Abstract
Background
Vascular dementia (VaD) is the second most common cause of dementia globally and is associated with a significant economic and social burden. Diet could represent an important tractable risk factor for VaD. We synthesised current evidence on associations between consumption of specific foods or dietary patterns and VaD risk.
Methods
Five databases were searched from inception to January 2024 for prospective cohort studies exploring associations between individual foods or dietary patterns and incident VaD.
Results
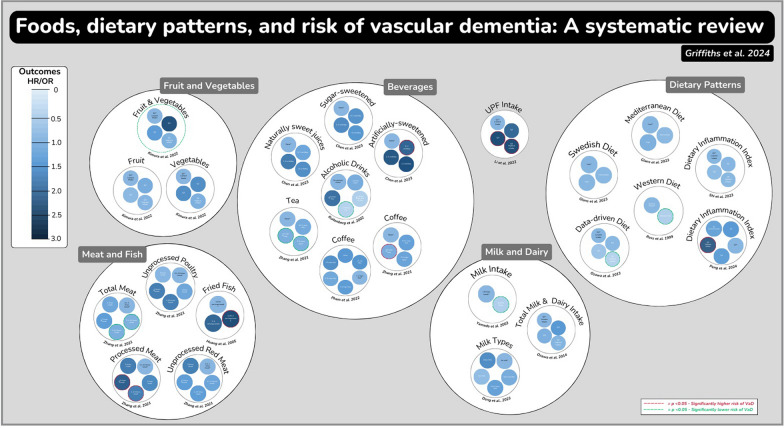
Sixteen studies were included. Compared with low intake reference groups, higher fruit and vegetable intake, moderate alcoholic drink intake (1–3 drinks/day), higher tea and coffee intake, and following a plant-based dietary pattern were associated with lower VaD risk. Conversely, moderate fried fish intake (0.25–2 servings/week), higher ultra-processed food intake (especially intake of sweetened beverages) and higher processed meat intake (≥ 2 servings/week) were associated with increased VaD risk. Inconsistent findings were observed for other dietary exposures.
Discussion
A healthy diet could lower VaD risk. However, evidence is characterised by a limited number of studies for specific dietary exposures. Further research is needed to inform personalised and population-based approaches to lower VaD risk.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-024-00880-2.
Keywords: Food, Nutrition, Dietary patterns, Vascular dementia, Vascular cognitive impairment
Background
Vascular dementia (VaD) is the second most common cause of dementia worldwide, accounting for ~ 15 to 20% of all dementia cases [1, 2], although estimates of prevalence are complicated by the fact that VaD frequently co-occurs alongside other causes of neurodegeneration (e.g., Alzheimer’s disease-related proteinopathies) [1, 2]. VaD results from vascular brain injury due to brain ischemia or haemorrhage [3], and has a considerable economic cost which is estimated to be greater than other dementias such as Alzheimer’s disease [4].
Identifying effective strategies to prevent or delay the onset of VaD is a major research and public health priority [5]. Lifestyle modifications have the potential to reduce the incidence of VaD by addressing underlying vascular risk factors such as hypertension and diabetes [6], and consumption of a healthy diet could represent an important lifestyle strategy to help lower VaD risk. However, there is currently limited knowledge or consensus on which dietary approaches are effective for preventing VaD. Over 10 years ago, Perez et al. [7] systematically reviewed the evidence on nutrition and VaD risk and identified certain nutritional factors which were associated with higher (lower levels of folate and B12, alongside higher fried fish intake) or lower (vitamin E and C, alongside fatty fish intake) risk of VaD. This review identified few studies which explored the impact of foods or healthy dietary patterns on VaD risk. However, exploring the impact of foods and dietary patterns on VaD risk is particularly important (compared with studying the impact of isolated nutrients/dietary compounds), as it accounts for the potentially cumulative and synergistic effects of different nutrients available within individual foods and across different foods within a dietary pattern [8–10]. Moreover, studying the impact of foods or dietary patterns better reflects ‘real world’ eating habits and allows for the development of clear and actionable dietary recommendations to lower VaD risk which can be disseminated to the public (e.g., via public health campaigns) and patient groups (e.g., through interactions with healthcare professionals) [11]. The review by Perez et al. [7] was also limited because an assessment of study quality/risk of bias was not conducted as per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines [12]. As such, the robustness of synthesised evidence is unclear. Included studies also comprised a mix of cross-sectional, retrospective and prospective studies (with the former study designs more prone to risk of reverse causality). These limitations, alongside the emergence of considerable new evidence in the past 10 years (e.g., [13–17]), provide the rationale for a new systematic review of the extant literature. Therefore, the aim of this study was to undertake a systematic review of prospective cohort studies exploring the associations between consumption of foods and dietary patterns with incidence of VaD.
Methods
This systematic review followed the PRISMA guidelines [12], and was pre-registered on PROSPERO (CRD42024504255).
Literature search
Literature searches were conducted in MEDLINE, PsycINFO and CINAHL via EBSCOhost, as well as The Cochrane Library and PubMed. All databases were searched from inception to 24th January 2024, and no language restrictions were applied during searches. Searches were conducted using a combination of synonyms, and relevant MeSH terms for vascular dementia, risk, diet and study design. Search terms can be found in the supplementary material. Search results were exported to EndNote where automated and manual deduplication was performed. Search results where then exported to Covidence, an online review platform, where further automated deduplication was performed prior to the commencement of screening (see ‘study selection’ below). Reference lists of eligible articles, alongside recently published review articles, were also searched for potentially relevant literature.
Study selection
Titles and abstracts of the retrieved studies were independently screened by two researchers (AG and OMS) to evaluate eligibility for inclusion in the review. Potential studies that could not be excluded from the review following the assessment of title and abstract were carried over to the full-text stage for further appraisal. Full texts of potentially relevant studies were assessed for eligibility by two investigators (AG and OMS). An additional reviewer (JM) was consulted if there was any disagreement between the two reviewers when appraising both the titles and abstracts and the full texts of potentially relevant papers. Studies were required to meet the following PECOS (Participant, Exposure, Comparator, Outcomes, Study Design) criteria to be eligible for inclusion in this review:
Participants: Adult participants aged ≥ 18 years who were free from dementia at study baseline.
Exposure: Self-reported intake of individual foods or dietary patterns. Data for individual foods were considered separately rather than grouping based around e.g., similarities in macronutrient/micronutrient content. There were no restrictions on dietary assessment tool (e.g., 24-h recall, food frequency questionnaire [FFQ], food diary). Studies focused on the intake of nutrients or consumption of specific dietary supplements were not eligible for inclusion, nor were studies reporting biomarker measures of food intake (e.g., carotenoids for vegetables or metabolic fingerprints), due to the challenges of distinguishing between food and supplement-based intake of measured metabolites. Studies reporting alcohol as an exposure were eligible for inclusion if they focused on intake of specific alcoholic drinks (e.g., wine, beer, spirits) and not if alcohol intake was considered in total gram ethanol intake (which we considered to be a nutrient).
Comparator: Lower intake reference groups (e.g., bottom tertile, quartile, quintile), as reported by the study authors, were used as the comparator. Where relevant, we also included studies exploring associations per unit (e.g., 1 standard deviation [SD], 1 point) increment in dietary intake of a specific food or dietary pattern score with VaD risk.
Outcomes: We included all relevant papers focusing on incident VaD, vascular cognitive impairment (VCI) and vascular cognitive impairment no dementia (VCIND). Studies reporting specifically on other dementia sub-types (e.g., Alzheimer’s, fronto-temporal or Lewy bodies dementia) were not eligible for inclusion. Studies providing data on all-cause dementia were included where information on VaD, VCI or VCIND incidence was presented separately. A clinical diagnosis was required and studies with self- or informant-reported diagnoses were excluded. No exclusions were applied with regarded to age of dementia onset.
Study design: Studies were required to have a prospective cohort design. There was no minimum duration of follow-up. Other types of study design (e.g., randomised controlled trials [due to a perceived lack of studies focusing on VaD incidence], cross-sectional studies, case-report studies, retrospective studies) were not eligible.
Data extraction and evidence synthesis
Data were extracted by one reviewer (OMS) and checked by a second reviewer (AG) for accuracy. Extracted data included study authors, publication year, study cohort, setting, participant characteristics (n, sex distribution, ethnicity and age), follow-up duration, number of VaD cases, VaD diagnosis method, details of the dietary exposure and assessment method, study outcomes (Hazard Ratio [HR] or odds ratio [OR] and associated confidence intervals [CIs]). Data are reported for the maximally adjusted statistical model reported by the authors to minimise risk of confounding. Where data were available and appropriate, sub-group analyses (e.g., stratified by genetic risk) are presented. A narrative synthesis of data was conducted due to the small number of studies identified for each dietary exposure. We specified a priori in our protocol that a minimum of 5 studies would be required for a specific dietary exposure to undertake meta-analysis (see PROSPERO: CRD42024504255).
