, with 25 metabolites up- and down-regulated in each of the low mental elasticity groups, and only 2 significant metabolites associated with depression were retrieved, both of which were down-regulated.
Among them, there were 26 co-differentiators between depression and childhood trauma, 23 co-differentiators between depression and rumination, and 15 co-differentiators between depression and resilience; further analysis revealed that there were two important co-metabolic differentiators between childhood trauma and depression: Dehydroepiandrosterone Sulfate (DHEAS), LPA(22:6) (Docosahexaenoic Acid (DHA)); and 2 important common metabolic differentiators between redundancy and depression were: N-Acetyl-L-aspartic acid, DHEAS; and 2 important common metabolic differentiators between resilience and depression were: LPA(22:6), Pseudouridine(See supplementary materials). This suggests that differential metabolites such as DHEAS, LPA (22:6), DHA, N-Acetyl-L-aspartic acid, and Pseudouridine may differ between depression and psychological factors.
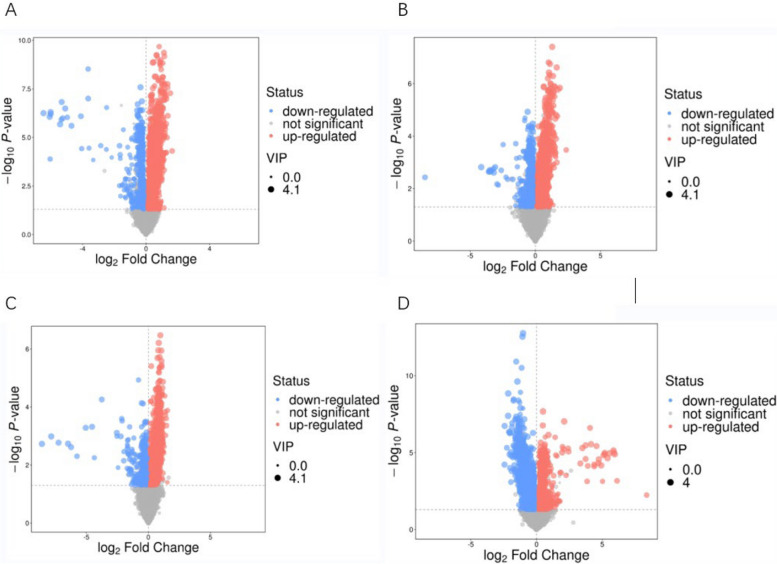
Based on the OPLS-DA scores, the -log10 q-values (y-axis) were graphed in relation to the corresponding log2 fold change (x-axis) in volcano plots (Fig. 4), and differential metabolites were filtered based on the set criteria (VIP > 1 and P < 0.05).
Fig. 4.
Volcano plot for group. A HC vs MDD. B LCT vs HCT. C LRR vs HRR. D LPR vs HPR. Note: Volcano plots indicate metabolites displaying sustaintial difference for each control vs. test. Scatters in red represent metabolites that have been significantly up-regulated, scatters in blue indicate metabolites that have been significantly down-regulated, and scatters in gray represent metabolites that do not exhibit a significant difference. Points that are further away from the origin and larger in size indicate a greater contribution to the difference between groups
Correlation between psychological factors and differential metabolism
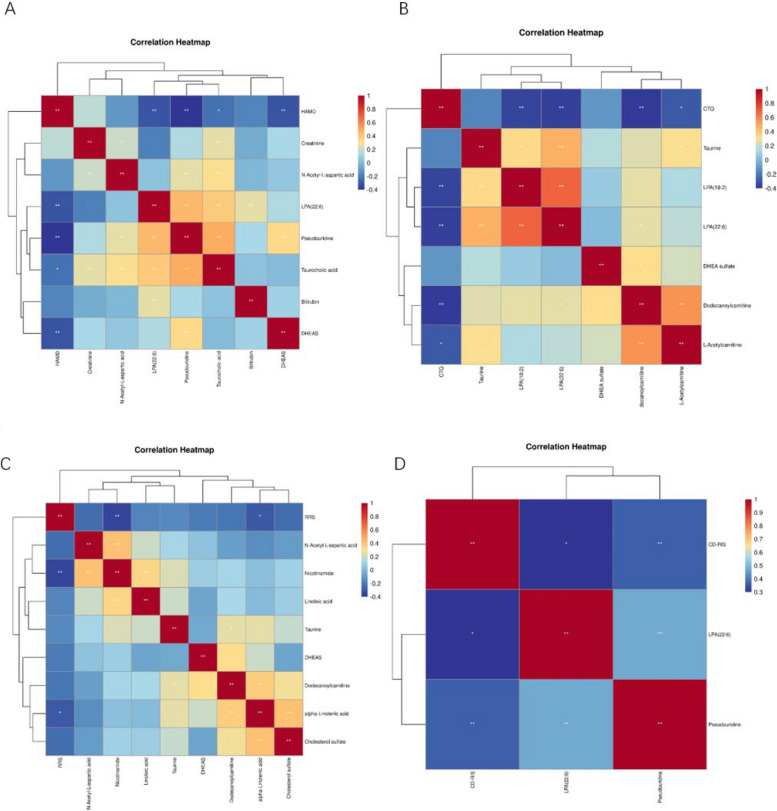
