Abstract
Background
Shorter courses of antimicrobial therapy have been shown to be non-inferior to longer durations for the management of several infections. However, data on critically ill patients with severe infections by multidrug-resistant Gram-negative bacteria (MDR-GNB) are scarce. In the duratiOn of theraPy in severe infecTIons by MultIdrug-reSistant gram-nEgative bacteria (OPTIMISE) trial, we assessed the non-inferiority of 7-day versus 14-day antimicrobial therapy for patients with intensive care unit (ICU)-acquired severe infections by MDR-GNB.
Methods
This was a randomised multicenter, open-label, parallel controlled, non-inferiority trial. Adult patients with severe infections by MDR-GNB initiated ≥ 48 h of ICU admission were eligible if they were hemodynamically stable and without fever > 48 h on the 7th day of appropriate antimicrobial therapy. Patients were 1:1 randomised to discontinue antimicrobial therapy on the 7th (± 1) day or to continue for a total of 14 (± 1) days. The primary outcome was clinical failure, defined as death or relapse of infection within 28 days of randomisation. An upper edge of the two-tailed 95% confidence interval (CI) of the delta between the clinical failure rate in the 7- and the 14-day lower than 10% in both intention-to-treat (ITT) and per protocol (PP) analyses was set as the non-inferiority criteria.
Results
A total of 106 patients composed the ITT population: 59 and 47 allocated to 7- and 14-day groups, respectively. The PP population included 75 patients: 47 and 28 in the 7- and 14-day groups, respectively. Clinical failure occurred in 42.4% and 44.7% of the ITT population in 7- and 14-day groups, respectively, (risk difference (RD) − 2.3, 95%CI − 21.3 to 16.7), and in 46.8% and 50.0% of the PP population in 7- and 14-day groups, respectively (RD − 3.2, 95%CI − 26.6 to 20.2). Most infections were of the respiratory tract (73/68.9%) and caused by carbapenem-resistant Enterobacterales (42/39.6%). The study was interrupted before reaching planned sample size due to low recruitment rate.
Conclusion
The OPTIMISE trial could not determine the non-inferiority of 7-day compared to 14-day therapy for severe infections caused by MDR-GNB due to early termination related to the low recruitment rate.
Trial registration: NCT05210387 on January 13, 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05178-6.
Keywords: Gram-negative bacteria, Antimicrobial therapy, Antimicrobial resistance, Enterobacterales, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa
Background
Antimicrobial resistance represents one of the greatest challenges in modern medicine, significantly increasing the morbidity and mortality of bacterial infections, especially in healthcare-associated infections Gram-negative bacteria (GNB) [1]. Alarming rates of multidrug-resistant (MDR)-GNB, particularly in Enterobacterales species, Acinetobacter baumannii and Pseudomonas aeruginosa, have been reported worldwide among intensive care units (ICU) patients [2], and have been commonly associated with increased mortality when compared with infections caused by their susceptible counterparts [3–5].
Randomised clinical trials have demonstrated that shorter durations of therapy (5–8 days) may be equally effective than “traditional” periods (14–21 days) for a variety of infections, with additional benefits of reduced selection of resistant isolates, lower incidence of adverse effects and reduction in overall hospital costs [6–8]. However, most studies focused on less severe infections and less vulnerable patient populations [9]. Moreover, difficult-to-treat MDR-GNB are still underrepresented in most of these randomised trials, impairing the ability to promptly implement shorter courses strategy of antimicrobial therapy into the clinical practice for the treatment of infections caused by these microorganisms, particularly in the critically ill patient [10].
In fact, the combination of severe infections and MDR-GNB could potentially be a case against shorter-duration therapies. First, infections presenting with sepsis may be associated with higher bacterial inoculum infections, increased target organ damage, which may affect the pharmacokinetics of antimicrobials, and immune dysfunction may impair pathogen clearance [9]. Second, infections by MDR-GNB more commonly affect immunocompromised patients or those with more comorbidities [10]. Third, adequate empirical therapy is usually delayed in infections by MDR-GNB, potentially aggravating organ dysfunction and worsening the prognosis [11]. Finally, some MDR-GNB are commonly treated with less potent and/or more toxic “second-line” antimicrobials, such as polymyxins and aminoglycosides. Although novel antimicrobials against MDR-GNB may change this landscape [12, 13], these new options are not widely available in low resource settings.
Recently, shorter courses based on favourable clinical response in the first days of therapy was found to be non-inferior to standard recommendations in critically ill patients with ventilator-associated pneumonia, with reduced exposure to antimicrobials [14], suggesting that this strategy may be effective and safe in critically ill patients with severe infections, if signs of clinical response to therapy are present. The duratiOn of theraPy in severe infecTIons by MultIdrug-reSistant gram-nEgative bacteria (OPTIMISE) trial accessed the non-inferiority of 7 versus 14 days of antimicrobial therapy for severe infections by MDR-GNB in ICU patients who were hemodynamically stable and afebrile on the 7th day of appropriate antimicrobial therapy.
Methods
Trial design
The OPTIMISE was an investigator-initiated, randomised, open-label trial, with parallel groups and 1:1 allocation ratio, conducted at 18 ICUs of Brazilian hospitals from January 27, 2022, to December, 20, 2023. Only hospitals in which either 7–8 days or 14–15 days of antimicrobial therapy for MDR-GNB infections would be acceptable as a possible duration of therapy were selected. The study protocol and amendments (Supplementary Stable 1) were approved by the research ethics committee (institutional review board, IRB) of the coordinating centre (Hospital Moinhos de Vento), as well as IRBs from all other participant sites (supplementary material). The trial was registered at https://clinicaltrials.gov (Unique Identifier: NCT05210387) on January 13, 2022. The study protocol was previously published elsewhere [15].
We followed the CONSORT Statement checklist for the reporting of randomised clinical trials [16]. An independent data and safety monitoring committee (DSMB; supplementary material) oversaw the conduct of the study and blindly reviewed predefined data.
Participants
Participants were eligible if they were ≥ 18 years; capable of providing written informed consent (or has a legal representative to do it); were admitted to the ICU for at least 48 h at the onset of infection (defined as the day the culture that yielded the growth of the isolate was collected); had a severe infection caused by a MDR-GNB; were hemodynamically stable and afebrile for at least 48 h on day 7 ± 1 of appropriate antimicrobial therapy since the onset of infection; and the patients’ care team consented inclusion of participant in the trial.
