Abstract
Current seasonal influenza vaccines are limited in that they need to be reformulated every year in order to account for the constant mutation of the virus. Hemagglutinin (HA) immunogens have been developed using computationally optimized broadly reactive antigens (COBRA) methodology that are able to elicit an antibody response that neutralizes antigenically distinct influenza strains; however, subunit proteins are not immunogenic enough on their own to generate a substantial immune response. Due to this, different delivery strategies and adjuvants can be used to improve immunogenicity. Recently, we have reported a new coordination polymer composed of the dipeptide carnosine and zinc (ZnCar) that is able to deliver protein antigens along with CpG to generate a potent immune response. In the present work, ZnCar was used to deliver the COBRA HA immunogen Y2 and the adjuvant CpG. We incorporated Y2 into ZnCar using two different methods to assess which would be the most immunogenic. Mice vaccinated with Y2 and CpG complexed with ZnCar showed an improved humoral and cellular response when compared to mice vaccinated with soluble Y2 and CpG. Further, we demonstrate in vitro that when Y2 and CpG are coordinated with ZnCar, they are protected from degradation at 40°C for 3 months or 24°C for 6 months. Overall, ZnCar shows promise as a delivery vehicle for subunit vaccines given its superior immunogenicity and in vitro storage stability.
Keywords: vaccine, coordination polymer, storage stability, influenza, broadly active antigen
Graphical Abstract

1. Introduction
Influenza is a major burden in the United States and is estimated to cause 52,000 deaths, 710,000 hospitalizations, and 41,000,000 illnesses annually 1. Furthermore, the economic burden of the disease has been estimated to be as high as $8 billion 2. Given this immense impact, vaccination against the virus is a major public health priority. Today, FDA approved influenza vaccines incorporate viral strains that are predicted to be the major circulating strains for the upcoming season; however, these predictions are months before they are applied, leading to mismatches months later when they are injected into patients. This is largely due to antigenic drift/shift of the immunodominant hemagglutinin (HA) protein located on the viral surface that changes every season rendering the previous season’s vaccine less effective 3.
Recently, development of a broadly active influenza vaccine that would not need reformulation every season has been a priority for vaccine researchers. One approach uses conserved influenza antigens, such as the matrix protein 2 ectodomain (M2e). While M2e does vary only slightly from season to season, there are shortcomings with using it as an antigen in vaccines. For example, anti-M2e antibodies are non-neutralizing, and instead provide protection via other effector-mediated antibody functions like antibody dependent cell-mediated cytotoxicity (ADCC) that largely cannot take place until the first round of replication has occurred 4. Another conserved antigen is the stalk region of HA. The shortcomings with the stalk antigen include vaccine induced disease enhancement due to cross-reactive anti-stalk antibodies, eliciting lower affinity antibodies binding to HA, increased potential for autoreactivity, and lack of reduced viral loads or lung inflammation after challenge 5–11. An alternative to targeting conserved epitopes is using a computationally optimized broadly reactive antigen (COBRA). COBRA is a methodology for generating HA proteins using multi-layered consensus sequencing that results in antigens that elicit broadly reactive antibodies that can neutralize influenza viruses across many seasons 12.
While the COBRA methodology has proven to be effective in protecting against influenza viruses across multiple influenza seasons in both mice and ferret models, there are some limitations. First, subunit vaccines often have low immunogenicity since there are no other danger signals delivered with subunit HA proteins that are usually present during natural infection. This means subunit vaccines often require the inclusion of an adjuvant 13. Second, these protein subunit vaccines usually have low thermal stability 14–16. This is a major problem when considering the distribution of vaccines to resource limited settings where low temperature storage may be cost prohibitive. Furthermore, the only FDA-approved adjuvant for use in an influenza vaccine, MF59, must be stored at 4°C 17.
Metal-organic coordination polymers are a new and emerging class of drug delivery vehicle that have the potential to provide a solution to these issues in the delivery of subunit vaccines 18. They are advantageous in that their synthesis is simpler than other delivery vehicles such as lipid nanoparticles or inactivated viruses, and the components needed to make these coordination polymers are generally inexpensive which is critical when designing a vaccine that requires mass production. Many different metals and linkers have been used to synthesize metal organic coordination polymers 18. For example, Yang et al. synthesized a metal-organic coordination polymer composed of iron and 2-aminoterephthalic acid (MIL-101-Fe-NH2). MIL-101-Fe-NH2 was used to deliver the model protein OVA along with the adjuvant cytosine-phosphate-guanosine (CpG) and produced a potent cellular immune response in mice.