Risk of bias assessment
Risk of bias was evaluated using the ROBINS-E tool [18]. This tool evaluates bias related to confounding, exposure measurement, participant selection, post-exposure interventions, missing data, outcome measurement, and selection of the reported result. An overall risk of bias was determined for each study, as well as a predicted direction of bias (if relevant), and whether this was likely to threaten conclusions of a given study. Signalling questions detailed in the Cochrane risk of bias tool were used to classify each domain as well as the overall risk of bias of each study, however further information on the judgement process in relation to our research question is available in supplementary material 2.
Results
Overview
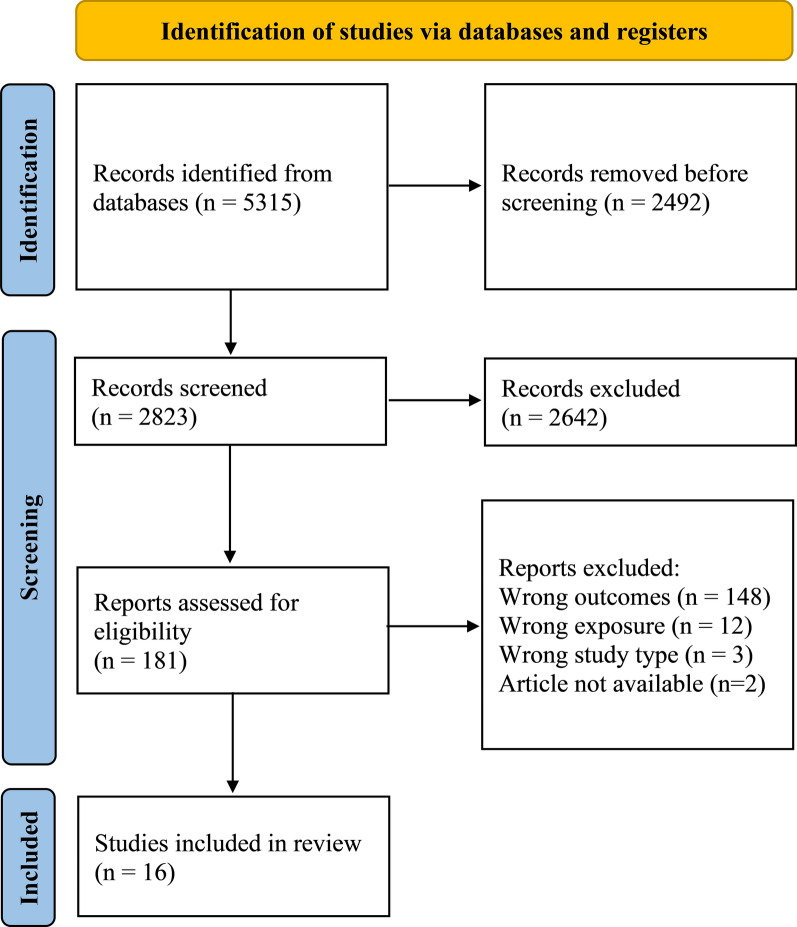
A total of 5315 articles were identified through electronic database searches. 2058 duplicates were automatically removed and a further 434 duplicates were manually identified and removed, after which 2823 titles and abstracts were screened for eligibility. 181 full texts were then retrieved for further appraisal, 16 of which were deemed to be eligible for inclusion in this review (Fig. 1, Supplementary Material 3). All eligible articles focused on risk of VaD. No studies focusing on risk of VCI or VCIND were identified.
Fig. 1.
PRISMA flow chart outlining the study selection process
Study characteristics
The characteristics of eligible studies are outlined in Table 1. The sum of sample sizes across studies was 2,242,736 participants, including 4616 VaD cases during a median follow-up of 11.4 years (range: 5.4 to 25.0 years). Half of the studies were conducted using the UK Biobank cohort (UK, 8 studies) [13–17, 19–21]. Other analytic cohorts included the Hisayama cohort (Japan, 3 studies) [22–24], Radiation Effects Research Foundation Adult Health Study (Japan, 1 study) [25], Cardiovascular Health Cognition Study (United States, 1 study) [26], Honolulu-Asia Aging Study (United States, 1 study) [27], Rotterdam study (Netherlands, 1 study) [28] and the Malmo diet and Cancer study (Sweden, 1 study) [29].
Table 1.
Summary of prospective cohort studies exploring associations between intake of individual foods and risk of VaD
| Author | Cohort and setting | Participant characteristics | Follow up duration | VaD cases | VaD diagnosis method | Dietary assessment method | Outcomes |
|---|---|---|---|---|---|---|---|
| Fruit and vegetables | |||||||
| Kimura et al. [24] | The Hisayama Study (Japan) | 1071 (452 men and 619 women) Japanese dementia-free participants aged 69 (6) years | 24 years | 144 | Physician and psychiatrist evaluation. DSM criteria used to diagnose dementia and the criteria of the NINDS was used to diagnose VaD | 70-item semiquantitative food frequency questionnaire |
Fruit: Quartile 1 (lowest): Reference group Quartile 2: 0.79 [0.51, 1.24] Quartile 3: 0.63 [0.39, 1.02] Quartile 4 (highest): 0.68 [0.43, 1.09] P-trend: 0.07 |
|
Vegetables: Quartile 1 (lowest): Reference group Quartile 2: 1.16 [0.73, 1.84] Quartile 3: 1.16 [0.72, 1.86] Quartile 4 (highest): 0.73 [0.44, 1.21] P-trend: 0.24 | |||||||
|
Combined fruit & Vegetables: Quartile 1 (lowest): Reference group Quartile 2: 1.94 (1.23, 3.07)* Quartile 3: 0.99 (0.60, 1.64) Quartile 4 (highest): 0.73 (0.42, 1.26) P-trend: 0.047* | |||||||
| Fish | |||||||
| Huang et al. [26] | Cardiovascular Health Cognition Study (United States) | 2233 (927 men and 1306 women) predominantly white dementia-free participants aged 72 years | 5.4 years | 50 | Committee of neurologists/psychiatrists using DSM criteria for dementia and the ADDTC criteria were used for VaD | The National Cancer Institute 99-item food frequency questionnaire |
Fried fish: < 0.25 servings/week: Reference group 0.25–2 servings/week: 2.6 [1.39,4.96]* 2–4 servings/week: 1.68 [0.74, 3.84] |
|
Fatty fish: No significant associations (data not reported) | |||||||
| Ultra-processed foods | |||||||
| Li et al. [15] | UK Biobank (UK) | 72,083 (33,940 men, 38,143 women) predominantly white dementia free individuals aged 62 (4) years | Median 10 years | 119 | Linkage to electronic healthcare records and use of ICD codes to identify dementia sub-types | 24-h dietary assessment tool (Oxford WebQ) |
UPF intake and VaD risk: Quartile 1 (lowest): Reference group Quartile 2: 1.66 [0.90, 3.05] Quartile 3: 2.18 [1.22, 3.92]* Quartile 4 (highest): 2.19 [1.21, 3.96]* Per 10% increase in UPF intake: Continuous: 1.28 [1.06, 1.55]* |
|
Replacing UPF intake with unprocessed/ minimally processed foods: 5% replacement: 0.88 [0.80, 0.97]* 10% replacement: 0.78 [0.65, 0.94]* 20% replacement: 0.61 [0.42, 0.89]* | |||||||
|
Individual UPFs and VaD risk (continuous HR per SD increase in intake): Beverages: 1.24 [1.08, 1.43]* Dairy: 1.06 [0.89, 1.27] Fruit/vegetables: 1.00 [0.84, 1.19] Meat/fish/eggs: 0.95 [0.78, 1.14] Starchy foods/cereals: 0.92 [0.75, 1.14] Sugary products:1.11 [0.92, 1.34] Fats and sauces: 1.01 [0.83, 1.22] Salty snacks: 1.12 [0.95, 1.32] | |||||||
| Sweet beverages | |||||||
| Chen et al. [19] | UK Biobank (UK) | 187,994 (103,495 men, 84,499 women) predominantly white dementia-free individuals aged 56 (8) years | Mean 9.5 years | 165 | Linkage to electronic healthcare records and use of ICD codes to identify dementia sub-types | 24-h dietary assessment tool (Oxford WebQ) |
Sugar-sweetened beverages and VaD None: Reference group 0 ~ 1 unit/d: 1.12 [0.76, 1.64] 1 ~ 2 unit/d: 1.20 [0.66, 2.18] > 2 unit/d: 1.05 [0.43, 2.58] |
|
Artificially-sweetened beverages and VaD: None: Reference group 0 ~ 1 unit/d: 1.69 [1.10, 2.61]* 1 ~ 2 unit/d: 1.85 [0.97, 3.54] > 2 unit/d: 1.90 [0.77, 4.68] | |||||||
|
Naturally sweet juices and VaD: None: Reference group 0 ~ 1 unit/d: 0.87 [0.61, 1.22] 1 ~ 2 unit/d: 1.40 [0.87, 2.26] > 2 unit/d: 1.16 [0.47, 2.90] | |||||||
| Meat | |||||||
| Zhang et al. [16] | UK Biobank (UK) | 493,888 (224,691 men and 269,197 women) predominantly white dementia-free individuals aged 57 (8) years | 8.0 (1.1) years | 490 | Linkage to electronic healthcare records and use of ICD codes to identify dementia sub-types | 47-item touchscreen food frequency questionnaire |
Processed meat: 0 times/week: 1.38 [0.92, 2.07] 0.1–0.9 times/week: Reference 1 time/week: 1.22 [0.95, 1.57] 2.0–4.9 times/week: 1.35 [1.05, 1.74]* ≥ 5.0 times/week: 1.76 [1.17, 2.65]* 25 g per day increments: 1.30 [0.90, 1.88] P-trend: 0.162 Unprocessed poultry: 0 times/week: 0.67 [0.38, 1.19] 0.1–0.9 times/week: Reference 1 time/week: 0.85 [0.64, 1.12] 2.0–4.9 times/week: 0.89 [0.68, 1.17] ≥ 5.0 times/week: 1.37 [0.85, 2.21] 25 g per day increments: 1.06 [0.83, 1.35] P-trend: 0.656 |