The participants were excluded if one or more of the following conditions were present: (1) participation in other experimental trials involving antimicrobial therapy; (2) the infection required longer therapy (supplemental material) (3) immunosuppression (supplementary material); (4) growth of the same bacteria under study in blood culture samples collected in the 48 h prior to randomisation (if cultures requested by the care team); (5) concomitant uncontrolled infection by another GNB (regardless of susceptibility profile); (6) prior participation in this trial; (7) known pregnancy; (8) palliative care or patients for whom initiation of antimicrobials, if necessary, or hemodynamic support measures (e.g., initiation or up-titration of vasopressors) had already been decided against.
Definitions
Bloodstream infections, pneumonia (with or without mechanical ventilation), or infections at any site (according to the criteria of the Brazilian Health Regulatory Agency—ANVISA [17]; supplementary material) accompanied sepsis or septic shock [18], were considered severe infections. Antimicrobial susceptibility testing and interpretation were performed according to the European Committee for Antimicrobial Susceptibility Testing [19], unless otherwise indicated. MDR-GNB isolates were defined as Enterobacterales species, P. aeruginosa or A. baumannii with in vitro resistance to carbapenems, regardless of susceptibility to other antimicrobials; Enterobacterales species with in vitro resistance to both ceftriaxone and cefepime; P. aeruginosa with in vitro resistance to at least one of the following: ceftazidime, cefepime or carbapenems; or A. baumannii with in vitro susceptibility to carbapenems, but not susceptible to other beta-lactams, including ampicillin/sulbactam (if tested and interpreted according to Clinical Laboratory Standards Institute [20]). If the microbiology laboratory did not carry out susceptibility testing for non-carbapenem beta-lactams and for ampicillin/sulbactam, the A. baumannii isolate was considered resistant to these antimicrobials. These MDR-GNB definitions encompass the bacteria listed by World Health Organization as critical priority antimicrobial resistance profile (i.e. carbapenem-resistant Enterobacterales, carbapenem-resistant A. baumannii, carbapenem-resistant P. aeruginosa, and third-generation cephalosporin-resistant Enterobacterales) [21], with the addition of carbapenem-susceptible but other first-line beta-lactams-resistant P. aeruginosa and A. baumannii).
Hemodynamic stability was defined as maintenance of mean arterial pressure ≥ 60 mmHg without vasopressors or fluid resuscitation in patients not on mechanical ventilation, not on sedatives, and not requiring dialysis. In mechanically ventilated patients, requiring renal replacement therapy, and/or in use of sedatives, low-dose norepinephrine (< 0.1 mcg/kg/min) was allowed, provided the dose remained stable in the 48 h preceding randomisation. Although a MAP ≥ 65 mmHg has been recommended as the target for initial resuscitation in patients with septic shock [22], it has been shown that MAPs ≥ 60 mm Hg are not associated with decreased organ perfusion in patients without vasopressors or fluid resuscitation in the context of clinical improvement. Fever was defined as axillary temperature ≥ 37.8 °C.
Treatment with at least one antimicrobial to which the MDR-GNB isolate exhibited in vitro was considered an appropriate antimicrobial therapy. Appropriate antimicrobial therapy should be initiated no more than 7 days of culture collection (the 7-day window was chosen to incorporate the expected delay in the final reports of microbiological reports). In the case of ceftazidime/avibactam, in the absence of specific susceptibility testing, Enterobacterales isolates in which phenotypic or genotypic testing indicated the presence of a class A carbapenemase was assumed to be in vitro susceptible. Tigecycline was accepted as an appropriate therapy if the isolated pathogen has a minimum inhibitory concentration ≤ 1 mg/L and the patient was treated with a dose of 100 mg every 12 h. If an appropriate antimicrobial was changed to another one also showing susceptibility result, the entire period was considered as appropriate treatment. In the case of polymicrobial infections, all pathogens must have presented in vitro susceptibility to antimicrobial(s) used for therapy to be considered appropriate. Empiric therapy was defined as that which was initiated before the results of cultures and susceptibility testing were available; and combination therapy as the use of at least two antimicrobials to which the MDR-GNB isolate exhibited in vitro susceptibility, initiated within 7 days of culture collection and concomitantly for at least 48 h.
Intervention
Patients were randomised to interrupt antimicrobial therapy for the infection that prompted the participant’s enrollment in the trial on the 7th day of appropriate antimicrobial therapy (intervention group) or to maintain antimicrobial therapy until the 14th day (control group). A variation of ± 1 day in both groups was acceptable for the per protocol (PP) analysis.
Outcomes
The primary outcome was treatment failure within 28 days of randomisation, defined as death or relapse of the infection. Relapse of infection was defined as infection at any site (as defined by ANVISA [17]; supplementary material) caused by the same GNB with the same susceptibility profile of antimicrobials used to define MDR. Relapse of infection was adjudicated by two independent infectious diseases physicians who were blinded to the intervention. The number of microbiological cultures collected over the number of follow-up days (from randomisation to 28 days, death or discharge) was evaluated to measure possible bias in investigating relapses.
Secondary outcomes, assessed within 28 days of participant randomisation, were days alive and free from hospitalisation; days alive and free of antimicrobial therapy; incidence of infections by other MDR-GNB and by other bacteria; length of ICU stay (assessed in survivors at 28 days); acute kidney injury [23]; cumulative incidence of all-cause diarrhoea; cumulative incidence of confirmed Clostridioides difficile infection; and cumulative incidence of other antimicrobial-related adverse events (supplementary material). Cumulative incidence of new hemodynamic instability lasting > 6 h within 14 days of randomisation was also assessed.
Sample size
A total of 520 participants was planned considering a clinical failure of 30% in both groups, a randomisation ratio of 1:1, and a non-inferiority margin of 10%, for an alpha of 0.05 and a beta of 0.20. Interim analyses were initially planned as detailed in the supplementary material. Considering the low recruitment rate, only one interim analysis was performed, as described in the results.