Of the many different metal-organic coordination polymers that have been investigated, one of the most widely used in vaccine delivery is zeolitic imidazolate framework-8 (ZIF-8) which is constructed from zinc and 2-methylimidazole (2-HMIM) 18. ZIF-8 has been used to encapsulate a variety of cargo for vaccine delivery such as adjuvants, antigens, whole viruses, virus-like particles (VLPs), and bacteria 19–22. For example, Teng et al. recently showed that when foot and mouth disease virus VLPs are encapsulated in ZIF-8 they are able to significantly enhance the humoral and cellular immune response in mice when compared to an unencapsulated control 21. ZIF-8 is also advantageous in that it has been shown to protect proteins from thermal degradation 23. Pateil et al. used ZIF-8 to encapsulate the enzyme laccase which was able to retain activity after 21 days storage at 30°C while an unencapsulated laccase control was not as stable 24.
One major disadvantage of ZIF-8 is poor biocompatibility. To address this, our group has recently developed a novel metal-organic coordination polymer composed of the dipeptide carnosine and zinc ions (ZnCar) 25. We showed that ZnCar is significantly more biocompatible compared to ZIF-8 in a variety of cell lines. We also incorporated the TLR9 agonist CpG and the model protein antigen ovalbumin into ZnCar without affecting their function. Furthermore, mice vaccinated with ovalbumin and CpG that had been incorporated into ZnCar showed a substantial humoral and cellular response when compared to ovalbumin and CpG that had not been incorporated 25.
Here, we sought to expand this work by comparing two different methods of antigen incorporation using a next generation H1N1 COBRA HA immunogen (Y2) as our antigen 12. In one method, Y2 was incorporated during the ZnCar synthesis process (Y2/ZnCar). In the other method, Y2 was added after the ZnCar synthesis process (ZnCar-Y2). We hypothesized that the second method would result in better activation of B cells due to B cell receptor (BCR) cross-linking and thus a more potent humoral response. Here, we evaluated this hypothesis in vivo by combing the two different incorporation methods adjuvanted with ZnCar-CpG and assessing the humoral and cellular immune responses in vaccinated mice.
Lastly, to determine the in vitro thermal stability of these platforms, we stored the vaccine at either 40°C for 3 months (similar to the FDA accelerated storage conditions) or 24°C for 6 months and evaluated the activity of CpG and the structure of Y2 after these storage conditions. Overall, this research aims to provide a shelf stable vaccine platform that can promote a potent immune response to a broadly active influenza antigen.
2. Materials and Methods
2.1. Materials
All chemicals were purchased from Sigma (St. Louis, MO) and used as purchased, unless otherwise indicated. Assays, biologics, and disposables were purchased from Thermo Fisher Scientific (Waltham, MA) unless otherwise indicated.
2.2. Cell Culture
DC2.4 Cells (MilliporeSigma, Burlington, MA) were cultured according to the manufacturer’s protocol in RPMI 1640 with L-glutamine and 25 mM HEPES containing 10% FBS and 1% penicillin/streptomycin. Madin-Darby canine kidney (MDCK) cells were cultured in DMEM (Corning, Corning, NY) containing 10% FBS and 1% penicillin/streptomycin. All cells were maintained at 37°C with 5% CO2 and 100% relative humidity.
2.3. Protein Expression
COBRA HA immunogen Y2 was expressed in HEK293T as previously described 12.
2.4. Mice
Female (6–8 week old) BALB/cJ mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal experiments were conducted with the approval of the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC).
2.5. Synthesis of ZnCar Materials
2.5.1. Synthesis of Batches used in Prime-Boost In Vivo Studies
Y2/ZnCar-
The following stock solutions were prepared: carnosine (51.3 mg, 0.227 mmol) in 5 mL of 0.1M HEPES buffer (pH = 7.4) (referred to below as “HEPES buffer”), and Zn(CH3COO)2·2H2O (50.0 mg, 0.227 mmol) in 10 mL of HEPES buffer. An aliquot of Y2 (366.75 μg in 75 μL) in 1X phosphate-buffered saline solution (PBS) was buffer-exchanged into HEPES buffer using 10K Amicon® centrifugal filter (95.7% recovery). To a 4 mL vial equipped with a magnetic stir bar, an aliquot of Zn(CH3COO)2·2H2O stock solution (1.254 mL, contains 6.3 mg) was added. To this solution, a Y2 aliquot (325.8 μg in 90 μL of HEPES buffer) was added while stirring. To this mixture, an aliquot of carnosine stock solution (0.627 mL, contains 6.4 mg) was added while stirring. The vial with the resulting reaction mixture was capped with a Teflon®-lined cap and the reaction mixture was stirred at 37°C for 18 h. The reaction mixture was cooled down to room temperature and the obtained precipitate (white powder) was isolated by centrifugation (21,100 x g, 20 min, 4°C). The pellet was washed with molecular biology grade water (1.5 mL) by re-suspending and pelleting again by centrifugation (21,100 x g, 20 min, 4°C). The wash was repeated one more time, and the obtained pellet was re-suspended in 0.5 mL of molecular biology grade water, frozen at −80°C for 30 min and lyophilized to produce Y2-loaded ZnCar material (Y2/ZnCar) as a white powder (6.93 mg).
