Abstract
At the onset of mitosis, the pericentriolar Golgi apparatus of mammalian cells is converted into small fragments, which are dispersed throughout the cytosol. The Golgi-associated protein GRASP65 is involved in this process. To address the role of GRASP65 in mitotic Golgi fragmentation, we depleted the protein from HeLa cells by RNAi. In the absence of GRASP65, the number of cisternae per Golgi stack is reduced without affecting the overall organization of Golgi membranes and protein transport. GRASP65-depleted cells entered mitosis, but accumulated in metaphase with condensed chromatin and multiple aberrant spindles and eventually died. Although Centrin2 and g-tubulin were detected in two of the spindle poles, the other spindle poles contained g-tubulin, but not Centrin2. Furthermore, we provide evidence that the expression of the C-terminus of GRASP65 interferes with entry of cells into mitosis. Our results suggest the requirement for GRASP65 in the regulation of spindle dynamics rather than a direct role in the stacking of Golgi cisternae. This novel function is in addition to the previously established negative role of GRASP65 at the G2/M transition, which is mediated by its C-terminus.
INTRODUCTION
It has been known since the 1960s that the pericentriolar Golgi apparatus of mammalian cells is in a fragmented form during mitosis (Robbins and Gonatas, 1964; Maul and Brinkley, 1970). The use of Golgi-specific antibodies confirmed these observations (Burke et al., 1982; Roth and Berger, 1982). In the past few years, an alternative model was put forth suggesting that Golgi membranes fuse with the ER during mitosis (Thyberg and Moskalewski, 1992; Zaal et al., 1999). However, recent evidence supports the proposal that Golgi membranes do not fuse with the ER and that they retain their identity during mitosis (Jesch et al., 2001; Pecot and Malhotra, 2004; Axelsson and Warren, 2004). Because mitotic Golgi fragmentation is only observed in cells that host Golgi membranes in the pericentriolar region (i.e., in mammalian cells), model organisms suitable for genetic analysis have not been of much use to address this important problem. Therefore, in vitro assays have been developed which either use Golgi-enriched fractions or permeabilized normal rat kidney (NRK) cells together with mitotic cytosol to reconstitute mitotic Golgi fragmentation (Misteli and Warren, 1995; Acharya et al., 1998). These in vitro systems have revealed the involvement of four kinases in this process: Cdc2, MEK1, a key player of the MAP kinase pathway, its upstream activator RAF-1 and Polo like kinase (Plk1; Acharya et al., 1998; Lowe et al., 1998; Sütterlin et al., 2001; Colanzi et al., 2003b). The MAP kinase pathway and Plk1 convert pericentriolar Golgi membranes first into smaller elements, which are converted further by a cdc2 kinase–dependent reaction (Kano et al., 2000; Colanzi et al., 2003a). In addition to the abovementioned protein kinases, the Golgi-associated protein GRASP65 is important for the first step of mitotic Golgi fragmentation (Sütterlin et al., 2001). Reagents that interfere with the C-terminus of GRASP65 block mitotic Golgi fragmentation in vitro and arrest cells at the G2/M transition of the cell cycle (Sütterlin et al., 2002). This cell cycle arrest is, however, only observed when Golgi membranes are positioned in the pericentriolar region of the cell. Incubating cells with reagents that artificially fragment Golgi membranes alleviate the GRASP65-induced cell cycle block in spite of the presence of GRASP65-specific inhibitory reagents. Based on these data, the organization of Golgi membranes in their pericentriolar position has been implicated with a role in cell cycle regulation (Sütterlin et al., 2002; Carcedo et al., 2004: Preisinger et al., 2005). GRASP65 was initially identified as a Golgi-localized protein that is required for the stacking of Golgi cisternae in vitro (Barr et al., 1997). This protein is heavily phosphorylated during mitosis and in vitro evidence has implicated the phosphorylation of GRASP65 with the unstacking of Golgi cisternae (Wang et al., 2005). According to this model, inhibiting GRASP65 function should lead to the unstacking of Golgi cisternae and result in Golgi fragmentation as observed during mitosis in intact cells. To test this working hypothesis, we depleted GRASP65 from HeLa cells by siRNA. Surprisingly, we observed no obvious defect in the organization (stacking) of Golgi cisternae or in protein transport along the secretory pathway. In GRASP65-depleted cells, there was, however, a defect in the overall organization of the mitotic spindle, which ultimately led to cell death. We discuss our findings on this novel function of GRASP65.
MATERIALS AND METHODS
Antibodies and Reagents
Antibodies used in this study were obtained from the following sources: GRASP65, GRASP55, giantin, β-COP (Malhotra lab), GM130 (BD Biosciences, San Diego, CA), Golgin 97 (Dr. Ed Chan, Scripps Research Institute La Jolla, CA), phospho-histone H3 (Upstate Biotechnology, Lake Placid, NY), α-tubulin (Accurate Chemical and Scientific, Westbury, NY), γ-tubulin (Sigma, St. Louis, NY), Centrin2 (Dr. Jeffrey Salisbury, Mayo Clinic), KDEL-Receptor (StressGen Biotechnologies, Victoria, British Columbia, Canada), V5 (Invitrogen, Carlsbad, CA), Z-VAD was kindly provided by Dr. Carrie Brachmann, UC Irvine.
Cell Culture and Transfections
HeLa cells were grown in complete medium consisting of α-DMEM (Life Technologies, Rockville, MD) containing 10% fetal calf serum and l-glutamine at 37°C in a 5% CO2 incubator. Knockdown transfections were performed with HeLa cells plated at 50% confluency in six-well dishes. To 175 μl Opti-MEM, 10 μl of a 20 μM siRNA stock (Qiagen, Chatsworth, CA) was added. In a separate tube, 4 μl Oligofectamine (Invitrogen) were mixed with 11 μl Opti-MEM and incubated for 10 min at RT. The two mixtures were combined, allowed to incubate at RT for 20 min, and then added to the cells in 0.8 ml Opti-MEM. Three to 6 h after the transfection, 3 ml of complete medium was added to each six-well dish. Twenty-four hours after the knockdown transfection, cells were split onto coverslips and analyzed for the absence of GRASP65 2–3 d after the knockdown transfection. For spindle analysis, knockdown or control cells grown in six-well tissue culture dishes were trysinized, washed with phosphate-buffered saline (PBS), plated onto poly-lysine (Sigma)-coated coverslips, and fixed. A cell line stably expressing Centrin2-GFP was generated by transfecting ATCC HeLa cells with the plasmid pEGFP-Centrin2 (kindly provided by Dr. Mikiko Takahashi, Biosignal Research Center, Kobe, Japan; Nishimura et al., 2005). An inducible stable cell line (60% of the cells inducibly expressing GRASP65) was generated by cotransfecting ATCC HeLa cells with the two plasmids pSwitch and pGene-hsΔ200-V5. This construct was generated by first cloning the human cDNA for GRASP65 (hsGRASP65) from a Hep3B library (kindly provided by Dr. Yusuke Maeda, Osaka, Japan) followed by subcloning the cDNA fragment that encodes for amino acids 201–453 via HindIII and EcoRI into the pGene vector. The initial transfection was followed by double selection for zeocin- and hygromycin-resistant clones that inducibly expressed hsΔ200-V5 (Gene Switch System, Invitrogen). Thirty-two hours after the siRNA transfection, control-transfected and GRASP65-depleted cells were treated with 20 μM Z-VAD for 16 h before fixation.