Randomisation
The patient allocation sequence in randomisation was created using the R studio program [24] by a statistician, respecting an individual randomisation in blocks of 2 and 4, in a 1:1 ratio and stratified by sites and by risk for mortality of infection (high versus low risk). Urinary tract infections and central-line associated bloodstream infections were defined as low-risk for mortality, all other infections were considered high-risk. Randomisation was performed by the investigator of each participating hospital through the REDCap data collection platform ensuring concealment of the randomisation list.
The study was open-label, as such, investigators and patients were not blinded to group allocation.
Statistical analysis
All statistical analyses were performed in R software version 4.3.1 [24]. A non-inferiority of the 7-day compared with 14-day course would be reached if the upper edge of the two-tailed 95% confidence interval (CI) of the difference between the failure rate in the intervention group and the control group were not more than 10% in the intention to treat (ITT) and in the PP analyses. The risk difference (95% CI) was calculated using the Wald method.
Secondary outcomes were analysed as described in the supplementary material. All tests were two-tailed and a P ≤ 0.05 was considered statistically significant.
Six subgroups analysis were defined for the primary outcome: bacterial aetiology; carbapenem susceptibility profile; severity of infection criteria; appropriate empirical therapy; use of combination antimicrobial therapy; and risk for mortality of infection.
Post hoc analysis
A sensitivity analysis was performed including in the PP population, those who were excluded because they received > 8 days or < 13 days of antimicrobial therapy in the 7- and 14-day groups, respectively. This sensitivity analysis assumed a worst-case scenario for the shorter therapy: 100% failure in patients newly included in the PP 7-day group and 0% failure in patients newly included in the PP 14-day group.
Results
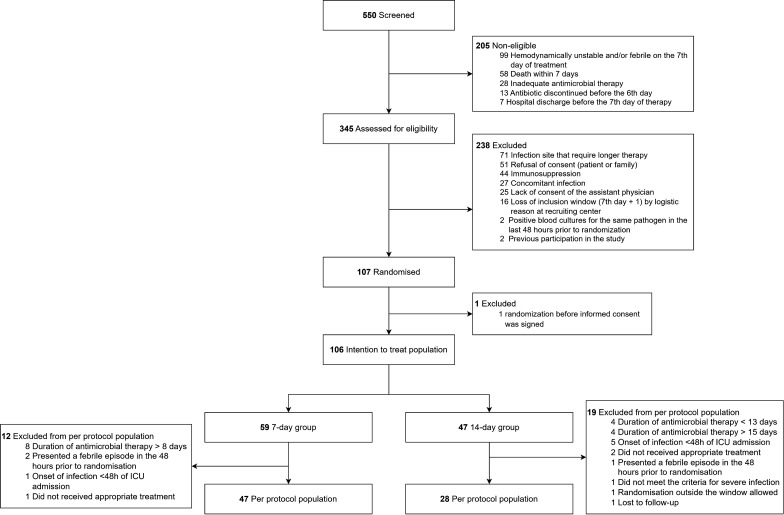
From January 1, 2022, to December 20, 2023, 550 patients with MDR-BGN severe infections at ICU were screened for eligibility, of whom 345 were eligible to participate in the study, but further excluded, resulting in a total of 107 participants randomised (Fig. 1). One patient was randomised before consenting participation and was excluded from ITT analysis, which was composed of 106 patients: 59 and 47 allocated to 7- and 14-day treatment groups, respectively (Fig. 1). Thirty-one participants (29.2%) were excluded from the PP analysis, resulting in: 47 and 28 patients in 7- and 14-day treatment, respectively (Fig. 1). The study follow-up was completed on December 25, 2023.
Fig. 1.
Flowchart of the study
The trial was interrupted at 20.6% of initially planned sample size due to low recruitment rate. Before interruption, the DSMB was formally requested to analyse the partial data and, considering the low recruitment rate and inability to attain the planned sample size within the previously planned timeframe of the study, to indicate whether the study should be stopped at that moment or it should be safe and ethical to continue the recruitment until the December 20, 2023 (end of study date approved by IRB). The DSMB analysed prespecified outcomes of 82 randomised patients and, on August 11, 2023, presented a report indicating that recruitment could continue until to the previously approved date for the end of the study.
Patients characteristics
Participants had a median age of 67.0 years (interquartile range [IQR], 52.3–75.3), and 36.8% (39 of 106) were female. The majority were respiratory infections (68.9%), with a median SOFA score of 5.0 (IQR 3.0–8.0) and caused by carbapenem-resistant Enterobacterales (39.6%) and Acinetobacter baumannii (25.5%). Most variables were well balanced between groups in both ITT (Table 1) and PP patients’ population (supplementary STable 2). There were proportionally more respiratory tract and fewer urinary infections in the 7-day group patients in the ITT population. The proportion of patients with high- and low-risk for mortality sites were similar. Carbapenem-resistant A. baumannii and carbapenem-resistant P. aeruginosa were also proportionally higher in the 7-day group.
Table 1.