Y2 loading in Y2/ZnCar was detected using Bradford’s assay reagent following the manufacturer’s protocol. For the assay, Y2/ZnCar sample was gently disintegrated by treatment with disintegration buffer (10X Tris-EDTA (TE) buffer) at 1 mg/mL Y2/ZnCar concentration while being rotated on a tube rotator until the full dissolution of the sample (1.5 h). Blank ZnCar sample was treated in identical way and served as a control sample (no signal was detected). Y2 loading in Y2/ZnCar was found to be 21.76±2.26 μg protein/mg total, or 2.18±0.23 mass % (average ± standard deviation, n = 3).
ZnCar-Y2-
ZnCar (6.35 mg) was re-suspended in 500 μL of HEPES buffer using a vortex and bath sonication. Y2 aliquots were thawed (10 aliquots, 24.45 μg/5 μL each = 244.5 μg total in 50 uL of PBS) and combined using 385 μL of HEPES buffer. Combined aliquots were added to the ZnCar suspension, and the resulting reaction mixture was swirled a few times, vortexed very lightly and placed on a tube rotator for 1.5 h. Concentrations based on the final volume of the reaction mixture were ZnCar at 6.79 mg/mL, and Y2 at 261.5 μg/mL. After that, rotation was stopped and the and the product was isolated by centrifugation (21,100 x g, 20 min, 4°C). The pellet was washed as described above, and then re-suspended in 0.5 mL of molecular biology grade water, frozen at −80°C for 20 min and lyophilized to produce Y2-loaded ZnCar material (ZnCar-Y2) as a white powder (5.14 mg). Y2 loading in ZnCar-Y2 was detected as with Y2/ZnCar above. Y2 loading in ZnCar-Y2 was found to be 20.07±1.36 μg protein/mg total, or 2.01±0.14 mass % (average ± standard deviation, n = 3).
ZnCar-CpG-
ZnCar (22.42 mg) was re-suspended in 2030 μL of HEPES buffer using a vortex and bath sonication. CpG (InvivoGen ODN 1826, MW 6365 g/mol, San Diego, CA) aliquots were thawed (4 μg/μL aliquots; 1160 μg total in 290 μL of sterile water) and combined using 2245 μL of HEPES buffer. Combined CpG aliquots were added to the ZnCar suspension, and the resulting reaction mixture was swirled a few times, vortexed very lightly and placed on a tube rotator for 70 min. Concentrations based on the final volume of the reaction mixture were ZnCar at 4.91 mg/mL, and CpG at 254 μg/mL. After that, the rotation was stopped and the product was isolated by centrifugation (21,100 x g, 15 min, 4°C). The pellet was washed as above, and then re-suspended in 0.5 mL of molecular biology grade water, frozen at −80°C for 25 min and lyophilized to produce CpG-loaded ZnCar material (ZnCar-CpG) as a white powder (21.30 mg).
CpG loading in ZnCar-CpG was detected using Quant-iT™ OliGreen™ ssDNA Assay Kit and Quant-iT OliGreen ssDNA Reagent following the manufacturer’s protocol. For the assay, ZnCar-CpG sample was gently disintegrated as described above. Blank ZnCar sample was treated in identical way and served as a control sample (no signal was detected). ZnCar-CpG sample was diluted 200 times to be within the linear range of the assay. CpG loading in ZnCar-CpG was found to be 55.94±6.19 μg CpG/mg total, or 5.59±0.62 mass % (average ± standard deviation, n = 4).
Formulation with Sucrose-
For intramuscular (IM) injections, Y2/ZnCar, ZnCar-Y2, and ZnCar-CpG were formulated with the cryoprotectant sucrose for better suspendability of the material. Y2/ZnCar (2.34 mg), ZnCar-Y2 (2.36 mg), and ZnCar-CpG (16.75 mg) were re-suspended in 1.285, 1.180, and 2.346 mL of isotonic sucrose solution (109 mg/mL solution of sucrose in molecular biology grade water), respectively. The suspensions were divided into aliquots, frozen at −80°C for 30 min, and lyophilized to produce final formulation for the injections (white powder). The samples were stored at −20°C until the day of injection. On the day of injection, tubes were resuspended with molecular biology grade water so that CpG or Y2 was at a 400 or 40 μg/mL respectively to correspond to a 10 μg CpG and 1 μg Y2 dose in 25 μL.
