Abstract
Ciliary neurotrophic factor (CNTF) is primarily known for its important cellular effects within the nervous system. However, recent studies indicate that its receptor can be highly expressed in denervated skeletal muscle. Here, we investigated the direct effect of CNTF on skeletal myoblasts of adult human. Surprisingly, we found that CNTF induced the myogenic lineage-committed myoblasts at a clonal level to dedifferentiate into multipotent progenitor cells—they not only could proliferate for over 20 passages with the expression absence of myogenic specific factors Myf5 and MyoD, but they were also capable of differentiating into new phenotypes, mainly neurons, glial cells, smooth muscle cells, and adipocytes. These “progenitor cells” retained their myogenic memory and were capable of redifferentiating into myotubes. Furthermore, CNTF could activate the p44/p42 MAPK and down-regulate the expression of myogenic regulatory factors (MRFs). Finally, PD98059, a specific inhibitor of p44/p42 MAPK pathway, was able to abolish the effects of CNTF on both myoblast fate and MRF expression. Our results demonstrate the myogenic lineage-committed human myoblasts can dedifferentiate at a clonal level and CNTF is a novel regulator of skeletal myoblast dedifferentiation via p44/p42 MAPK pathway.
INTRODUCTION
Adult skeletal myoblasts have long been considered as myogenic lineage-committed cells with either self-renewing or differentiating into multinucleated myotubes in vitro (Jankowski et al., 2002; Tamaki et al., 2003). Myoblast fate is critically dependent on the myogenic regulatory factors (MRFs): MyoD, Myf5, myogenin, and Mrf4, which have a well-defined role in orchestrating skeletal muscle development and differentiation. Gene targeting in mice has illustrated the essential role for MRFs: Myf5 and MyoD is critical to establishing the myogenic cell lineage and producing committed, undifferentiated myoblasts (Kablar et al., 1999), whereas myogenin has an important role in initiating differentiation (Hasty et al., 1993); Mrf4 regulates terminal differentiation and myofiber maintenance (Patapoutian et al., 1995). Gene targeting in mice has illustrated the essential role for Myf5 and MyoD in myoblast fates. In MyoD–/– and/or Myf5–/– knockout mice, muscle precursors acquire nonmuscle cell fates (Kablar et al., 1999) and become multipotential stem cells or a progenitor compartment (Parker et al., 2003). Early histological studies indicated that muscle fibers at the amputation plane of urodele amphibians might dedifferentiate by budding off mononucleate cells from syncytial muscle cells (Echeverri and Tanaka, 2002). Recent studies suggest that dedifferentiation may be also possible in mammalian system (Odelberg et al., 2000; Tsai et al., 2002; Chen et al., 2004). Especially, the recent reports have shown that ectopic expression of msx1 in C2C12 myotubes can induce dedifferentiation of the myotubes to produce multipotent mononucleated cells (Odelberg et al., 2000), and a 2,6-disubstituted purine, reversine, can also induce dedifferentiation of C2C12 myoblasts to become mesenchymal progenitor cells (Chen et al., 2004). However, the C2C12 myogenic line may be heterogeneous because there is this novel evidence for a resident subset of cells with side population (SP) phenotype in the C2C12 line (Benchaouir et al., 2004), whereas SP cells themselves are growth-arrested and delayed in their ability to differentiate with behaving as multipotent stem cells (Zhou et al., 2001; Jankowski et al., 2002; Tamaki et al., 2003). In addition, whether human skeletal myoblasts can dedifferentiate is unclear. Therefore, further studies will be needed to elucidate the plasticity of myoblast dedifferentiation at a clonal level, especially in the human myoblasts.
Ciliary neurotrophic factor (CNTF) was initially identified by its ability to support the survival of parasympathetic neurons of the chick ciliary ganglion in vitro (Adler et al., 1979). CNTF activity has subsequently been found to be important in regulating numerous other processes within the nervous system, including neural stem cell proliferation (Shimazaki et al., 2001; Weinelt et al., 2003) and gliogenesis (Aberg et al., 2001; Song and Ghosh, 2004), as well as rod differentiation (Ezzeddine et al., 1997; Schulz-Key et al., 2002). In addition, CNTF can substitute for leukemia inhibitory factor (LIF) in maintaining pluripotentiality of embryonic stem (ES) cells in culture (Wolf et al., 1994) and also bind with the LIF receptor (LIFR) in mature astrocytes (Monville et al., 2001). Beside its activities on the nervous system, CNTF also can reduce body fat (Lambert et al., 2001) and is a regulator of muscular strength in aging (Guillet et al., 1999). The expression of CNTF is restricted to Schwann cells in the peripheral and astrocytes in the CNS (Oppenheim et al., 1991; Stockli et al., 1991). Recent studies indicated that CNTF receptor (CNTFR) localized predominantly within neural tissue has relatively high expression in skeletal muscle (Sleeman et al., 2000) and in denervated skeletal muscle CNTF exerts myotrophic effects (Helgren et al., 1994), and the expression of CNTF α-receptor (CNTFRα) also significantly increases (Weis et al., 1998). However, the direct effect of CNTF on skeletal myoblasts is not yet well understood.
CNTF belongs to the interleukin (IL)-6 family cytokines: IL-6, IL-11, LIF, oncostatin M (OSM), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine (CLC). Cellular responses to CNTF and IL-6 type cytokines are elicited by different multiunit receptor complexes (CNTFRα,gp130, LIFR). Binding of CNTF to the CNTFRα induces a heterodimer of the signal transducing β-receptors gp130 and LIFR, which triggers intracellular signaling cascades (Groötzinger et al., 1999; Senaldi et al., 1999; Shi et al., 1999). The variety of CNTFR complexes, however, funnels into activation of an only limited number of intracellular signaling cascades mainly including the JAK-STAT and ERK-MAPK or p44/p42 MAPK pathways (Boulton et al., 1994; Dziennis and Habecker, 2003; Rhee and Yang, 2003; Zvonic et al., 2003), whereas the latter has been implicated in regulating myoblast differentiation in vitro, but its functions seem to be controversial with either a positive (Gredinger et al., 1998; Al-Khalili et al., 2004) or a negative (Tortorella et al., 2001; Khurana and Dey, 2002) role in myogenesis. Finally, although CNTF can cause activation of p44/p42 MAPK in neural cells (Prithi and Ronald, 1998; Kuroda et al., 2001; Dziennis and Habecker, 2003) and adipocytes (Zvonic et al., 2003), whether CNTF may activate p44/p42 MAPK in skeletal myoblasts is still unclear.
Here, we investigate the direct effect of CNTF on skeletal myoblasts of adult human and its p44/p42 MAPK signaling mechanism in vitro. We first isolated and obtained the clones of individual myoblasts from adult human skeletal muscle. The clonal myoblasts could be induced to dedifferentiate into multipotent progenitor cells when treated with exogenous CNTF. Furthermore, CNTF was able to cause p44/p42 MAPK activation and significantly down-regulate the expression of MRFs in the myoblasts. Finally, PD98059, a specific inhibitor of p44/p42 MAPK pathway, could abolish the effects of CNTF on myoblast fate and MRF expression. This work for the first time provides evidence that skeletal myoblasts of adult human can be induced to dedifferentiate at a clonal level by CNTF via p44/p42 MAPK pathway in vitro.
MATERIALS AND METHODS
Myoblast Isolation and Culture
Human skeletal myoblasts were obtained from the triceps brachii muscle biopsies of a 46-year-old male volunteer. The procedure of myoblast isolation and purification was performed as previously described (Rando and Blau, 1994). The cells were propagated on culture plates coated with collagen (Sigma-Aldrich, St. Louis, MO) in growth medium (GM). GM consists of Ham's F10 supplemented with 10% horse serum (HS), 10% fetal bovine serum (FBS), 1% chick embryo extract, 1% penicillin-streptomycin (Hyclone, Logan, UT), and 2.5 ng/ml bFGF (R&D Systems, Minneapolis, MN).
For experiment on clones of individual myoblasts, the purified myoblasts were serially diluted in GM and plated onto collagen-coated 96-well plates and allowed to adhere overnight after which the positions of the individual cells were marked. The medium was then supplemented to 50% with filtered myoblast-conditioned medium. The individual cells allowed to further grow to ∼50 cells. After this time sterile cloning discs (Sigma Chemical), which had been soaked in 0.25% trypsin (Boehringer Mannheim, Indianapolis, IN), were placed on each single clone until the cells detached, and the discs with attached cells were placed in four wells of a 24-well plate for 10–14 d, and then were passaged into four wells of a 6-well plate. After grown to subconfluence, these monoclonal cells were stained with anti-Desmin antibody (see Immunocytochemistry and Cytochemical Assay) and only the Desmin+ clones were passaged into four 25-cm2 tissue-culture flasks. Cultures were incubated in 37°C/5% CO2 incubator. The clones of individual myoblasts were maintained in GM for further proliferation and then passaged.