|
Unprocessed red meat: 0 times/week: 1.35 [0.80, 2.28] 0.1–1 times/week: Reference 1.1–1.9 times/week: 0.88 [0.64, 1.20] 2.0–2.9 times/week: 0.83 [0.60, 1.15] ≥ 3.0 times/week: 0.97 [0.71, 1.32] 50 g per day increments: 0.72 [0.48, 1.08] P-trend: 0.119 | |||||||
|
Total meat: 0 times/week: 0.85 [0.47, 1.54] 0.1–3.0 times/week: Reference 3.1–4.9 times/week: 0.73 [0.54, 0.98]* 5.0–6.9 times/week: 0.76 [0.58, 0.99]* ≥ 7.0 times/week: 0.97 [0.75, 1.26] 50 g per day increments: 1.01 [0.81, 1.26] P-trend: 0.936 | |||||||
|
Interaction between APOE genotype and meat intake on VaD risk: Processed meat * APOE genotype: p = 0.198 Unprocessed poltry * APOE genotype: p = 0.617 Unprocessed red meat * APOE genotype: p = 0.563 Total meat * APOE genotype: p = 0.263 | |||||||
| Milk and dairy | |||||||
| Deng et al. [20] | UK Biobank (UK) | 307,271 (147,641 men, 159,630 women) predominantly white dementia-free individuals aged 56 (8) years | Median (IQR): 12.3 (11.6–13.1) years | 834 | Linkage to electronic healthcare records and use of ICD codes to identify dementia sub-types | 47-item touchscreen food frequency questionnaire |
Comparison against no milk reference group: No milk: Reference group Skimmed milk: 0.79 [0.56, 1.13] Full cream milk: 0.92 [0.60, 1.40] Soy milk: 0.59 [0.34, 1.02] Other milk: 1.17 [0.65, 2.12] |
|
Soy milk versus other milk varieties: Versus all non-soy milk: 0.72 [0.46, 1.12] Versus full cream milk: 0.65 [0.40, 1.08] Versus skimmed milk: 0.74 [0.47, 1.14] Versus other milk: 0.51 [0.26, 0.97]* | |||||||
| Ozawa et al. [22] | The Hisayama Study (Japan) | 1,081 (457 men, 624 women) Japanese dementia-free individuals aged 69 (6) years | 17 years | 98 | Physician and psychiatrist evaluation using DSM criteria to define dementia and the NINDS criteria were used to diagnoses VaD |
70-item semiquantitative food frequency questionnaire |
Total milk and dairy intake: Quartile 1 (lowest): Reference group Quartile 2: 1.02 [0.59–1.77] Quartile 3: 0.74 [0.42–1.33] Quartile 4 (highest): 0.69 [0.37–1.29] |
| Yamada et al. [25]# | Radiation Effects Research Foundation Adult Health Study (Hiroshima, Japan) | 1774 (475 men, 1299 women) Japanese dementia-free participants age not specified | Range: 25–30 years | 38 |
Neurological examination. Clinical diagnosis of dementia and its subtypes was carried out using DSM criteria |
Self-administered basic dietary questionnaire examining consumption of milk, fish, meat and tofu |
Milk intake and VaD risk: < 4 times/week: Reference group Almost daily: 0.35 [0.14, 0.77]*# |
|
Fish, meat, tofu: Not significantly associated with VaD risk (data not reported) | |||||||
| Alcoholic drinks | |||||||
| Ruitenberg et al. [28] | Rotterdam study (Netherlands) | 5395 (2212 men and 3183 women) Dutch dementia-free participants aged 55 years or above | 6.0 years | 29 | Neurologist examination during health checks and additional cases detected via linkage to computerised medical records | 170-item food frequency questionnaire |
Total number of alcoholic drinks (wine, beer, liquor, and fortified wine) and VaD: No alcoholic drinks: Reference group < 1 drink/week: 0.79 [0.30, 2.08] ≥ 1 drinks/week but < 1 drink/day: 0.36 [0.12, 1.08] 1–3 drinks/day: 0.30 [0.10, 0.92]* ≥ 4 drinks/day: 1.53 [0.31, 7.56] |
|
Men only: No alcoholic drinks: Reference group < 1 drink/week: 1.19 [0.30, 4.80] ≥ 1 drinks/week but < 1 drink/day: 0.33 [0.07, 1.46] 1–3 drinks/day: 0.29 [0.07, 1.18] ≥ 4 drinks/day: 1.17 [0.31, 9.51] | |||||||
|
Women only: No alcoholic drinks: Reference group < 1 drink/week: 0.54 [0.13, 2.16] ≥ 1 drinks/week but < 1 drink/day: 0.46 [0.09, 2/29] 1–3 drinks/day: 0.40 [0.05, 3.34] ≥ 4 drinks/day: N/A [no women in this category developed dementia] | |||||||
|
APOE4 carriers: No alcoholic drinks: Reference group < 1 drink/week: 0.78 [0.19, 3.27] ≥ 1 drinks/week but < 1 drink/day: 0.26 [0.05, 1.37] 1–3 drinks/day: 0.26 [0.06, 1.17] ≥ 4 drinks/day: N/A [limited number of cases to conduct stratified analyses] | |||||||
|
APOE4 non-carriers: No alcoholic drinks: Reference group < 1 drink/week: 0.91 [0.24, 3.43] ≥ 1 drinks/week but < 1 drink/day: 0.55 [0.13, 2.42] 1–3 drinks/day: 0.17 [0.02, 1.55] ≥ 4 drinks/day: N/A [limited number of cases to conduct stratified analyses] | |||||||
| Tea and coffee | |||||||
| Pham et al. [13]# | UK Biobank (UK) | 398,646 (182,370 men and 216,276 women) predominantly white dementia-free participants aged 37–73 years | ~ 7.3 to 11.9 years | 451 | Linkage to electronic healthcare records and death registries and use of ICD codes to identify dementia sub-types | 47-item touchscreen food frequency questionnaire |
Cups of coffee and VaD risk: None: 1.04 [0.84, 1.29] Decaf: 1.09 [0.86, 1.38] < 1 cup/day: 0.85 [0.57, 1.24] 1–2 cups/day: Reference group 3–4 cups/day: 0.98 [0.76, 1.26] 5–6 cups/day: 0.83 [0.57, 1.22] > 6 cups/day: 1.14 [0.70, 1.86] |
| Zhang et al. [17] | UK Biobank (UK) | 365,682 (167,060 men and 198,622 women) predominantly white dementia-free participants aged 60 (5) years | Median 11.4 years | 1223 | Linkage to electronic healthcare records and death registries and use of ICD codes to identify dementia sub-types | 47-item touchscreen food frequency questionnaire |
Cups of coffee and VaD risk: None: Reference group 0.5–1 cups/day: 0.93 [0.79, 1.09] 2–3 cups/day: 0.89 [0.76, 1.04] ≥ 4 cups/day: 0.78 [0.64, 0.94]* |
|
Cups of tea and VaD risk: None: Reference group 0.5–1 cups/day: 0.80 [0.63, 1.01] 2–3 cups/day: 0.74 [0.61, 0.88]* ≥ 4 cups/day: 0.75 [0.63, 0.89]* | |||||||
|
Combined cups of coffee and tea and VaD risk: 0 cups/day coffee, 0 cups/day tea: Reference 0 cups/day coffee, 0.5–1 cups/day tea: 0.93 [0.52, 1.64] 0 cups/day coffee, 2–3 cups/day tea: 0.59 [0.38, 0.89]* 0 cups/day coffee, ≥ 4 cups/day tea: 0.55 [0.38, 0.79]* 0.5–1 cups/day coffee, 0 cups/day tea: 0.90 [0.53, 1.52] 0.5–1 cups/day coffee, 0.5–1 cups/day tea: 0.53 [0.31, 0.90]* 0.5–1 cups/day coffee, 2–3 cups/day tea: 0.56 [0.38, 0.83]* 0.5–1 cups/day coffee, ≥ 4 cups/day tea: 0.53 [0.37, 0.76]* 2–3 cups/day coffee, 0 cups/day tea: 0.73 [0.48, 1.11] 2–3 cups/day coffee, 0.5–1 cups/day tea: 0.51 [0.32, 0.80]* 2–3 cups/day coffee, 2–3 cups/day tea: 0.50 [0.34, 0.73]* 2–3 cups/day coffee, ≥ 4 cups/day tea: 0.54 [0.37, 0.79]* ≥ 4 cups/day coffee, 0 cups/day tea: 0.52 [0.35, 0.77]* ≥ 4 cups/day coffee, 0.5–1 cups/day tea: 0.50 [0.31, 0.81]* ≥ 4 cups/day coffee, 2–3 cups/day tea: 0.44 [0.28, 0.69]* ≥ 4 cups/day coffee, ≥ 4 cups/day tea: 0.59 [0.39, 0.90)* | |||||||