2.5.2. Synthesis of Batches Used in Storage Studies
Y2/ZnCar, ZnCar-Y2 and ZnCar-CpG batches for storage studies were synthesized following the same protocols as described above. Y2 and CpG loadings were detected following the same protocols as described above. Y2 loading in Y2/ZnCar was found to be 24.42±1.85 μg protein/mg total, or 2.44±0.19 mass % (average ± standard deviation, n = 3). Y2 loading in ZnCar-Y2 was found to be 6.65±1.15 μg protein/mg total, or 0.67±0.11 mass % (average ± standard deviation, n = 3). CpG loading in ZnCar-CpG was found to be 49.23±0.67 μg CpG/mg total, or 4.92±0.07 mass % (average ± standard deviation, n = 3). Y2/ZnCar, ZnCar-Y2 and ZnCar-CpG batches for storage studies were formulated with sucrose in identical way it was formulated for in vivo studies.
2.5.3. Endotoxin Quantification
Endotoxin was evaluated using the Pierce LAL chromogenic endotoxin quantitation kit in accordance with the manufacturer’s instructions. Y2/ZnCar, ZnCar-Y2, and ZnCar-CpG samples had undetectable levels of endotoxin (<0.1 EU/mg of material). Soluble Y2 had 0.80±0.04 EU/μg of Y2 (average ± standard deviation, n = 3).
2.5.4. Imaging
SEM (Hitachi S-4700 with EDS, Tokyo, Japan) was carried out at Chapel Hill Analytical and Nanofabrication Laboratory (UNC CHANL) facility.
2.6. In Vivo Vaccine Efficacy
Female BALB/cJ mice (n = 18) were vaccinated IM on a prime + boost schedule (day 0 and 21) with the following formulations: 109 mg/mL sucrose (negative control), soluble Y2 (1 μg) + soluble CpG (1 μg) in 109 mg/mL sucrose, Y2 (1 μg) + Addavax (InvivoGen), Y2/ZnCar (1 μg Y2) + ZnCar-CpG (1 μg CpG), or ZnCar-Y2 (1 μg Y2) + ZnCar-CpG (1 μg CpG). Blood was collected from mice on days 14, 28, and 42 via a submandibular bleed and then spun down in serum collection tubes (Greiner, Monroe, NC). Sera was stored at −80°C until later analysis.
2.7. Humoral Response to Vaccination
2.7.1. Quantification of Y2 Specific Antibody Titers
Y2 specific antibody titers were quantified as previously described 25. Briefly, high-binding 384-well plates coated with 0.3 μg/mL of Y2 were blocked with 3% milk in PBS (Dry powder, Food Lion, Salisbury, NC) and then incubated with sera for 2 hours. After two hours antigen specific IgG, IgG1, and IgG2a was detected using a goat anti-mouse HRP conjugated secondary antibody (SouthernBiotech, Birmingham, AL) followed by addition of the HRP substrate TMB and quenching with 2N sulfuric acid.
2.7.2. Determination of Antibody Mediated Complement Deposition (ADCD) Activity
The ADCD activity was determined as previously reported 26, 27. Briefly, Y2 was conjugated to carboxylate modified fluorescent microspheres via sulfo-N-hydroxysuccinimide (NH) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC). Y2 conjugated microspheres were incubated with heat inactivated sera followed by incubation with guinea pig complement (Cedarlane Laboratories, Burlington, Canada) and subsequent incubation with a goat anti-guinea pig complement C3 FITC conjugated polyclonal antibody (MP Biomedicals, Solon, Ohio). Microspheres were analyzed via flow cytometry (ThermoFisher Attune NxT). The complement deposition score was calculated as follows: (percent FITC positive microspheres x geometric mean intensity of the FITC channel)/10,000.
2.7.3. Hemagglutination Inhibition (HAI) Titers
HAI titers were determined as previously described against the following viruses: A/California/07/2009(H1N1), A/Michigan/45/2015(H1N1), A/Brisbane/02/2018(H1N1), and A/Guangdong-Maonan/SWL/1536/2019(H1N1) 28.
2.8. Cellular Response to Vaccination
On day 35, mice (n = 5) were humanely euthanized for the collection of spleens and draining lymph nodes (inguinal + popliteal pooled). Lymphocytes were processed into single cell suspensions and then stained with fluorescent antibodies (Biolegend, San Diego, CA) for the quantification of germinal center (GC) B-cells (B220+, GL7+, and CD38-) via flow cytometry on a ThermoFisher Attune NxT.
