Abstract
A major clinical manifestation of infection with Bacteroides fragilis is the formation of intra-abdominal abscesses, which are induced by the capsular polysaccharides of this organism. Transposon mutagenesis was used to locate genes involved in the synthesis of capsular polysaccharides. A 24,454-bp region was sequenced and found to contain a 15,379-bp locus (designated wcf) with 16 open reading frames (ORFs) encoding products similar to those encoded by genes of other bacterial polysaccharide biosynthesis loci. Four genes encode products that are similar to enzymes involved in nucleotide sugar biosynthesis. Seven genes encode products that are similar to sugar transferases. Two gene products are similar to O-acetyltransferases, and two products are probably involved in polysaccharide transport and polymerization. The product of one ORF, WcfH, is similar to a set of deacetylases of the NodB family. Deletion mutants demonstrated that the wcf locus is necessary for the synthesis of polysaccharide B, one of the two capsular polysaccharides of B. fragilis 9343. The virulence of the polysaccharide B-deficient mutant was comparable to that of the wild type in terms of its ability to induce abscesses in a rat model of intra-abdominal infection.
Intra-abdominal infections present serious clinical problems that are difficult to diagnose and treat, frequently require surgical intervention, and often result in complications, including death. Bacteroides fragilis accounts for only 0.5% of the normal human colonic flora; however, it is the anaerobic species most frequently isolated from clinical infections, particularly within the abdominal cavity (16, 37). The capsular polysaccharides of B. fragilis are the most important virulence factors in the formation of intra-abdominal abscesses by this organism, and purified capsule is able to induce abscesses in experimental animal models (27, 31).
The B. fragilis capsule is composed of two distinct high-molecular-weight polysaccharides, termed polysaccharide A (PS A) and polysaccharide B (PS B) (34), that are coexpressed (56). The chemical composition and structure of each polysaccharide have been determined for one B. fragilis strain, NCTC 9343 (56). PS A is composed of the following repeating unit: [→3)α-d-AATp(1→4)[β-d-Galf(1→3)]α-d-GalpNAc(1→3) β-d-Galp(1→], where AAT is 2-acetamido-4-amino-2,4,6-trideoxygalactose. A pyruvate substituent having the R configuration spans O-4 and O-6 of the β-d-galactopyranosyl residue. PS B is composed of the following repeating unit: [→4)α-l-QuipNAc(1→3)β-d-QuipNAc(1→4)[α-l-Fucp(1→2) β-d-GalpA(1→3)β-d-GlcpNAc(1→3)]α-d-Galp(1→], with a 2-aminoethylphosphonate substituent on O-4 of the N-acetyl-β-d-glucopyranosyl residue. The most striking feature of PS A and PS B is the presence of both positively and negatively charged groups on each repeating unit. Of the many bacterial capsular polysaccharides whose structures have been determined, relatively few contain both positively and negatively charged substituent groups. The charge motif of these polysaccharides is essential for abscess formation (54).
The capsule of B. fragilis initiates a unique immune response in the host: the formation of abscesses. This process is dependent upon T cells (46, 53) and therefore is distinct from responses to most other polysaccharide antigens, which are considered to be T cell independent. Studies in our laboratory have shown that the B. fragilis capsule acts as an immunomodulator regulating the formation of intra-abdominal abscesses (53).
Despite the importance of B. fragilis capsular polysaccharides as virulence factors, as unique immunomodulators, and as interesting bacterial molecules with a rare charge motif, nothing is known about the genetics of their biosynthesis or regulation. This study describes the characterization of the first such locus of B. fragilis.
MATERIALS AND METHODS
Bacteria, plasmids, and media.
Bacterial strains and plasmids are described in Table 1. Escherichia coli strains were grown in Luria broth or on Luria agar plates. B. fragilis strains were grown anaerobically in supplemented brain heart infusion (BHI) broth (BHIS; Randolph Biomedical, West Warwick, R.I.) or on BHI agar plates supplemented with hemin (50 μg/ml) and menadione (0.5 μg/ml). The following concentrations of antibiotic supplements were added when appropriate: ampicillin, 100 μg/ml; kanamycin, 20 μg/ml; erythromycin, 7.5 μg/ml; trimethoprim, 100 μg/ml; gentamicin, 200 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| HB101 | F′hsdS20(rB−mB−)recA13 leuB6 ara-14 proA2 lacY1 galK2 rpsL20 (Smr) xyl-5 mtl-1 supE44λ− | 42 |
| DH5α | FΦ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17 (rK−mK+) deoR thi-1 supE44 gyrA96 relA1 λ− | 5 |
| B. fragilis | ||
| 638R (TM4000) | Clinical | 38 |
| NCTC 9343 | Type strain; appendix abscess | 22, ATCC |
| 9343ΔwcfF | ΔwcfF | This study |
| 9343ΔwcfD-L | Δ(wcfD wzy wcfE wcfF wcfG wcfH wcfI wcfJ wcfK wcfL orf5) | This study |
| Plasmids | ||
| pHC79 | Cosmid vector; Apr | 20 |
| pBluescript II SK | Phagemid cloning vector; Apr | Stratagene |
| pNJR6 | Bacteroides suicide vector; mob+ Tra− Kmr | 51 |
| R751 | Mobilizable plasmid used to move plasmids from E. coli to B. fragilis; Tra+ Tpr | 48 |
| R751Ω4 | Plasmid R751 containing duplicate copies of Tn4351 (EmrBacteroides) (TprE. coli) | 47 |
| pNJR6Ω4 | Plasmid pNJR6 containing duplicate copies of Tn4351 (EmrBacteroides) (Km4E. coli) Tra+ | This study |
| pLEC6 | Junctional clone of B. fragilis 638R mutant 2-42 containing a portion of pNJR6 and 900 bp of B. fragilis DNA; Kmr | This study |
| pLEC6.1 | Subclone of pLEC6 containing 900-bp EcoRI-HindIII fragment (B. fragilis DNA) cloned in pBluescript; Apr | This study |
| pMJC2 | B. fragilis 9343 gene bank clone containing PS B biosynthesis region (wcf) cloned into pHC79; Apr | This study |
| pMJC2.1 | 4.3-kb EcoRI subclone of pMJC2 in pBluescript; Apr | This study |
| pMJC2.2 | 10.2-kb PvuII subclone of pMJC2 in pBluescript; Apr | This study |
| pMJC2.3 | 2.1-kb PvuII-SmaI subclone of pMJC2 in pBluescript; Apr | This study |
| pMJC2.4 | 7.5-kb SmaI subclone of pMJC2 in pBluescript; Apr | This study |
| pMJC2.5 | 4.6-kb SmaI-PvuII subclone of pMJC2 utilizing the ori of pHC79; Apr | This study |
| pMJC2.6 | 4.2-kb SmaI-EcoRI subclone of pMJC2 in pBluescript; Apr | This study |
| pMJC2Δ | Subclone of pMJC2 containing DNA from bp 3 through bp 24454, with bp 9277–19823 deleted; utilizes ori and Apr of pHC79 | This study |
| pMJC2Δ.1 | pMJC2Δ cloned into the StuI site of pNJR6; Apr Kmr | This study |
Transposon mutagenesis and screening.