Immunoprecipitation and Immunofluorescence Microscopy
To determine the level of GRASP65, control or GRASP65-depleted cells derived from two six-well dishes were lysed in 1% Triton X-100, 150 mM NaCl, 50 mM Tris, pH 7.0, and GRASP65 was immunoprecipitated using a polyclonal antibody to GRASP65. The immunoprecipitations were analyzed by Western blot. Alternatively, the total cell lysates were analyzed by Western blotting for the presence of various Golgi proteins (GM130, Golgin 97, and GRASP55) using specific antisera. For immunofluorescence studies, cells were fixed either with 4% formaldehyde in PBS or with methanol at –20°C, and the samples were processed as described previously.
Electron Microscopy
HeLa cells grown in six-well dishes were transfected with GR65–1 or its scrambled counterpart. Sixty hours after the knockdown transfection, cells were fixed with 1% glutaraldehyde in HEPES buffer, embedded in Epon 812, and cut into thin sections. Analysis of thin sections was performed under Tecnai-12 electron microscope (FEI, Philips, Einhoven, The Netherlands). Images were taken using a Ultra View (Olympus, California) CCD digital camera. Morphometric analysis of Golgi stacks was performed in 30 cells for each experimental condition using the ANALYSIS software.
VSV-G Transport
HeLa cells grown in six-well dishes were transfected with GRASP65-specific or control siRNA. Twelve hours after the knockdown transfection, the cells were split and plated onto coverslips. Twenty-four hours after the knockdown transfection, cells on coverslips were transfected with the GFP-VSV-G (tsO45) plasmid (Hirschberg et al., 1998). The cells were first incubated at 37°C for 24 h and then shifted to 40°C for 8 h to accumulate GFP-VSV-G in the ER. The cells were then released from the temperature block and the localization of VSV-G was analyzed by immunofluorescence microscopy at several time points after release from the temperature block.
FACS Analysis
Cells were fixed in ice-cold 70% ethanol, followed by extraction with 45 mM Na2HPO4, 2.5 mM citric acid, and 0.1% Triton X-100, pH 7.8, and staining with 10 mM propidium iodide, 10 mM PIPES, 100 mM NaCl, 2 mM Mg2Cl, 0.2% Triton X-100, and 50 U/ml RNAse, pH 6.8) for 30 min at room temperature. Cells were then subjected to FACS at the UC San Diego Cancer Center FACS facility.
RESULTS
Depletion of GRASP65 Results in Cell Death
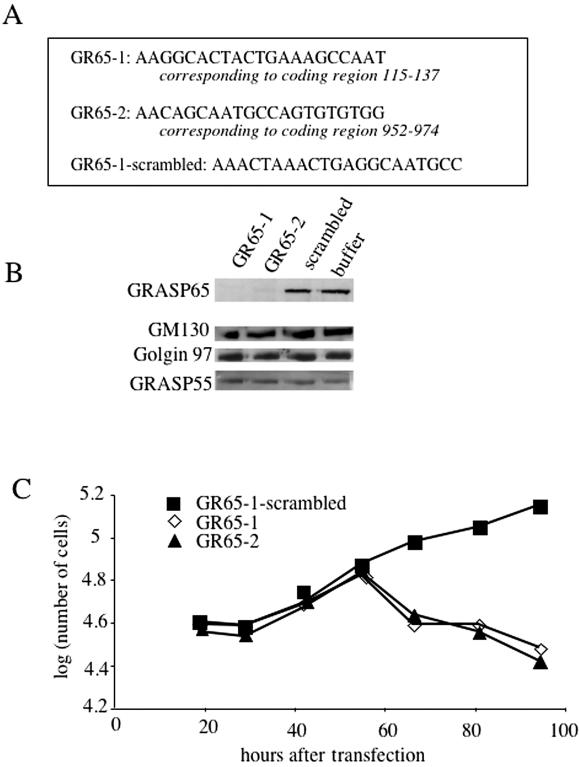
We depleted GRASP65 from HeLa cells using the two GRASP65-specific siRNAs GR65–1 and GR65–2 (Figure 1A). A scrambled version of GR65–1 (control siRNA) and buffer were used as controls. Forty-eight to 72 h after transfection, total cell lysates were analyzed for the levels of GRASP65 by immunoprecipitation and the Golgi proteins GM130, Golgin 97, and GRASP55 by Western blotting. As shown in Figure 1B, GRASP65 is efficiently depleted from HeLa cells by both siRNAs. Transfection of the scrambled control siRNA showed no effect on the level of GRASP65 when compared with the buffer control. The levels of the Golgi proteins GM130, Golgin 97, and GRASP55 were unaffected under these conditions.
Figure 1.
Depletion of GRASP65 by siRNA promotes cell death. (A) The sequences of the human GRASP65 gene used for designing SiRNA probes are shown (exact location is indicated). GR65–1–scrambled represents a scrambled version of GR65–1 used as control. (B) HeLa cells were transfected with GR65–1, GR65–2, GR65–1–scrambled or buffer. After 48 h, the level of GRASP65 was analyzed by immunoprecipitation/western blotting from total lysate, whereas the levels of the Golgi proteins GM130, Golgin 97, and GRASP55 were detected by Western blot analysis of the same lysates. (C) HeLa cells grown in six-well dishes were transfected with GR65–1, GR65–2, or GR65–1–scrambled. At the indicated time points, cells from two independent wells/per transfection were trypsinized and stained with trypan-blue to identify dead cells, followed by counting the live (i.e., trypan-blue negative) cells.
Because a higher rate of cell death was observed in GRASP65-specific siRNA transfections than in control transfections, we analyzed the growth rate of GRASP65-depleted and control cells (Figure 1C). Initially, cells transfected with control or GRASP65-specific siRNAs grew at similar rates. However, 48–72 h after transfection, GRASP65-depleted cells were dying, suggesting that GRASP65 is necessary for cell growth and survival. The timing of cell death in GRASP65-specific siRNA transfections correlates with the timing of efficient GRASP65 depletion. Because in these initial experiments, both GRASP65-specific siRNAs removed GRASP65 and caused cell death to a similar extent, GR65–1 and its scrambled control counterpart were used for all experiments described below.