To identify myogenic specificity, the myoblast clones were seeded on collagen-coated glass coverslips at a density of 2 × 104 cells/cm2, cultured in GM for 24 h to proliferate or in differentiation medium (DM) for 72 h to differentiate into multinucleated myotubes, and then subjected to immunocytochemical analysis. DM consists of DMEM (GIBCO-BRL, Rockville, MD) supplemented with 1% penicillin-streptomycin and 2% HS. To exclude the presence of fibroblasts and mesenchymal stem cells (MSCs) in the myoblast clones, NIH3T3 and human MSCs were also cultured in the same myoblast GM or DM to determine the myogenic specificity of these cells. Furthermore, the Nestin+ NSCs (neural stem cells) and CD45+/CD34+ HSCs (hemopoietic stem cells), which were used as the positive control of the immunocytochemical analysis with anti-Nestin, anti-CD34, and anti-CD45 antibodies to exclude the presence of previously reported progenitor/stem cells isolated from adult skeletal muscle, such as Nestin+ neuronal differentiation of stem cells (Romero-Ramos et al., 2002), and CD45+/CD34+ SP cells/MDSCs (Asakura et al., 2002; Jankowski et al., 2002; Qu-Petersen et al., 2002; Tamaki et al., 2003) were plated onto the collagen-coated glass coverslips for 12 h to adhere. The control human MSCs and HSCs were gifts from Professor XiaoDan Guo and Xiaoxia Jiang, Ph.D., whereas NSCs of adult rat were isolated as previously described (Xu et al., 2003).
To observe the effect of CNTF on myoblast differentiation in vitro, DM with 0–50 ng/ml CNTF (R&D Systems) was directly added to the subconfluent myoblasts for 72 h, and the medium was replaced every 12 h with medium containing fresh CNTF. To determine whether p44/p42 MAPK pathway was involved in the CNTF regulatory mechanism, the myoblasts were treated with DM containing 30–50 ng/ml CNTF in the presence or absence of 20 μM PD98059 (P-215; Sigma-Aldrich) for 72 h as previously described (Mellott et al., 2002). The extent of myoblast myogenic differentiation was measured by the number of multinucleated myotube formation and the expression levels of myogenic regulatory factors (MRFs: Myf5, MyoD, and myogenin). Cells containing more than three nuclei were regarded as multinucleated myotubes. Each group was examined in at least three independent experiments.
Isolation and Culture of Myoblast-derived Progenitor Cells
In the cultures treated with 30–50 ng/ml CNTF for 72 h, there were many small, spherical cells (termed MBPCs for myoblast-derived progenitor cells). MBPCs were dissociated with a plastic pipette, centrifuged at 350 × g, resuspended in GM0 medium, and cultured in 50-cm2 flasks in a 37°C/5% CO2 incubator. GM0 consists of DMEM-F12, 3:1, 1% penicillin/streptomycin (Hyclone), 2% B27, 20 ng/ml epidermal growth factor (GIBCO-BRL), and 100 ng/ml bFGF. To passage MBPCs, medium containing floating MBPCs was centrifuged, resuspended, and reseeded in fresh GM0 medium. Cells were passaged every 7–10 d.
For experiment on the clones of individual MBPCs, the floating MBPCs (≥3 passages) cultured in GM0 were plated at serially limiting dilution into 48-well uncoated tissue-culture plates. Each individual chamber was assessed by microscope for the presence or absence of a single cell. The chambers of individual cells were marked and then supplemented to 50% with filtered MBPCs-conditioned medium. At 5 wk, the clones from a single cell were respectively passaged into four wells of a 24-well plate and then 2–3 wk later were passaged into four wells of a 6-well plate. After 3 wk of expansion the clones were passaged into four 25-cm2 tissue-culture flasks. The cells were passaged every 7–10 d.
The floating MBPCs can adhere onto collagen-coated coverslips after resuspended and plated in DM0 medium for 12 h. DM0 consists of DMEM-F12, 3:1, 1% penicillin/streptomycin, 2% B27, and 3% FBS.
To identify the cellular and molecular characterization of MBPCs, the proliferating MBPCs in GM0 were switched into the NSC growth medium (Clarke et al., 2000) for 7 d to compare the proliferation ability of MBPCs in both GM0 and NSC growth medium. In addition, the adherent MBPCs, NSCs, and HSCs were subjected to the immunocytochemical assays.
Multipotency Assay for MBPCs
To assess the ability of MBPCs to produce neurons, the adherent MBPCs in DM0 were switched into DM1 for 14–20 d. DM1 consists of DMEM-F12, 3:1, 1% penicillin/streptomycin, 2% B27, 3% FBS, and 50 ng/ml NGF (Sigma-Aldrich). Medium was changed every day.
To assess the ability of MBPCs to produce glia cells, the adherent MBPCs in DM0 were switched into DM2 for 10–14 d. DM2 consists of DMEM-F12, 3:1, 1% penicillin/streptomycin, 2% B27, 3% FBS, and 30 ng/ml CNTF (R&D Systems). Medium was changed every day.
Adipogenic potential was studied in the DM3 medium for 15–20 d, and the medium was changed every 2–3 d. DM3 consists of DMEM-F12, 3:1, 1% penicillin/streptomycin, 2% B27, and 10% FBS.
During inducing the multipotent differentiation as above, we continuously monitored the morphological signs of neurons, glia, smooth muscle cells, adipocytes, and myotubes and then exposed them to immunocytochemical or cytochemical assays.
Immunocytochemistry and Cytochemical Assay
Cells grown on collagen-coated coverslips were fixed with 4% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for 30 min, washed three times with PBS, and permeabilized with 0.1% Triton X-100 in PBS (PBST) for 20 min. The cells were blocked overnight at 4°C with 5% normal goat serum (NGS) in PBST and then exposed to primary antibodies: anti-Desmin (18–0016, 1:500; Zymed, South San Francisco, CA), anti-Myosin (18–0105, 1:500; Zymed), anti-Vimentin (18–0052, 1:500; Zymed), anti-Nestin (AB5922 or MAB353, 1:200; Chemicon, Temecula, CA), anti-neurofilament-160 (NF160; N-5264, 1:2000; Sigma-Aldrich), anti-tyrosine hydroxylase (TH; MAB318, 1:1000; Chemicon), anti-choline acetyl transferase (chAT; AB143, 1:2000; Sigma-Aldrich), anti-CNPase (C-5922, 1:500; Sigma-Aldrich), anti-SMA (A-2547, 1:2000; Sigma-Aldrich), anti-CD34 (555822, 1:100; BD PharMingen, San Diego, CA), and anti-CD45 (347723, 1:100; BD PharMingen), anti-Myf5 (sc-22825, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-MyoD (sc-760, 1:1000; Santa Cruz Biotechnology), anti-neuron-specific enolase (NSE; MAB324, 1:2000; Chemicon), and anti-GFAP (G-9269, 1:500; Sigma-Aldrich) antibodies in PBST/2% NGS overnight at 4°C. Cells were washed three times with PBS and incubated with the secondary antibodies, the Alexa 488–conjugated goat anti-mouse (A11001) or anti-rabbit (A11008) IgG antibodies and Texas Red–conjugated goat anti-mouse (T862) or anti-rabbit (T2767) IgG antibodies (1:1000; Molecular Probes, Eugene, OR), in PBST for 2 h at 4°C. The cells were washed three times with PBS and then counterstained with Hoechst 33342 (B2261, Sigma-Aldrich) to show nuclei. The cells were observed with a RADIANCE 2100 confocal microscope (Bio-Rad, Richmond, CA) using FITC and Texas Red filters. Specially, when exposed to anti-M-cadherin (sc-6470, 1:50; Santa Cruz Biotechnology) antibody, the cells were blocked with 5% normal rabbit serum (NRS) and incubated with the Alexa 488–conjugated rabbit anti-goat IgG antibody (A11078, 1:1000; Molecular Probes).
Pluripotent differentiation was confirmed using the specific markers of anti-Nestin, anti-NF160, anti-TH, anti-NSE, anti-chAT, anti-CNPase, anti-GFAP, anti-SMA, and anti-Myosin antibodies or Oil Red O stain (Raff, et al., 1989) for immunocytochemical or cytochemical assays. Before staining of Oil Red O, the cells were fixed for 10 min at RT in 4% formaldehyde and washed with 70% ethanol. The cells were incubated in 2% Oil Red O reagent for 5 min at RT. Excess stain was removed by washing with 70% ethanol, followed by several changes of distilled water. The cells were counterstained for 2 min with hematoxylin.
For quantification of the percentage of cells producing a given marker protein, in any given experiment at least five fields were photographed at 200× magnification, and the number of positive cells was determined relative to the total number of labeled nuclei.