Plasmid R751Ω4 has been used previously to create transposon insertions in the chromosomes of Bacteroides species; the result has often been the insertion of the entire plasmid (47). Because R751Ω4 is a large plasmid, its integration was not advantageous for these studies. Therefore, the Tn4351 tandem transposon was excised from R751Ω4 with SalI and ligated into the unique SalI site of the Bacteroides suicide vector pNJR6, resulting in pNJR6Ω4. This plasmid is smaller and, when integrated with the transposon, permits cloning of the Bacteroides DNA at the junction of the transposon with various restriction enzymes by using the E. coli origin of replication and the Kmr gene of pNJR6. E. coli DH5α containing pNJR6Ω4 and the conjugal helper plasmid R751 was mated with B. fragilis 638R by a modification of a previously described protocol (49). B. fragilis was grown to an optical density at 550 nm of 0.2, centrifuged, and mixed with DH5α containing pNJR6Ω4 and R751. These strains were mated aerobically overnight on BHIS plates and then plated onto BHIS plates containing erythromycin and gentamicin and incubated anaerobically. The resulting colonies were screened by immunoblotting with monoclonal antibody (MAb) 4D5 (638R capsule specific).
Cloning the DNA at the transposon junction of mutant 2-42.
The chromosome of mutant 2-42 (MAb 4D5 negative) yielded a 15-kb HindIII fragment that contained the origin of replication and the Kmr gene of pNJR6 as well as ∼900 bp of B. fragilis DNA. This fragment was cloned by ligation of HindIII-digested 2-42 chromosomal DNA (favoring intramolecular ligations), transformation of DH5α, and selection with kanamycin. The resulting plasmid (pLEC6) has a very low copy number; therefore, the 900-bp B. fragilis DNA of pLEC6 was subcloned into pBluescript II SK (Stratagene, La Jolla, Calif.) by using the EcoRI site at the end of the transposon and the HindIII site at the end of the cloned B. fragilis DNA; the result was plasmid pLEC6.1. The insert of pLEC6.1 was sequenced with primers complementary to vector sequences.
Construction of plasmids pMJC2 to pMJC2.6.
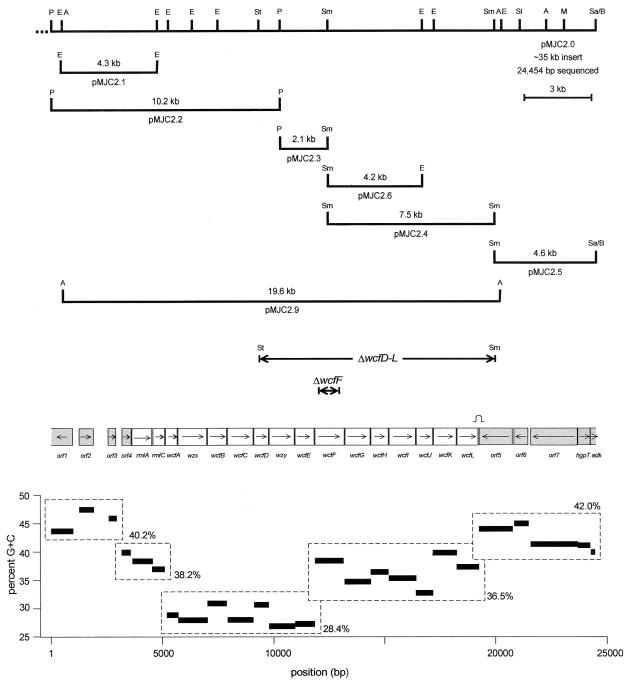
Plasmid pMJC2 was selected from a B. fragilis 9343 gene bank with the insert of pLEC6.1 as a probe. The gene bank was constructed with Sau3AI partial digests of chromosomal DNA cloned into the BamHI site of pHC79. For construction of pMJC2 subclones (pMJC2.1 to pMJC2.6), restriction fragments of pMJC2 were separated on agarose gels and eluted with a Qiagen gel extraction kit (<10 kb) or electroeluted (>10 kb). All subclones except pMJC2.5 were cloned into pBluescript II SK. The restriction sites used and the sizes and locations of the subclones constructed are illustrated in Fig. 1 and Table 1. Plasmid pMJC2.5 is an EcoRI fragment of pMJC2 which was circularized and utilizes the β-lactamase gene and origin of replication of pHC79.
FIG. 1.
ORF map of a 24,454-bp region of the 9343 chromosome contained on cosmid pMJC2. Restriction sites within this region are shown for the following enzymes: PvuII (P), EcoRI (E), Asp718 (A), StuI (St), SmaI (Sm), SalI (Sl), MluI (M), and Sau3AI/BamHI junction with the pHC79 vector (Sa/B). ΔwcfD-L and ΔwcfF represent the regions that were deleted in the chromosomes of mutants 9343ΔwcfD-L and 9343ΔwcfF. The arrows indicate direction of transcription of each ORF. The ORFs that do not demonstrate homology with genes involved in polysaccharide biosynthesis (outside the wcf locus) are shaded. The lower diagram demonstrates the average G+C content for each ORF of the region. The dashed-line boxes represent a clustering of adjacent ORFs with similar G+C contents. A transcriptional terminator-like stem-loop region downstream of wcfL is indicated.
Creation of deletion mutants 9343ΔwcfD-L and 9343ΔwcfF.
Mutant 9343ΔwcfD-L is devoid of 10 of the 16 genes in the wcf region, beginning at the StuI site in wcfD and continuing to the SmaI site just downstream of the wcf locus in orf5 (Fig. 1). To create this mutant, pMJC2 was digested with PvuII and StuI, and the 9.2-kb fragment representing bp 3 through bp 9277 was recovered. pMJC2 was separately digested with SmaI and PvuII, and the 7.3-kb fragment representing bp 19,823 through bp 27,125 was eluted. The PvuII site of the 7.3-kb SmaI-PvuII fragment lay within the pHC79 cloning vector, and this 7.3-kb fragment contained the ori and the Apr gene of pHC79. The 9.2-kb fragment was ligated with this 7.3-kb fragment, and the ligation was transformed into DH5α and plated with ampicillin selection. The resulting colonies were tested by PCR for the correct ligation of the two PvuII ends and the StuI end of the 9.2-kb fragment with the SmaI end of the 7.3-kb fragment. The correct clone, designated pMJC2Δ, was linearized with PvuII and cloned into the unique StuI site of the mobilizable Bacteroides suicide vector pNJR6, creating pMJC2Δ.1. Plasmid pMJC2Δ.1 was transferred into the chromosome of wild-type 9343 with the conjugal helper plasmid R751, and cointegrates were selected by Emr encoded by the suicide vector. The cointegrate was passaged three times in BHIS to allow for resolution of the cointegrate and plated onto BHIS. The resulting colonies were replica plated onto BHIS containing erythromycin, and the Ems colonies were tested for the mutant genotype by PCR. The loss of the 10.5-kb region of wcfD to -L in the 9343 chromosome was confirmed by Southern blotting. This mutant was designated 9343ΔwcfD-L.