GRASP65 Depletion Does Not Perturb Golgi Organization or Protein Transport
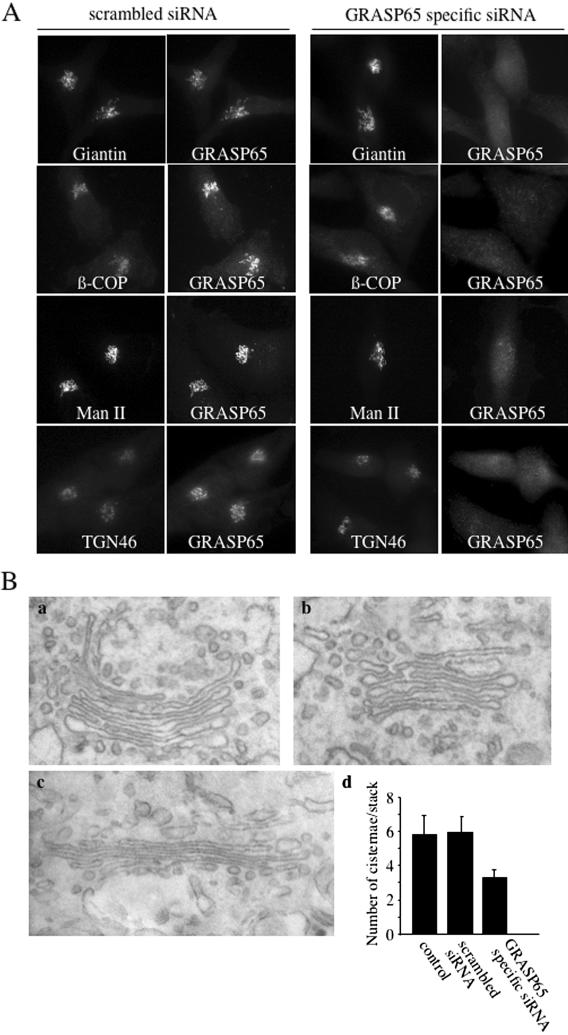
GRASP65 has been proposed to function as a structural component of the Golgi apparatus (Barr et al., 1997; Wang et al., 2004). To investigate the reason for cell death in the absence of GRASP65, we first tested the effect of GRASP65 depletion on the organization of the Golgi apparatus. HeLa cells and HeLa cells stably expressing a GFP-tagged form of mannosidase II grown on coverslips were transfected with GRASP65-specific or scrambled control siRNA. Then 2.5 d after the transfections, cells were fixed and processed for fluorescence microscopy using antibodies to the bona fide Golgi markers giantin, β-COP, and TGN46 to monitor the organization of Golgi membranes in a cis-to-trans direction (Figure 2A). Although GRASP65 colocalized with the Golgi proteins giantin, β-COP, mannosidase II, and TGN46 in control cells, GRASP65 depleted in 85% of the cells transfected with the GRASP65-specific siRNA 48–72 h after siRNA transfection. However, in GRASP65 knockdown cells, marker proteins of the cis/medial and trans-Golgi cisternae were localized to the pericentriolar region of the cell. It thus appears that GRASP65 is not required for the overall organization of the Golgi apparatus in HeLa cells. Depletion of GRASP65 in another cell line, U2OS cells, also revealed no obvious defect in the overall Golgi organization (unpublished data). We analyzed the organization of Golgi membranes by electron microscopy in HeLa cells and found stacked Golgi membranes in control and GRASP65-depleted cells (Figure 2B). Interestingly, however, the number of cisternae per stack was reduced from an average of 6 to an average of 3 per stack. Because there is no apparent loss of the components of early (cis/medial) and late (TGN) Golgi cisternae, we entertain the possibility that in the absence of GRASP65, Golgi cisternae fuse. The overall number of cisternae would thus be reduced without perturbing the stacked organization of Golgi membranes. This observation could help explain previous reports, which showed that interfering with GRASP65 in an in vitro Golgi reassembly assay produced single or isolated Golgi cisternae (less Golgi cisternae) instead of stacked cisternae (Barr et al., 1997).
Figure 2.
GRASP65 depletion does not affect the organization of Golgi membranes. (A) HeLa cells were transfected with GR65–1–scrambled (scrambled siRNA) and GR65–1 (GRASP65-specific siRNA) and plated on coverslips. Forty-eight to 60 h after transfection, cells were fixed and processed for immunofluorescence microscopy using the indicated antibodies. To detect mannosidase II, a HeLa cell line that stably expresses mannosidase II-GFP was used for transfection. (B) HeLa cells untreated (a, control), transfected with GR65–1 (b), or GR65–1–scrambled (c) were cultured for 48 h. The cells were processed for electron microscopy as described in Materials and Methods. The number of Golgi cisternae per stack was quantified from 30 cells per condition (d).
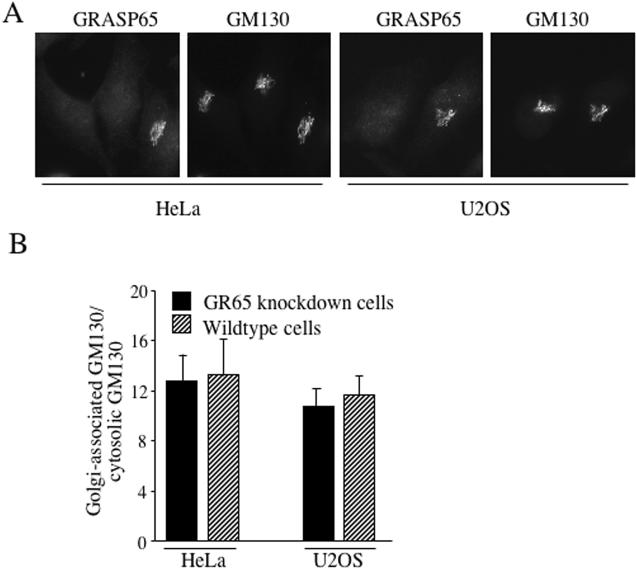
The Golgi-associated protein GM130 tightly binds to GRASP65 and its localization is proposed to depend on membrane-bound GRASP65 (Barr et al., 1998). We therefore analyzed the distribution of GM130 in control and GRASP65-depleted HeLa and U2OS cells (Figure 3A) and found that the Golgi localization of GM130 is not affected by GRASP65 depletion. The quantification of Golgi-bound and cytosolic GM130 in GRASP65-depleted and control cells confirmed this finding for both cell lines, HeLa and U2OS (Figure 3B). Therefore, binding to GRASP65 cannot be the sole mechanism of GM130 localization to Golgi membranes.