Data are presented for maximally adjusted statistical models. Data reflect HR [95% CI] unless otherwise stated. #Data for Yamada et al. [25] and Pham et al. [13] reflect OR [95% CI]. *Denotes statistical significance. ICD = International Statistical Classification of Diseases and Related Health Problems, DSM = Diagnostic and Statistical Manual of Mental Disorders (DSM), NINDS = National Institute of Neurological Disorders and Stroke, ADDTC = State of California Alzheimer’s Disease Diagnostic and Treatment Centre. A list of confounders used in these analyses can be found in supplementary material 4.
Associations between individual foods and VaD risk
Eleven studies explored associations between individual food groups and risk of VaD, including fruit and vegetables [24], fish [26], ultra-processed foods [15], sweet drinks [19], meat [16], alcoholic drinks [28], tea and/or coffee [13, 17], and milk/dairy [20, 22, 25] (Table 1).
Fruit and vegetables
Kimura et al. [24] explored the associations between fruit and vegetable intake (alone and combined) on risk of VaD in 1071 participants from the Hisayama study in Japan. Over a 24-year follow-up period, 144 participants developed VaD. There were no significant associations between intake of either fruit or vegetables alone and VaD risk (Table 1). However, a higher combined intake of fruit and vegetables was associated with lower VaD risk (P-trend = 0.047).
Fish
Huang et al. [26] investigated associations between fried and fatty fish intake with VaD incidence in 2233 participants from the Cardiovascular Health Cognition Study (United States). During a 5.4 year follow-up, 50 participants developed VaD. Consumption of 0.25 to 2 servings/week of fried fish was associated with a 260% increased risk of VaD (HR: 2.60, 95% CI 1.39 to 4.96) versus consumption of little or no (< 0.25 servings/week) fried fish. There was a similar pattern for higher intake of fried fish (2–4 servings/week) and VaD risk, although associations were not significant (Table 1). Higher intake of fatty fish in this study tended to be associated with lower risk of VaD, although associations were not significant (data not reported).
Ultra-processed foods (UPF)
Li et al. [15] explored associations between UPF intake and VaD risk in 72,083 participants from the UK Biobank. UPF intake was classified using the NOVA framework, which groups foods into one of four different categories (Group 1: Unprocessed or minimally processed foods; Group 2: Processed culinary ingredients Group 3: Processed foods; and Group 4: Ultra-processed foods) based on the level of processing. During a median follow-up of 10 years, 119 individuals developed VaD. Compared with the reference group (quartile 1) consuming the lowest level of UPFs, there was a significantly greater risk of VaD for quartiles 3 (HR: 2.18, 95%CI 1.22, 3.93) and 4 (HR: 2.19, 95% CI 1.22, 3.96). When intake of UPFs was expressed continuously rather than categorically, each 10% increase in UPF intake was associated with a 28% increase in VaD risk (HR: 1.28, 95%CI 1.06, 1.55). Replacing 5%, 10% and 15% UPF intake by weight with unprocessed or minimally processed foods was associated with a 12% (HR: 0.88, 95% CI 0.80, 0.97), 22% (HR: 0.78, 95% CI 0.65, 0.94) and 39% (HR 0.61, 95% CI 0.42, 0.89) lower risk of VaD. When the authors explored associations between individual UPFs and risk of VaD, associations were only significant for UPF beverages (e.g., sugary fruit-based beverages, industrial chocolate powder beverages, squash, diet sodas, artificially sweetened ice teas), with each SD increase in intake associated with a 24% increase in VaD risk (HR: 1.24, 95% CI 1.08, 1.43).
Sweet drinks
Chen et al. [19] explored associations between sugar-sweetened, artificially sweetened and naturally sweet (i.e., fruit and vegetable) juices and VaD risk in 187,994 participants from the UK Biobank cohort. During a mean follow-up of 9.5 years, 165 individuals developed VaD.
There were no significant associations between the intake of sugar-sweetened beverages or naturally sweet juices and VaD risk (Table 1). However, higher intake of artificially sweetened beverages was associated with increased VaD risk, with 0–1 unit/day increasing VaD risk by 69% (HR 1.69, 95% CI 1.10–2.61) versus the no intake reference group. Intake of 1–2 and > 2 units per day also tended to be associated with higher VaD risk versus no intake, but these associations were not significant (Table 1).
Meat
Zhang et al. [16] explored associations between different types of meat intake (processed meat, unprocessed poultry, unprocessed red meat and total meat) and VaD incidence in 493,888 participants from the UK Biobank. During a median follow-up of 8 years, 490 developed VaD. Moderate (2.0–4.9 times per week) and high (≥ 5.0 times/week) frequency of processed meat consumption were associated with a 35% (HR: 1.35, 95% CI 1.05 to 1.74) and 76% (HR: 1.76, 95% CI 1.17, 2.65) increased risk of VaD versus infrequent (0.1–0.9 times/week) processed meat consumption. There were no significant associations between unprocessed poultry and unprocessed red meat on VaD risk (Table 1). Meanwhile, moderate (3.1–4.9 times/week) and higher (5.0–6.9 times/week) frequency of consumption for total meat (the sum of all meat types combined) was associated with a 27% (HR: 0.73, 95% CI 0.54 to 0.98) and 24% (HR: 0.76, 95% CI 0.58 to 0.99) lower risk of VaD versus lower consumption (0.1–3 times/week). However, very high total meat intake was not significantly associated with VaD risk versus the lower consumption reference group (HR: 0.97, 95% CI 0.75 to 1.26). When exploring potential diet*gene interactions, the authors found no significant interactions between any of the meat types and APOE genotype on VaD risk (Table 1).
Milk and dairy
Three studies explored associations between milk types and/or other dairy consumption and VaD risk. Deng et al. [20] investigated associations between milk type and risk of VaD in 307,271 individuals from the UK Biobank cohort. During a median 12.3 years follow-up, 834 individuals developed VaD. Compared with individuals who did not consume milk, there was no significant difference in the associations between intake of skimmed milk (HR: 0.79, 95% CI 0.56, 1.13), full-cream milk (HR: 0.92, 95%CI 0.60, 1.40), soy milk (HR: 0.59, 95%CI 0.34–1.02) and other milk (HR: 1.17, 95%CI 0.65, 2.12) with VaD risk. However, when risk of VaD was compared between individuals who primarily consumed soy milk versus alternative milk varieties, intake of soy milk tended to be associated with a lower risk of VaD, which was statistically significant when compared to intake of other milk (e.g., milk excepting full-cream and skimmed milk) (HR: 0.51, 95% CI 0.26, 0.97).