Mutant 9343ΔwcfF was created by a modification of this procedure. Primers internal to and oriented outward from wcfF (primer 1, 5′ CATGACCGGAATTCAAAGCATCAAC, and primer 3, 5′ CTTGGGCGAATTCGGCTAAAGTG) were used in PCR with primers located approximately 3 kb upstream of primer 1 (primer 2, 5′ GGAAAACGTCGACTTGAAAGATTGG) or downstream of primer 3 (primer 4, 5′ GCAATGCTCTCTGTCGACATTTTAT). PCRs with primers 1 and 2 and primers 3 and 4 were performed with high-fidelity DNA polymerase (Life Technologies, Gaithersburg, Md.). The PCR products were digested with EcoRI and SalI and gel purified. The products were used together in a ligation with SalI-digested pNJR6. A clone containing the correct orientation of the PCR products, resulting in a 945-bp deletion of wcfF, was selected by PCR. The conjugal transfer and cointegrate selection and resolution were done as described for 9343ΔwcfD-L.
DNA sequencing and analysis.
DNA sequencing was performed with an automated DNA sequencer (Prism model 377; Applied Biosystems, Inc.) with AmpliTaq FS DyeDeoxy terminator cycle sequencing kits (Applied Biosystems) at the Automated Sequencing and Genotyping Center at Brigham and Women’s Hospital.
The Genetics Computer Group (GCG; Madison, Wis.) suite of programs (Wisconsin Package version 9.1) was used for the majority of routine sequence analyses, including fragment assembly, restriction analysis, open reading frame (ORF) detection, and conceptual translation. Prediction of the membrane-spanning topology and secondary structure was aided by TMpred (19a), with a 19-residue minimum helix length and a 25-residue maximum helix length. To compare DNA and protein sequences against the GenBank, (Protein Information Resource (PIR) and SwissProt databases, the BLAST (1) and FASTA (36) clients as implemented in the GCG package and the BLAST clients (2) accessible from the Internet at the National Center for Biotechnology Information were used. Alignment of protein and DNA sequence sets was routinely investigated with the Bestfit and Gap programs of the GCG package; default values for extension and gapping and the BLOSUM62 matrix (19) were used. Hydrophobic cluster analysis was examined with the drawhca program (7a).
SDS-polyacrylamide gel electrophoresis and Western blotting (immunoblotting).
Bacterial cell lysates were run on discontinuous sodium dodecyl sulfate (SDS)-polyacrylamide gels (10% acrylamide; ESA, Inc. Chelmsford, Mass.) and transferred to nitrocellulose by standard techniques. The blots were blocked in Tris-buffered saline (TBS) containing 5% nonfat dry milk (TBS-milk) and then incubated for 1 h in TBS-milk containing primary antibody. The blots were washed with TBS and incubated with alkaline phosphatase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G (Sigma Chemical Co.) at 1:1,000 in TBS for 1 h. The blots were washed with TBS and developed with a colorimetric detection solution. Ascitic fluid containing MAb CE3 (9343 PS A specific) was used at 1:1,000. Culture supernatant containing MAb 4D5 (638R capsule specific) was used at 1:10.
9343ΔwcfD-L-adsorbed serum was prepared by the following procedure. The starting antiserum for adsorption was raised in rabbits to formalin-fixed, whole-cell, wild-type B. fragilis 9343. A 100-μl volume of this antiserum was diluted with 10 ml of phosphate-buffered saline and mixed with formalin-fixed 9343ΔwcfD-L from a 100-ml overnight culture. The bacteria were mixed with the antiserum for 1 h at 37°C and then removed by centrifugation. This adsorption was repeated. The resulting 9343ΔwcfD-L-adsorbed antiserum was used at a dilution of 1:30 (final dilution, 1:3,000).
Capsular polysaccharide extraction, purification, and analysis.
A 16-liter culture of B. fragilis 9343ΔwcfD-L was grown in a fermentor, and the capsular polysaccharide was extracted with phenol and water as previously described (34). The resulting material was resuspended in 274 ml of deoxycholate buffer (3% sodium deoxycholate, 50 mM glycine, 10 mM EDTA [pH 9.8]). One-quarter of the sample was applied to a Sepharose S400 column equilibrated with deoxycholate buffer. This procedure completely separated lipopolysaccharide from capsular polysaccharide as determined by silver-stained SDS-polyacrylamide gels. Fractions containing capsular material were pooled, concentrated, precipitated, resuspended in 20 ml of distilled H2O (dH2O) (pH 9.0), and dialyzed against pH 9.0 dH2O for 48 h to remove the detergent. The material was then dialyzed against pH 7.0 dH2O and lyophilized, and the total weight of the capsular polysaccharide was determined. The capsular material was resuspended in 50 mM Tris (pH 7.4) and applied to an anion-exchange column (Q-Sepharose; Pharmacia). The column was washed with 2 bed volumes of buffer and eluted with a linear gradient to 2 M NaCl. Column fractions were analyzed by immunoelectrophoresis as described previously (34). Fractions appearing identical by immunoelectrophoresis were pooled, concentrated, dialyzed, lyophilized, and analyzed by nuclear magnetic resonance to confirm the identity of the polysaccharide.
Competitive ELISA inhibition.
A competitive enzyme-linked immunosorbent assay (ELISA) was performed as previously described (35). Immulon-2 96-well plates (Dynatech) were coated with purified PS B and frozen at −20°C until use. Antigens to be tested were dissolved at a concentration of 100 μg/ml in phosphate buffer, diluted in triplicate, and added to PS B-coated plates. Polyclonal rabbit antiserum enriched for antibodies to PS B was added to all wells at a 1:4,000 dilution. The plates were thoroughly washed, and a goat anti-rabbit horseradish peroxidase conjugate was added for 1 h. After thorough washing, substrate was added for 1 h. Absorbance was read at 405 nm. Percent inhibition calculations for each antigen were based on comparisons with uninhibited control wells.
Rat abscess model.