Figure 3.
GM130 binds Golgi membranes independent of GRASP65. (A) HeLa or U2OS cells were transfected with GR65–1 or its scrambled counterpart. Sixty hours after transfection, cells were fixed and processed for immunofluorescence microscopy with antibodies to GRASP65 and GM130. Images show transfected cells depleted of GRASP65 and for comparison a nontransfected wild-type cell on the same coverslip. (B) Images obtained for the experiment described in A were quantified using the Axiovert Imaging Software (Zeiss, Thornwood, NY) by determining the average signal density of GM130 on the Golgi and in the cytosol in GR65–1–transfected cells or nontransfected control cells observed on the same coverslip. The ratio of Golgi-associated versus cytosolic signal for GM130 is shown from seven independent cells on four different coverslips.
GRASP65 Is Not Required for Protein Transport or Drug-induced Fragmentation/Reassembly
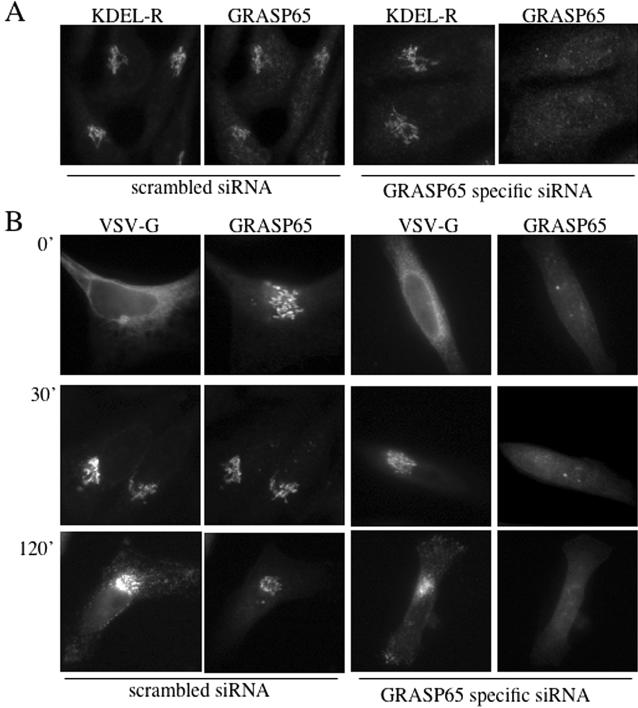
Does GRASP65 depletion affect protein transport along the secretory pathway? KDEL-receptor cycles between the ER and the early Golgi apparatus and can thus be used to monitor this step of the secretory pathway (Lewis and Pelham, 1992). In both, control and GRASP65-depleted cells, KDEL-receptor was localized mainly in the Golgi membranes, suggesting no apparent defect in protein transport between the ER and the Golgi apparatus (Figure 4A). To address whether GRASP65 is required for protein transport across the Golgi stack and between the Golgi and the cell surface, we analyzed the transport of VSV-G from the ER via the Golgi to the cell surface (Figure 4B). Control and GRASP65-depleted cells grown on coverslips were transfected with a plasmid encoding for VSV-G (tsO45)-GFP (Hirschberg et al., 1998). Twenty-four hours after transfection, cells were shifted to 40°C for 8 h to accumulate the temperature sensitive VSV-G in the ER. Cells were then shifted to 32°C to allow transport out of the ER and at various time points, cells were fixed and analyzed for the distribution of GFP and GRASP65. VSV-G was transported to the cell surface with similar kinetics in control and GRASP65 knockdown cells, suggesting that GRASP65 was not required for transport of VSV-G along the secretory pathway (Figure 4, A and B).
Figure 4.
GRASP65 depletion does not perturb protein traffic along the secretory pathway. (A) The localization of KDEL-receptor, a marker of protein traffic between ER and the early Golgi, was analyzed in HeLa cells 48 h after transfection with scrambled control or GRASP65-specific siRNA. (B) The transport of the vesicular stomatitis virus G-protein (VSV-G) was analyzed in control and GRASP65-depleted cells. Cells were transfected with scrambled control or GRASP65-specific siRNA, followed by the transfection with a plasmid encoding for VSV-G (tsO45)-GFP. After a 24-h incubation at 37°C, the cells were shifted to 40°C for 8 h to arrest VSV-G in the ER. Cells were then shifted to the permissive temperature of 32°C to allow progression of VSV-G-GFP along the secretory pathway. Samples were taken at the indicated time and fixed for immunofluorescence microscopy to localize VSV-G-GFP and GRASP65, respectively.
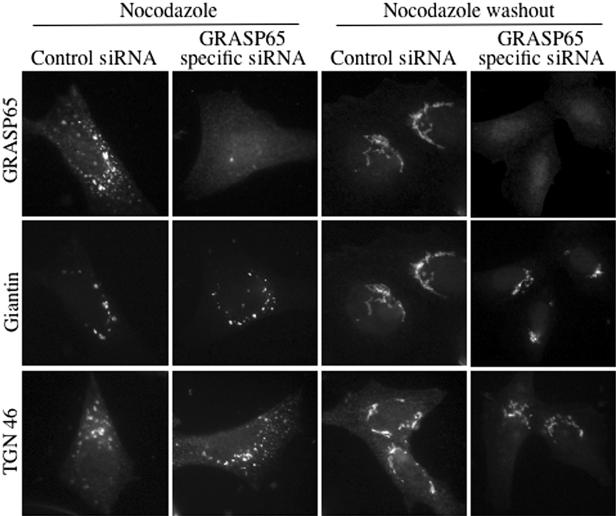
Nocodazole treatment depolymerizes microtubules resulting in reorganization of the pericentriolar Golgi apparatus into small stacks that are dispersed throughout the cytosol (Cole et al., 1996). This drug-induced perturbation of Golgi organization is reversible. Cells transfected with GRASP65-specific siRNA or its scrambled counterpart were treated with nocodazole (Figure 5). One set of coverslips was fixed for immunofluorescence and another washed to remove nocodazole to monitor recovery of the Golgi apparatus. In the absence of GRASP65, Golgi proteins were found in the form of ministacks in the cytosol after treatment with nocodazole. After washout of nocodazole, Golgi membranes reassembled in the pericentriolar region of the cells, just as seen in control cells (Figure 5). The same set of experiments was carried out with brefeldin A (BFA), which causes Golgi membranes to reversibly fuse with the ER (Lippincott-Schwartz et al., 1989). We observed that BFA-induced Golgi dynamics were normal in the absence of GRASP65 (unpublished data) and conclude that GRASP65 is not required for Golgi disassembly or reassembly after treatment of cells with BFA or nocodazole.