Baseline characteristics of intention to treat population
| Baseline characteristics | All n = 106 |
7-day group n = 59 |
14-day group n = 47 |
|---|---|---|---|
| Age, years | 67.0 (52.3–75.3) | 67.0 (55.0–76.0) | 67.0 (49.5–75.5) |
| Female | 39 (36.8) | 22 (37.3) | 17 (36.2) |
| Race/Skin color | |||
| White/Caucasian | 68 (64.2) | 38 (64.4) | 30 (63.8) |
| Black/Brown | 36 (34.0) | 20 (33.9) | 16 (34.0) |
| Asian | 2 (1.9) | 1 (1.7) | 1 (2.1) |
| Body Mass Index (kg/m2) a | 25.5 (23.0–29.0) | 25.0 (23.0–29.0) | 26.0 (23.0–29.0) |
| Charlson comorbidity index | 1 (0–3) | 2 (0–3) | 1 (1–3) |
| Infection Site | |||
| Bloodstream infection of unknown source | 6 (5.7) | 2 (3.4) | 4 (8.5) |
|
Central Line-associated bloodstream infection |
4 (3.8) | 3 (5.1) | 1 (2.1) |
| Respiratory Tract | 73 (68.9) | 44 (74.6) | 29 (61.7) |
| Urinary | 23 (21.7) | 10 (17.0) | 13 (27.7) |
| Infection risk | |||
| Low risk | 27 (25.5) | 14 (23.7) | 13 (27.7) |
| High risk | 79 (74.5) | 45 (76.3) | 34 (72.3) |
| Severity criteria | |||
| Bloodstream infection | 8 (7.6) | 4 (6.8) | 4 (8.5) |
| Pneumonia | 51 (48.1) | 28 (47.5) | 23 (48.9) |
| Sepsis or septic shock | 47 (44.3) | 27 (45.8) | 20 (42.6) |
| SOFA score at the onset of infection | 5.0 (3.0–8.0) | 5.0 (3.0–7.0) | 6.0 (3.5–8.0) |
| Vasoactive drugs | 40 (37.7) | 22 (37.3) | 18 (38.3) |
| WBC count at randomisation (/mm3)b | 10,860 (8352 −15,825) | 12,375 (9162–17,137) | 9025.0 (6935–13,470) |
| Creatinine at randomization (mg/dL)c | 1.3 (0.8–2.0) | 1.2 (0.8–2.0) | 1.3 (0.9–2.0) |
| C-reactive protein at the onset of infection (mg/L)d | 119.6 (64.7–216.3) | 103.3 (56.6–190.0) | 147.9 (75.3–265.9) |
|
C-reactive protein at randomization (mg/L)e |
72.9 (28.8–156.1) | 74.6 (24.9–128.2) | 70.7 (39.5–185.1) |
| Mean arterial pressure at randomization (mmHg) | 88.5 (± 14.4) | 88.1 (± 13.8) | 89.1 (± 15.2) |
| Temperature at randomization (°C) | 36.4 (36.0–36.7) | 36.4 (36.0–36.8) | 36.4 (36.0–36.7) |
| Heart rate (beats per minute) | 91.6 (± 16.7) | 94.1 (± 17.1) | 88.5 (± 15.9) |
| Respiratory frequency (breaths per minute) | 20.0 (17.3–23.8) | 20.0 (16.5–24.5) | 20.0 (18.0–22.0) |
| Peripheral O2 saturation (%) | 96.5 (94.0–98.0) | 97.0 (94.0–98.5) | 96.0 (94.0–97.0) |
| Mechanical ventilation | 83 (78.3) | 48 (81.4) | 35 (74.5) |
| Antibiotic therapy | |||
| Appropriate empirical therapy | 50 (47.2) | 26 (44.1) | 24 (51.1) |
| Combination therapy | 27 (25.5) | 14 (23.7) | 13 (27.7) |
| Microbiology | |||
| Enterobacterales | 67 (63.2) | 34 (57.6) | 33 (70.2) |
| Carbapenem-resistant Enterobacteralesf | 42 (39.6) | 23 (39.0) | 19 (40.4) |
|
3rd and 4th Generation-resistant Enterobacteralesg |
25 (23.6) | 11 (18.6) | 14 (29.8) |
| Pseudomonas aeruginosa | 18 (17.0) | 12 (20.3) | 6 (12.8) |
|
Carbapenem-resistant Pseudomonas aeruginosa |
13 (12.3) | 11 (18.6) | 2 (4.3) |
|
3rd or 4th G-resistant Pseudomonas aeruginosa |
5 (4.7) | 1 (1.7) | 4 (8.5) |
| Acinetobacter baumannii | 29 (27.4) | 19 (32.2) | 10 (21.3) |
|
Carbapenem-resistant Acinetobacter baumannii |
27 (25.5) | 18 (30.5) | 9 (19.2) |
|
Carbapenem-susceptible Acinetobacter baumannii |
2 (1.9) | 1 (1.7) | 1 (2.1) |
| Polymicrobial infections | 15 (14.2) | 11 (18.6) | 4 (8.5) |
| Concomitant infection in another site | 20 (18.9) | 11 (18.6) | 9 (19.2) |
Data presented as n (%), mean (± standard deviation) or median (Interquartile range p25–p75)
SOFA sequential organ failure assessment, WBC white blood count, G generation
aTotal (n = 102), 7-day group (n = 57), 14-day group (n = 45)
bTotal (n = 100), 7-day group (n = 56), 14-day group (n = 44)
cTotal (n = 90), 7-day group (n = 51), 14-day group (n = 39)
dTotal (n = 63), 7-day group (n = 38), 14-day group (n = 25)
eTotal (n = 64), 7-day group (n = 38), 14-day group (n = 26)
fKlebsiella pneumoniae (total 36, 18 on 7-day group and 18 on 14-day group), Serratia marcescens (total 5, 3 on 7-day group and 2 on 14-day group) and Klebsiella oxytoca (1 on 7-day group)
gKlebsiella pneumoniae (total 15, 7 on 7-day group and 8 on 14-day group), Escherichia coli (total 4, 1 on 7-day group and 3 on 14-day group), Klebsiella oxytoca (total 2, 1 on 7-day group and 1 on 14-day group), Proteus mirabilis (total 2, 1 on 7-day group and 1 on 14-day group) and Morganella morganii (1 on 14-day group)
Intervention
In the ITT population, the median duration of antimicrobial treatments were 7 days (IQR, 7–8) and 13 days (IQR, 12–14) in the 7- and 14-day groups, respectively. Fourteen (23.7%) in the 7-day and 13 (27.7%) in the 14-day group received combination therapy. In the PP population, the median duration of antimicrobials were 7 days (IQR, 7–8) and 13 days (IQR, 13–14) in the 7- and 14-day groups, respectively. Other characteristics of interventions in the ITT and PP populations are shown in Table 1 and supplementary STable 2, respectively. The antimicrobials administered to patients in each group in ITT and PP populations are shown in supplementary STable 3.
Outcomes
Clinical failure within 28 days of randomisation occurred in 42.4% and 44.7% of patients in 7- and 14-day groups, respectively, in the ITT population (risk difference − 2.3, 95%CI − 21.3 to 16.7), and in 46.8% and 50.0% of patients in 7- and 14-day groups, respectively, in the PP populations (risk difference − 3.2, 95%CI − 26.57 to 20.19) (Table 2). There was no difference statistically significant in days of culture collections / days of follow-up neither in the ITT population: 0.08 (IQR 0.0–0.17) versus 0.05 (IQR 0.0–0.14) in 7- and in 14-day groups, respectively, P = 0.17; nor in the PP population: 0.07 (IQR 0.02–0.16) versus 0.04 (IQR 0.0–0.12) in 7- and in 14-day groups, respectively, P = 0.224.