An animal model for intra-abdominal sepsis was utilized for these studies (30). Male Wistar rats (180 to 200 g; Charles River Laboratories, Wilmington, Mass.) were anesthetized with a single intraperitoneal injection of 0.15 ml of pentobarbitalsodium (Nembutal) (50 mg/ml; Abbott Laboratories, North Chicago, Ill.). The inocula, containing serial 10-fold dilutions of B. fragilis 9343 or 9343ΔwcfF, were mixed with sterile rat cecal contents and 10% BaSO4 (wt/vol) as previously described (32, 53). An anterior midline incision (0.5 cm) was made through the abdominal wall and peritoneum, and 0.5 ml of inoculum was inserted into the pelvis with a pipette. The incisions were closed with 3.0 silk sutures. The animals that did not survive the surgical procedure were removed from the experiment. Six days postchallenge, the surviving animals were necropsied in an observer-blinded fashion and examined for the presence of intra-abdominal abscesses. The presence of one or more abscesses in an animal was scored as a positive result. The results are reported as the number of animals with one or more abscesses per total number of animals. The number of organisms necessary to cause abscesses in 50% of the animals (AD50) was calculated by the method of Reed and Muench (39).
Nucleotide sequence accession numbers.
The sequence discussed in this paper has been assigned GenBank accession no. AF048749.
RESULTS
Cloning of a polysaccharide biosynthesis locus of 9343.
The prototypical B. fragilis strain used to study abscess formation, NCTC 9343, is somewhat resistant to the introduction of foreign DNA; thus, transposon mutagenesis of this strain is difficult. To locate genes involved in capsular polysaccharide biosynthesis in strain 9343, transposon mutants were generated with B. fragilis 638R. MAb 4D5 (638R capsule specific [33]) was used to screen the mutant bank. The DNA at the junction of the transposon insertion of mutant 2-42 (4D5 negative) was homologous to rmlA, a gene known to be present in various bacterial polysaccharide biosynthesis loci. This rmlA probe hybridized with 9343 chromosomal DNA; therefore, this region was further studied in the 9343 chromosome. The 638R rmlA probe (pLEC6.1 insert) was used to select the 9343 gene bank clone pMJC2 (Fig. 1). This cosmid clone was mapped with restriction enzymes and subcloned as diagrammed in Fig. 1. The subclones were used as sequencing templates. In total, 24,454 bp were sequenced. Analysis of this sequence revealed 23 complete ORFs and two partial frames at the ends. The central 15,379 bp of this sequence was found to contain 16 ORFs, all of which encode putative products with varying degrees of similarity to proteins implicated in polysaccharide biosynthesis in other organisms (see Table 3). In accordance with changes in the nomenclature of polysaccharide biosynthesis genes (40), the ORFs were named wcfA through wcfL. Four genes were given previously designated names due to a high degree of homology or conserved sequence characteristics: rmlA, rmlC, wzx, and wzy.
TABLE 3.
Similarity of products encoded by wcf to sequences in the database
| ORFa | % G+C | Size (aa) | Organism and gene | Gene product | % Identical/similarb | Accession no. |
|---|---|---|---|---|---|---|
| orf1 | 43.7 | 1–322 | None | |||
| orf2 | 47.5 | 211 | Nicotiana tabacum | S-Adenosyl-methionine-sterol-C-methyltransferase | 28/45 (aa 47–191) | U71108 |
| Lactococcus lactis | Putative menaquinone biosynthesis methyltransferase | 32/45 (aa 51–158) | L14679 | |||
| orf3 | 45.9 | 113 | None | |||
| orf4 | 39.9 | 130 | None | |||
| rmlA | 38.5 | 295 | Shigella flexneri rmlA | Glucose-1-phosphate thymidyltransferase | 67/80 (aa 2–289) | D55213 |
| Salmonella enterica rmlA | Glucose-1-phosphate thymidyltransferase | 66/79 (aa 2–289) | P55254 | |||
| rmlC | 37.0 | 182 | Salmonella enterica rmlC | dTDP-4-keto-6-deoxyglucose 3,5-epimerase | 58/70 (aa 1–171) | S23343 |
| Escherichia coli rmlC | dTDP-4-keto-6-deoxyglucose 3,5-epimerase | 55/70 (aa 1–169) | P37745 | |||
| wcfA | 28.9 | 173 | Escherichia coli lacA | Galactoside O-acetyltransferase | 42/59 (aa 56–167) | P07464 |
| Staphylococcus aureus capG | Galactoside O-acetyltransferase | 53/71 (aa 102–167) | P39856 | |||
| wzx | 28.0 | 511 | Salmonella enterica C1 wzx | Flippase | 24/44 (aa 5–510) | G47677 |
| Salmonella enterica E1 wzx | Putative flippase | 27/46 (aa 13–426) | S23344 | |||
| wcfB | 31.0 | 287 | Yersinia enterocolitica O:8 wbcH | Putative galactoside 2-l-fucosyltransferase | 36/48 (aa 106–229) | U46859 |
| Homo sapiens | α(1,2) Fucosyltransferase | 25/43 (aa 8–218) | AB004860 | |||
| wcfC | 28.1 | 386 | Erwinia amylovora amsD | Glycosyltransferase galactose α(1,6) linkages | 30/50 (aa 206–315) | Q46634 |
| Yersinia enterocolitica O:8 wbcl | Putative galactosyltransferase | 24/42 (aa 78–337) | U46859 | |||
| wcfD | 30.7 | 218 | Escherichia coli lacA | Galactoside acetyltransferase | 34/49 (aa 47–218) | U73857 |
| Escherichia coli | Putative O-acetyltransferase | 38/52 (aa 64–215) | U73857 | |||
| wzy | 26.9 | 365 | Salmonella enterica | O antigen polymerase | 24/44 (aa 3–362) | A47677 |
| Klebsiella pneumoniae | Unknown | 24/42 (aa 1–364) | Q48457 | |||
| wcfE | 27.3 | 291 | Streptococcus pneumoniae cps141 | β-1,3-N-acetylglucosaminyltransferase | 34/55 (aa 10–100) | X85787 |
| Streptococcus pneumoniae cps19bQ | Putative rhamnosyltransferase | 24/46 (aa 10–236) | AF004325 | |||
| wcfF | 38.5 | 425 | Salmonella typhi vipA | UDP-glucose/GDP-mannose dehydrogenase family | 53/73 (aa 1–425) | Q04972 |
| Burkholderia solanacearum epsD | NDP-N-acetyl-d-galactosaminuronic acid dehydrogenase | 30/50 (aa 6–417) | Q45410 | |||
| wcfG | 34.8 | 377 | Staphylococcus aureus capM | CapM | 22/47 (aa 195–375) | P39862 |
| Zea mays | Sucrose synthase (d-fructose 2-glucosyltransferase) | 27/42 (aa 194–360) | P49036 | |||
| wcfH | 36.5 | 259 | Rhizobium galegae nodB | Chitooligosaccharide deacetylase | 25/40 (aa 77–200) | P50354 |
| Azorhizobium caulinodans nodB | Chitooligosaccharide deacetylase | 34/46 (aa 151–199) | Q07740 | |||
| wcfI | 35.4 | 407 | Escherichia coli wcaC | Putative glycosyltransferase | 23/39 (aa 4–361) | P71237 |
| Klebsiella pneumoniae rfbF | Putative galactosyltransferase | 26/51 (aa 239–363) | L41518 | |||
| wcfJ | 32.8 | 254 | Escherichia coli wcaE | Putative glycosyltransferase | 27/46 (aa 1–211) | P71239 |
| Streptococcus pneumoniae cps14J | β-1,4-galactosyltransferase | 29/52 (aa 3–113) | X85787 | |||
| wcfK | 39.9 | 339 | Pseudomonas aeruginosa wbpK | Putative dehydratase | 25/43 (aa 2–271) | U50396 |
| Escherichia coli galE | UDP-galactose 4-epimerase | 22/39 (aa 1–266) | None given | |||
| wcfL | 37.4 | 318 | Escherichia coli rfe | Undecaprenyl-phosphate N-acetylglucosaminyltransferase | 23/39 (aa 94–302) | M76129 |
| orf5 | 44.2 | 504 | None | |||
| orf6 | 45.0 | 214 | None | |||
| orf7 | 41.4 | 687 | Vibrio cholerae irgA | Receptor for ferric vibriobactin | 22/39 (aa 41–681) | P27772 |
| Salmonella typhimurium btuB | Vitamin B12/E colicin receptor | 25/40 (aa 41–686) | P37409 | |||
| hgpT | 41.2 | 178 | Salmonella typhimurium hprt | Hypoxanthine-guanine phosphoribosyl transferase | 35/58 (aa 13–171) | AF008931 |
| adk | 40.0 | 1–55 | Escherichia coli adk | Adenylate kinase | 61/83 (aa 1–55) | P05082 |
orf1 and adk are partial sequences.