Figure 5.
Nocodazole-induced changes in Golgi organization are not perturbed by GRASP65 depletion. HeLa cells were transfected with the scrambled control or the GRASP65-specific siRNA. Forty-eight hours after transfection, cells were treated with 25 μg/ml nocodazole 90 min (Nocodazole) and either fixed or washed with medium to remove nocodazole and allowed to recover for 3 h (Nocodazole washout). The cells were processed for immunofluorescence microscopy using antibodies to GRASP65 to confirm the knockdown, to the cis-Golgi marker giantin and to the TGN marker TGN 46 to monitor the disassembly and reassembly of the cis- and trans-specific cisternae of the Golgi apparatus.
GRASP65 Depletion Perturbs Spindle Dynamics
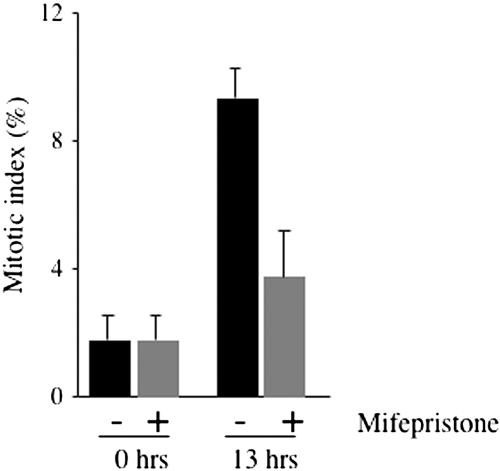
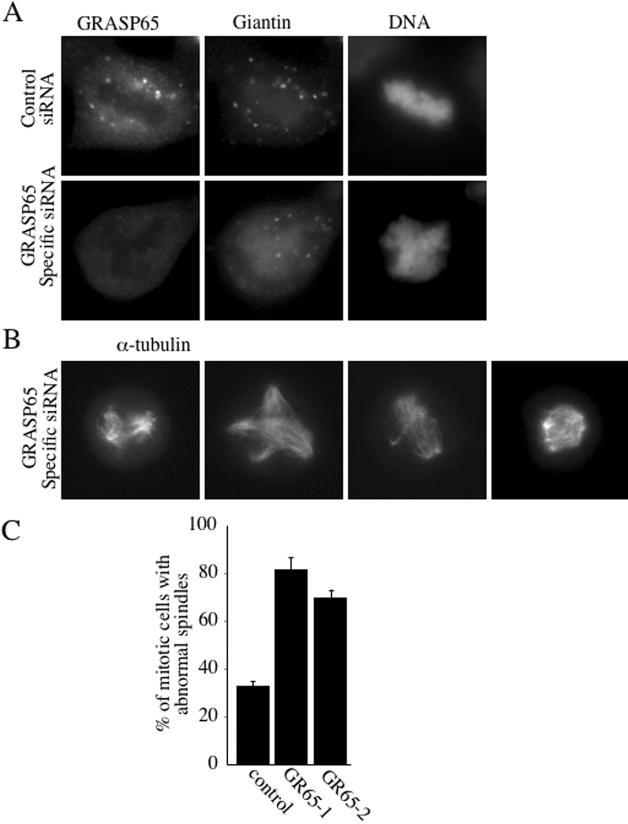
Why do GRASP65-depleted cells die? We have previously shown that interfering with the C-terminus of GRASP65 prevented mitotic Golgi fragmentation and arrested cells in the G2 phase of the cell cycle (Sütterlin et al., 2002). These experiments had been carried out by microinjecting recombinant GRASP65 C-terminus into NRK cells. We extended these studies to HeLa cells and generated a stable cell line, which expresses a V5-tagged form of the C-terminus of human GRASP65 (hsΔ200-V5) under the control of an inducible promotor. This cell line provides a convenient means to analyze the involvement of the C-terminus of GRASP65 in mitotic progression as similar quantities of the protein are expressed in an inducible manner in 60% of the cells. This approach is better than our previous and more tedious approach of microinjecting cells with purified recombinant Δ200 GRASP65 and the recently used assay of transient transfection of NRK cells with Δ200 GRASP65 (Sütterlin et al., 2002; Preisinger et al., 2005). Cells were arrested in S-phase by thymidine treatment for 12 h in the absence or presence of mifepristone, a specific inducer of V5-tagged hsΔ200 expression. Thymidine was removed and the cells were allowed to enter the cell cycle in the absence or presence of the inducer. Thirteen hours after thymidine washout, cells were fixed and processed for immunofluorescence using antibodies to phospho-histone H3 to detect mitotic cells and to V5 to identify hsΔ200-expressing cells. Although at this time point, ∼9% of the cells were in mitosis in uninduced cells, only ∼4% of the induced cells stained positive for phospho-histone H3 (Figure 6). This finding confirmed our previous result that the C-terminus of GRASP65 blocks mitotic Golgi fragmentation and mitotic entry, suggesting that GRASP65-depleted cells may die as a consequence of a cell cycle block at the G2/M transition. To address whether cells enter mitosis in the absence of GRASP65, nonsynchronous populations of control and GRASP65-depleted cells were fixed and processed for immunofluorescence using antibodies to GRASP65 to confirm the knockdown, to the Golgi marker giantin to monitor Golgi fragmentation and the DNA dye Hoechst. We observed mitotic cells with condensed chromatin and fragmented Golgi membranes in control and GRASP65-depleted cells (Figure 7A). Staining with an antibody to α-tubulin revealed that control cells contained predominantly bipolar spindles that aligned the DNA in the metaphase plate, whereas multiple disorganized and nonfunctional spindle asters were observed in GRASP65-depleted cells (Figure 7B). These aberrant spindles were unable to align condensed chromatin for subsequent segregation into the daughter cells and were observed in ∼80 or 75% of the mitotic cells transfected with GR65–1 or GR65–2, respectively (Figure 7C). We defined and counted abnormal spindles as those that were monopolar, multipolar, or mislocalized or had unfocused poles. The number of 30% of cells with abnormal spindles for control cells may appear high, and it is conceivable that it contains, at least in control cells, spindles that mature into normal bipolar spindles. A similar defect in spindle formation was observed in U2OS cells (unpublished data). Because we did not observe any defect in the organization of tubulin during interphase (unpublished data), we propose that GRASP65 regulates microtubule dynamics only during entry into mitosis.
Figure 6.