Splenocytes were also processed into single cell suspensions and then cultured with or without soluble Y2 (10 μg/mL) for 36 h to assess antigen specific cellular responses. After 36 h the supernatant was collected to measure the production of IL-2 and IFN-γ via an ELISA kit (Biolegend) according to the manufacturer’s protocol. Data is reported as cytokine concentration in stimulated samples minus cytokine concentration in unstimulated samples. Antigen specific IL-2 and IFN-γ production was also assessed via ELISpot (BD Biosciences, San Jose, CA) on the stimulated splenocytes according to the manufacturer’s protocol. Spots were enumerated on an ImmunoSpot® Analyzer (Shaker Heights, OH). Data is reported as spots in stimulated samples minus spots in unstimulated samples.
2.9. Lethal Influenza Challenge
On day 56, mice (n = 13) were anesthetized with isoflurane and challenged intranasally with 50 μL A/California/07/2009 (H1N1) (100k pfu/mouse). Mice were monitored daily for 14 days and those that lost over 20% of their initial weight were humanely euthanized.
2.10. Quantification of Lung Viral Titers
On day 59 (day 3 after infection), mice (n = 3) were humanely euthanized to quantify lung viral titers as previously described 29, 30. Briefly, lungs were collected and homogenized. Lung homogenates were incubated on a monolayer of MDCK cells for 2 h. After 2 h the homogenate was aspirated and overlay media (DMEM + 1.2% Avicel medium + 2.4 μg/mL N-Ac trypsin) was placed on the monolayer and incubated for 72 h. After 72 h the monolayer was stained with 0.5% crystal violet for 30 minutes. After washing off excess crystal violet solution the plaques were visually enumerated.
2.11. Long-term Storage of Vaccine Formulations
To assess the stability of ZnCar and its ability to protect the cargo, ZnCar-CpG, ZnCar-Y2, and Y2/ZnCar were stored at −20°C or 24°C (under vacuum at −20 inHg) for 3 months or at −20°C or 24°C (in a desiccator cabinet) for 6 months. Y2 without ZnCar that had been lyophilized in 10mM phosphate buffer + 8% (w/v) (1:1 trehalose:mannose) was also stored at the same conditions 31. After storage, the morphology of the coordination polymers was assessed via SEM (Hitachi S-4700 with EDS).
2.12. In Vitro Analysis of ZnCar-CpG Stability
To determine the in vitro activity of ZnCar-CpG stored at various conditions, an in vitro stimulation was performed. DC2.4 cells were seeded at 25,000 cells/well in a TC treated 96-well plates and allowed to adhere overnight. ZnCar-CpG stored at various conditions as above was then added to wells along with soluble CpG and blank ZnCar stored at −20°C as controls. After 24 h, the supernatant was removed, and the TNF-α concentration was determined via ELISA (Biolegend) according to the manufacturer’s protocol. The viability and proliferation of the cells was determined by adding 0.06 mM resazurin and incubating for 2 h. After incubation, the fluorescence intensity (λexcitation = 530 nm and λemission = 630 nm) was measured on a SpectraMax M2 microplate reader. Percent viability was determined by subtracting the background signal of wells treated with lysis buffer and normalizing to cells treated with media only.
2.13. Y2 Stability Assessment via a Competitive ELISA
The stability of Y2 after storage was assessed by using a competitive ELISA. ZnCar-Y2 and Y2/ZnCar stored at various conditions were disintegrated as above. These samples along with soluble Y2 as a control were then incubated with a library of monoclonal antibodies that bind to distinct Y2 epitopes for 30 minutes in blocking buffer 32. 25 μL of the mixture was then added to a 384-well high binding plate that had been coated overnight with 25 μL of 300 ng/mL Y2 overnight, washed 3x with PBST, blocked for 1 h, and again washed 3x with PBST. After a 2 h incubation, the plates were washed 3x with PBST and 25 μL goat anti-human IgG Fc (SouthernBiotech) was added to the wells. After 1 h, the plates were washed 5x with PBST and 25 μL TMB was added to the wells. The reaction was then stopped with 12.5 μL 2N sulfuric acid, and absorbance was measured at 450 and 570 nm on a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA). Data is reported by first subtracting the background absorbance at 570 nm from the absorbance at 450 nm and then normalizing this value to native Y2 as 100% via GraphPad Prism 9.
2.14. Statistical Analysis
All figures are generated using GraphPad Prism 9. To determine statically significant differences ANOVA was used followed by Tukey’s pairwise comparisons.