Table 2.
Primary and secondary outcomes (Intention to Treat and Per Protocol populations)a
| Outcomes | Intention to treat population | Per protocol population | ||||||
|---|---|---|---|---|---|---|---|---|
| 7-day group N = 59 |
14-day group N = 47 |
Risk difference (95% CI) |
P value | 7-day group N = 47 |
14-day group N = 28 |
Risk Difference (95% CI) |
P value | |
| Primary outcome | ||||||||
| Clinical failure | 25 (42.4) | 21 (44.7) | − 2.3 (− 21.3 to 16.7) | 0.98 | 22 (46.8) | 14 (50) | 3.2 (− 26.57 to 20.19) | 0.98 |
| 28-day mortality | 22 (37.3) | 19 (40.4) | − 3.1 (− 21.8 to 15.5) | 0.90 | 19 (40.4) | 12 (42.9) | − 2.4 (− 25.51 to 20.65) | 0.99 |
| Relapse | 5 (8.5) | 2 (4.3) | 4.2 (− 4.93 to 13.37) | 0.64 | 5 (10.6) | 2 (7.1) | 3.5 (− 9.49 to 16.49) | 0.93 |
| Secondary outcomes | ||||||||
| Days alive and free from hospitalisationb | 0 (0–4) | 0 (0–3) | – | 0.79 | 0.0 (0.0–1.0) | 0.0 (0.0–4.0) | – | 0.80 |
| Days alive and free from any antibiotic therapyb | 11.5 (0–25) | 3 (0–17) | – | 0.08 | 12.0 (0.0–25.3) | 0.0 (0.0–20.0) | – | 0.09 |
| New infections caused by other bacteria (independent of susceptibility profile) | 18 (30.5) | 17 (36.2) | − 5.7 (− 23.74 to 12.42) | 0.68 | 17 (36.2) | 7 (25) | 11.2 (− 9.95 to 32.29) | 0.46 |
| New infections caused by other MDR-GNB | 11 (18.6) | 9 (19.2) | − 0.5 (− 15.51 to 14.51) | 0.99 | 11 (23.4) | 1 (3.6) | 19.8 (5.91 to 33.75) | 0.05 |
| Length of ICU stayc | 8.0 (2.8–22.0) | 7 (3.0–20.3) | – | 0.81 | 8.0 (4.0–21.0) | 4.0 (3.0–22.0) | – | 0.76 |
| Adverse events | ||||||||
| Acute Kidney Injury | 30 (58.8) | 22 (52.4) | 6.4 (− 13.82 to 26.7) | 0.68 | 25 (58.2) | 13 (48.2) | 10.0 (− 13.94 to 33.92) | 0.5684 |
| KDIGO 1d,e | 9 (17.65) | 6 (14.29) | 8 (18.6) | 3 (11.1) | ||||
| KDIGO 2d,e | 10 (19.6) | 2 (4.8) | 7 (16.3) | 2 (7.4) | ||||
| KDIGO 3d,e | 11 (21.6) | 14 (33.3) | 10 (23.3) | 8 (29.6) | ||||
| Diarrhoea | 27 (45.8) | 20 (42.6) | 3.2 (− 15.8 to 22.22) | 0.89 | 21 (44.7) | 13 (46.4) | − 1.8 (− 25.06 to 21.56) | 0.99 |
|
Clostridioides difficile confirmed infection |
3 (5.1) | 1 (2.1) | 3.0 (− 4 to 9.92) | 0.78 | 3 (6.4) | 0 (0) | 6.4 (− 0.61; 13.37) | 0.45 |
| Othersf | 1 (0.94) | 0 (0) | 1.7 (− 1.6 to 4.98) | 0.99 | 1 (2.1) | 0 (0) | 2.1 (− 2 to 6.26) | 0.99 |
| New hemodynamic instability | 16 (27.1) | 8 (17.0) | 10.1 (− 5.52 to 25.72) | 0.32 | 13 (27.7) | 3 (10.7) | 17.0 (− 0.22 to 34.12) | 0.15 |
Data presented as n (%) or median (IQR p25-p75). MDR-GNB Multidrug-resistant Gram-negative bacteria, ICU intensive care unit
aPrimary and secondary outcomes were assessed within 28 days of randomisation, with exception of new hemodynamic instability which were assessed in 14 days. All but one patient was followed-up until the 28th day
bTotal (N = 103), 7-day group (n = 58), 14-day group (n = 45)
cTotal (N = 62), 7-day group (n = 36, 14-day group (n = 26)
dIntention to treat population: Total (N = 93), 7-day group (n = 51), 14-day group (n = 42)
ePer protocol population: Total (N = 70), 7-day group (n = 43), 14-day group (n = 27)
fSeizure (n = 1)
No statistically significant differences were found between groups in secondary outcomes, including adverse effects, in the ITT analysis (Table 2). In the PP population, there was a higher rate of other GNB infections (23.4%) in the 7-compared to the 14-day (3.6%) group (STable 2).
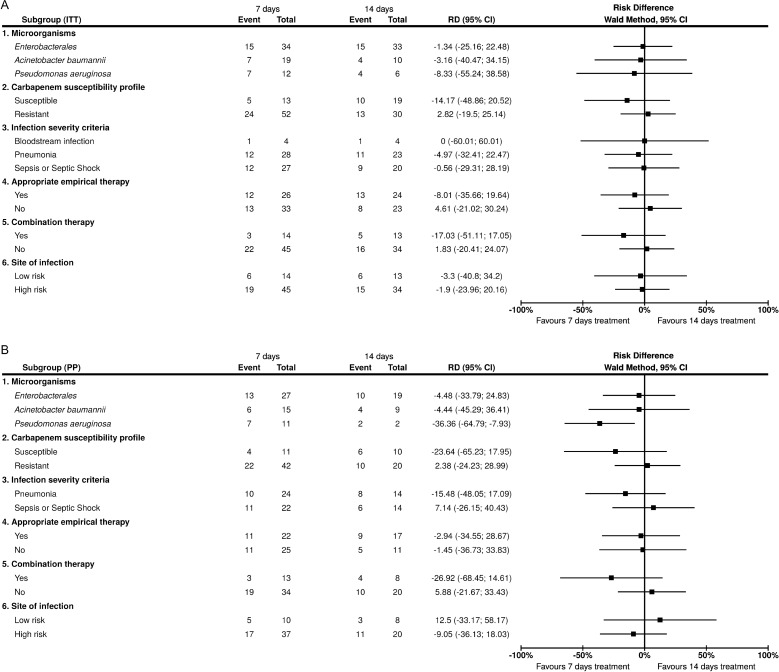
Subgroup analyses
Similar results were found in the six pre-specified subgroups in both ITT and PP analysis (Fig. 2). In the PP population, significantly lower clinical failure rates were found in the 7-day group of patients infected by P. aeruginosa (Fig. 2).