Spearman's correlation analysis was employed to examine the connection between the scores of the scales and the distict metabolites (Fig. 5).Hamilton Depression Scale scores exhibited a notable inverse correlation (P < 0.05) with Taurocholic acid, LPA(22:6), DHEAS, and Pseudouridine.Childhood Trauma Questionnaire scores were significantly and negatively correlated with LPA(22:6), LPA(18:2), Dodecanoylcarnitine, and L-Acetylcarnitine (p < 0.05). Rumination response scale scores were substaintially negatively correlated with Nicotinamide and alpha-Linolenic acid (P < 0.05). Resilience scale scores were significantly and positively correlated with LPA (22:6) and Pseudouridine (p < 0.05). This suggests that differential metabolites such as DHEAS, LPA (22:6), DHA, N-Acetyl-L-aspartic acid, and Pseudouridine may differ between depression and psychological factors.
Fig. 5.
Heat map of correlation between scale scores and important differential metabolites. A HAMD. B CTQ. C RRS. D CD-RIS
Discussions
This study aims to explore the interconnectedness of biological and psychosocial factors in adolescent depression by examining childhood trauma, rumination, and resilience and their metabolic correlations. Most of the research on adolescent depression has been limited to one side of the coin, such as biological or psychosocial factors, and fails to explain the interconnectedness of multiple influences.
In this research, we detected notable disparities in HAMD, CTQ, RRS, and CD-RISP scores between MDD and HC groups (P < 0.001), and HAMD scores were significantly different in LCT versus HCT, LRR versus HRR, and LPR versus HPR groups (P < 0.001). This suggests that depressed patients have lower resilience higher rumination and childhood trauma. Correlation analysis in this study showed a significant positive effect of rumination, childhood trauma, and depression. And negative correlation between resilience and both rumination, childhood trauma, and depression. The findings suggest that childhood trauma, redundancy, and mental resilience are interrelated and collectively influence the onset and progression of adolescent depression. Research suggest that resilience reduces the effects of childhood trauma, rumination on depression, and that resilience significantly mediates the relationship between childhood trauma and depression, and rumination and depression, respectively [36, 37]. Therefore, the present study concluded that childhood trauma and rumination are risk factors for adolescent depression, and that resilience may act as a safeguard for the occurrence of adolescent depression.