Western Blot Analysis
Cells were rinsed three times with cold PBS and harvested at the time indicated. Cells were lysed and whole cell extracts were collected as described previously (Gredinger et al., 1998). Equal amounts of extracted proteins (100 μg) were loaded and separated by SDS-polyacrylamide gels (SDS-PAGE), and then transferred onto nitrocellulose membranes using a semidry transfer (Bio-Rad). Membranes were blocked overnight at 4°C in blocking buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween 20, and 2% [wt/vol] nonfat dry milk) and then incubated with the same buffer with the first and second antibodies. Between the second and third incubations, membranes were washed three times in 0.1% Tween 20 and 1× Tris-buffered saline (20 mM Tris-HCl, pH 7.4, 150 mM NaCl). Immunodetection was performed using the following antibodies: anti-Myf5, anti-MyoD, anti-myogenin (sc-13137, Santa Cruz Biotechnology; 1:1000) and anti-phospho- (9101s) or anti-nonphospho-(9102) p44/p42 MAPK (1:1000; Cell Signaling Technology) followed by HRP-conjugated antibodies (1:3000; Sigma), and ECL kit (Amersham Biosciences, Piscataway, NJ) detection. All antibodies were diluted in this blocking solution. The membranes were incubated in primary antibodies for 3 h at RT and washed with Tris-buffered saline/0.1% Tween 20 before incubation in HRP-conjugated secondary antibodies for 2 h at RT. Proteins were quantitated using a Molecular Dynamics Densitometer (Sunnyvale, CA) with ScionImage software (Frederick, MD).
RT-PCR
Total RNA was obtained from MBPCs, from the negative control NIH3T3 and from the positive control myotubes of the myoblasts cultured in DM for 7 d using TRIzol reagent according the manufacturer's protocol (Invitrogen). RT-PCR was performed by standard methodology by using the following DNA primers: Myf5, 5′-CTC AGG AAT GCC ATC CGC TAC A-3′ (forward) and 5′-GGT GCT GGC AAC TGG AGA GAG AGA A-3′ (reverse); MyoD, 5′-GGG AAG AGT GCG GCG GTG TCG AG-3′ (forward) and 5′-TCC GAG AAG GGT GCT GCG TGG AA-3′ (reverse); GAPDH, 5′-GAA GGT GAA GGT CGG AGT-3′ (forward) and 5′-GAA GAT GGT GAT GGG ATT TC-3′ (reverse). The following parameters were used: 94°C 30 s, 68°C 90 s for Myf5 and MyoD; 94°C 30 s, 56°C 30 s and 72°C 60 s for GAPDH, 72°C 10 min for 35 cycles. PCR products were checked by 1% agarose-TBE-ethidium bromide gels. The expected products sizes are: Myf5, 339 base pairs; MyoD, 445 base pairs; and GAPDH, 226 base pairs.
Statistical Analysis
All values are reported as means ± SE. Differences were assessed by Student' t test using standard spreadsheet software. P value of < 0.05 was considered to be statistically significant.
RESULTS
Myogenic Lineage-committed Myoblast Clones
Individual myoblasts from the skeletal muscle of adult human were isolated into collagen-coated 96-well plates by limiting dilution. Microscopy revealed that many wells contained no cells, but that ∼5% wells contained a single cell. After these individual cells were cultured in growth medium (GM; see Materials and Methods) for 4 wk, >50% of these single cells proliferated to generate clones. These clones were subjected to immunocytochemical analysis by staining with Desmin, a marker of myogenic lineage-committed myoblasts. Only the Desmin+ clones were allowed to further propagated and five of these clones were analyzed.
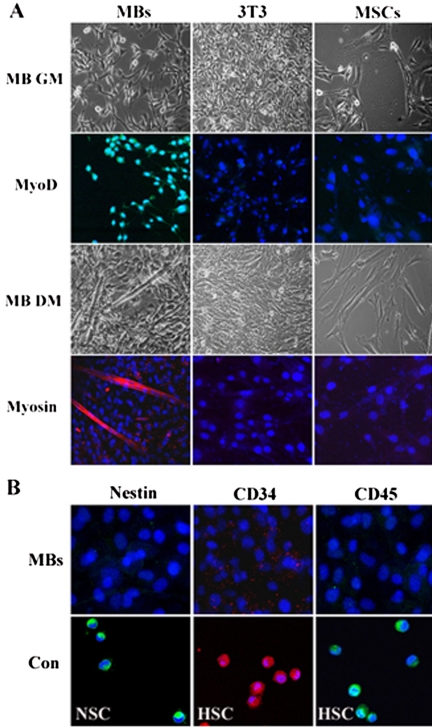
To characterize the Desmin+ clones better, we analyzed them for production of markers associated with other progenitor/stem cells which have been isolated and identified from adult skeletal muscle, such as muscle satellite cells (Jankowski et al., 2002), the precursor cells of myoblasts, SP (side population) cells (Asakura et al., 2002; Jankowski et al., 2002; Tamaki et al., 2003), MDSCs (muscle-derived stem) cells (Jankowski et al., 2002; Qu-Petersen et al., 2002), and NSCs (neural stem cells; Romero-Ramos et al., 2002). The Desmin+ clones were dispersed and plated on collagen-coated glass coverslips in GM for 24 h to adhere and proliferate, or in differentiation medium (DM; see Materials and Methods) for 72 h to induce myogenic differentiation. Immunocytochemistry revealed that, of the five clones analyzed, all of them grown for 12 h in GM were positive for MyoD (Figure 1A), the markers of myogenic lineage-committed myoblasts (Kablar et al., 1999; Jankowski et al., 2002; Tamaki et al., 2003), but were negative for Nestin (Figure 1B), a muscle-derived NSC marker (Romero-Ramos et al., 2002). They also were negative for CD34 (Figure 1B) and CD45 (Figure 1B), the markers of muscle-derived CD45+/CD34+ SP cells/MDSCs (Asakura et al., 2002; Jankowski et al., 2002; Qu-Petersen et al., 2002; Tamaki et al., 2003). Furthermore, these possible heterogeneous progenitor/stem cells including satellite cells in the adult skeletal muscle never express myogenic specific protein MyoD. On the basis of these results, we concluded that the clonal myoblasts were myogenic specificity and might never contain the satellite cells, NSCs, SP cells, and MDSCs.
Figure 1.
Myogenic lineage-committed clones of individual myoblasts. (A) Phase micrographs of clonal myoblasts (MBs), NIH3T3 (3T3), and mesenchymal stem cells (MSCs) cultured in myoblast GM (MB GM) for 24 h or in myoblast DM (MB DM) for 72 h. Immunocytochemistry assay of these cells cultured in the GM or DM by using the anti-MyoD (MyoD) and anti-Myosin (Myosin) antibodies. (B) Immunocytochemistry assay of clonal myoblasts (MBs) and its positive control (Con) NSC and HSC by using anti-Nestin, anti-CD34, and anti-CD45 antibodies. This data presented are representative of five clones with similar results. (The myoblasts are randomly selected from 3 to 25 passage clones of the individual Desmin+ myoblasts.)
In addition, two compartments in adult skeletal muscle that might contaminate the myoblast clones are fibroblasts and mesenchymal stem cells (MSCs). To exclude the heterogeneous possibility, we cultured the myoblast clones, NIH3T3, and MSCs in the same GM or DM of myoblasts for 24 or 72 h. Immunocytochemical analysis revealed that NIH3T3 and MSCs in the GM were uniformly negative for MyoD (Figure 1A). We then determined whether NIH3T3 and MSCs would differentiate into multinucleated myotubes as the myoblast clones when cultured under conditions used to induce myoblasts to produce multinucleated myotubes. Our results clearly demonstrated that grown for 72 h in DM of myoblasts, the clonal myoblasts could differentiate into multinucleated myotubes expressing muscle contractile protein Myosin (Figure 1A), a marker of terminal differentiation myotubes. Under the same DM condition, however, NIH3T3 and MSCs did not produce any multinucleated myotubes and detectable Myosin (Figure 1A). These shown data could drive us to exclude the heterogeneous contamination possibility of NIH3T3 and MSCs in the myoblast clones.
We have also determined that cells in the clones were capable of dividing for more than 25 passages. The myoblasts shown in Figure 1 were randomly selected from 3 to 25 passages of the five selected clones and showed similar results. Thus, myoblasts isolated from skeletal muscle of adult human can generate clones of individual myoblasts, and the myoblast clones can divide for more than 25 passages, while preserving their homogeneous identity with myogenic lineage-committed phenotypes.
CNTF-induced Myogenic Inhibition
The in vitro myogenic differentiation of myoblasts is usually monitored by the fusion of mononucleated myoblasts into multinucleated myotubes and by the expression of MyoD and myogenin proteins (Hasty et al., 1993; Odelberg et al., 2000; Parker et al., 2003). To investigate effect of CNTF on myoblast differentiation, the increasing concentrations (0–50 ng/ml) of exogenous CNTF were added to subconfluent myoblasts cultured in DM for 72 h, and then the cells were analyzed for their ability to undergo myogenic differentiation.