Expression of hsΔ200 GRASP65 arrests cells at the G2/M transition in HeLa cells. A cell line expressing the V5-tagged C-terminus of human GRASP65 (hsΔ200-V5) under an inducible promotor (Switch System, Invitrogen) was arrested in S-phase by double thymidine treatment. The second thymidine incubation was carried out in the absence or presence of mifepristone, which is an inducer of hsΔ200-V5 expression. Thymidine was removed to allow progression into mitosis, in the absence or the presence of the inducer. Cells were fixed 13 h after thymidine washout. Cells were processed for immunofluorescence microscopy using antibodies to V5 to detect hsΔ200-V5–expressing cells and phospho-histone H3 to identify mitotic cells. The quantification of four independent coverslips is shown.
Figure 7.
GRASP65 depletion promotes spindle abnormalities. (A) A nonsynchronous population of cells was processed for immunofluorescence microscopy 48 h after transfection with scrambled control or GRASP65-specific siRNA, using antibodies to GRASP65 to verify the knockdown, giantin to monitor the organization of Golgi membranes, and with the DNA dye Hoechst to identify mitotic cells with condensed chromatin. (B) Mitotic GRASP65-depleted HeLa cells were stained with anti-α-tubulin antibody to reveal the organization of the mitotic spindle. Four different mitotic cells are shown. (C) The percentage of mitotic cells with abnormal spindles (monopolar, multipolar, misaligned, unfocused pole) observed 48 h after transfection with GR65–1–scrambled, GR65–1 or GR65–2. These results are derived from three independent experiments, each sample derived from two independent transfections.
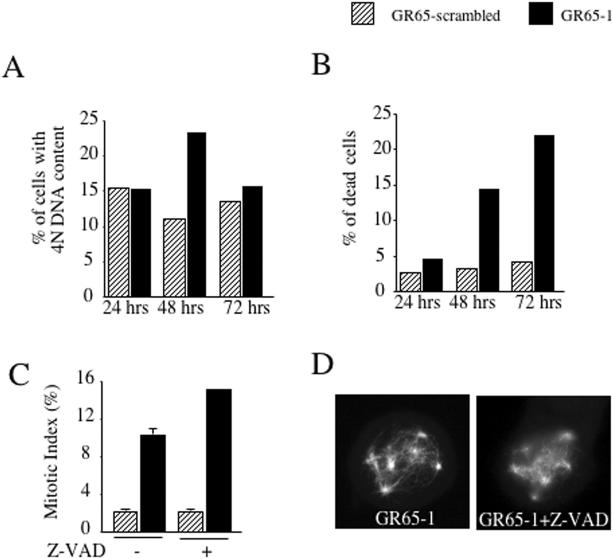
We then asked whether cells with defective spindles exit mitosis. Knockdown and control cells were subjected to propidium iodide–based FACS analysis at various time points after knockdown transfection. Although 24 h posttransfection, no difference between control and knockdown cells could be detected, the 48-h time point revealed an accumulation of cells with 4N DNA content and an increasing number of dead cells (Figure 8, A and B). At 72 h after transfection, the accumulation of 4N DNA content cells was less prominent, whereas more than 20% of the propidium iodide signal corresponded to dead cells (sub-G1), suggesting that the accumulated cells were unable to progress further in the cell cycle and died. The additional comparison of the mitotic index of control and knockdown cells at the 48-h time point demonstrated that the accumulated cells were in mitosis (Figure 8C). We also addressed whether defective spindles are the cause or the consequence of cells undergoing apoptosis. Cells depleted of GRASP65 were treated for 16 h with the generic apoptosis inhibitor Z-VAD. Immunofluorescence analysis with antibodies to phospho-histone H3 and α-tubulin revealed an increase in the mitotic index from 10 to 15% in Z-VAD–treated GRASP-depleted cells, whereas no change was observed in control transfected cells. Seventy-five percent of mitotic Z-VAD–treated GRASP65 knockdown cells contained aberrant spindles, demonstrating that depletion of GRASP65 leads to defects in spindle formation, which causes a metaphase block and subsequent cell death.
Figure 8.
GRASP65-depleted cells are arrested in mitosis before cell death. (A) HeLa cells transfected with scrambled control or GRASP65-specific siRNA were trypsinized at various time after transfection, stained with propidium iodide, and subjected to FACS analysis. The percentage of cells with 4N DNA content is shown. (B) The propidium iodide–stained cells prepared for the experiment described in A were analyzed for their sub-G1 (dead or damaged cells) content. The percentage of propidium iodide signal corresponding to dead or damaged cells is shown. (C) The mitotic index (positive staining with an antibody to phospho-histone H3) was determined by immunofluorescence 48 h after knockdown transfection from scrambled control-transfected or GRASP65-depleted cells. The mitotic index was also determined from knockdown and control-transfected cells that have been incubated for 16 h with 20 μM Z-VAD. (D) Mitotic GRASP65-depleted HeLa cells were left untreated or incubated with 20 μM Z-VAD for 16 h and stained with anti-α-tubulin antibody to reveal the organization of the mitotic spindle.
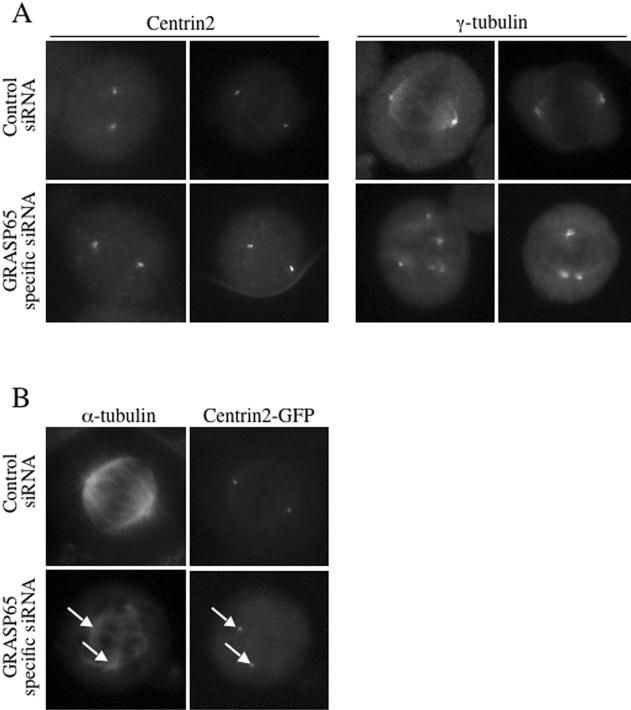
To characterize the aberrant spindle asters in GRASP65 knockdown cells, we stained control and GRASP65-depleted cells with antibodies to Centrin2 or γ-tubulin (Figure 9A). We also stained these cells with the mitotic marker phospho-histone H3 to verify that they were in mitosis (unpublished data). Centrin2, a small calcium-binding protein and a component of the centriole and is required for centriolar duplication (Salisbury et al., 2002). Only ∼20% of the cellular γ-tubulin localizes to the centrosome during interphase, the remainder is cytosolic and is found in the form of γ-tubulin complexes (γ-TuRC; Moudjou et al., 1996). The recruitment of these complexes to the centrosome at the onset of mitosis contributes to a significant increase in microtubule nucleation activity required for spindle formation (Khodjakov and Rieder, 1999). Control cells contained Centrin2 and γ-tubulin at the poles of each spindle aster (Figure 9A). GRASP65-depleted cells revealed that 79% of the mitotic cells contained two Centrin2-positive poles with two centrioles each. In contrast, multiple γ-tubulin foci were observed in 80% of the mitotic GRASP65 knockdown cells, suggesting that the multiple aberrant spindle poles do not contain Centrin2. To address the organization of α-tubulin and Centrin2 in the same mitotic cell, we generated a cell line, which stably expresses Centrin2-GFP. Again, in GRASP65-depleted cells, we observed multiple disorganized spindle asters of which only two contained Centrin2-GFP (Figure 9B). We conclude that depletion of GRASP65 interferes with γ-tubulin recruitment to the mitotic centrosome, which is required for bipolar spindle formation. Furthermore, the absence of GRASP65 does not cause defects in centrosome duplication during S-phase or centrosome fragmentation because two Centrin2-containing–based asters are seen in control and GRASP65-depleted cells.