The identity and similarity are based on the stated amino acid region (in parentheses) of the wcf product.
These 16 ORFs are all transcribed from the same DNA strand and are tightly clustered. A 60-bp sequence containing a perfect 23-bp inverted repeat is present 82 bp downstream of the stop codon of the last gene of the region, wcfL, and likely terminates transcription. These genetic characteristics suggest that this region is an operon, as is typical of other bacterial polysaccharide biosynthesis loci (8, 10, 44).
The G+C content of the B. fragilis chromosome has been reported to be 41 to 44% (22). This range is consistent with DNA flanking the wcf region (Fig. 1). The wcf locus, as is typical of regions involved in polysaccharide biosynthesis, is comparatively A+T rich, with G+C content values for the coding regions ranging from 26.9 (wzy) to 39.9% (wcfF) and averaging 33.2%. Moreover, the G+C content of wcf is variable and clusters into three regions: a central portion with genes of the lowest G+C content surrounded on both sides by genes of higher G+C content (Fig. 1).
Phenotype conferred by the wcf locus.
Because the transposon insertion of mutant 2-42 was in strain 638R rather than 9343, and because MAb 4D5 does not react with 9343, no phenotype was ascribed to the wcf locus. Therefore, to determine which polysaccharide is synthesized by this region, 10.5 kb of wcf were deleted from the 9343 chromosome, removing the last 10 genes of the region (Fig. 1). The resulting strain, 9343ΔwcfD-L, was then tested for phenotype. MAbs that react with 9343 PS A are available; however, no MAbs that are specific to 9343 PS B are available. The MAb G9, which was previously shown to react with PS B (33), also cross-reacts slightly with purified PS A. The PS A-specific MAb CE3 (34) reacted with 9343ΔwcfD-L (data not shown). Given this result, as well as the many studies showing that 9343 does not express an O antigen and is therefore a rough strain (24, 57), it seemed most likely that this region synthesizes PS B.
To test this assumption, two experiments were performed. In the first, polyclonal antiserum to the wild-type organism was adsorbed with 9343ΔwcfD-L so that the remaining antibodies in the adsorbed serum were specific to the molecule encoded by the wcf locus. In Western blot analysis, these antibodies recognized a high-molecular-weight molecule of the wild-type strain (Fig. 2). To prove that 9343ΔwcfD-L was devoid of PS B, total capsular polysaccharide was isolated from a 16-liter broth culture of the mutant strain. The capsular composition of wild-type 9343 is largely PS B, with a PS B/PS A molar ratio of 3:1 (56). Approximately 99% of the polysaccharide isolated from 9343ΔwcfD-L was composed of PS A, as determined by nuclear magnetic resonance spectroscopy. The remaining 1% (non-PS A material) was unable to prevent binding of PS B-enriched antiserum to a PS B-coated plate in a competitive ELISA (data not shown), demonstrating that wcf contains genes necessary for the synthesis of PS B.
FIG. 2.
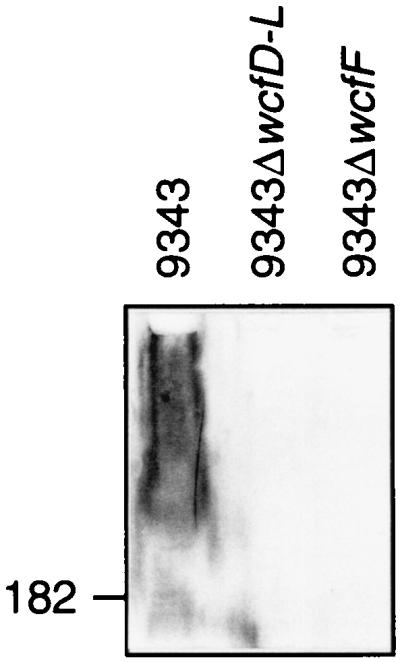
Western immunoblot of cell lysates of B. fragilis 9343 and mutants probed with 9343ΔwcfD-L-adsorbed antiserum. The location of the 182-kDa marker is shown.
Abscess induction by PS B mutant.
To test the effect of loss of PS B on the ability of 9343 to cause abscesses in the animal model, a second mutant was created (9343ΔwcfF) that is mutated in only one gene, wcfF. This mutant was created to ensure that only PS B was lost from the mutant strain. The 9343ΔwcfD-L mutant was lacking wcfH, a gene whose product may be involved in synthesis of the free amino group of PS A. In addition, orf5 was mutated in the large deletion strain, and the product of this gene is undefined. WcfF is very similar to dehydrogenases, which would be necessary for the synthesis of PS B but not PS A. The 9343ΔwcfD-L-adsorbed antiserum is unable to react with 9343ΔwcfF (Fig. 2), demonstrating that the 9343ΔwcfF mutant contains no immunoreactive molecule compared to 9343ΔwcfD-L, which has been shown to be devoid of PS B. When 9343ΔwcfF and the wild type were compared in the animal model, the ability of the mutant to cause abscesses was not attenuated (Table 2). The AD50s were calculated to be 3.9 × 105 for the wild type and 3.5 × 105 for the mutant.