3. Results and Discussion
3.1. Humoral Response to a ZnCar Based Vaccine
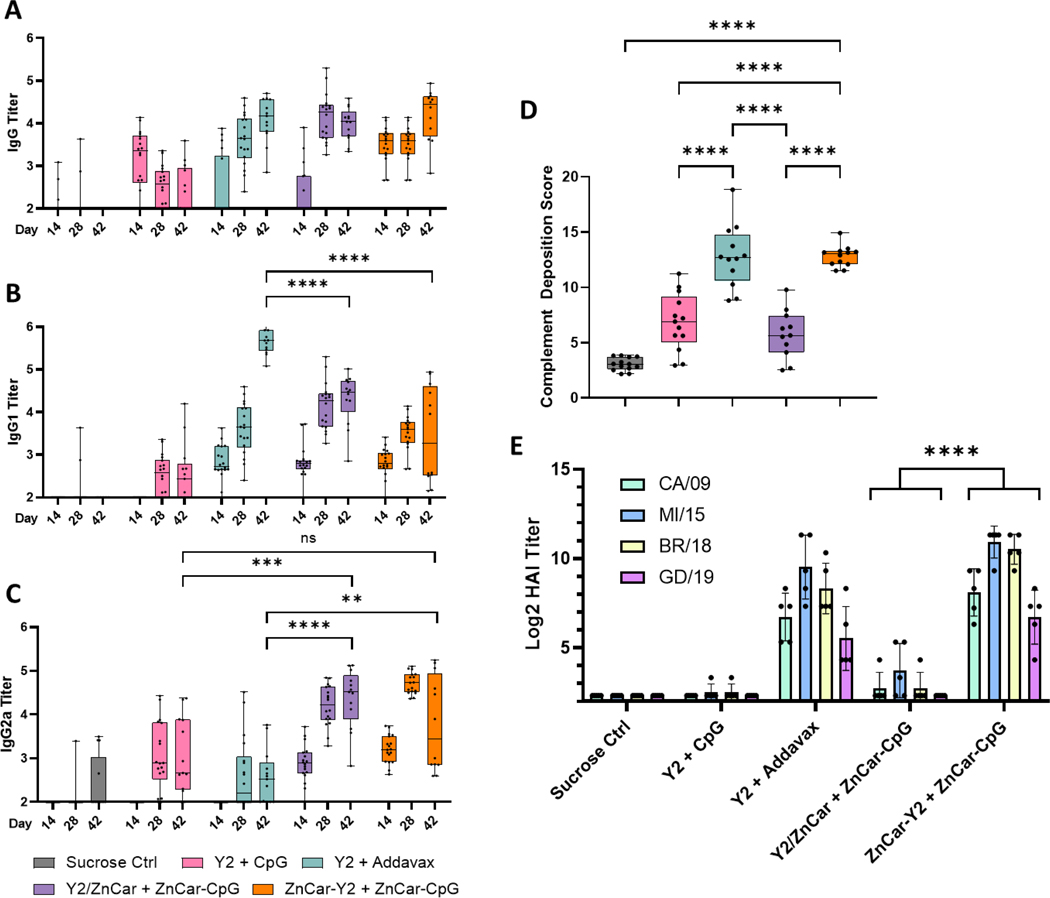
We first evaluated the humoral response of the ZnCar vaccine with Y2 HA incorporated during coordination polymer synthesis (ZnCar/Y2) or after coordination polymer synthesis (ZnCar-Y2) (Figure S1). CpG was incorporated after ZNCar synthesis (ZnCar-CpG) (Figure S1). Y2 and CpG were loaded at high loading efficiencies (21.76 μg/mg for ZnCar/Y2, 20.07 μg/mg for ZnCar-Y2, and 55.94 μg/mL for ZnCar-CpG). This high loading efficiency is likely due to the coordination of the zinc ions with either phosphate groups on CpG or histidine groups on the his-tag of Y2. Mice were vaccinated on a prime-boost schedule with isotonic sucrose in water, soluble Y2 + CpG, Y2 + Addavax (similar to the FDA-approved adjuvant MF59), Y2/ZnCar + ZnCar-CpG, and ZnCar-Y2 + ZnCar-CpG. On days 14, 28, and 42 sera was collected to measure Y2-specific IgG titers (Figure 1A–C). No significant differences were observed in total Y2-specific IgG production between Addavax, Y2/ZnCar, and ZnCar-Y2 (Figure 1A). Apart from total IgG, it is also important to consider IgG1 and IgG2a titers since these isotypes indicate Th2 and Th1 skewing of the immune response, respectively 33. Addavax produced significantly higher (p ≤ 0.0001) Y2-specific IgG1 titers when compared to both ZnCar groups (Figure 1B); however, both Y2/ZnCar and ZnCar-Y2 produced significantly higher (p ≤ 0.01 and p ≤ 0.0001 respectively) Y2-specific IgG2a titers compared to Addavax (Figure 1C). Furthermore, when looking at the ratio of IgG1/IgG2a titer the Addavax group produced a ratio near 2 for the day 42 sera while both ZnCar-CpG groups produced a ratio near 1 (Figure S3). Overall, these data demonstrate the ability of both Y2/ZnCar and ZnCar-Y2 adjuvanted with ZnCar-CpG to produce a more balanced Th1/Th2 response when compared to Addavax. This improved balanced response over Addavax is important given that IgG1 and IgG2 have been found to play separate crucial roles in protection against influenza 34.