Figure 9.
GRASP65 depletion perturbs γ-tubulin recruitment to the centrosome. (A) A nonsynchronous population of scrambled control or GRASP65-specific siRNA-transfected HeLa cells was processed for immunofluorescence microscopy 48 h after siRNA transfection using antibodies to the centrosomal marker protein Centrin2 or the pericentriolar protein γ-tubulin to monitor the composition of the aberrant spindle poles (2 cells are shown for each phenotype). These cells were positive for the mitotic marker phospho-histone H3 (unpublished data). (B) A HeLa cell line stably expressing Centrin2-GFP was generated, transfected with scrambled control or GRASP65-specific siRNA and processed for immunofluorescence microscopy to monitor Centrin2-GFP and α-tubulin in the same mitotic cell. The α-tubulin staining reveals a number of disorganized spindle asters of which only two contained Centrin2-GFP (arrows).
DISCUSSION
At the onset of mitosis, the interphase centrosome undergoes a dramatic reorganization to promote spindle formation (see for review: Bornens, 2002). Several factors contribute to this conversion: the microtubule nucleating activity is significantly enhanced by recruitment of cytosolic γ-tubulin ring complexes (γ-TuRCs) to the centrosome (Khodjakov and Rieder, 1999). γ-TuRC-recruitment to the centrosome itself does not require intact microtubules, but is dependent on pericentrin and other centrosomal proteins, which anchor γ-TURCs at the spindle poles (Khodjakov and Rieder, 1999; Zimmerman et al., 2004). In addition to the recruitment of γ-tubulin complexes to the centrosome, noncentrosome-associated microtubules move toward the spindle and contribute to spindle formation (Tulu et al., 2003).
We have found that the Golgi-associated protein GRASP65 is important for bipolar spindle formation and suggest that this is through the regulation of γ-tubulin recruitment to the centrosome at the onset of mitosis. Although the exact function of GRASP65 in centrosomal reorganization during mitosis remains to be determined, our data demonstrates that the Golgi apparatus and the centrosome are tightly linked, not only spatially, but also functionally. Additional evidence in support of this proposal stems from the findings that ZW10, a component of the spindle checkpoint and RIN-1, which participates in the radiation-induced G2/M checkpoint, were recently shown to interact with the ER t-SNARE syntaxin 18 and to regulate ER-to-Golgi transport (Hirose et al., 2004). The yeast homolog of the mammalian GRASP proteins has been identified as component of the spindle checkpoint in Saccharomyces cerevisiae (Norman et al., 1999).
The Role of Golgi Locale/Organization and GRASP65 in Cell Cycle Progression
We have previously shown that inhibiting Golgi fragmentation by microinjecting GRASP65-specific reagents prevented entry into mitosis. The cells as a result were arrested in G2 (Sütterlin et al., 2002). Artificial fragmentation of the Golgi membranes under these conditions alleviated this G2-specific block. In other words, GRASP65-specific reagents block entry into mitosis by preventing fragmentation and dispersal of the pericentriolar Golgi apparatus. This proposal is strengthened by the data shown here: inducible expression of the C-terminus of GRASP65 in HeLa cells prevented entry into mitosis. Inhibiting Golgi fragmentation by interfering with the function of BARS50 also arrests cells in G2 (Carcedo et al., 2004). More recently, Preisinger and colleagues have reported that transient transfection of the C-terminal portion of GRASP65 delayed mitotic progression rather than an arrest in G2. We think this minor difference is likely due to unequal expression of the C-terminal GRASP65 by transient transfection. Regardless, evidence is accumulating for a “locale and organization,” specific role of Golgi membranes in cell cycle progression. GRASP65, when localized to the pericentriolar Golgi apparatus has a negative role with respect to Golgi fragmentation. This inhibitory function has to be alleviated, e.g., by phosphorylation or interaction with another protein, to allow Golgi fragmentation. In addition to this negative function, GRASP65 is required during mitosis for the formation of a bipolar spindle, as demonstrated in this study. In the absence of GRASP65, Golgi membranes fragment and cells enter mitosis, but the cells are arrested in metaphase and die most likely because γ-tubulin recruitment is defective and multiple nonfunctional spindle asters are formed. The study presented here provides further evidence for the emerging role of the pericentriolar Golgi membranes and its components in controlling cell cycle progression and affecting signaling events in addition to a protein sorting and transport compartment.
In vitro assays have revealed important players of the mitosis-specific Golgi dynamics. However, these assays do not permit a distinction between the role of a given protein as a structural component, trafficking component, or a signaling molecule that connects the process of Golgi fragmentation to other cell cycle–specific physiological processes. As shown here, GRASP65 is not a structural component or required for protein trafficking in nondividing cells. GRASP65 thus belongs to a special class of proteins, which reside on the Golgi cisternae and undergo a specific modification such as phosphorylation, whereas the cells are in G2 for its functional role later in mitosis. Analysis of Golgi-associated proteins in intact cells is thus necessary to decipher the function of proteins in the multitask compartment such as the Golgi apparatus.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–12–1065) on May 11, 2005.