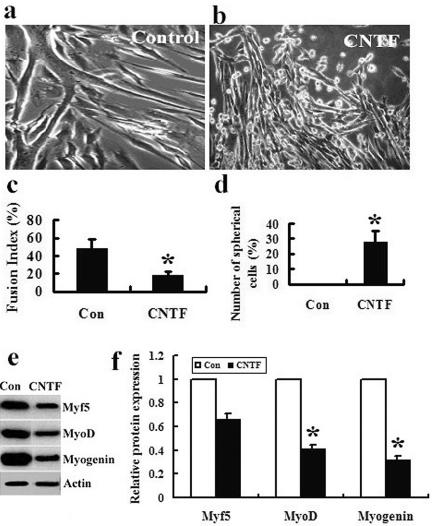
After myoblast were cultured with (CNTF) or without (Control) 30 ng/ml CNTF in DM for 72 h, the control cells normally underwent myogenic differentiation to form multinucleated myotubes (Figure 2, a and c). In contrast, in the CNTF-treated cells there were twofold less multinucleated myotubes than the control (Figure 2, b and c), whereas there were many small, spherical cells (Figure 2, b and d). Western blot analysis also indicated that the CNTF treatment resulted in significant decrease in the expression levels of MyoD and myogenin compared with the control cells (Figure 2, e and f). In our experiments, the myoblasts treated with 30–50 ng/ml CNTF shared the similar results as shown in Figure 2.
Figure 2.
Exogenous CNTF inhibits myoblast differentiation and induces a novel cell population in vitro. (a and b) Phase micrographs of myoblasts treated with (CNTF) or without (Control) 30 ng/ml CNTF in DM for 72 h. The data presented are representative of the experiments that are treated with 30–50 ng/ml CNTF with similar results. (c) The differentiation extent of myoblasts in a and b is expressed as fusion index indicating a percentage of the number of nuclei in the fused cells among the total number of nuclei in five randomly chosen fields at 200× magnification. Cells containing more than three nuclei are regarded as fused cells. (d) The number of spherical cells is expressed as a percentage of the number of nuclei in the spherical cells among the total number of nuclei in five randomly chosen fields at 200× magnification. Nestin+ cells are regarded as spherical cells in a and b. (e) Western blot analysis by using the anti-Myf5, -MyoD, and -myogenin antibodies. Extracts are prepared from the cells as described in a and b, and 100 μg of each extract is separated by SDS-PAGE and transferred to nitrocellulose. The data presented are representative of three separate experiments with similar results. (f) The intensities of the protein bands in e are quantified by densitometry with ScionImage software. *p < 0.05.
Thus, the exogenous CNTF can inhibit the myogenic differentiation of skeletal myoblasts in vitro and induce a novel cell population.
Dedifferentiated Myoblast Clones
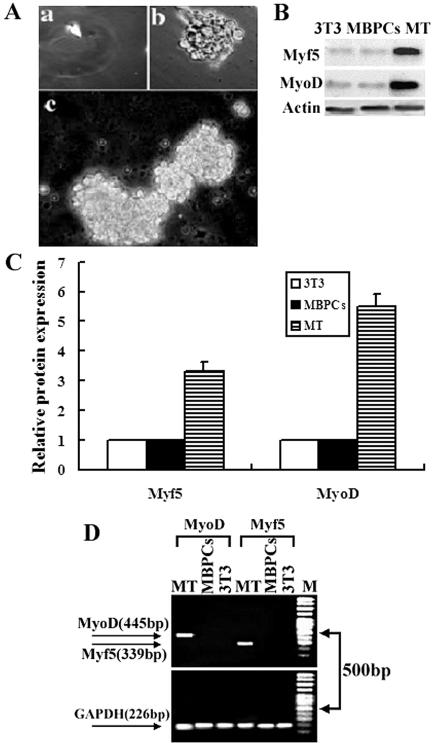
There were ∼30% small, spherical cells (termed MBPCs for myoblast-derived progenitor cells) in the myoblasts treated with 30–50 ng/ml concentrations of exogenous CNTF (Figure 2, b and d). MBPCs can be dissociated with a plastic pipette from the exogenous CNTF-treated cultures. Individual MBPCs were isolated into uncoated 48-well plates by limiting dilution. Microscopy revealed that ∼7% wells contained a single cell (Figure 3Aa). After these individual cells were cultured in growth medium GM0 (see Materials and Methods) for 5 wk, >45% of these single cells proliferated to generate clones in suspension (Figure 3Ab). These clone were further expanded in 25-cm2 uncoated tissue-culture flasks to grow as spheres in suspension over the course of 8 wk (Figure 3Ac). We determined that cells in these clones could divide for more than 20 passages.
Figure 3.
Dedifferentiated myoblast clones. (A) Phase micrographs of individual MBSC isolated by limiting dilution (a). Individual MBSC clone proliferated in 48-well uncoated tissue-culture plates for 5 wk in suspension (b). The clone is further expanded in 25-cm2 uncoated tissue-culture flasks to grow as spheres in suspension over the course of 8 wk (c). (B) Western blot analysis using anti-Myf5, -MyoD, and -myogenin antibodies. Extracts were prepared from MBPCs, from the positive control myotubes (MT) of the myoblasts cultured in DM for 7 d, and from the negative control NIH3T3 (3T3). One hundred micrograms of each extract was separated by SDS-PAGE and transferred to nitrocellulose. The data presented are representative of three separate experiments with similar results. (C) The intensities of the protein bands in B are quantified by densitometry with ScionImage software. (D) Semiquantitative RT-PCR analysis of Myf5 and MyoD expression in MBPCs. Total RNA was obtained from MBPCs*, from the negative control NIH3T3 (3T3), and from the positive control myotubes (MT) of the myoblasts cultured in DM for 7 d. The data presented are representative of three separate experiments with similar results. PCR products were checked by 1% agarose-TBE-ethidium bromide gels after 35 cycles. M, 100-bp molecular size marker. (The MBPCs were randomly selected from 3 to 20 passage clones of the individual MBPCs.)
One biochemical indicator of myoblast dedifferentiation would be the expression loss of Myf5 and MyoD, the markers of myogenic lineage-committed myoblasts (Odelberg et al., 2000; Parker et al., 2003; Chen et al., 2004). To investigate whether MBPCs are, in fact, the dedifferentiated myoblasts induced by CNTF, we investigated the expression levels of Myf5 and MyoD in the MBPCs. MBPCs (randomly selected from 3–20 passages of individual MBPC clones) were subjected to western blot and RT-PCR analysis. The results demonstrated that both protein and mRNA expression of Myf5 and MyoD were reduced to undetectable levels in the MBPCs compared with either the positive control myotubes (MT) or the negative control NIH3T3 (3T3; Figure 3, B–D). Therefore, we suggest that 30–50 ng/ml concentration of exogenous CNTF can induce myoblast dedifferentiation.
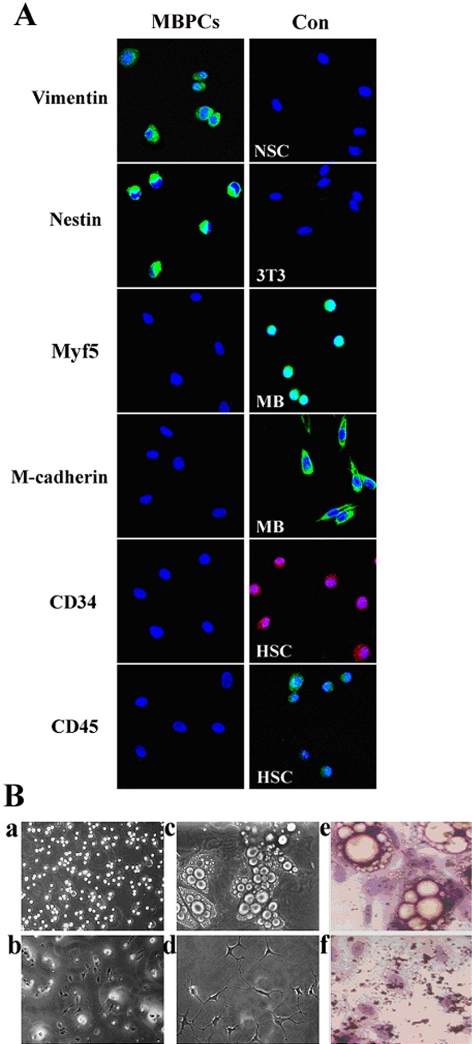
To determine the molecular characterization of MBPCs, the MBPC clones were dispersed and plated on collagen-coated glass coverslips in DM0 (see Materials and Methods) for 12 h to adhere. Immunocytochemistry revealed that MBPCs were uniformly positive for Vimentin, a marker of mesenchymal cells, and Nestin, a marker of NSCs (Figure 4A), whereas all of them were negative for Myf5 and M-cadherin (Figure 4A), the markers of satellite cells (Jennifer and Terence, 2003), and they also were negative for CD34 and CD45 (Figure 4A), the markers of HSCs (Jackson et al., 1999). We next asked how similar the MBPCs were to NSCs, which also express Nestin but which, unlike MBPCs, were negative for Vimentin. In addition, our results revealed that MBPCs, which were cultured under conditions used to proliferate NSCs (Clarke et al., 2000) for 7 d, did not survive and proliferate in suspension (Figure 4Bb) compared with those cultured in GM0 (Figure 4Ba). Furthermore, NSCs have never been reported to generate adipocytes (Clarke et al., 2000). In the present study, we also found that when the MBPCs and NSCs were cultured under the same conditions of adipogenic differentiation (see Materials and Methods), only the MBPCs can differentiate into adipocytes (Figure 4B, c and e) while the NSCs did not show the adipogenic potency to adipocytes (Figure 4B, d and f). Significantly, the MBPCs are different from NSCs although they expressed the NSC marker Nestin.