TABLE 2.
Ability of B. fragilis 9343 and PS B mutant to induce abscessesa
| Challenge dose | No. of animals with abscesses/total no. challenged
|
|
|---|---|---|
| 9343 | 9343 ΔwcfF | |
| 108 | 14/14 | 12/15 |
| 107 | 13/17 | 13/17 |
| 106 | 9/17 | 10/17 |
| 105 | 14/27 | 14/25 |
| 104 | 0/10 | 0/10 |
One of 10 rats developed abscesses when challenged with sterile cecal contents alone.
DISCUSSION
The importance of the capsular polysaccharides of B. fragilis in the formation of abscesses has been recognized for decades (31). The two capsular polysaccharides of the prototype strain NCTC 9343 have been purified, the structures have been determined (56), and the charge motif necessary for abscess formation has been well studied (54, 55). The host response to these polysaccharides leading to the formation of abscesses is beginning to be understood at the molecular level (14). Despite these advances, this is the first description of the cloning and sequencing of a B. fragilis capsular polysaccharide biosynthesis locus.
We have shown that the wcf region is necessary for the synthesis of PS B. To determine the contribution of PS B to abscess formation in the natural context of the bacterial cell surface, a defined mutation that abolished the synthesis of PS B was made. Animal studies showed that this mutant was able to cause abscesses in the animal model at the same level as the wild type. This result is not surprising, considering that each of the B. fragilis 9343 capsular polysaccharides has been demonstrated to be a potent abscess-inducing polysaccharide. In the rat intra-abdominal abscess model, the AD50 of purified PS A is 0.67 μg, in contrast to that of purified PS B (AD50 = 25 μg) or capsular polysaccharide complex (AD50 = 22 μg). Although PS B is not necessary for abscess formation when PS A is present, it is likely that synthesis of either PS A or PS B alone is sufficient to cause abscesses, although a PS A mutant may be somewhat attenuated. It is possible that abrogation of both polysaccharides will be necessary to completely attenuate abscess formation by B. fragilis.
The wcf locus is typical of other bacterial polysaccharide biosynthesis loci in regard to gene composition, G+C content, and genetic organization. Putative functions for some of the gene products encoded by the region are assigned based on similarity to other known products.
Genes upstream and downstream of wcf.
It is unclear whether either of the two small ORFs upstream of rmlA (orf3 and orf4) is involved in the synthesis of PS B. These ORFs exhibit no significant homology to other sequences, and they are relatively small. The DNA upstream of orf3 is intergenic, with no ORF of significant size in either direction for 892 bp. Upstream of this gap is a 636-bp ORF that is similar over its central portion to S-adenosyl-sterol-C-methyltransferases from plant species (6) and to methyltransferases involved in menaquinone biosynthesis in bacteria (25).
Downstream of wcfL is a 102-bp gap before the end of an unidentified ORF transcribed from the opposite DNA strand. This ORF (orf5) is the final gene in what may be an operon composed of three genes. The most upstream gene of this region (orf7) is the only ORF of the three that encodes a product similar to other proteins in the database. ORF7 is similar to a family of TonB-dependent outer membrane proteins that are receptors for a variety of molecules, including ferric vibriobactins (15), ferric enterobactins, vitamin B12, many types of colicins, and some bacteriophages (18, 29).
rml genes.
The first two genes of the wcf locus, rmlA and rmlC, are easily recognized by the high degree of similarity of their predicted products to other glucose-1-phosphate thymidyltransferase (RmlA) and dTDP-4-keto-6-deoxy-d-glucose 3,5 epimerase (RmlC) protein sequences (Table 3). The products encoded by rmlA and rmlC may not be involved in the synthesis of PS B. These highly conserved proteins are usually described as rhamnose biosynthesis enzymes, but neither of the structurally characterized B. fragilis 9343 capsular polysaccharides contains rhamnose as a substituent component. Homologues of some or all of the four rml genes (rmlA, -B, -C, and -D) are frequently found at the margins of polysaccharide biosynthesis loci.
Gene products involved in the formation of nucleotide sugar precursors (WcfF and WcfK).
wcfF encodes a 425-amino-acid (aa) product that is similar to 6-dehydrogenase enzymes of other bacterial polysaccharide loci (Table 3). The amino-terminal portion of WcfF contains the 28-aa consensus NAD-binding domain found in enzymes of this class (9, 59). The gene product of wcfF may be responsible for the synthesis of UDP-galacturonic acid (the only uronic acid of PS B) from UDP-galactose.
The predicted product of wcfK is similar to two classes of enzymes: the dTDP-glucose-4,6-dehydratases encoded by rmlB and the galactose-4-epimerases encoded by galE. WcfK contains two NAD-binding domains: one at the amino terminus and a second near the middle of the product. Genes whose products are similar to both GalE and RmlB have been found in other polysaccharide biosynthesis loci (7, 12, 43, 50). Some of these products are proposed to be involved in the synthesis of N-acetylfucosamine from N-acetylgalactosamine (7, 43, 50). If WbfK has similar 6-dehydratase activity, it may convert UDP-d-GlcNAc to UDP-d-QuiNAc.
Although wcf contains 16 genes, only two gene products (excluding RmlA and RmlC) are similar to products involved in nucleotide sugar formation. Gene products responsible for the synthesis of the GDP-l-Fuc and the NDP-l-QuiNAc residues of PS B may not be encoded by wcf. In the biosynthesis of colanic acid of E. coli, the l-fucose residue in the repeating unit has been demonstrated to be derived from mannose-6-phosphate by the products of five genes of the wca locus (4, 52). No such homologues are present in wcf that could account for the formation of GDP-l-Fuc from mannose-6-phosphate.
Sugar transferase genes (wcfB, wcfC, wcfE, wcfG, wcfI, wcfJ, and wcfL).
The predicted product of wcfL is similar to several Rfe proteins, which catalyze the transfer of an N-acetylglucosamine residue to undecaprenylphosphate as the initial step in the synthesis of enterobacterial common antigen and of some E. coli lipopolysaccharide O antigens (41). WcfL displays a hydrophobicity profile indicative of an integral membrane protein. It is likely that WcfL transfers the first monosaccharide to a lipid carrier in the synthesis of B. fragilis PS B.
The products of six genes (wcfB, wcfC, wcfE, wcfG, wcfI, and wcfJ) are similar to various proteins involved in the transfer of monosaccharide constituents in the synthesis of bacterial polysaccharides (Table 3). Hydrophobic cluster analysis (13) of the amino acid sequences of each of these putative glycosyltransferases has demonstrated that each product contains the two strictly conserved aspartic acid residues surrounded by hydrophobic stretches typical of glycosyltransferases (3, 45).