Fig. 2.
Subgroup analysis. A Intention to treat population; B Per protocol population
Post hoc analysis
Eight patients of the 7-day group received antimicrobial therapy for > 8 days and four in the 14-day group for < 13 days and were excluded for the PP population. Two (25%) of eight and two (50%) of four of the 7- and 14-days groups presented clinical failure in 28 days. In the sensitivity analysis including these patients in the PP population, and considering 100% and 0% of clinical failure rates for these eight and four patients of 7- and 14-day groups, respectively, the failure rates were as follows: 54.6% (30/55) and 43.8% (14/32) in 7- and 14-day groups, respectively, risk difference 10.8 (95%CI − 10.85 to 32.45).
Discussion
In this open-label, randomised clinical trial, it was not feasible to demonstrate the non-inferiority of 7 days compared to 14 days of appropriate antimicrobial therapy for the treatment of severe infections by MDR-GNB in critically ill patients who were afebrile and haemodynamic stable on the 7th day of therapy. Although rates of clinical failure were similar in 7- and 14-days groups, the upper limits of the 95% CI of the risk differences were higher than the pre-defined criteria for non-inferiority of 10% in both ITT (16.7%) and PP (20.2%) analyses. Each component of the composite outcome rates separately were also similar between groups. Unfortunately, the study had to be interrupted without reaching the planned sample size, a fact that made this study underpowered to meet the non-inferiority criteria.
Similar rates of clinical failure were also found in the prespecified subgroups. Of note, clinical failure and mortality rates were numerically lower in the 7-day compared to 14-day arm, while relapse was in the opposite direction. This finding was likely related to the naturally longer time at risk for relapse in the 7-day arm, which falsely inflated the relapse differences between arms. Moreover, as observed in previous trials [14, 25, 26], these relapses were not associated with increased mortality, which corroborates this hypothesis. The statistically significant finding of lower rate of clinical failure in the 7-day arm in the prespecified subgroup of patients with P. aeruginosa infections of the PP population was based on a very low number of events, which makes it a fragile association. Likewise, the statistical significance found in the number of other GNB infections in the favouring the 14-day group in the PP population should be interpreted with caution, because there was only one event in this group, which was much lower than the number observed in the ITT population in which no significantly difference was observed, and, in contrast to the component of the primary outcome, relapse of MDR-GNB infections, these other GNB infections were not adjudicated by blinded infectious diseases physicians. Noteworthy, there were higher proportions of carbapenem-resistant A. baumannii and carbapenem-resistant P. aeruginosa in the 7-day group, which could have adversely impacted on the outcomes of this group. Despite of some differences in the proportion of sites of infection theoretically favouring the 14-day group, this has not affected the overall findings since the proportion of patients with sites of infection with high- and low-risk for mortality (76.2% and 72.3% of high-risk sites in 7- and 14-day groups respectively, were similar, and there were non-statistically significant differences in clinical failure rates in these subgroups.
Although there have been randomised trials evaluating shorter duration of antimicrobial therapy in severe infections, such as bloodstream infections [27, 28] and ventilator-associated pneumonia [14, 25, 26, 29], no previous study has been specifically designed to address these difficult-to-treat pathogens. In addition, MDR-GNB were either absent [26, 29] or not described [25, 27] in these trials. In the Yahav et al. study, 109 patients with uncomplicated MDR-GNB bloodstream infections, and no difference was found in clinical failure [28]. However, this study may not be directly compared to ours, since it was neither restricted to hospital-acquired infections nor ICU patients, potentially including less severe infections and less ill patients, and most (105 of 109) MDR-GNB isolates were third-generation cephalosporin-resistant Enterobacterales [28], contrasting with the predominance of carbapenem-resistant isolates (77%) included in ours. The study with the largest proportion of MDR-GNB, actually carbapenem-resistant GNB isolates, was the recently published REGARD-VAP trial, which assessed individualised short-course antimicrobial treatment (≤ 7 days) or usual care (≥ 8 days) in 461 patients with ventilator-associated pneumonia [14]. A total of 33% (76 patients) and 28% (65) of patients allocated to individualised short-course and usual care groups had ventilator-associated pneumonia caused by carbapenem-resistant GNB, and the 60-day composite primary outcome of death or pneumonia recurrence were similar in the short-course and usual care in the ITT (49% and 55%, respectively) as in the PP population (50% and 55%, respectively) [14], which were clinical failure rates comparable to those found in our study, in both 7- and 14-day therapy arms. Taken together, these results might indicate that shorter courses of antimicrobial therapy for critically ill patients with severe infections by MDR-GNB may be effective and safe, provided the patient presents clinical signs of response to therapy and is hemodynamically stable.
In addition to the specific design which included only severe infections caused by MDR-GNB, a strength of the OPTIMISE trial is that, as in REGARD-VAP study, randomisation was performed in critically ill patients who have received appropriate therapy for a certain short period of time and presented clinical signs of improvement for at least 48 h, in contrast to that carried out soon after the infection diagnosis, before any clinical indication of improvement, potential leading to therapy interruption when the clinical condition might indicate a poor response in the first 7 days of treatment. The trial by Yahav et al. [28], not specifically addressing critically ill patients, also had this design, which, in fact, seems to better represent the clinical practice, in which clinicians’ decision on stopping therapy take into account the presence of some evidence that the condition improved and the patient is clinically stable. Moreover, this design does not consider events occurring before day 7 of therapy, when groups actually did not yet differentiate in relation to the intervention, as it happens when randomisation is made after infection diagnosis.