Ozawa et al. [22] explored associations between overall intake of milk and dairy and VaD risk in 1081 participants in the Hisayama study (Japan). During a 17 year follow-up, 98 participants developed VaD. There were no significant differences in VaD risk between different quartiles of milk and dairy intake (Table 1).
Finally, Yamada et al. [25] explored associations between milk intake and VaD risk in 1774 adults from the Radiation Effects Research Foundation Adult Health Study (Hiroshima, Japan). During 25 to 30 years of follow-up, 38 individuals developed VaD. Individuals who reported consuming milk almost daily at baseline, versus less than twice per week, had a 65% lower risk of VaD (OR: 0.35, 95% CI 0.14–0.77) at follow-up. These authors also explored associations between fish, meat and tofu intake with VaD risk but found no significant associations (data not reported).
Alcoholic drinks
Ruitenberg et al. [28] explored associations between total number of alcoholic drinks (comprising wine, beer, liquor and fortified wines) and VaD incidence in 5395 participants from the Rotterdam study (Netherlands). During a mean 6-year follow-up, 29 individuals developed VaD. The authors found that, in the entire cohort, consumption of 1 to 3 alcoholic drinks/day was associated with 70% lower VaD risk (HR: 0.30, 95% CI 0.10 to 0.92) compared with consuming no alcoholic drinks. Lower alcohol intakes (i.e., < 1 drink/day) also tended to be associated with reduced VaD incidence versus no consumption, but these associations were not significant. There were no significant associations between high alcohol intake (> 4 drinks/day) and VaD risk and associations were not significant when analyses were stratified by sex or APOE4 carrier status (Table 1).
Tea and coffee
Pham et al. [13] explored associations between coffee intake and VaD incidence in 398,646 participants from the UK Biobank cohort. Participants were followed for ~ 7.3 to 11.9 years, during which time 451 individuals developed VaD. There were no significant associations between cups of coffee consumed per day and odds of VaD (Table 1).
In another analysis of data from UK Biobank involving 365,682 participants, Zhang et al. [17] explored associations between cups/day of both tea and coffee (individually and combined) on risk of VaD. Participants were followed for a median of 11.4 years during which time 1223 individuals developed VaD. Compared with no coffee intake, consumption of ≥ 4 cups/day was associated with a 22% reduced risk of VaD (HR: 0,78, 95% CI 0.64 to 0.94). Meanwhile, compared with no tea intake, consumption of 2–3 or ≥ 4 cups/day tea was associated with 26% (HR: 0.74, 95% CI 0.61 to 0.88) and 25% (HR: 0.75, 95% CI 0.63 to 0.89) lower risk of VaD. When intake of coffee and tea were considered together, higher combined intake of tea and coffee was typically associated with lower VaD risk versus no consumption of either drink (Table 1).
Associations between dietary patterns and VaD risk
Five studies investigated the associations between dietary patterns and VaD risk, including Swedish and Mediterranean dietary patterns [29], consumption of a Western versus Oriental diet [27], a data-driven dietary pattern [23] and a pro-inflammatory diet, as quantified using the dietary inflammatory index (DII) [14, 21].
Swedish and Mediterranean dietary patterns
Glans et al. [29] explored associations between adherence to a Swedish diet and a Mediterranean diet with risk of VaD in 28,025 participants from the Malmo diet and Cancer study in Sweden. Over 19.8 years of follow-up, 461 individuals developed VaD. The authors observed no significant associations between adherence to either the Swedish or Mediterranean dietary patterns and risk of VaD. Associations were consistent across a range of sensitivity analyses, including use of an alternative Mediterranean diet score, and there was no interaction between either dietary pattern and APOE4 carrier status (Table 2).
Table 2.
Summary of prospective cohort studies exploring associations between dietary patterns and risk of VaD
| Author | Cohort and setting | Participant characteristics | Follow up duration | VaD cases | VaD diagnosis method | Dietary assessment method | Outcomes |
|---|---|---|---|---|---|---|---|
| Swedish and Mediterranean diets | |||||||
| Glans et al. [29] | Malmo Diet and Cancer Study (Sweden) | 28,025 (11,033 men and 16,992 women) Swedish dementia-free participants aged 58 (8) years | Median 19.8 (4.8) years | 461 | Physician review of registered dementia diagnoses from the Swedish National Patient Register |
Diet assessed through a combination of 7-day diet diary and 168-item FFQ Swedish diet assessed via the Swedish Dietary Guidelines Score Mediterranean diet assessed using a modified version of the MEDAS score |
Swedish dietary guidelines and VaD: Low (0–1 points): Reference group Intermediate (2–3 points): 0.97 [0.79, 1.18] High (4–5 points): 1.00 [0.73, 1.35] Continuous score: 1.00 [0.93, 1.08] Interaction with APOE status: P = 0.88 |
|
Mediterranean diet adherence and VaD: Low (0–1 points): Reference group Intermediate (2–4 points): 0.95 [0.74, 1.22] High (5–10 points): 1.09 [0.70, 1.70] Continuous score: 1.06 [0.98, 1.15] Interaction with APOE status: P = 0.63 Alternative Mediterranean diet score (continuous): 0.99 [0.97, 1.01] | |||||||
| Western vs. Oriental diet | |||||||
| Ross et al. [27]# | Honolulu-Asia Aging Study (Hawaii, USA) | 3509 (all male) Japanese American dementia-free participants average age ~ 53 at baseline | 25 years | 68 | Physician review of clinical and laboratory information. VaD was diagnosed based on the ADDTC criteria | Participants asked whether their diet was mostly Oriental, Western or mixed |
Preference for a Western versus Oriental/mixed diet and VaD risk: Participant cohort including those with VaD and those with no stroke, no dementia: 0.54 [0.30–0.98]* Participants cohort including those with VaD and those with stroke but no dementia: 0.43 [0.22, 0.86]* |
| Data driven dietary patterns | |||||||
| Ozawa et al. [23] | Hisayama study (Japan) | 1006 (433 men and 573 women) Japanese dementia-free participants with mean age 68 years | Median 15 years | 88 | Physician and psychiatrist evaluation. DSM criteria used to diagnose dementia and the criteria of the NINDS was used to diagnose VaD |
Diet assessed via a 70-item semiquantitative food frequency questionnaire A dietary pattern was constructed using reduced rank regression |
Data driven dietary pattern and VaD risk: Quartile 1: Reference Quartile 2: 0.97 [0.56, 1.68] Quartile 3: 0.74 [0.41, 1.34] Quartile 4: 0.45 [0.22, 0.91]* P trend: 0.02 |
|
Stratified analysis in participants without diabetes: Quartile 1: Reference Quartile 2: 0.94 [0.53, 1.69] Quartile 3: 0.82 [0.45, 1.50] Quartile 4: 0.32 [0.13, 0.76]* P trend: 0.01 | |||||||
|
Stratified analysis in participants with diabetes: Quartile 1: Reference Quartile 2: 0.63 [0.14, 2.93] Quartile 3: 0.12 [0.01, 1.30] Quartile 4: 0.42 [0.09, 2.04] P trend: 0.30 | |||||||
| Pro-inflammatory dietary pattern | |||||||
| Shi et al. [21] | UK Biobank (UK) | 166,377 (77,575 men and 88,802 women) predominantly white dementia-free participants aged 56 (8) years | Median 9.5 years | 267 | Linkage to electronic healthcare records and death registries and use of ICD codes to identify dementia sub-types |
Diet assessed via a 24-h dietary assessment tool (Oxford WebQ) Dietary inflammatory index generated using published methods |
Dietary inflammation index (DII) and VaD risk: Quartile 1: Reference group Quartile 2: 1.01 [0.72, 1.42] Quartile 3: 0.916 [0.64, 1.32] Quartile 4: 0.972 [0.66, 1.44] Per 1 point increase in DII score: 0.98 [0.91, 1.06] Non-linear trend analysis: P = 0.449 |
| Peng et al. [14] | UK Biobank (UK) | 207,301 (93,034men and 114,267 women) predominantly white dementia-free participants average age 57 years | Average 11.4 years | 91 | Linkage to electronic healthcare records and death registries and use of ICD codes to identify dementia sub-types |
24-h dietary assessment tool (Oxford WebQ) Dietary inflammatory index generated using published methods |
Dietary inflammation index (DII) and VaD risk: Quintile 1: 1.34 [0.62, 2.87] Quintile 2: 1.50 [0.72, 3.17] Quintile 3: Reference group Quintile 4: 1.44 [0.68, 3.05] Quintile 5: 2.27 [1.13, 4.53]* Continuous score: 1.13 [0.96, 1.32] P trend: 0.120 |
|
Comparison against quintile 1 as a reference group: VaD risk did not significant differ between quintiles (data not reported) Non-linear analyses: No significant associations between DII score and VaD risk (data not reported) | |||||||
Data are presented for maximally adjusted statistical models. Data reflect HR [95% CI] unless otherwise stated. #Data for Ross et al. [27] reflect OR [95% CI]. *Denotes statistical significance. ICD = International Statistical Classification of Diseases and Related Health Problems, DSM = Diagnostic and Statistical Manual of Mental Disorders (DSM), NINDS = National Institute of Neurological Disorders and Stroke, ADDTC = State of California Alzheimer’s Disease Diagnostic and Treatment Centre. A list of confounders used in these analyses can be found in supplementary material 4. Definitions and construction of each dietary pattern can be found in supplementary material 5.