Polymerization and export genes (wzy and wzx).
The characteristics of low G+C content, multiple membrane-spanning domains, and sequence similarity were used to putatively identify the genes encoding Wxy and Wzx within the wcf locus. The eighth gene of the operon, wzy, has a G+C content of 26.9% (the lowest of the region) and encodes a protein of 365 aa. This putative protein is 44% similar to the O-antigen polymerase of Salmonella enterica C1 (26) and is extremely hydrophobic, with nine potential membrane-spanning domains.
The fourth gene is designated wzx. This gene has a G+C content of 28% and encodes a protein of 511 aa. The gene product displays significant similarity (44%) to the flippase of S. enterica C1, whose function has been proven experimentally (28) (Table 3). Wzx of B. fragilis is extremely hydrophobic, displaying 11 potential membrane-spanning regions.
Acetyltransferase genes (wcfA and wcfD).
The gene products of wcfA and wcfD are both similar to galactoside-O-acetyltransferases of the CysE-LacA-NodL family, which acetylate a variety of substrates. A motif has been described for this family of acetyltransferases that has 21 conserved amino acids over a 31-aa stretch (11). WcfA aligns with this consensus sequence with just two mismatches, and WcfD aligns with five mismatches. Although the published structure of PS B does not contain O-acetylated sugars, some lots of purified PS B have been shown to be O acetylated to varying degrees (56a), which may explain the presence of these genes in the wcf locus.
WcfH, a putative deacetylase.
The presence of both positively and negatively charged groups on the repeating-unit structures of PS A and PS B has been shown to be critical to the virulence of the B. fragilis capsular polysaccharides (54). The positively charged free amino group of PS A may be formed by removal of an acetyl group from an N-acetylated precursor sugar by a deacetylase. WcfH is 40% similar over half of the protein to NodB of Rhizobium meliloti. NodB proteins are deacetylases involved in the hydrolysis of the N-acetamido group of N-acetyl-d-glucosamine during the formation of the lipo-chitin-oligosaccharides (21). There is ample precedent for a gene of one polysaccharide biosynthesis locus encoding a product that is involved in the synthesis of a different polysaccharide (3, 17, 23, 58). Due to the rare occurrence of genes encoding deacetylase-like proteins in polysaccharide biosynthesis loci and the importance of the free amino group to the abscess-inducing capabilities of both PS A and PS B, future studies will include analysis of the enzymatic activity of WcfH.
ACKNOWLEDGMENTS
We are grateful to Katharine McQuilkin and Pamela Russell for assistance with polysaccharide purification, Andrea DuBois for assistance with mutant screening, Ron Cisneros and Matthew Lawlor for assistance with the animal model, and Julie McCoy for editorial services.
This work was supported in part by grants AI34073 and AI39576 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amor P A, Whitfield C. Molecular and functional analysis of genes required for expression of group IB K antigens in Escherichia coli: characterization of the his-region containing gene clusters for multiple cell-surface polysaccharides. Mol Microbiol. 1997;26:145–161. doi: 10.1046/j.1365-2958.1997.5631930.x. [DOI] [PubMed] [Google Scholar]
- 4.Andrianipoulos K, Wang L, Reeves P R. Identification of the fucose synthetase gene in the colanic acid gene cluster of Escherichia coli K-12. J Bacteriol. 1998;180:998–1001. doi: 10.1128/jb.180.4.998-1001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bethesda Research Laboratories. BRL pUC host: E. coli DH5 alpha competent cells. Focus. 1986;8:9. [Google Scholar]
- 6.Bouvier-Nave P, Husselstein T, Desprez T, Benveniste P. Identification of cDNAs encoding sterol methyl-transferases involved in the second methylation step of plant sterol biosynthesis. Eur J Biochem. 1997;246:518–529. doi: 10.1111/j.1432-1033.1997.t01-1-00518.x. [DOI] [PubMed] [Google Scholar]
- 7.Burrows L L, Charter D F, Lam J S. Molecular characterization of the Pseudomonas aeruginosa serotype O5 (PAO1) B-band lipopolysaccharide gene cluster. Mol Microbiol. 1996;22:481–495. doi: 10.1046/j.1365-2958.1996.1351503.x. [DOI] [PubMed] [Google Scholar]
- 7a.Conard, L. Drawhca Program. [Online.] http://www.lmcp.jussieu.fr/∼soyer/www_hca/hca-form.html. [November 1998, last date accessed.]
- 8.Chitnis C E, Ohman D E. Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence of an operonic structure. Mol Microbiol. 1993;8:583–590. doi: 10.1111/j.1365-2958.1993.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 9.Comstock L E, Johnson J A, Michalski J M, Morris J G, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty B A, van de Rijn I. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J Biol Chem. 1994;269:169–175. [PubMed] [Google Scholar]
- 11.Downie J A. The nodL gene from Rhizobium leguminosarum is homologous to acetyltransferases encoded by lacA and cysE. Mol Microbiol. 1989;3:1649–1651. doi: 10.1111/j.1365-2958.1989.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 12.Fallarino A, Maurangelos C, Stroeher U H, Manning P A. Identification of additional genes required for O-antigen biosynthesis in Vibrio cholerae O1. J Bacteriol. 1997;179:2147–2153. doi: 10.1128/jb.179.7.2147-2153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaboriaud C, Bissery V, Benchetrit T, Mornon J P. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 1987;224:149–155. doi: 10.1016/0014-5793(87)80439-8. [DOI] [PubMed] [Google Scholar]
- 14.Gibson F C R, Onderdonk A B, Kasper D L, Tzianabos A O. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J Immunol. 1998;160:5000–5006. [PubMed] [Google Scholar]
- 15.Goldberg M B, Boyko S A, Caldrewood S B. Transcriptional regulation by iron of a Vibrio cholerae virulence gene and homology of the gene to the Escherichia coli fur system. J Bacteriol. 1990;172:6863–6870. doi: 10.1128/jb.172.12.6863-6870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorbach S L, Bartlett J G. Anaerobic infections. N Engl J Med. 1974;290:1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- 17.Hammerschmidt S, Birkholz C, Zahringer U, Robertson B D, van Putten J, Ebeling O, Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994;11:885–896. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 18.Heller K, Kadner R J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985;161:904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henikoff S, Henikoff J G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Hoffman, K., and W. Stoffel. TMpred. [Online.] http://ulrec3.unil.ch/software/TMPRED_form.html. [November 1998, last date accessed.]