Metabolites, as products of the body's metabolism, may help us to better explore the pathogenesis of depression in adolescents. The findings of the current research revealed that DHEAS, N-acetyl-L-aspartate, pseudouridine, bilirubin, docosahexaenoic acid LPA(22:6), taurocholic acid were noticeably lower, and creatinine was markedly higher in MDD group in comparison to HC group. Researches have demonstrated that DHEAS may have a crucial impact on cortical development and plasticity, preventing negative effects and depression [38, 39]. This suggests that DHEAs may ameliorate adolescent depression by modulating cortical plasticity. Animal models of depression have also confirmed that N-acetyl-L-aspartate is downregulated in the brain [40]. The ratio of N-acetylaspartate to creatine in prefrontal white matter areas is a significant predictor of the onset and development of depression in adolescents [41]. The results of this study also support the idea that a decrease in N-acetyl-L-aspartate is associated with depression in adolescents. Pseudouridine is currently thought to be linked to the initiation of depression after stroke, and our experiments, which first found differences in adolescent depressed patients, warrant further exploration [42]. Bilirubin may be modulating the development of depression by acting on TNF-α to prevent the extinction of dopaminergic neurons [43]. In conjunction with the aforementioned study, the findings suggest that the incidence of adolescent depression may be associated with inflammatory processes, which merits further investigation. Studies have shown a link between adult depressive symptoms and a decline in renal function, with creatinine being one of the main indicators for evaluating renal function [44]. The findings of this study point to a potential correlation between adolescent depression and renal function. LPA 22:6 stands for docosahexaenoic acid (DHA) containing 1- or 2-acyl LPAs. In the previous work of our group, taurocholic acid and DHA were found to be significantly decreased in depressed patients and may be potential markers of depression [45]. Previous studies have also found that DHA is significantly decreased in human cerebrospinal fluid, and taurocholic acid may be ameliorating depression by inhibiting inflammatory responses [46]. In combination with this study, the evidence supporting the close relationship between inflammation and adolescent depression is further substantiated. Therefore, we suggest that DHEAS, N-acetyl-L-aspartate, pseudouridine, bilirubin, creatinine, LPA(22:6), and taurocholic acid may be involved in the pathogenesis of depression in adolescents.
The combined application of psychosocial factors and metabolomics is a good way to explore the pathogenesis of depression in adolescents. DHEAS and LPA(22:6) were significant differential metabolites in both depressed and normal groups, as well as high and low childhood trauma groups, suggesting their potential role in the metabolic pathways associated with depression.. N-Acetyl-L-aspartic acid and DHEAS were significant differential metabolites in both depressed and normal groups, as well as high and low rumination groups, suggesting their potential role in the metabolic pathways associated with depression. Pseudouridine and LPA(22:6) were identified as significant differential metabolites in both the depressed and normal groups, as well as in the high and low childhood resilience groups. The findings indicate that resilience and adolescent depression may be associated with a common set of metabolic pathways. In this study, we further employed spearman correlation analysis to examine the correlation between the scale scores and the differential metabolites to intuitively find that taurocholic acid, docosahexaenoic acid LPA(22:6), DHEAS, and pseudouridine were substantially negatively connected to depression (P < 0.05). Childhood trauma was notably negatively linked to LPA(22:6), octadecadienoic acid, dodecanoylcarnitine and L-acetylcarnitine (P < 0.05). Rumination was significantly negatively correlated with nicotinamide and alpha-linolenic acid (P < 0.05). Psychological elasticity was significantly positively correlated with LPA(22:6) and pseudouridine (P < 0.05). The extant literature indicates that childhood trauma is significantly correlated with depressive symptoms in adulthood. Moreover, this relationship appears to be mediated by functional connectivity in cognitively relevant brain regions [47]. ruminative thinking is often used as part of negative cognition in depressed patients to influence the onset of depression [48]. Studies have shown that people with high resilience have a lower risk of depression [49]. Resilience alleviates negative cognitions in depression and correlates with therapeutic efficacy in depression [50, 51]. These studies suggest that psychological factors may modulate the development of depression by affecting cognitively related brain regions. Dehydroepiandrosterone, as one of the key hormones of the hypothalamic–pituitary–adrenal axis (HPA), has been shown that high levels of DHEAS concentrations correlate with cognitive levels. Research has demonstrated that DHEAS can influence the release of glutamate, acetylcholine, and norepinephrine in the brain, which can impact cognitive function [52]. Decreased levels of DHA affects the HPA axis and can lead to cognitive decline, thus acting as an antidepressant, and DHA may be involved in the modulation of depression through the production of lipid mediators of lipoxygenase and cytochrome P450 [53–55]. DHA has been demonstrated to mitigate the cognitive decline associated with a high-fat diet in male rats. This effect is thought to be mediated by a reduction in the density of 5-hydroxytryptamine receptors and gamma-aminobutyric acid receptors in the brain [56]. DHEAS, DHA are collectively involved in regulating HPA activity thereby influencing the onset of depression [57]. This indicates that the incidence of adolescent depression is associated with the activity of the HPA axis. Regarding the mechanism by which N-acetyl-L-aspartate and pseudouridine affect depression is still unclear, so more studies are needed to explore it. In summary, we suggest that childhood trauma, rumination, and low resilience may be contributing to depression by affecting levels of DHEAS and LPA(22:6), which in turn affect an individual's cognitive functioning. In addition, the HPA axis can be used as an important target to explore the interaction between psychological factors and adolescent depression.