Figure 1.
Mice (n = 18) (BALB/cJ) were vaccinated on a prime + boost schedule (days 0 and 21) (IM) with the following: sucrose control, Y2 + CpG, Y2 + Addavax, Y2/ZnCar + ZnCar-CpG, or ZnCar-Y2 + ZnCar-CpG. Y2 and CpG were dosed at 1 and 10 μg, respectively. On days 14, 28, and 42 Y2- specific (A) IgG, (B) IgG1, and (C) IgG2a titers were assessed. (D) The ADCD activity of the sera was also analyzed for day 42. (E) The HAI titer was determined against four H1N1 viruses. (A-D) Data is represented as median ± range. (E) Data is represented as mean ± SD. ** = p ≤ 0.01, *** = p ≤ 0.001, and **** = p ≤ 0.0001.
Apart from measuring antigen specific IgG titers, the functionality of these antibodies, as well as the broadly neutralizing activity of the antibodies elicited by the COBRA HA antigen is also important to measure as many of these functions are correlates of protection 35. One significant way of preventing virus infection of cells is known as viral neutralization, which prevents pathogen entry. To measure the neutralizing titers produced by our vaccine, we utilized the hemagglutination inhibition (HAI) assay. HAI titers were measured with day 42 sera against four antigenically distinct strains of H1N1 influenza (CA/09, MI/15, Bris/18, and GD/19) (Figure 1E). Both Addavax and ZnCar-Y2 produced high HAI titers against all four tested strains (Figure 1E). Previous work has shown that this broad reactivity would not be possible with current seasonal influenza vaccines that would only protect against 1–2 strains, if it was included in that year’s vaccine and if circulating strains had not mutated too significantly from the vaccine strain 12. Interestingly, mice vaccinated with Y2/ZnCar produced significantly lower (p ≤ 0.0001) HAI titers for all four viruses when compared to ZnCar-Y2 (Figure 1E). Antibody dependent complement deposition (ADCD) is another antibody function that is important in the clearance of influenza viruses 36. Similar to HAI titers, the Addavax and ZnCar-Y2 groups both had high ADCD activity; however, Y2/ZnCar produced significantly lower (p ≤ 0.0001) ADCD activity (Figure 1D).
Together these data reveal the superior humoral response of ZnCar-Y2 over Y2/ZnCar which could be due to a couple of factors. First, cross-linking of BCRs is critical to the activation of B cells. The incorporation of Y2 after the coordination polymer synthesis could have led to a higher amount of Y2 on the surface of ZnCar for ZnCar-Y2 which could lead to this BCR cross linking 37. For example, the surface display of HA molecules on liposomes containing cobalt-porphyrin phospholipid (CoPoP) elicits a potent humoral response following vaccination 38. Second, although ZnCar is uniquely prepared in HEPES buffer over harsh solvents, the shorter incubation time of Y2 to form ZnCar-Y2 (compared to Y2/ZnCar) could result in a less denatured protein antigen.