Figure 4.
Molecular characterization of MBPCs. (A) Immunocytochemistry assay of MBPCs, and its control (Con) NSCs (NSC), NIH3T3 (3T3), myoblasts (MB) and HSCs (HSC) by using the anti-Vimentin, -Nestin, -Myf5, -M-cadherin, -CD34 and -CD45 antibodies. Cells are counterstained with Hoechst 33342 (blue) to show nuclei. (B) Phase micrographs of MBPCs in GM0 (a) and in NSC growth medium (b). The adipogenic potency of MBPCs (c) and NSCs (d) under the same conditions of adipogenic differentiation to MBPCs, and their cytochemistry stain by Oil Red O in cultures of c (e) and d (f).
Thus, MBPCs are myoblast-dedifferentiated progenitor cells with the expression absence of Myf5 and MyoD, and distinct from previously identified adult stem cells such as satellite cells, HSCs and NSCs with regard to their selective expression of the Vimentin and Nestin, their growth factor requirements and their potency of adipogenic differentiation.
Multipotency of MBPCs
To determine whether the MBPCs can exhibit signs of multipotency, we plated clones of the individual MBPCs in DM0 or control myoblasts (MBs) on collagen-coated glass coverslips and cultured these cells under conditions that were favorable for differentiating into neural, glial and adipose lineages in vitro.
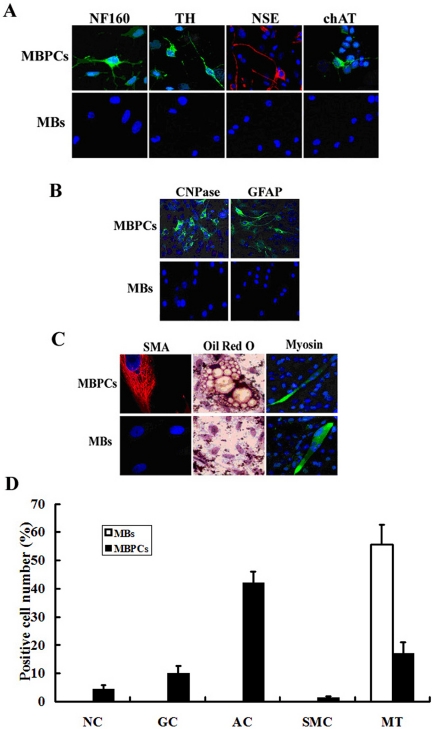
These MBPCs and MBs were cultured in neurogenic differentiation medium DM1 (see Materials and Methods) for 14–20 d. About 3–5% of MBPCs readily differentiated into cells that expressed the specific markers of neurons, NF160, TH, chAT, and NSE (Figure 5, A and D), suggesting that MBPCs had differentiated into neurons. No control MBs could be induced to produce these markers of neural lineage under the same conditions (Figure 5, A and D).
Figure 5.
MBPCs show signs of multipotency. Immunocytochemistry assay of MBPCs, and its control myoblasts (MBs) by using (A) the specific makers of neurons: anti-NF160, TH, NSE, and chAT antibodies; (B) the specific glial makers: anti-CNPase and -GFAP antibodies and (C) the anti-SMA and -Myosin antibodies. Cells were counterstained with Hoechst 33342 (blue) to show nuclei. In addition, (C) MBPCs and MBs are stained by the lipophilic dyes, Oil Red O. (D) The percentage of cells producing a given marker protein in A–C is expressed as positive cell number indicating a percentage of the number of nuclei in the cells stained a given marker among the total number of nuclei in five randomly chosen fields at 200× magnification.
When induced to differentiate into glial lineage by treatment with DM2 (see Materials and Methods) for 10–14 d, ∼8–12% of MBPCs produced cells expressing glial markers (Raff et al., 1989) CNPase and GFAP (Figure 5, B and D). No control MBs could be induced to produce these markers of glial lineage under the same conditions (Figure 5, B and D).
When cultured in adipogenic differentiation medium DM3 (see Materials and Methods) for 15–20 d, 45% of MBPCs could generate cells characterized by typical adipocyte morphology and numerous vacuoles that stained bright orange by the lipophilic dyes, Oil Red O (Zuk et al., 2001; Figure 5, C and D). In the study of adipogenic potency, we found there also were a few smooth muscle cells expressing SMA (Figure 5, C and D) and ∼20% myotubes expressing Myosin (Figure 5, C and D) in the cultures. Control MBs only differentiated into myotubes (Figure 5, C and D) with no signs of adipocyte or smooth muscle differentiation under the same conditions (Figure 5).
These results clearly demonstrate the evidence that MBPCs are multipotent progenitor cells that can differentiate into more than one lineage cells in vitro when cultured in different differentiation medium, whereas myoblasts show no signs of multipotency under the same conditions. Thus, CNTF induces dedifferentiation of skeletal myoblasts rather than transdifferentiation to neural, glial, smooth muscle, and adipose lineages in vitro.
p44/p42 MAPK-mediated CNTF Effects
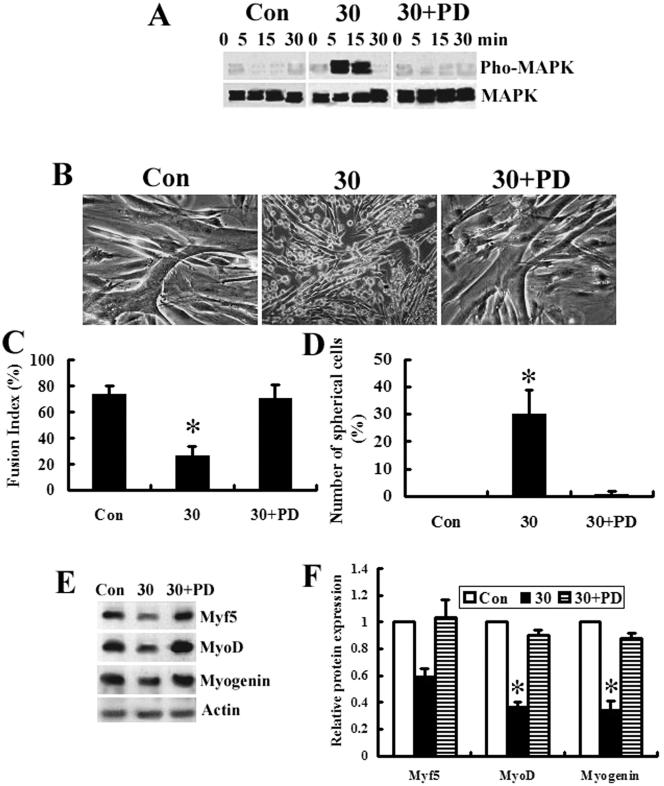
To determine the relevance of the exogenous CNTF and p44/p42 MAPK activation in regulating the myoblast differentiation, the effects of CNTF treatment on p44/p42 MAPK phosphorylation and the expression of myogenic differentiation markers Myf5 and MyoD were examined. Western blot was performed on the myoblast extracts that were prepared from the cells following a treatment without (Con) or with (30) 30 ng/ml exogenous CNTF at the time indicated. Our results clearly demonstrated that the cultures of myoblasts treated with CNTF (Figure 6A, 30) resulted in a markedly increase in phosphorylated p44/p42 MAPK detection compared with the control cultures (Figure 6A, Con). A time course indicated that this p42/p44 MAPK activation was transient and peaked at 5–15 min and that the activation levels could return to baseline by 30 min after CNTF exposure (Figure 6A, 30), indicating that CNTF can activate p44/p42 MAPK during myoblast differentiation and the effect of CNTF on myoblast differentiation may be via or in concert with p44/p42 MAPK activity.