The research also has some shortcomings: In order to address the potential for recall bias, when confronted with some of the participants' unclear memories, we chose to ask their family members and retest if necessary, while ensuring the participants' privacy. some of the data in this study came from questionnaires, and it is difficult to avoid recall bias, so that the accuracy of the data is affected to some extent. In order to address the potential for recall bias, when confronted with some of the participants' unclear memories, we chose to ask their family members and retest if necessary, while ensuring the participants' privacy. Furthermore, the study was limited by several factors, including a relatively small sample size, geographic constraints within the Guizhou region, lack of generalizability, and the use of a single metabolic measure. Our next study will employ a longitudinal research design to investigate causal relationships and temporal patterns. The combined effect of psychosocial and biological interventions will be investigated. Targeted metabolomic studies will be conducted to validate the identified metabolites. The sample size will be expanded and multi-center, cross-regional validation studies will be increased. The clinical application of this study lies in the potential use of the identified metabolites as possibility biomarkers for depression. It is also conceivable that resilience training could be incorporated into therapeutic approaches with the objective of mitigating the effects of childhood trauma and rumination.. Furthermore, the genetic basis of rumination and resilience will be explored.
Conclusions
The present study corroborates the biological correlation between psychological factors and adolescent depression, as evidenced by both scale assessment and metabolomic analysis. The findings of this study indicate that psychological factors (childhood trauma, rumination, resilience) are biologically linked to the development of depression in adolescents. The impact of rumination on adolescent depression may be associated with DHEAs. The impact of childhood trauma and resilience on adolescent depression may be associated with LPA (22:6). These findings underscore the significance of psychological elements in the biological underpinnings of adolescent depression. Nevertheless, further investigation is required to elucidate the precise mechanisms underlying the association between psychological factors and adolescent depression.
Supplementary Information
Abbreviations
- HC
Adolescent healthy control group
- MDD
Adolescent depression group
- ICD-10
The World Health Organization Classification of Mental and Behavioral Disorders
- CTQ
Childhood trauma questionnaire
- CD-RISC
Connor- Davidson resilience scale
- RRS
Ruminative Responses Scale
- LCT
Low childhood trauma group
- HCT
High childhood trauma group
- LPR
Low resilience group
- HPR
High resilience group
- LRR
Low redundancy group
- HRR
High redundancy group
- HAMD
Hamilton depression scale
- OPLS-DA
Orthogonal projection latent structure discriminant analysis
- VIP
Variable importance
- BMI
Body mass index
- DHEAS
Dehydroepiandrosterone Sulfate
- DHA
Docosahexaenoic Acid
- HPA
Hypothalamic-pituitary-adrenal axis
Authors’ contributions
XYG is the frst author and drafted the manuscript for submission. XYG and GT were responsible for data collection, the study s topic and scope, research of evidence as well as analysis and interpretation of data. FL and HYFwere involved in data collection, coding of data, and provided intellectual input into the article. TZ and JC supervised the study and provided intellectual input into the article. All authors read and approved the final manuscript.
Funding
This case report received funding from the National Natural Science Foundation of China(82260280) and the Technology Program of Guizhou Province (QianKeHeJiChu-ZK[2023]386).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from all participants for this study. In the case of participants under the age of 16, informed consent was obtained from both the participants themselves and their legal guardians. The study was reviewed and approved by the Medical Ethics Committee of The Affiliated Hospital of Guizhou Medical University and was in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xunyi Guo and Gan Tang contributed equally to this work.
Contributor Information
Jing Chen, Email: 494205690@qq.com.
Tao Zou, Email: zoutaozou@gmail.com.