- 20.Hohn B, Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980;11:291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 21.John M, Rohrig H, Schmidt J, Wienede U, Schell J. Rhizobium Nod B protein involved in nodulation signal synthesis is a chitooligosaccharide deacetylase. Proc Natl Acad Sci USA. 1993;90:625–629. doi: 10.1073/pnas.90.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J L. Taxonomy of the Bacteroides. I. Deoxyribonucleic acid homologies among Bacteroides fragilis and other saccharolytic Bacteroides species. Int J Syst Bacteriol. 1978;28:245–268. [Google Scholar]
- 23.Kao C C, Sequeira L. A gene cluster required for coordinated biosynthesis of lipopolysaccharide and extracellular polysaccharide also affects virulence of Pseudomonas solanacearum. J Bacteriol. 1991;173:7841–7847. doi: 10.1128/jb.173.24.7841-7847.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasper D L, Weintraub A, Lindberg A A, Lonngren J. Capsular polysaccharides and lipopolysaccharides from two Bacteroides fragilis reference strains: chemical and immunochemical characterization. J Bacteriol. 1983;153:991–997. doi: 10.1128/jb.153.2.991-997.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P T, Hsu A Y, Ha H T, Clarke C F. A C-methyltransferase involved in both ubiquinone and menaquinone (vitamin K2) biosynthesis: isolation and identification of the Escherichia coli ubiE gene. J Bacteriol. 1997;179:1748–1754. doi: 10.1128/jb.179.5.1748-1754.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S J, Romano L K, Reeves P R. Sequence and structural analysis of the rfb (O antigen) gene cluster from a group C1 Salmonella enterica strain. J Gen Microbiol. 1992;138:1843–1855. doi: 10.1099/00221287-138-9-1843. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg A A, Weintraub A, Kasper D L, Lonngren J. Virulence factors in infections with Bacteroides fragilis: isolation and characterization of capsular polysaccharide and lipopolysaccharide. Scand J Infect Dis. 1982;35:45–52. [PubMed] [Google Scholar]
- 28.Liu D L, Cole R A, Reeves P R. An O-antigen processing function of Wzx (RfbX): a promising candidate for O-unit flippase. J Bacteriol. 1996;178:2102–2107. doi: 10.1128/jb.178.7.2102-2107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundrigan M D, Kadner R J. Nucleotide sequence of the gene for the ferrienterochelin receptor fepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986;261:10797–10801. [PubMed] [Google Scholar]
- 30.Onderdonk A B, Bartlett J G, Louie T, Sullivan-Seigler N, Gorbach S L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976;13:22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onderdonk A B, Kasper D L, Cisneros R L, Bartlett J G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977;136:82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- 32.Onderdonk A B, Weinstein W M, Sullivan N M, Bartlett J G, Gorbach S L. Experimental intra-abdominal abscesses in rats: quantitative bacteriology of infected animals. Infect Immun. 1974;10:1256–1259. doi: 10.1128/iai.10.6.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantosti A, Colangeli R, Tzianabos A O, Kasper D L. Monoclonal antibodies to detect capsular diversity among Bacteroides fragilis isolates. J Clin Microbiol. 1995;33:2647–2652. doi: 10.1128/jcm.33.10.2647-2652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantosti A, Tzianabos A O, Onderdonk A B, Kasper D L. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect Immun. 1991;59:2075–2082. doi: 10.1128/iai.59.6.2075-2082.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paoletti L C, Kasper D L, Michon F, DiFabio J, Holme K, Jennings H J, Wessels M R. An oligosaccharide-tetanus toxoid conjugate vaccine against type III group B streptococcus. J Biol Chem. 1990;265:18278–18283. [PubMed] [Google Scholar]
- 36.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polk B F, Kasper D L. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med. 1977;86:569–571. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- 38.Privitera G, Dublanchet A, Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979;139:97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- 39.Reed L J, Muench H. A simple method for determining 50 per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 40.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R H, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 41.Rick P D, Silver R P. Enterobacterial common antigen and capsular polysaccharides. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 104–122. [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 44.Sau S, Sun J, Lee C Y. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J Bacteriol. 1997;179:1614–1621. doi: 10.1128/jb.179.5.1614-1621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxena I M, Brown R M, Jr, Fevre M, Geremia R A, Henrissat B. Multidomain architecture of beta-glycosyl transferases: implications for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shapiro M E, Kasper D L, Zaleznik D F, Spriggs S, Onderdonk A B, Finberg R W. Cellular control of abscess formation: role of T cells in the regulation of abscesses formed in response to Bacteroides fragilis. J Immunol. 1986;137:341–346. [PubMed] [Google Scholar]
- 47.Shoemaker N B, Getty C, Gardner J F, Salyers A A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986;165:929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoemaker N B, Getty C, Guthrie E P, Salyers A A. Two Bacteroides plasmids, pBFTM10 and pB8-51, contain transfer regions that are recognized by broad-host-range IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986;166:959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoemaker N B, Getty C E, Salyers A A, Gardner J F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985;162:626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skurnik M, Venho R, Toivanen P, Al-Hendy A. A novel locus of Yersinia enterocolitica serotype O:3 involved in lipopolysaccharide outer core biosynthesis. Mol Microbiol. 1995;17:575–594. doi: 10.1111/j.1365-2958.1995.mmi_17030575.x. [DOI] [PubMed] [Google Scholar]
- 51.Stevens A M, Shoemaker N B, Salyers A A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990;172:4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevenson G, Andriaopoulos K, Hobbs M, Reeves P R. Organization of the Escherichia coli K-12 gene cluster responsible for production of the extracellular polysaccharide colonic acid. J Bacteriol. 1996;178:4885–4893. doi: 10.1128/jb.178.16.4885-4893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzianabos A O, Kasper D L, Cisneros R L, Smith R S, Onderdonk A B. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J Clin Investig. 1995;96:2727–2731. doi: 10.1172/JCI118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 55.Tzianabos A O, Onderdonk A B, Smith R S, Kasper D L. Structure-function relationships for polysaccharide-induced intra-abdominal abscesses. Infect Immun. 1994;62:3590–3593. doi: 10.1128/iai.62.8.3590-3593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tzianabos A O, Pantosti A, Baumann H, Brisson J R, Jennings H J, Kasper D L. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]
- 56a.Tzianabos, A. O. Unpublished data.
- 57.Weintraub A, Larsson B E, Lindberg A A. Chemical and immunochemical analysis of Bacteroides fragilis lipopolysaccharides. Infect Immun. 1985;49:197–201. doi: 10.1128/iai.49.1.197-201.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitfield C, Valvano M A. Biosynthesis and expression of cell-surface polysaccharides in Gram-negative bacteria. Adv Microb Physiol. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 59.Wyk P, Reeves P. Identification and sequence of the gene for abequose synthase, which confers antigenic specificity on group B salmonellae: homology with galactose epimerase. J Bacteriol. 1989;171:5687–5693. doi: 10.1128/jb.171.10.5687-5693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]