References
- Acharya, U., Mallabiabarrena, A., Acharya, J. K., and Malhotra, V. (1998). Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell 92, 183–192. [DOI] [PubMed] [Google Scholar]
- Axelsson, M. A., and Warren, G. (2004). Rapid, endoplasmic reticulum-independent diffusion of the mitotic Golgi haze. Mol. Biol. Cell 15, 1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, F. A., Nakamura, N., and Warren, G. (1998). Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 17, 3258–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, F. A., Puype, M., Vandekerckhove, J., and Warren, G. (1997). GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91, 253–262. [DOI] [PubMed] [Google Scholar]
- Bornens, M. (2002). Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34. [DOI] [PubMed] [Google Scholar]
- Burke, B., Griffiths, G., Reggio, H., Louvard, D., and Warren, G. (1982). A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1, 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcedo, C. H., Bonazzi, M., Spano, S., Turacchio, G., Colanzi, A., Luini, A., and Corda, D. (2004). Mitotic Golgi partitioning is driven by the membrane-fissioning protein CtBP3/BARS. Science 305, 93–96. [DOI] [PubMed] [Google Scholar]
- Chabin-Brion, K., Marceiller, J., Perez, F., Settegrana, C., Drechou, A., Durand, G., and Pous, C. (2001). The Golgi complex is a microtubule-organizing organelle. Mol. Biol. Cell 12, 2047–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colanzi, A., Suetterlin, C., and Malhotra, V. (2003a). Cell-cycle-specific Golgi fragmentation: how and why? Curr. Opin. Cell Biol. 15, 462–467. [DOI] [PubMed] [Google Scholar]
- Colanzi, A., Sutterlin, C., and Malhotra, V. (2003b). RAF1-activated MEK1 is found on the Golgi apparatus in late prophase and is required for Golgi complex fragmentation in mitosis. J. Cell Biol. 161, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, N. B., Sciaky, N., Marotta, A., Song, J., and Lippincott-Schwartz, J. (1996). Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 7, 631–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg, K., Miller, C. M., Ellenberg, J., Presley, J. F., Siggia, E. D., Phair, R. D., and Lippincott-Schwartz, J. (1998). Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 143, 1485–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose, H., Arasaki, K., Dohmae, N., Takio, K., Hatsuzawa, K., Nagahama, M., Tani, K., Yamamoto, A., Tohyama, M., and Tagaya, M. (2004). Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 23, 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesch, S. A., Mehta, A. J., Velliste, M., Murphy, R. F., and Linstedt, A. D. (2001). Mitotic Golgi is in a dynamic equilibrium between clustered and free vesicles independent of the ER. Traffic 2, 873–884. [DOI] [PubMed] [Google Scholar]
- Kano, F., Takenaka, K., Yamamoto, A., Nagayama, K., Nishida, E., and Murata, M. (2000). MEK and Cdc2 kinase are sequentially required for Golgi disassembly in MDCK cells by the mitotic Xenopus extracts. J. Cell Biol. 149, 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., and Rieder, C. L. (1999). The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, M. J., and Pelham, H. R. (1992). Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 68, 353–364. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L. C., Bonifacino, J. S., and Klausner, R. D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, M., Rabouille, C., Nakamura, N., Watson, R., Jackman, M., Jamsa, E., Rahman, D., Pappin, D. J., and Warren, G. (1998). Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell 94, 783–793. [DOI] [PubMed] [Google Scholar]
- Maul, G. G., and Brinkley, B. R. (1970). The golgi apparatus during mitosis in human melanoma cells in vitro. Cancer Res. 30, 2326–2335. [PubMed] [Google Scholar]
- Misteli, T., and Warren, G. (1995). Mitotic disassembly of the Golgi apparatus in vivo. J. Cell Sci. 108(Pt 7), 2715–2727. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., Bordes, N., Paintrand, M., and Bornens, M. (1996). gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109(Pt 4), 875–887. [DOI] [PubMed] [Google Scholar]
- Nishimura, T., Takahashi, M., Kim, H.-S., Mukai, H., and Ono, Y. (2005). Centrosome-targeting region of CG-NAP causes centrosome amplification by recruiting cyclin E-cdk2 complex. Genes Cells 10, 75–86. [DOI] [PubMed] [Google Scholar]
- Norman, T. C., Smith, D. L., Sorger, P. K., Drees, B. L., O'Rourke, S. M., Hughes, T. R., Roberts, C. J., Friend, S. H., Fields, S., and Murray, A. W. (1999). Genetic selection of peptide inhibitors of biological pathways. Science 285, 591–595. [DOI] [PubMed] [Google Scholar]
- Pecot, M. Y., and Malhotra, V. (2004). Golgi membranes remain segregated from the endoplasmic reticulum during mitosis in mammalian cells. Cell 116, 99–107. [DOI] [PubMed] [Google Scholar]
- Preisinger, C., Korner, R., Wind, M., Lehmann, W. D., Kopajtich, R., and Barr, F. A. (2005). Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signaling. EMBO J. 24, 753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, E., and Gonatas, N. K. (1964). Histochemical and ultrastructural studies on Hela cell cultures exposed to spindle inhibitors with special reference to the interphase cell. J. Histochem. Cytochem. 12, 704–711. [DOI] [PubMed] [Google Scholar]
- Roth, J., and Berger, E. G. (1982). Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J. Cell Biol. 93, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury, J. L., Suino, K. M., Busby, R., and Springett, M. (2002). Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 12, 1287–1292. [DOI] [PubMed] [Google Scholar]
- Sütterlin, C., Hsu, P., Mallabiabarrena, A., and Malhotra, V. (2002). Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell 109, 359–369. [DOI] [PubMed] [Google Scholar]
- Sütterlin, C., Lin, C. Y., Feng, Y., Ferris, D. K., Erikson, R. L., and Malhotra, V. (2001). Polo-like kinase is required for the fragmentation of pericentriolar Golgi stacks during mitosis. Proc. Natl. Acad. Sci. USA 98, 9128–9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg, J., and Moskalewski, S. (1992). Reorganization of the Golgi complex in association with mitosis: redistribution of mannosidase II to the endoplasmic reticulum and effects of brefeldin A. J. Submicrosc. Cytol. Pathol. 24, 495–508. [PubMed] [Google Scholar]
- Tulu, U. S., Rusan, N. M., and Wadsworth, P. (2003). Peripheral, non-centrosome-associated microtubules contribute to spindle formation in centrosome-containing cells. Curr. Biol. 13, 1894–1899. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Satoh, A., and Warren, G. (2005). Mapping the functional domains of the Golgi stacking factor GRASP65. J. Biol. Chem. 280, 4921–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal, K. J. et al. (1999). Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell 99, 589–601. [DOI] [PubMed] [Google Scholar]
- Zimmerman, W. C., Sillibourne, J., Rosa, J., and Doxsey, S. J. (2004). Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 15, 3642–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]