References
- 1.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J PSYCHIATR RES. 2020;126:134–40. [DOI] [PubMed] [Google Scholar]
- 2.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. The lancet. 2012;379(9820):1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA PEDIATR. 2021;175(11):1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalin NH. Anxiety, depression, and suicide in youth. Am Psychiatric Assoc. 2021;178(4):275–79. [DOI] [PubMed]
- 5.Zheng K, Chu J, Zhang X, Ding Z, Song Q, Liu Z, Peng W, Cao W, Zou T, Yi J. Psychological resilience and daily stress mediate the effect of childhood trauma on depression. CHILD ABUSE NEGLECT. 2022;125: 105485. [DOI] [PubMed] [Google Scholar]
- 6.Colodro-Conde L, Couvy-Duchesne B, Zhu G, Coventry WL, Byrne EM, Gordon S, Wright MJ, Montgomery GW, Madden PA, Ripke S. A direct test of the diathesis–stress model for depression. MOL PSYCHIATR. 2018;23(7):1590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Gong H, Sun W, Ma P, Chen Y, Gao Y. The health context paradox in the relationship between victimization, classroom bullying attitudes, and adolescent depression: an analysis based on the hlm model. J AFFECT DISORDERS. 2024;354:694–701. [DOI] [PubMed] [Google Scholar]
- 8.Deighton S, Neville A, Pusch D, Dobson K. Biomarkers of adverse childhood experiences: A scoping review. Psychiat Res. 2018;269:719–32. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, He C, Fan D, Liu X, Zhang H, Zhang H, Zhang Z, Xie C. Neural effects of childhood maltreatment on dynamic large-scale brain networks in major depressive disorder. PSYCHIAT RES. 2022;317: 114870. [DOI] [PubMed] [Google Scholar]
- 10.LeMoult J, Humphreys KL, Tracy A, Hoffmeister J, Ip E, Gotlib IH. Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J Am Acad Child Adolesc Psychiatry. 2020;59(7):842–55. [DOI] [PubMed] [Google Scholar]
- 11.Watkins ER, Roberts H. Reflecting on rumination: Consequences, causes, mechanisms and treatment of rumination. Behav Res Ther. 2020;127: 103573. [DOI] [PubMed] [Google Scholar]
- 12.He J, Liu Y, Cheng C, Fang S, Wang X, Yao S. Psychometric properties of the Chinese version of the 10-item ruminative response scale among undergraduates and depressive patients. FRONT PSYCHIATRY. 2021;12: 626859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdolini N, Amoretti S, Montejo L, García-Rizo C, Hogg B, Mezquida G, Rabelo-da-Ponte FD, Vallespir C, Radua J, Martinez-Aran A. Resilience and mental health during the COVID-19 pandemic. J AFFECT DISORDERS. 2021;283:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalisch R, Russo SJ, Müller MB. Neurobiology and systems biology of stress resilience. Physiol Rev. 2024;104(3):1205–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestler EJ, Russo SJ. Neurobiological basis of stress resilience. Neuron. 2024;112(12):1911–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thumfart KM, Jawaid A, Bright K, Flachsmann M, Mansuy IM. Epigenetics of childhood trauma: Long term sequelae and potential for treatment. Neurosci Biobehav Rev. 2022;132:1049–66. [DOI] [PubMed] [Google Scholar]
- 17.De Bellis MD, Zisk A. The biological effects of childhood trauma. Child and Adolescent Psychiatric Clinics. 2014;23(2):185–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson DP, Rhee SH, Friedman NP, Corley RP, Munn-Chernoff MA, Hewitt JK, Whisman MA. A twin study examining rumination as a transdiagnostic correlate of psychopathology. CLIN Psychol Sci. 2016;4(6):971–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misaki M, Tsuchiyagaito A, Guinjoan SM, Rohan ML, Paulus MP. Whole-brain mechanism of neurofeedback therapy: predictive modeling of neurofeedback outcomes on repetitive negative thinking in depression. TRANSL PSYCHIAT. 2024;14(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghulam A, Bonaccio M, Costanzo S, Bracone F, Gianfagna F, de Gaetano G, Iacoviello L. Psychological resilience, cardiovascular disease, and metabolic disturbances: a systematic review. Front Psychol. 2022;13: 817298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whipp AM, Heinonen-Guzejev M, Pietiläinen KH, van Kamp I, Kaprio J. Branched-chain amino acids linked to depression in young adults. Front Neurosci-Switz. 