Western versus oriental diet
Ross et al. [27] evaluated associations between preference for a Western versus Oriental or mixed diet (as determined by asking participants a single question on whether they perceived their diet to be mostly Oriental, Western, or mixed) and VaD risk in 3509 Japanese-American men from the Honolulu-Asia Aging Study. During a ~ 25-year observation period, 68 individuals developed VaD. Two sets of analyses were conducted, the first of which included participants with VaD and individuals without stroke or dementia, and the second of which included the same participants with VaD and participants with stroke but no dementia. Preference for a Western diet, instead of an Oriental or mixed diet, was associated with 46% lower odds of VaD (OR: 0.54, 95% CI 0.30 to 0.98) in the cohort including participants with VaD and no stroke, no dementia. There was 57% lower odds of VaD (OR: 43, 95% CI 0.22 to 0.86) in the cohort including participants who developed VaD and those with stroke but not dementia.
Data driven dietary patterns
Ozawa et al. [23] explored associations between dietary patterns and risk of VaD in 1006 participants from the Hisayama study (Japan). During 15 years of follow-up, 88 participants developed VaD. Using reduced rank regression, the authors identified a potential dietary pattern for dementia prevention which broadly corresponded to a customary Japanese diet. This diet was rich in soybeans and soybean-derived foods alongside vegetables (especially green vegetables), algae, and milk/dairy products, and contained a low amount of rice. Individuals with the highest adherence to this dietary pattern (quartile 4) had a 55% lower risk of VaD (HR: 0.45, 95% CI 0.22 to 0.91) than a low adherence reference group (quartile 1). When analyses were stratified by diabetes status, higher adherence to this dietary pattern was only significantly associated with lower VaD risk in participants without diabetes (Table 2).
Pro-inflammatory diet
Two studies explored the associations between the Dietary Inflammatory Index (DII) score, which classifies an individual’s dietary pattern according to its inflammatory potential (higher scores reflect a more pro-inflammatory diet) and VaD risk. Firstly, Shi et al. [21] explored the associations between DII score and VaD risk in 166,377 participants from the UK biobank. Individuals were followed for a median of 9.5 years, during which time 267 developed VaD. There were no significant associations between DII score and risk of VaD in linear or non-linear analyses (Table 2).
A second study also explored associations between DII score and risk of VaD in the UK Biobank dataset. Peng et al. [14] followed 207,301 participants for an average 11.4 years during which time 91 individuals developed VaD. Participants with the highest DII scores had a 227% (quintile 5, HR: 2.27, 95% CI 1.13 to 4.53) increased risk of VaD versus those with moderate DII scores (quintile 3, reference group). When VaD risk was compared against quintile 1 as a reference group (rather than quintile 3, as per the main analyses) associations between higher DII score and VaD risk were not significant. Similarly, in non-linear analyses there were no significant associations between DII score and VaD risk.
Risk of bias assessment
Risk of bias across studies was mixed (Supplementary Material 6). There was a high risk of bias in three studies [26–28]due to covariate selection for statistical models, which could increase the risk of confounding, and also in one study due to concerns over the accuracy of measurement of the dietary exposure (measured using a single question) [27]. There were also some concerns with the risk of misclassification of the dietary exposure for alcohol [28], milk [25], UPFs [15], and the pro-inflammatory dietary pattern [21], which further categorised two studies with some concerns.
Discussion
This systematic review of prospective cohort studies explored associations between intake of foods and dietary patterns with risk of VaD. We identified sixteen studies which were eligible for inclusion, with 11 focusing on individual food groups and 5 focusing on dietary patterns and VaD risk.
Several foods/dietary patterns were associated with a decreased risk of VaD including a higher total intake of fruits and vegetables, moderate-to-high total meat intake, moderate intake of alcoholic drinks (1–3 drinks/day), preference for a Western versus an Oriental diet, and adherence to a (data-driven) plant-based dietary pattern. Some, but not all, data also suggested that higher consumption of tea and/or coffee, daily consumption of milk, and a preference for soy milk versus ‘other’ milks were associated with lower VaD risk. Some of the foods/dietary patterns suggested to reduce risk of VaD have been associated with better cardiovascular health and could plausibly reduce VaD risk by mitigating vascular risk factors for this condition. For example, high intake of fruit and vegetables has been inversely associated with risk of coronary heart disease and stroke [30, 31]. Plant-based dietary patterns, similar to the data-driven dietary pattern derived by Ozawa et al. [23], have been consistently linked with lower CVD risk factors [32–34] and CVD incidence [35, 36]. Moderate, but not high, intake of coffee has typically been associated with lower CVD risk (i.e., a ‘J’ shaped relationship) [37–39], although individuals with uncontrolled hypertension are advised to limit caffeine intake [39, 40]. A ‘J’ shaped relationship has also been posited between alcohol intake and vascular health [41], although others (including recent large-scale Mendelian randomisation studies [42]) have suggested intake of any amount of alcohol is deleterious towards cardiovascular health.
Perhaps the most surprising finding is that preference for a Western diet—often considered to be a model of unhealthy eating—was suggested to be protective against VaD when compared with an ‘Oriental’ diet in a study by Ross et al. [27] in Japanese American men. We feel that considerable caution should be taken when interpreting findings from this study, which were deemed to be at high risk of bias. A particular challenge is that the authors captured dietary preference via a single question enquiring about whether participants consumed a mostly Oriental, Western, or mixed diet. As such, there was no information given on the potentially protective or harmful components of these diets, which would be detected using more granular dietary assessment tools. There is also potential risk of misclassification of participants with such simple dietary assessment. It is unlikely that a Western diet is optimal for reducing VaD risk. Indeed, when compared with other ‘healthier’ diets, such as the Mediterranean diet, a Western diet has typically been associated with worse health outcomes, including increased risk of stroke [43], coronary heart disease [44], and Alzheimer’s disease [45]. Several individual foods which are common in a typical Western diet were also individually linked to VaD risk in this study (e.g., processed meat, fried fish, and UPFs [15, 16, 26]). Future studies are recommended to use more accurate dietary assessment tools (rather than a single question on dietary preference) and provide clearer definitions for the components included within dietary patterns.
UPFs have attracted considerable attention recently in the scientific literature and mainstream media. Exploring the links between UPF consumption and VaD risk is therefore particularly timely and relevant to these ongoing discussions. Higher intake of UPFs was associated with increased VaD risk in the study by Li et al. [15]. However, when exploring individual UPF groups, only UPF beverages (e.g., sweetened/carbonated drinks) were significantly associated with VaD risk. Chen et al. [19] also reported that consumption of 0–1 serving per day of artificially sweetened beverages was associated with a 69% increased risk of VaD versus abstention. Interestingly, some studies exploring links between UPF intake and other health conditions have reported similar findings, with associations between UPF intake and multimorbidity of cancer and cardiometabolic diseases, and type II diabetes, largely driven by intake of sugar- or artificially-sweetened drinks and/or processed meats [46, 47]. Although more research is needed, particularly randomised controlled trials [48], more nuanced recommendations may be required that focus on specific foods with established links to adverse health outcomes rather than making recommendations to avoid an entire food category (i.e., UPFs) based on processing status.
Strengths and limitations
This systematic review provides new insight into the current state of the knowledge on diet and VaD risk, building upon the earlier review in this area by Perez et al. [7]. There are several strengths. Firstly, by focusing on prospective cohort studies, we reduced risk of reverse causality compared with the inclusion of cross-sectional or retrospective studies. Nevertheless, it is acknowledged that it can be difficult to draw causal inferences from one single study design, and triangulation across many different study designs (including Mendelian Randomisation studies and randomised controlled trials, of which there are currently very few in this area) with orthogonal sources of bias would yield more robust inferences [49]. This could also provide better insights into which foods/patterns would be more reliable targets for intervention. Secondly, we conducted extensive database searches which were designed by an information specialist to ensure comprehensive coverage of the available literature. Thirdly, we followed the PRISMA guidelines (including conducting RoB assessments), and pre-registering this review on PROSPERO to maximise transparency.