The major limitation of the OPTIMISE trial was its early interruption, limiting its power to determine the non-inferiority of shorter duration therapies despite the similar rates in clinical failures in both groups. The study activated 36 sites along the study period, but only half actually recruited patients (supplemental SFigure 1). The Covid-19 pandemics substantially delayed the IRBs approvals and activation processes of the sites and compromised local teams capacity of recruiting patients during the first year of the study. Additionally, we had an unexpected high rate of non-eligibility among patients with severe infections by MDR-GNB at ICU, either because of haemodynamic instability or fever in the 48 h period before randomisation window (n = 99 [48.3%]/205) or because of death within the first 7 days of the onset of infection (n = 58 [28.3%]/205). Another limitation was that the number of patients in the PP analysis was further restricted, for reasons including patients with longer durations in the 7-day group (n = 8 [13.6%]/59), and shorter durations in the 14-day group (n = 4 [8.3%]/48). Although, the median and IQR of the ITT were within the time period of the PP population, it could be the case that the 8 patients excluded from PP analysis had more severe diseases, and, in contrast, the 4 patients with shorter durations in the 14-day arm had less severe illness. This would lead to a bias in the PP population that might favour the non-inferiority [30]. In fact, two (25%) of eight and two (50%) of four of the 7- and 14-days groups presented clinical failure in 28 days. In a post hoc analysis, considering these patients in the PP population and assuming a worst-case scenario of 100% and 0% of clinical failure in the former and later groups, respectively, if they followed the protocol assigned for each arm, the risk difference for clinical failure would be 10.8% higher in the 7-day group. Although the 95%CI of this worst case scenario for 7-day therapy sensitivity analysis increased the uncertainty on the effect of the intervention, the overall rate of clinical failure in this group (54.6) may be still comparable to that found in the short duration arm of the subgroup with MDR-GNB in PP analysis of the REGARD-VAP [14]. Also, a total of 25 patients were excluded because the assistant physician did not consent to patient participation. Although it might have caused undue exclusions of patients resulting in a selection bias, since these exclusions could be due to an initial evaluation that the infection was not severe enough or too severe for longer or shorter therapy durations, respectively. This unlikely affected the overall results since it is expected that randomisation would allocate these patients evenly to both groups. Another limitation is that the presence of fever was evaluated by axillary temperature, which may underestimate the body temperature compared to oesophageal temperature, for example. This might have caused more classifications of “clinical stability” in both groups, since some patients could be still febrile if measurements were made by oesophageal temperature. This potential misclassification at the 7th day could be indicative for the need of more prolonged treatment. However, if this bias had occurred it would have favoured the null hypothesis. Finally, the open design may predispose physicians to seek for a new infection diagnosis in patients without antimicrobial therapy. Although culture collections per days of follow up were similar between groups and blind adjudication of relapses was done, the asymmetric (longer) time exposure to develop relapse in the 7-day group may still lead to higher rates of relapses in the shorter therapy group [31].
In summary, despite similar clinical failure rates, including the 28-day mortality component, observed in both groups, the OPTIMISE trial could not determine the non-inferiority of 7-day compared to 14-day therapy for severe infections caused by MDR-GNB due to early termination related to the low recruitment rate. Since pathogen and susceptibility profile-specific randomised clinical trials are extremely difficult to be carried out, future trials addressing duration of therapy in MDR-GNB will remain a challenge to be conducted. Therefore, OPTIMISE trial’s new results bring an important contribution to the scarce existing evidence on the duration of therapy in severe MDR-GNB infection in the critically ill patient.
Supplementary Information
Acknowledgements
We are grateful to Carlos Sérgio Luna Gomes Duarte (post mortem) from Hospital Tricentenário, Olinda, Brazil, for his significant contribution in/on the study execution. We would like to thank Bruna Genro, David Van Duin and Suzi Camey for their qualified participation in the DSMB.
OPTIMISE Study Group
Jaysa Pizzi1, Thaissa Torrezini2, Euclimeire da Silva Neves2, Jamile Freire Barreto dos Santos2, Jaime Paula Pessoa Linhares Filho3, Marcos de Almeida e Pontes Vieira3, René Rodrigues Pereira3, Cassia Righy4, Ricardo Turon4, Bruno Gonçalves4.
1Hospital Nossa Senhora da Conceição, Porto Alegre, Brazil; 2Instituto Couto Maia, Salvador, Brazil; 3Hospital OTO clinica, Fortaleza, Brazil; 4Instituto Estadual do Cérebro Paulo Niemeyer, Rio de Janeiro, Brazil.
Abbreviations
- ANVISA
Brazilian Health Regulatory Agency
- CI
Confidence interval
- DSMB
Data and safety monitoring committee
- GNB
Gram-negative bacteria
- ICU
Intensive care units
- IQR
Interquartile range
- IRB
Institutional review board
- ITT
Intention to treat
- MDR
Multidrug-resistant
- PP
Per protocol
Author contributions
BA and APZ conceived the study. ACK, BMT, BAMPB, AJP, VCV, GMN contributed to the study design. BA, JDCH, GPS, CAJOA, TMS, LSBB and APZ contributed to the study execution and verified all data. GGLS, EB, ACPA, JPA, DCB, DHL, FCA, GCR, VFF, VCSD, GFSM, VFDR, FCP, ALNG, VPL, FHL, CMDMC, KDNOC, VMI, PK included participants, followed up them and acquired data. BA, BSR and APZ performed the statistical analysis. BA, APZ and ACK interpreted the results and drafted the manuscript. All authors read, contributed to the manuscript and approved the final version.
Funding
The research was funded by the Ministry of Health (MH) of Brazil through the Support Program for Institutional Development of the Unified Health System (PROADI-SUS). The funder had no role in collection, analysis, and interpretation of data, nor in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol and amendments were approved by the research ethics committee (institutional review board, IRB) of the coordinating centre (Hospital Moinhos de Vento), as well as IRBs from all other participant sites. Written informed consent was obtained from the patients (or from a legal representative, if the patient was not capable of providing it at that moment).