2022;16: 935858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Qin M, Teng T, Li X, Yu Y, Wang J, Wu H, He Y, Zhou X, Xie P. Identification of sex-specific plasma biomarkers using metabolomics for major depressive disorder in children and adolescents. Front Psychiatry. 2022;13: 929207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Yieh L, Yang T, Drinkenburg W, Peeters P, Steckler T, Narayan VA, Wittenberg G, Ye J. Metabolomic biosignature differentiates melancholic depressive patients from healthy controls. BMC Genomics. 2016;17:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Wang J, Chen S, Chen X, Liu J, Tang H, Zhou J, Tian Y, Wang X, Cao X. Abnormal energy metabolism, oxidative stress, and polyunsaturated fatty acid metabolism in depressed adolescents associated with childhood maltreatment: A targeted metabolite analysis. Psychiat Res. 2024;335:335. [DOI] [PubMed] [Google Scholar]
- 25.Karnib N, El-Ghandour R, El Hayek L, Nasrallah P, Khalifeh M, Barmo N, Jabre V, Ibrahim P, Bilen M, Stephan JS. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacol. 2019;44(6):1152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akimoto H, Oshima S, Sugiyama T, Negishi A, Nemoto T, Kobayashi D. Changes in brain metabolites related to stress resilience: Metabolomic analysis of the hippocampus in a rat model of depression. BEHAV BRAIN RES. 2019;359:342–52. [DOI] [PubMed] [Google Scholar]
- 27.Kelley TL. The selection of upper and lower groups for the validation of test items. J Educ Psychol. 1939;30(1):17. [Google Scholar]
- 28.Bech P. The responsiveness of the different versions of the Hamilton Depression Scale. World Psychiatry. 2015;14(3):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Li Y, Yu C, Li L. A systematic review of the reliability studies of the Chinese version of the Depression Scale. Chinese Journal of Epidemiology. 2017;38(1):7. [Google Scholar]
- 30.Petrikova M, Kascakova N, Furstova J, Hasto J, Tavel P. Validation and adaptation of the Slovak version of the Childhood Trauma Questionnaire (CTQ). Int J Environ Res Public Health. 2021;18(5):2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Zhang Y, Li L, Zhou Y. Evaluation on reliability and validity of Chinese version of childhood trauma questionnaire. Chin J Tissue Eng Res. 2005;53:209–11.
- 32.Connor KM, Davidson JR. Development of a new resilience scale: The Connor-Davidson resilience scale (CD-RISC). Depress anxiety. 2003;18(2):76–82. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Zhang J. Factor analysis and psychometric evaluation of the Connor-Davidson Resilience Scale (CD-RISC) with Chinese people. Soc Behav Personal Int J. 2007;35(1):19–30. [Google Scholar]
- 34.Roberts RE, Andrews JA, Lewinsohn PM, Hops H. Assessment of depression in adolescents using the Center for Epidemiologic Studies Depression Scale. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990;2(2):122. [Google Scholar]
- 35.Han X, Yang H. Chinese version of Nolen-Hoeksema ruminative responses scale (RRS) used in 912 college students: reliability and validity. Chin J Clin Psychol. 2009;17(5)550–51.
- 36.Watters ER, Aloe AM, Wojciak AS. Examining the associations between childhood trauma, resilience, and depression: a multivariate meta-analysis. Trauma Violence Abuse. 2023;24(1):231–44. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Wang Y, Xie P, Deng H, Qiu L, Liu W, Huang D, Xia B, Liu S, Zhang X. Rumination and depression in Chinese adolescents with mood disorders: The mediating role of resilience. J Clin Psychiatry. 2023;84(5):48097. [DOI] [PubMed] [Google Scholar]
- 38.Apter-Levy Y, Zagoory-Sharon O, Feldman R. Chronic depression alters mothers’ DHEA and DEHA-to-cortisol ratio: implications for maternal behavior and child outcomes. FRONT PSYCHIATRY. 2020;11:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malkesman O, Asaf T, Shbiro L, Goldstein A, Maayan R, Weizman A, Kinor N, Okun E, Sredni B, Yadid G. Monoamines, BDNF, dehydroepiandrosterone, DHEA-sulfate, and childhood depression—an animal model study. Adv Pharm Pharm Sci. 2009;2009:405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pu J, Liu Y, Gui S, Tian L, Yu Y, Song X, Zhong X, Chen X, Chen W, Zheng P. Metabolomic changes in animal models of depression: a systematic analysis. Mol Psychiatr. 2021;26(12):7328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao N, Fang J, Xie H, Liu X, Jiang X, Wang G, Cui M, Wang B, Liu Q. Correlation between neurochemical metabolism and memory function in adolescent patients with depression: A multi-voxel 1 H magnetic resonance spectroscopy study. Psychiat Clin Neuros. 2016;70(4):167–74. [DOI] [PubMed] [Google Scholar]