Figure 6.
p44/p42 MAPK-mediated CNTF effects. (A) Western blot assays using anti-phospho (pho-MAPK) or anti-nonphospho (MAPK)-p44/p42 MAPK antibodies. Extracts are prepared from the cells after a treatment without (Con) or with 30 ng/ml CNTF in the absence (30) or presence (30+PD) of 20 μM PD98059 at the time indicated. The graph presented is representative of three separate experiments with similar results. (B) Phase micrographs of myoblasts treated without (Con) or with 30 ng/ml CNTF in the absence (30) or presence (30+PD) of 20 μM PD98059 in DM for 72 h. (C) The differentiation extent of myoblasts in B is expressed as fusion index indicating a percentage of the number of nuclei in the fused cells among the total number of nuclei in five randomly chosen fields at 200× magnification. Cells containing more than three nuclei were regarded as fused cells. (D) The number of spherical cells is expressed as a percentage of the number of nuclei in the spherical cells among the total number of nuclei in five randomly chosen fields at 200× magnification. Nestin+ cells are regarded as spherical cells in B. (E) Western blot analysis by using the anti-Myf5, -MyoD, and -myogenin antibodies. Extracts were prepared from the cells as described in B, and 100 μg of each extract was separated by SDS-PAGE and transferred to nitrocellulose. The data presented are representative of three separate experiments with similar results. (F) The intensities of the protein bands in E are quantified by densitometry with ScionImage software. *p < 0.05.
To confirm involvement of p44/p42 MAPK pathway in the effect of CNTF on myoblast differentiation in vitro, the myoblasts were also cultured in the presence of 20 μM PD98059 with 30 ng/ml CNTF (30+PD) in DM for 72 h. As can be seen in Figure 6A, this CNTF-induced p42/p44 MAPK activation (Figure 6A, 30) was reversed by PD98059 treatment (Figure 6A, 30+PD). We also found only in the cultures treated in the absence of 20 μM PD98059 with 30 ng/ml CNTF (30), there were many MBPCs (Figure 6B, 30, and 6D, 30), whereas myotube formation was remarkably inhibited (Figure 6B, 30; and 6C, 30) compared with the control (Figure 6B, Con; and 6C, Con) or the cultures treated in the presence of 20 μM PD98059 with 30 ng/ml CNTF (Figure 6B, 30+PD; and 6C, 30+PD). Furthermore, Western blot analysis demonstrated that the CNTF-induced down-regulation of Myf5, MyoD, and myogenin expression was restored by PD98059 (Figure 6, E and F).
These data indicate that PD98059, a specific inhibitor of MAPKK that is the upstream activator of p44/p42 MAPK pathway, can block the CNTF-induced myogenic inhibition of myoblasts and lead to an abolition of the CNTF-induced MBPCs. Thus, the CNTF-induced myogenic inhibition and dedifferentiation of myoblasts are dependent on the activation of the p44/p42 MAPK pathway.
DISCUSSION
The data presented here supported the following conclusions. First, the clones of individual myoblasts used for experiments are homogeneous that can divide for more than 25 passages with preserving their myogenic lineage-committed phenotypes. Second, exogenous CNTF can induce the monoclonal myoblasts in vitro to dedifferentiate into multipotent progenitor cells—they not only can proliferate at a clonal level for >20 passages with the expression absence of MRFs, but they are also capable of differentiating into new phenotypes, mainly neurons, glial cells, adipocytes, smooth muscle cells, and myotubes. Finally, we suggest that the effects of CNTF on the myoblast fate may dependent on the activation of p44/p42 MAPK pathway. Thus, the myogenic lineage-committed human myoblasts can dedifferentiate at a clonal level, and CNTF may be thought as a novel regulator of skeletal myoblast dedifferentiation in vitro, whereas the p44/p42 MAPK pathway mediated its regulatory mechanism.
All the experiments described here are performed on Desmin+ clones of individual myoblasts. The myoblasts in these clones can divide for more than 25 passages with preserving their myogenic specificity because the myoblasts randomly selected from 3 to 25 passages were uniformly positive for MyoD, the key marker of myogenic lineage-committed myoblasts (Kablar et al., 1999; Parker et al., 2003; Chen et al., 2004); while grown in DM to induce myogenic differentiation, they can form multinucleated myotubes stained positive for Myosin, a marker of myogenic terminal differentiation myotubes (Gal-Levi et al., 1998; Odelberg et al., 2000; Langley et al., 2002). The data shown in Figure 1 let us exclude the possibility that multiple distinct stem/progenitor cells identified in adult skeletal muscle present in the myoblast clones: first, although muscle satellite cells, the precursor cells of myoblasts, may express Myf5, the expression of MyoD is not detectable in the cells (Jennifer et al., 2003); second, because the myoblast clones are uniformly negative for Nestin, CD34, and CD45, there are no such heterogeneous contamination cells as previously identified Nestin+ NSCs (Romero-Ramos et al., 2002), CD34+/CD45+ HSCs (Jackson et al., 1999), CD45+ SP cells (Asakura et al., 2002), and CD34+ MDSCs (Qu-Petersen et al., 2002) in adult skeletal muscle; in addition, there is also not the presence of fibroblasts and MSCs because they do not differentiate to form multinucleated myotubes expressing Myosin when grown under the same DM conditions to the myoblasts; finally, these possible heterogeneous cells in the adult skeletal muscle never express myogenic specific protein MyoD. Taken together, these results provide strong evidence against the interpretation that the myoblast culture system contains multiple distinct cells. Rather, these results support the conclusion that the present myoblast clones are homogeneous by morphological criteria and immunocytochemistry identification. Thus, it is an excellent cell culture system to study the effect of exogenous factors on adult human skeletal myoblasts in vitro.
Although some factors such as HGF, bFGF, Myostatin and msx1 are involved in regulating the expression of MRFs and myogenic differentiation (Gal-Levi et al., 1998; Odelberg et al., 2000; Langley et al., 2002; Al-Khalili et al., 2004), the involvement of CNTF has never been reported. In the present study we use the culture system of monoclonal myoblasts to investigate the effect of CNTF on skeletal myoblast plasticity of adult human. Our results clearly demonstrate that the human myoblasts can be induced to dedifferentiate by exogenous CNTF. CNTF can inhibit myogenic differentiation by significantly down-regulating the fusion index and MRF expression of the myoblasts and induce the myoblasts to produce a novel cell population (MBPCs). Both mRNA and protein expression of Myf5 and MyoD, which are involved in the determination of myogenic lineage (Kablar et al., 1999; Parker et al., 2003; Chen et al., 2004; Delfini and Duprez, 2004), are reduced to undetectable levels in the MBPCs, whereas has high expression in the positive control myotubes of the myoblasts and has no expression in the negative control 3T3, indicating that the myoblasts have dedifferentiated to become unspecialized cells MBPCs because of the loss of their lineage-committed key markers Myf5 and MyoD (Kablar et al., 1999; Parker et al., 2003; Wagers and Weissman, 2004). Furthermore, immunocytochemistry revealed that MBPCs do not express Myf5 and M-cadherin, which are the markers of myoblasts and satellite cells, the precursors of myoblasts, whereas MBPCs do express Nestin and Vimentin. Although the MBPCs expressed the NSC marker Nestin, they clearly differ. Because NSCs do not produce Vimentin and have never been demonstrated to generate adipocytes in the present study, and the results reported by Clarke et al. (2000). Finally, MBPCs do not express CD45 and CD34, so that the possibility of HSCs is able to be excluded (Jackson et al., 1999). Thus, we suggest that MBPCs represent a novel progenitor cells derived from the skeletal myoblasts of adult human.
MBPCs are able to renew themselves at a clonal level for more than 20 passages in suspension, whereas the cloned MBPCs are capable of differentiating into not only mesenchymal cells, but also neuroectodermal cells in vitro, mainly including adipocytes, smooth muscle cells and myotubes, as well as neurons and glial cells. On the basis of the above results, we suggest that MBPCs are multipotent progenitor cells from the dedifferentiated myoblast population induced by CNTF. Significantly, the CNTF-induced MBPCs show stronger plasticity without being confined to mesenchymal differentiation potential than that previously reported (Odelberg et al., 2000; Asakura et al., 2001; Chen et al., 2004). Similarly, we also find that rat myoblasts are just like the human myoblasts, which are capable of producing myogenic inhibition and MBPCs when treated with the exogenous CNTF (Xiaoping Chen et al., unpublished data). However, whether the CNTF-induced MPSCs are also this multipotent in vivo remains to be determined.