The principal limitation is that there are only one or two studies available exploring associations between most individual foods/dietary patterns with VaD risk. Moreover, some of these studies, despite having very similar methodology, report conflicting findings. This makes it difficult to draw firm conclusions about the links between specific foods/dietary patterns and VaD risk at present. For example, two studies using data from the same cohort (UK Biobank), and published in the same year, explored associations between coffee intake and VaD risk, with different findings. Pham et al. [13] found no significant associations between coffee intake and VaD risk, whereas Zhang et al. [17] reported significantly (22%) lower risk of VaD with intake of four or more cups of coffee per day. Zhang et al. [17] also observed significantly lower risk of VaD with higher (≥ 2 cups/day) intake of tea, and higher combined intake of coffee and tea. There are several possible explanations for the discrepancy in findings between these two studies. Perhaps most relevant is the fact that the two studies used different reference groups in their analyses. Pham et al. [13] compared risk of VaD for different coffee intake groups against a reference group consuming 1–2 cups/day coffee. In contrast, Zhang et al. [17] compared against a reference group consuming no coffee per day. A no intake reference group may seem the more obvious choice and is consistent with widespread practice in nutritional epidemiology in which researchers tend to compare higher intakes against lower/no intake reference groups. However, Pham et al. [13] reasoned that comparison against light coffee drinkers may be more appropriate to avoid bias from individuals with poor health, who may avoid consuming coffee due to existing health conditions/co-morbidities. There was similar ambiguity between two studies exploring associations between DII score (which reflects the inflammatory potential of a diet) and VaD risk in the UK Biobank cohort. Shi et al. [21] found no significant associations between DII score and VaD risk, whereas Peng et al. [14] reported higher VaD risk in participants with the highest DII scores. The discrepancy in findings between these studies is also likely to be due differences in the DII reference group used for comparison in analyses. Shi et al. [21] compared risk of VaD in higher DII quartiles against the lowest DII quartile (signifying the most anti-inflammatory diet) as a reference group, whilst Peng et al. [14] compared risk of VaD between higher and lower DII groups against the middle quintile as a reference group. When Peng et al. [14] used quintile 1 as an alternative reference group, associations between higher DII scores and VaD risk were not significant. Agreeing upon standardised, best practice methodology (including most appropriate reference groups) for future research could help overcome some of these issues. A further limitation is that approximately half of the studies which make up this systematic review were conducted using the UK Biobank cohort. This dataset has several strengths, including granular dietary, lifestyle, sociodemographic and genetic data. However, it also has certain limitations, including the healthy volunteer selection bias and predominantly white participant group could limit generalisability [50]. Moreover, dietary data via the Oxford WebQ was captured during a relatively narrow window of time (2009–2012) and it is possible that participants diets may have changed during the follow up period which cannot currently be accounted for based on available data. One additional limitation relates to the use of hospital in patient and/or death record for the diagnosis of VaD, which may lead to underreporting and misclassification of dementia subtypes [51]. This is an issue for studies using the UK Biobank cohort, where it has been suggested that there is a positive predictive value (i.e. the proportion of cases identified that were true positives) of 43.8% for VaD [52].
Conclusions
This study outlines current state of the knowledge around intake of foods and dietary patterns with VaD risk. The findings suggest that an appropriate diet—such as consumption of higher intake of fruits and vegetables and adherence to a plant-based dietary pattern, which is somewhat consistent with dementia prevention recommendations by the World Health Organization (WHO) [53]—can lower VaD risk. However, evidence is characterised by a limited number of studies with some inconsistencies in the findings. More research is needed to build a critical mass of evidence to inform personalised and population-based approaches to mitigate VaD risk. This could include substantiating findings for the aforementioned foods/dietary patterns in other populations/cohorts (including those with a greater proportion of participants from minority ethnic and lower socioeconomic backgrounds) and exploring new dietary approaches which might help lower VaD risk such as those with established cardioprotective properties (e.g., nitrate-rich vegetables [54–56], nuts [57–59], fermented dairy foods [60] and the DASH diet [61]).
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- CI
Confidence interval
- DII
Dietary inflammatory index
- FFQ
Food frequency questionnaire
- HR
Hazard ratio
- OR
Odds Ratio
- PECOS
Participant, exposure, comparator, outcomes, study design
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- SD
Standard deviation
- UPF
Ultra-processed food
- VaD
Vascular dementia
- VCI
Vascular cognitive impairment
- VCIND
Vascular cognitive impairment no dementia
- WHO
World Health Organisation
Author contributions
This study was conceived by OMS, MS and BCMS, with additional input from EYHT. JM developed and conducted the database searches. AG and OMS carried out the titles and abstracts screening, full text screening, data extraction/checking and risk of bias appraisal/checking. AG, JM, EYHT, SG, EA, AF, RT, ES, BCMS, MS and OMS critically interpreted the results and drafted the manuscript, with AG and OMS taking lead roles. RT created the graphical abstract. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
ET’s salary is supported by an NIHR Clinical Lectureship. SG has received research funding to her institution from the Royal Society of Edinburgh, Scottish Neurological Research Fund and Alzheimer’s Association, alongside an ISTAART Travel Fellowship. SG has also received consulting fees from Scottish Brain Sciences and is a part time employee of Scottish Brain Sciences. SG is vice chair of an NHS research ethics committee, and her salary was funded during part of this project by a grant from the UK Medical Research Council. OMS has received research funding to his institution from the Fruit Juice Science Centre. All other authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forrest SL, Kovacs GG. Current concepts of mixed pathologies in neurodegenerative diseases. Can J Neurol Sci J Can Sci Neurol. 2023;50:329–45. [DOI] [PubMed] [Google Scholar]
- 2.Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol. 2019;39:1542–9. [DOI] [PubMed] [Google Scholar]
- 3.Vascular G-R, Impairment C. Vascular cognitive impairment. Contin Lifelong Learn Neurol. 2019;25:147–64. [Google Scholar]
- 4.Fillit H, Hill J. The costs of vascular dementia: a comparison with Alzheimer’s disease. J Neurol Sci. 2002;203–204:35–9. [DOI] [PubMed] [Google Scholar]
- 5.Dementia: a public health priority. https://www.who.int/publications-detail-redirect/dementia-a-public-health-priority. Accessed 18 May 2024.