- 42.Liang Z, Jia Y, Li Z, Li M, Wang M, Yun Y, Yu L, Shi L, Zhu R. Urinary biomarkers for diagnosing poststroke depression in patients with type 2 diabetes mellitus. Diabetes, Metab Syndr Obes Targets and Therapy. 2019;12:1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jayanti S, Dalla Verde C, Tiribelli C, Gazzin S. Inflammation, dopaminergic brain and bilirubin. Int J Mol Sci. 2023;24(14):11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, He P, Liu M, Zhou C, Liu C, Li H, Zhang Y, Li Q, Ye Z, Wu Q. Association of depressive symptoms with rapid kidney function decline in adults with normal kidney function. Clinical journal of the American Society of Nephrology: CJASN. 2021;16(6):889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai S, Xie J, Bai H, Tian T, Zou T, Chen J. Gut microbiota-derived inflammation-related serum metabolites as potential biomarkers for major depressive disorder. J Inflamm Res. 2021;14:3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omori W, Kano K, Hattori K, Kajitani N, Okada-Tsuchioka M, Boku S, Kunugi H, Aoki J, Takebayashi M. Reduced cerebrospinal fluid levels of lysophosphatidic acid docosahexaenoic acid in patients with major depressive disorder and schizophrenia. Int J Neuropsychoph. 2021;24(12):948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wan Z, Rolls ET, Feng J, Cheng W. Brain functional connectivities that mediate the association between childhood traumatic events, and adult mental health and cognition. EbioMedicine. 2022;79:104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Velden AM, Scholl J, Elmholdt E, Fjorback LO, Harmer CJ, Lazar SW. O Toole MS, Smallwood J, Roepstorff A, Kuyken W: Mindfulness training changes brain dynamics during depressive rumination: A randomized controlled trial. Biol Psychiat. 2023;93(3):233–42. [DOI] [PubMed] [Google Scholar]
- 49.Lin L, Chien Y, Chen Y, Wu C, Chiou H. Bullying experiences, depression, and the moderating role of resilience among adolescents. Front Public Health. 2022;10: 872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Min J, Yu JJ, Lee C, Chae J. Cognitive emotion regulation strategies contributing to resilience in patients with depression and/or anxiety disorders. Compr Psychiat. 2013;54(8):1190–7. [DOI] [PubMed] [Google Scholar]
- 51.Hoorelbeke K, Marchetti I, De Schryver M, Koster EH. The interplay between cognitive risk and resilience factors in remitted depression: A network analysis. J Affect Disorders. 2016;195:96–104. [DOI] [PubMed] [Google Scholar]
- 52.Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89(2):134–52. [DOI] [PubMed] [Google Scholar]
- 53.Carlson SE, Colombo J. DHA and Cognitive Development. Oxford University Press. 2021;151(11): 3265–66. [DOI] [PMC free article] [PubMed]
- 54.Borsini A, Nicolaou A, Camacho-Muñoz D, Kendall AC, Di Benedetto MG, Giacobbe J, Su K, Pariante CM. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatr. 2021;26(11):6773–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang L, Liang Q, Shi Y. Pure docosahexaenoic acid can improve depression behaviors and affect HPA axis in mice. Eur Rev Med Pharmacol Sci. 2012;16(13):1765–73. [PubMed] [Google Scholar]
- 56.Yu Y, Wu Y, Patch C, Wu Z, Szabo A, Li D, Huang X. DHA prevents altered 5-HT1A, 5-HT2A, CB1 and GABAA receptor binding densities in the brain of male rats fed a high-saturated-fat diet. J Nutr Biochem. 2013;24(7):1349–58. [DOI] [PubMed] [Google Scholar]
- 57.Ter Horst DM, Schene AH, Figueroa CA, Assies J, Lok A, Bockting C, Ruhé HG, Mocking R. Cortisol, dehydroepiandrosterone sulfate, fatty acids, and their relation in recurrent depression. Psychoneuroendocrino. 2019;100:203–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.