It has been well known that CNTF and its receptor complex expression and function are mainly restricted to nervous system. However, recent studies have demonstrated that its receptor has relatively high expression in skeletal muscle (Sleeman et al., 2000), and in denervated skeletal muscle CNTF exerts myotrophic effects (Helgren et al., 1994) and the expression of CNTFRα significantly also increases (Weis et al., 1998). On the basis of these findings and our previous results, we suppose that CNTF may possibly have direct effects on skeletal myoblasts through its signal mechanism. There are some documents to reveal that CNTF signaling is primarily accomplished through the Jak-STAT and p44/p42 MAPK pathways (Boulton et al., 1994; Dziennis and Habecker, 2003; Rhee and Yang, 2003; Zvonic et al., 2003). The latter has been implicated in the control of skeletal myoblast myogenesis, but its functions seem to be controversial, as some reports have proposed a positive regulatory function through up-regulating MRF expression (Gredinger et al., 1998; Al-Khalili et al., 2004), whereas other reports proposed an inhibitory role at the beginning of the myogenic program through down-regulating MRF expression (Tortorella et al., 2001; Khurana and Dey, 2002). In addition, the p44/p42 MAPK pathway also plays biphasic but opposite roles in skeletal myoblast differentiation (Adi et al., 2002). Furthermore, although CNTF treatment can cause activation of the p44/p42 MAPK pathway in neural cells (Prithi and Ronald, 1998; Kuroda et al., 2001; Dziennis and Habecker, 2003) and adipocytes (Zvonic et al., 2003), whether CNTF may activate the p44/p42 MAPK pathway in skeletal myoblasts is still unclear. In the present study, we find that CNTF treatment can activate p44/p42 MAPK and down-regulate the expression of MRFs in the human skeletal myoblasts. Moreover, PD98059, a specific inhibitor of MAPKK, which is the upstream activator of the p44/p42 MAPK pathway, is able to block the CNTF-induced inhibition of myotube formation and lead to an abolition of the CNTF-induced MBPCs. On the other hand, the CNTF-induced down-regulation of MRF expression is also restored by PD98059. Thus, our results not only for the first time reveal that CNTF can activate p44/p42 MAPK in skeletal myoblasts of adult human, but also consistent with the results reported by Tortorella et al. (2001) and Khurana and Dey, (2002) that p44/p42 MAPK activation plays an inhibitory role in the myogenic differentiation of skeletal myoblasts through down-regulating MRF expression. Finally, the CNTF-induced myoblast dedifferentiation is also dependent on the activation of the p44/p42 MAPK pathway.
In summary, we find that skeletal myoblasts of adult human can be induced to dedifferentiate into multipotent progenitor cells by CNTF via the p44/p42 MAPK pathway in vitro. This work elucidates that the myoblast-derived progenitor cells show not only mesenchymal but also neuroectodermal plasticity at a clonal level. In addition, CNTF can regulate the myoblast fate because it can either inhibit myogenic differentiation of the myoblasts into multinucleated myotubes or stimulate dedifferentiation of the myoblasts into multipotent progenitor cells. Furthermore, CNTF is capable of causing p44/p42 MAPK activation and the activated pathway may mediate the CNTF-induced myogenic inhibition and dedifferentiation. Thus, CNTF, a neurotrophic factor that is well known for its numerous and important cellular effects in the nervous system, is a novel dedifferentiation regulator of human skeletal myoblasts. Finally, because few factors are known to induce adult myoblast dedifferentiation in vitro, our finding may have important implications for degenerative disease treatment of the adult human.
Acknowledgments
We thank Dr. Xiao Xiao in Pittsburgh University, Dr. Yi Yao in Washington University, Dr. Bai Lu in National Institutes of Health, Lin Mei in Alabama University, Dr. Mellita Schachener Camartin in Hamburg University, and Dr Zhichong Xiao in Singapore General Hospital for their scientific suggestion and stylistic help. This work was supported by Chinese National Hi-tech project (2003AA205004), Chinese national key basic research project (2001CB510100), Chinese natural science foundation (30470833), Beijing Hitech project (H010210190123), and Beijing natural science foundation (7021002).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–03–0218) on April 20, 2005.
References
- Aberg, M. A., Ryttsen, F., Hellgren, G., Lindell, K., Rosengren, L. E., MacLennan, A. J., Carlsson, B., Orwar, O., and Eriksson, P. S. (2001). Selective introduction of antisense oligonucleotides into single adult CNS progenitor cells using electroporation demonstrates the requirement of STAT3 activation for CNTF-induced gliogenesis. Mol. Cell Neurosci. 17, 426–443. [DOI] [PubMed] [Google Scholar]
- Adler, R., Landa, K. B., Manthorpe, M., and Varon, S. (1979). Cholinergic neuronotrophic factors. Intraocular distribution of soluble trophic activity for ciliary neurons. Science 204, 143–146. [DOI] [PubMed] [Google Scholar]
- Adi, S., Bin-Abbas, B., Wu, N. Y., and Rosenthal, S. M. (2002). Early stimulation and late inhibition of extracellular signal-regulated kinase 1/2 phosphorylation by IGF-I: a potential mechanism mediating the switch in IGF-I action on skeletal muscle cell differentiation. Endocrinology 143, 511–516. [DOI] [PubMed] [Google Scholar]
- Al-Khalili, L., Kramer, D., Wretenberg, P., and Krook, A. (2004). Human skeletal muscle cell differentiation is associated with changes in myogenic markers and enhanced insulin-mediated MAPK and PKB phosphorylation. Acta Physiol. Scand. 180, 395–403. [DOI] [PubMed] [Google Scholar]
- Asakura, A., Komaki, M., and Rudnicki, M. (2001). Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68, 245–253. [DOI] [PubMed] [Google Scholar]
- Asakura, A., Seale, P., Girgis-Gabardo, A., and Rudnicki, M.A. (2002). Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 159, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchaouir, R., Rameau, P., Decraene, C., Dreyfus, P., Israeli, D., Pietu, G., Danos, O., and Garcia, L. (2004). Evidence for a resident subset of cells with SP phenotype in the C2C12 myogenic line: a tool to explore muscle stem cell biology. Exp. Cell Res. 294, 254–268. [DOI] [PubMed] [Google Scholar]
- Boulton, T. G., Stahl, N., and Yancopoulos, G. D. (1994). Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J. Biol. Chem. 269, 11648–11655. [PubMed] [Google Scholar]
- Chen, S. B., Zhang, Q. S., Wu, X., Schultz, P. G., and Ding, S. (2004). Dedifferentiation of lineage-committed cells by a small molecule. J. Am. Chem. Soc. 126, 410–411. [DOI] [PubMed] [Google Scholar]
- Clarke, D. L., Johansson, C. B., Wilbertz, J., Veress, B., Nisson, E., Karlstrom, H., Lendahl, U., and Frisen, J. (2000). Generalized potential of adult neural stem cells. Science 288, 1660–1663. [DOI] [PubMed] [Google Scholar]
- Delfini, M. C. and Duprez, D. (2004). Ectopic Myf5 or MyoD prevents the neuronal differentiation program in addition to inducing skeletal muscle differentiation, in the chick neural tube. Development 131, 713–723. [DOI] [PubMed] [Google Scholar]
- Dziennis, S., and Habecker, B. A. (2003). Cytokine suppression of dopamine-beta- hydroxylase by extracellular signal-regulated kinase-dependent and -independent pathways. J. Biol. Chem. 278, 15897–15904. [DOI] [PubMed] [Google Scholar]
- Echeverri, K., and Tanaka, E. M. (2002). Mechanisms of muscle dedifferentiation during regeneration. Cell Dev. Biol. 13, 353–360. [DOI] [PubMed] [Google Scholar]
- Ezzeddine, Z. D., Yang, X., DeChiara, T., Yancopoulos, G., and Cepko, C. L. (1997). Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development 124, 1055–1067. [DOI] [PubMed] [Google Scholar]
- Gal-Levi, R., Leshem, Y., Aoki, S., Nakamura, T., and Halevy, O. (1998). Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochem. Biophys. Acta 1402, 39–51. [DOI] [PubMed] [Google Scholar]
- Gredinger, E., Gerber, A. N., Tamir, Y., Tapscott, S. J., and Bengal, E. (1998). Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J. Biol. Chem. 273, 10436–10444. [DOI] [PubMed] [Google Scholar]
- Grötzinger, J., Kernebeck, T., Kallen, K. J., and Rose-John, S. (1999). IL-6 type cytokine receptor complexes: hexamer, tetramer or both? Biol. Chem. 380, 803–813. [DOI] [PubMed] [Google Scholar]