- 6.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43:3137–46. [DOI] [PubMed] [Google Scholar]
- 7.Perez L, Helm L, Sherzai AD, Jaceldo-Siegl K, Sherzai A. Nutrition and vascular dementia. J Nutr Health Aging. 2012;16:319–24. [DOI] [PubMed] [Google Scholar]
- 8.Siervo M, Shannon OM, Llewellyn DJ, Stephan BC, Fontana L. Mediterranean diet and cognitive function: from methodology to mechanisms of action. Free Radic Biol Med. 2021;176:105–17. [DOI] [PubMed] [Google Scholar]
- 9.Shannon OM, Ranson JM, Gregory S, Macpherson H, Milte C, Lentjes M, et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: findings from the UK Biobank prospective cohort study. BMC Med. 2023;21:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shannon OM, Ashor AW, Scialo F, Saretzki G, Martin-Ruiz C, Lara J, et al. Mediterranean diet and the hallmarks of ageing. Eur J Clin Nutr. 2021;75:1–17. [DOI] [PubMed] [Google Scholar]
- 11.Townsend R, Fairley A, Gregory S, Ritchie C, Stevenson E, Shannon OM. Nutrition for dementia prevention: a state of the art update for clinicians. Age Ageing. 2024;53(2):30–8. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham K, Mulugeta A, Zhou A, O’Brien JT, Llewellyn DJ, Hyppönen E. High coffee consumption, brain volume and risk of dementia and stroke. Nutr Neurosci. 2022;25:2111–22. [DOI] [PubMed] [Google Scholar]
- 14.Peng M, Yuan S, Lu D, Ling Y, Huang X, Lyu J, et al. Dietary inflammatory index, genetic susceptibility and risk of incident dementia: a prospective cohort study from UK biobank. J Neurol. 2024;271:1286–96. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Li S, Yang H, Zhang Y, Zhang S, Ma Y, et al. Association of ultraprocessed food consumption with risk of dementia: a prospective cohort study. Neurology. 2022;99:e1056–66. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Greenwood DC, Risch HA, Bunce D, Hardie LJ, Cade JE. Meat consumption and risk of incident dementia: cohort study of 493,888 UK Biobank participants. Am J Clin Nutr. 2021. 10.1093/ajcn/nqab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Yang H, Li S, Li W, Wang Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: a cohort study in the UK Biobank. PLOS Med. 2021;18: e1003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA, et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int. 2024;186: 108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Chen J, Cao Y, Sun Y, Huang L, Ji JS, et al. Sugary beverages and genetic risk in relation to brain structure and incident dementia: a prospective cohort study. Am J Clin Nutr. 2023;117:672–80. [DOI] [PubMed] [Google Scholar]
- 20.Deng Z, Xie D, Cai J, Jiang J, Pan D, Liao H, et al. Different types of milk consumption and the risk of dementia: analysis from a large-scale cohort study. Clin Nutr Edinb Scotl. 2023;42:2058–67. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Lin F, Li Y, Wang Y, Chen X, Meng F, et al. Association of pro-inflammatory diet with increased risk of all-cause dementia and Alzheimer’s dementia: a prospective study of 166,377 UK Biobank participants. BMC Med. 2023;21:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozawa M, Ohara T, Ninomiya T, Hata J, Yoshida D, Mukai N, et al. Milk and dairy consumption and risk of dementia in an elderly japanese population: the Hisayama study. J Am Geriatr Soc. 2014;62:1224–30. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama study. Am J Clin Nutr. 2013;97:1076–82. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y, Yoshida D, Ohara T, Hata J, Honda T, Hirakawa Y, et al. Long-term association of vegetable and fruit intake with risk of dementia in Japanese older adults: the Hisayama study. BMC Geriatr. 2022;22:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the radiation effects research foundation adult health study. J Am Geriatr Soc. 2003;51:410–4. [DOI] [PubMed] [Google Scholar]
- 26.Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE ε4. Neurology. 2005;65:1409–14. [DOI] [PubMed] [Google Scholar]
- 27.Ross GW, Petrovitch H, White LR, Masaki KH, Li CY, Curb JD, et al. Characterization of risk factors for vascular dementia. Neurology. 1999;53:337–337. [DOI] [PubMed] [Google Scholar]
- 28.Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, et al. Alcohol consumption and risk of dementia: the Rotterdam study. The Lancet. 2002;359:281–6. [DOI] [PubMed] [Google Scholar]
- 29.Glans I, Sonestedt E, Nägga K, Gustavsson A-M, González-Padilla E, Borne Y, et al. Association between dietary habits in midlife with dementia incidence over a 20-year period. Neurology. 2023;100:e28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke. Stroke. 2014;45:1613–9. [DOI] [PubMed] [Google Scholar]
- 31.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shannon OM, Mendes I, Köchl C, Mazidi M, Ashor AW, Rubele S, et al. Mediterranean diet increases endothelial function in adults: a systematic review and meta-analysis of randomized controlled trials. J Nutr. 2020. 10.1093/jn/nxaa002. [DOI] [PubMed] [Google Scholar]
- 33.Cowell OR, Mistry N, Deighton K, Matu J, Griffiths A, Minihane AM, et al. Effects of a Mediterranean diet on blood pressure: a systematic review and meta-analysis of randomized controlled trials and observational studies. J Hypertens. 2020. 10.1097/HJH.0000000000002667. [DOI] [PubMed] [Google Scholar]
- 34.Bruns A, Greupner T, Nebl J, Hahn A. Plant-based diets and cardiovascular risk factors: a comparison of flexitarians, vegans and omnivores in a cross-sectional study. BMC Nutr. 2024;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gan ZH, Cheong HC, Tu Y-K, Kuo P-H. Association between plant-based dietary patterns and risk of cardiovascular disease: a systematic review and meta-analysis of prospective cohort studies. Nutrients. 2021;13:3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Liu B, Han H, Hu Y, Zhu L, Rimm EB, et al. Associations between plant-based dietary patterns and risks of type 2 diabetes, cardiovascular disease, cancer, and mortality - a systematic review and meta-analysis. Nutr J. 2023;22:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kouli G-M, Panagiotakos DB, Georgousopoulou EN, Mellor DD, Chrysohoou C, Zana A, et al. J-shaped relationship between habitual coffee consumption and 10-year (2002–2012) cardiovascular disease incidence: the ATTICA study. Eur J Nutr. 2018;57:1677–85. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-Artalejo F, López-García E. Coffee consumption and cardiovascular disease: a condensed review of epidemiological evidence and mechanisms. J Agric Food Chem. 2018;66:5257–63. [DOI] [PubMed] [Google Scholar]
- 40.Teramoto M, Yamagishi K, Muraki I, Tamakoshi A, Iso H. Coffee and green tea consumption and cardiovascular disease mortality among people with and without hypertension. J Am Heart Assoc. 2023;12: e026477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson PL. J-curve revisited: cardiovascular benefits of moderate alcohol use cannot be dismissed. Med J Aust. 2013;198:419–22. [DOI] [PubMed] [Google Scholar]
- 42.Biddinger KJ, Emdin CA, Haas ME, Wang M, Hindy G, Ellinor PT, et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5: e223849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherzai A, Heim LT, Boothby C, Sherzai AD. Stroke, food groups, and dietary patterns: a systematic review. Nutr Rev. 2012;70:423–35. [DOI] [PubMed] [Google Scholar]
- 44.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men123. Am J Clin Nutr. 2000;72:912–21. [DOI] [PubMed] [Google Scholar]
- 45.Więckowska-Gacek A, Mietelska-Porowska A, Wydrych M, Wojda U. Western diet as a trigger of Alzheimer’s disease: from metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. 2021;70: 101397. [DOI] [PubMed] [Google Scholar]
- 46.Cordova R, Viallon V, Fontvieille E, Peruchet-Noray L, Jansana A, Wagner K-H, et al. Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. Lancet Reg Health-Eur. 2023;35: 100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, Khandpur N, Desjardins C, Wang L, Monteiro CA, Rossato SL, et al. Ultra-processed food consumption and risk of type 2 diabetes: three large prospective us cohort studies. Diabetes Care. 2023;46:1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawlor DA, Tilling K, Davey SG. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45:1866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson T, Ly A, Schnier C, Rannikmäe K, Bush K, Brayne C, et al. Identifying dementia cases with routinely collected health data: a systematic review. Alzheimers Dement. 2018;14:1038–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson T, Schnier C, Bush K, Rannikmäe K, Henshall DE, Lerpiniere C, et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Risk reduction of cognitive decline and dementia: WHO guidelines. https://www.who.int/publications-detail-redirect/9789241550543. Accessed 1 Mar 2022. [PubMed]
- 54.Shannon OM, Gregory S, Siervo M. Dietary nitrate, aging and brain health: the latest evidence. Curr Opin Clin Nutr Metab Care. 2022;25:393–400. [DOI] [PubMed] [Google Scholar]
- 55.Shannon OM, Easton C, Shepherd AI, Siervo M, Bailey SJ, Clifford T. Dietary nitrate and population health: a narrative review of the translational potential of existing laboratory studies. BMC Sports Sci Med Rehabil. 2021;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siervo M, Shannon O, Kandhari N, Prabhakar M, Fostier W, Köchl C, et al. Nitrate-rich beetroot juice reduces blood pressure in tanzanian adults with elevated blood pressure: a double-blind randomized controlled feasibility trial. J Nutr. 2020;150:2460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glenn AJ, Aune D, Freisling H, Mohammadifard N, Kendall CWC, Salas-Salvadó J, et al. Nuts and cardiovascular disease outcomes: a review of the evidence and future directions. Nutrients. 2023;15:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shannon OM, Stephan BCM, Minihane A-M, Mathers JC, Siervo M. Nitric oxide boosting effects of the Mediterranean diet: a potential mechanism of action. J Gerontol A Biol Sci Med Sci. 2018. 10.1093/gerona/gly087. [DOI] [PubMed] [Google Scholar]
- 59.Dikariyanto V, Smith L, Robertson M, Kusaslan E, O’Callaghan-Latham M, Chowienczyk P, et al. Effects of daily intake of almonds on cardiac autonomic functions measured by heart rate variability in response to acute stress: a randomized controlled trial. Curr Dev Nutr. 2020;4(Suppl 2):20. [Google Scholar]
- 60.Zhang K, Chen X, Zhang L, Deng Z. Fermented dairy foods intake and risk of cardiovascular diseases: a meta-analysis of cohort studies. Crit Rev Food Sci Nutr. 2020;60:1189–94. [DOI] [PubMed] [Google Scholar]
- 61.Chiavaroli L, Viguiliouk E, Nishi SK, Mejia SB, Rahelić D, Kahleová H, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.