Consent for publication
Not applicable.
Competing interests
BA received support for attending meetings and/or travel by MSD. G.M.N received payment for lectures for Pfizer and MSD and received support for attending meetings and/or travel by Pfizer. A.P.Z. is a research fellow of the National Council for Scientific and Technological Development (CNPq), Ministry of Science and Technology, Brazil. All other authors have no conflicts to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Beatriz Arns, Email: beatriz.arns@hmv.org.br.
OPTIMISE Study Group:
Jaysa Pizzi, Thaissa Torrezini, Euclimeire da Silva Neves, Jamile Freire Barreto dos Santos, Jaime Paula Pessoa Linhares Filho, Marcos de Almeida e Pontes Vieira, René Rodrigues Pereira, Cassia Righy, Ricardo Turon, and Bruno Gonçalves
References
- 1.Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabah A, Buetti N, Staiquly Q, Ruckly S, Akova M, Aslan AT, et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2023;49:178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falcone M, Tiseo G, Carbonara S, Marino A, Di Caprio G, Carretta A, et al. Mortality attributable to bloodstream infections caused by different carbapenem-resistant gram-negative bacilli: results from a nationwide study in Italy (ALARICO Network). Clin Infect Dis. 2023;76:2059–69. [DOI] [PubMed] [Google Scholar]
- 4.Stewardson AJ, Marimuthu K, Sengupta S, Allignol A, El-Bouseary M, Carvalho MJ, et al. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): a multinational prospective cohort study. Lancet Infect Dis. 2019;19:601–10. [DOI] [PubMed] [Google Scholar]
- 5.Vincent J-L, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323:1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee RA, Stripling JT, Spellberg B, Centor RM. Short-course antibiotics for common infections: what do we know and where do we go from here? Clin Microbiol Infect. 2023;29:150–9. [DOI] [PubMed] [Google Scholar]
- 7.Spellberg B, Rice LB. The shorter is better movement: past, present, future. Clin Microbiol Infect. 2023;29:141–2. [DOI] [PubMed] [Google Scholar]
- 8.Wald-Dickler N, Spellberg B. Short-course antibiotic therapy-replacing constantine units with “shorter is better.” Clin Infect Dis. 2019;69:1476–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yek C, Lawandi A, Evans SR, Kadri SS. Which trial do we need? Optimal antibiotic duration for patients with sepsis. Clin Microbiol Infect. 2023;29:1232–6. [DOI] [PubMed] [Google Scholar]
- 10.Haddad SF, Allaw F, Kanj SS. Duration of antibiotic therapy in Gram-negative infections with a particular focus on multidrug-resistant pathogens. Curr Opin Infect Dis. 2022;35:614–20. [DOI] [PubMed] [Google Scholar]
- 11.Ohnuma T, Chihara S, Costin B, Treggiari MM, Bartz RR, Raghunathan K, et al. Association of appropriate empirical antimicrobial therapy with in-hospital mortality in patients with bloodstream infections in the US. JAMA Netw Open. 2023;6: e2249353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28:521–47. [DOI] [PubMed] [Google Scholar]
- 13.Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Esbl K, Amp C, Enterobacterales C-R, Aeruginosa P, CRAB, et al. IDSA 2024 guidance on the treatment of antimicrobial resistant gram-negative infections. [cited 2024 Aug 1]. Available from: https://www.idsociety.org/practice-guideline/amr-guidance/
- 14.Mo Y, Booraphun S, Li AY, Domthong P, Kayastha G, Lau YH, et al. Individualised, short-course antibiotic treatment versus usual long-course treatment for ventilator-associated pneumonia (REGARD-VAP): a multicentre, individually randomised, open-label, non-inferiority trial. Lancet Respir Med. 2024;12:399–408. [DOI] [PubMed] [Google Scholar]
- 15.Arns B, Horvath JDC, Rech GS, Sesin GP, Agani CAJO, da Rosa BS, et al. A randomized, open-label, non-inferiority clinical trial assessing 7 versus 14 days of antimicrobial therapy for severe multidrug-resistant gram-negative bacterial infections: the OPTIMISE trial protocol. Infect Dis Ther. 2024;13:237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caderno 2 - Critérios Diagnósticos de Infecção Relacionada à Assistência à Saúde.pdf [Internet]. [cited 2024 Jul 25]. Available from: https://www.gov.br/anvisa
- 18.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ESCMID-European Society of Clinical Microbiology, Diseases I. eucast: EUCAST [Internet]. [cited 2024 Jul 25]. Available from: https://www.eucast.org/
- 20.Clsi. M100: Performance Standards for Antimicrobial Susceptability Testing. 2021.
- 21.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–27. [DOI] [PubMed] [Google Scholar]
- 22.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii T, Uchino S, Takinami M, Bellomo R. Validation of the Kidney Disease Improving Global Outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol. 2014;9:848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The R Project for Statistical Computing [Internet]. [cited 2024 Jul 25]. Available from: https://www.r-project.org/
- 25.Bouglé A, Tuffet S, Federici L, Leone M, Monsel A, Dessalle T, et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia in adults: a randomized, controlled, open-label trial. Intensive Care Med. 2022;48:841–9. [DOI] [PubMed] [Google Scholar]
- 26.Chastre J, Wolff M, Fagon J-Y, Chevret S, Thomas F, Wermert D, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–98. [DOI] [PubMed] [Google Scholar]
- 27.von Dach E, Albrich WC, Brunel A-S, Prendki V, Cuvelier C, Flury D, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA. 2020;323:2160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis. 2019;69:1091–8. [DOI] [PubMed] [Google Scholar]
- 29.Capellier G, Mockly H, Charpentier C, Annane D, Blasco G, Desmettre T, et al. Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS ONE. 2012;7: e41290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mo Y, Lim C, Watson JA, White NJ, Cooper BS. Non-adherence in non-inferiority trials: pitfalls and recommendations. BMJ. 2020;370: m2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metersky ML, Klompas M, Kalil AC. Less is more: a 7-day course of antibiotics is the evidence-based treatment for pseudomonas aeruginosa ventilator-associated pneumonia. Clin Infect Dis. 2023;76:750–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.