- Guillet, C., Auguste, P., Mayo, W., Kreher, P., and Gascan, H. (1999). Ciliary neurotrophic factor is a regulator of muscular strength in aging. J. Neurosci. 19, 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty, P., Bradley, A., Morris, J. H., Edmondson, D. G., Venuti, J. M., Olson, E. N., and Klein, W. H. (1993). Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364, 501–506. [DOI] [PubMed] [Google Scholar]
- Helgren, M. E., Squinto, S. P., Davis, H. L., Parry, D. J., Boulton, T. G., Heck, C. S., Zhu, Y., Yancopoulos, G. D., Lindsay, R. M., and DiStefano, P. S. (1994). Trophic effect of ciliary neurotrophic factor on denervated skeletal muscle. Cell 11, 493–504. [DOI] [PubMed] [Google Scholar]
- Jackson, K. A., Mi, T., and Goodell, M. A. Hematopoietic potential of stem cells isolated from murine skeletal muscle. (1999). Proc. Natl. Acad. Sci. USA 96, 14482–14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski, R. J., Deasy, B. M., and Huard, J. (2002). Muscle-derived stem cells. Gene Ther. 9, 642–647. [DOI] [PubMed] [Google Scholar]
- Jennifer, E. M., and Terence, A. P. (2003). Muscle satellite cells. Int. J. Biochem. Cell Biol. 35, 1151–1156. [DOI] [PubMed] [Google Scholar]
- Kablar, B., Krastel, K., Ying, C., Tapscott, S. J., Goldhamer, D. J., and Rudnicki, M. A. (1999). Myogenic determination occurs independently in somites and limb buds. Dev. Biol. 206, 219–231. [DOI] [PubMed] [Google Scholar]
- Khurana, A., and Dey, C. S. (2002). Subtype specific roles of mitogen activated protein kinases in L6E9 skeletal muscle cell differentiation. Mol. Cell Biochem. 238, 27–39. [DOI] [PubMed] [Google Scholar]
- Kuroda, H., Sugimoto, T., Horii, Y., and Sawada, T. (2001). Signaling pathway of ciliary neurotrophic factor in neuroblastoma cell lines. Med. Pediatr. Oncol. 36, 118–121. [DOI] [PubMed] [Google Scholar]
- Lambert, P. D. et al. (2001). Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc. Natl. Acad. Sci. USA 98, 4652–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley, B., Thomas, M., Bishop, A., Sharma, M., Gilmour, S., and Kambadur, R. (2002). Myostatin inhibits myoblast differentiation by down regulating MyoD expression. J. Biol. Chem. 277, 49831–49840. [DOI] [PubMed] [Google Scholar]
- Mellott, T., Lopez-Coviella, I., Blusztajn, J. K., and Berse, B. (2002). Mitogen-activated protein kinase kinase negatively modulates ciliary neurotrophic factor-activated choline acetyltransferase gene expression. Eur. J. Biochem. 269, 850–858. [DOI] [PubMed] [Google Scholar]
- Monville, C., Coulpier, M., Conti, L., De-Fraja, C., Dreyfus, P., Fages, C., Riche, D., Tardy, M., Cattaneo, E., and Peschanski, M. (2001). Ciliary neurotrophic factor may activate mature astrocytes via binding with the leukemia inhibitory factor receptor. Mol. Cell Neurosci. 17(2): 373–384. [DOI] [PubMed] [Google Scholar]
- Odelberg, S. J., Kollhoff, A., and Keating, M. T. (2000). Dedifferentiation of mammalian myotubes induced by msx1. Cell 103, 1099–1109. [DOI] [PubMed] [Google Scholar]
- Oppenheim, R. W., Prevette, D., Yin, Q. W., Collins, F., and MacDonald, J. (1991). Science 251, 1616–1618. [DOI] [PubMed] [Google Scholar]
- Parker, M. H., Seale, P., and Rudnicki, M. A. (2003). Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat. Rev. Genet. 4, 497–507. [DOI] [PubMed] [Google Scholar]
- Patapoutian, A., Yoon, J. K., Miner, J. H., Wang, S., Stark, K., and Wold, B. (1995). Disruption of the mouse MRF4 gene identifies multiple waves of myogenesis in the myotome. Development 121, 3347–3358. [DOI] [PubMed] [Google Scholar]
- Prithi, R., and Ronald, D. (1998). Multiple routes to astrocytic differentiation in the CNS. J. Neurosci. 18, 3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen, Z. et al. (2002). Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 157, 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff, M. C. (1989). Glial cells diversification in the rat optic nerve. Science 243, 1450–1455. [DOI] [PubMed] [Google Scholar]
- Rando, T. A., and Blau, H. M. (1994). Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, K. D., and Yang, X. J. (2003). Expression of cytokine signal transduction components in the postnatal mouse retina. Mol. Vis. 9, 715–722. [PMC free article] [PubMed] [Google Scholar]
- Romero-Ramos, M. et al. (2002). Neuronal differentiation of stem cells isolated from adult muscle. J. Neurosci. Res. 69, 894–907. [DOI] [PubMed] [Google Scholar]
- Schulz-Key, S., Hofmann, H. D., Beisenherz-Huss, C., Barbisch, C., and Kirsch, M. (2002). Ciliary neurotrophic factor as a transient negative regulator of rod development in rat retina. Invest. Ophthalmol. Vis. Sci. 43, 3099–3108. [PubMed] [Google Scholar]
- Senaldi, G. et al. (1999). Novel neurotrophin-1/B cell-stimulating factor-3, a cytokine of the IL-6 family. Proc. Natl. Acad. Sci. USA 96, 11458–11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., Wang, W., Yourey, P. A., Gohari, S., Zukauskas, D., Zhang, J., Ruben, S., and Alderson, R. F. (1999). Computational EST database analysis identifies a novel member of the neuropoietic cytokine family. Biochem. Biophys. Res. Commun. 262, 132–138. [DOI] [PubMed] [Google Scholar]
- Shimazaki, T., Shingo, T., and Weiss, S. (2001). The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J. Neurosci. 21, 7642–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman, M. W., Anderson, K. D., Lambe, P. D., Yancopoulos, G. D., and Wiegand, S. J. (2000). The ciliary neurotrophic factor and its receptor, CNTFRα. Phamaceutica Acta Helvetiae 74, 265–272. [DOI] [PubMed] [Google Scholar]
- Song, M. R., and Ghosh, A. (2004). FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat. Neurosci. 7, 229–235. [DOI] [PubMed] [Google Scholar]
- Stockli, K. A., Lillien, L. E., Naher Noe, M., Breitfeld, G., Hughes, R. A., Raff, M. C., Thoenen, H., and Sendtner, M. (1991). Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J. Cell Biol. 115, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki, T., Akatsuka, A., Okada, Y., Matsuzaki, Y., Okano, H., and Kimura, M. (2003). Growth and differentiation potential of main- and side-population cells derived from murine skeletal muscle. Exp. Cell Res. 291, 83–90. [DOI] [PubMed] [Google Scholar]
- Tortorella, L., Milasincic, D. J., and Polch, P. F. (2001). Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J. Biol. Chem. 276, 13709–13717. [DOI] [PubMed] [Google Scholar]
- Tsai, R. Y., Kittappa, R., and McKay, R. D. (2002). Plasticity, niches, and the use of stem cells. Dev. Cell 2, 707–712. [DOI] [PubMed] [Google Scholar]
- Wagers, A. J., and Weissman, I. L. (2004). Plasticity of adult stem cells. Cell 116, 639–648. [DOI] [PubMed] [Google Scholar]
- Weinelt, S. et al. (2003). Ciliary neurotrophic factor overexpression in neural progenitor cells (ST14A) increases proliferation, metabolic activity, and resistance to stress during differentiation. J. Neurosci. Res. 71, 228–236. [DOI] [PubMed] [Google Scholar]
- Weis, J., Lie, D. C., Ragoss, U., Zuchner, S. L., Schroder, J. M., Karpati, G., Farruggella, T., Stahl, N., Yancopoulos, G. D., and DiStefano, P. S. (1998). Increased expression of CNTF receptor alpha in denervated human skeletal muscle. J. Neuropathol. Exp. Neurol. 57, 850–857. [DOI] [PubMed] [Google Scholar]
- Wolf, E., Kramer, R., Polejaeva, I., Thoenen, H., and Brem, G. (1994). Efficient generation of chimaeric mice using embryonic stem cells after long-term culture in the presence of ciliary neurotrophic factor. Transgenic Res. 3, 152–158. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Kimura, K., Matsumoto, N., and Ide, C. (2003). Isolation of neural stem cells from the forebrain of deceased early postnatal and adult rats with protracted post-mortem intervals. J. Neurosci. Res. 74, 533–540. [DOI] [PubMed] [Google Scholar]
- Zhou, S. et al. (2001). The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7, 1028–1034. [DOI] [PubMed] [Google Scholar]
- Zuk, P. A., Zhu, M., Mizuno, H., Huang, J., Futrell, J. W., Katz, A. J., Benhaim, P., Lorenz, H. P., and Hedrick, M. H. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228. [DOI] [PubMed] [Google Scholar]
- Zvonic, S., Cornelius, P., Stewart, W.C., Mynatt, R. L., and Stephens, J. M. (2003). The regulation and activation of ciliary neurotrophic factor signaling proteins in adipocytes. J. Biol. Chem. 278, 2228–2235. [DOI] [PubMed] [Google Scholar]