3.2. Cellular Response to a ZnCar Based Vaccine
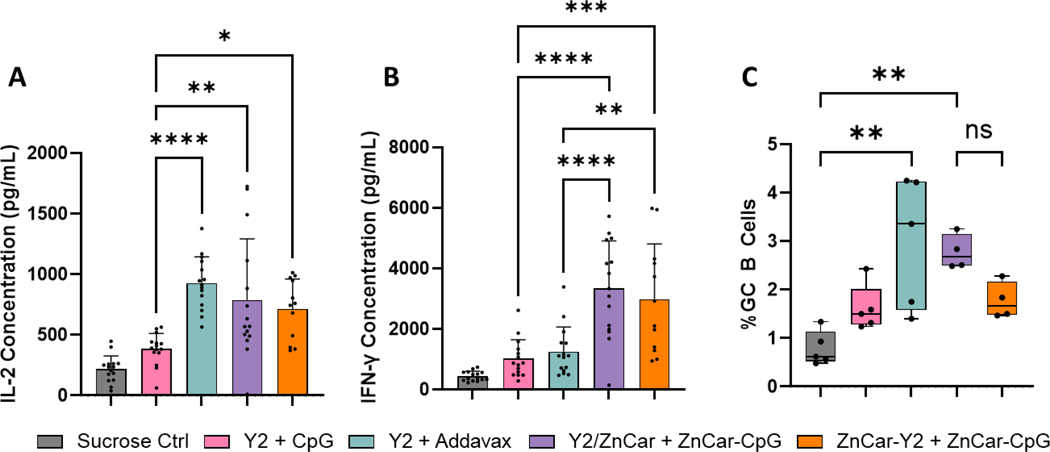
To assess the cellular response to vaccination, mice were euthanized on day 35 to collect spleens and draining lymph nodes (dLNs). Upon antigen restimulation, Addavax, Y2/ZnCar, and ZnCar-Y2 all produced similarly high levels of IL-2 (Figure 2A), while Y2/ZnCar and ZnCar-Y2 produced significantly higher (p ≤0.0001 and p ≤ 0.001, respectively) IFN-γ when compared to Addavax. It should be noted that this trend was not observed in IL-2 and IFN-γ ELISpot data (Figure S2AB). This difference in ELISpot and ELISA data indicates that Addavax and ZnCar-CpG treated mice both had the same number of IFN-γ secreting splenocytes, but the mice treated with ZnCar-CpG produced more total IFN-γ. dLNs were used to measure the frequency of GC B cells post vaccination via flow cytometry. Only Addavax and Y2/ZnCar produced a significantly higher (p ≤ 0.01) frequency of GC B cells when compared to sucrose control; however, the frequency of GC B cells produced by Y2/ZnCar was not statistically different from ZnCar-Y2 (Figure 2C). Overall, these data reveal that ZnCar-CpG produces a more robust cellular immune response when compared to Addavax which is important in generating protection especially in those who are immunocompromised 39, 40.
Figure 2.
On day 35, mice (n = 5) were humanely euthanized to collect spleens and draining lymph nodes. Splenocytes were incubated with Y2 for 36 h after which the supernatant was collected to measure antigen specific (A) IL-2 and (B) IFN-γ secretion. (C) Lymph nodes were processed and stained with fluorescent antibodies to quantify GC B-cells (B220+, GL7+, and CD38-) via flow cytometry. (A-B) Data is represented as mean ± SD. (C) Data is represented as median ± range. * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, and **** = p ≤0.0001.
3.3. Protective Efficacy of a ZnCar Based Vaccine
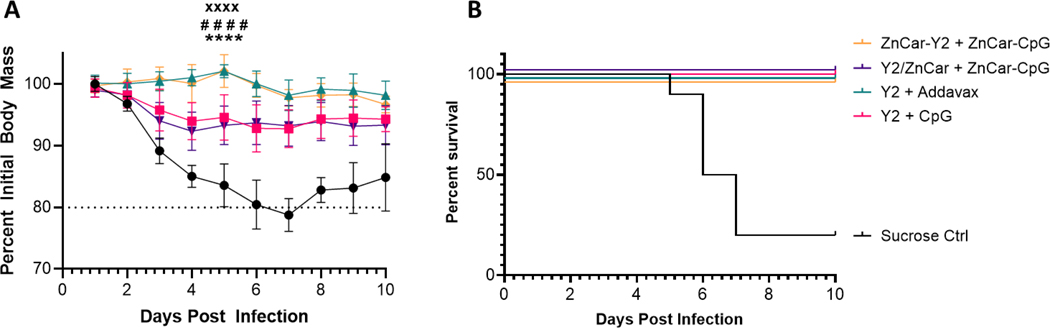
The protective efficacy of the different vaccine formulations was determined by challenging mice with a lethal dose of CA/09 (H1N1). For influenza, weight loss is a metric of infection and there was no substantial weight loss for the mice treated with Addavax or ZnCar-Y2; however, while mice treated with Y2/ZnCar and soluble Y2 + CpG did not fall below 80% of their initial weight they did lose substantially more weight than Addavax and ZnCar-Y2 treated mice (Figure 3AB). For example, on day 5 both soluble Y2 + CpG and Y2/ZnCar treated mice lost significantly (p ≤0.0001) more weight than mice treated with ZnCar-Y2. To further verify these findings, mice (n = 3) were humanely euthanized on day 3 after infection to assess viral lung titers via a plaque assay. Mice treated with Addavax or ZnCar-Y2 both had significantly lower (p ≤ 0.05) lung viral titers when compared to soluble Y2 + CpG while Y2/ZnCar did not have significantly different lung viral titers when compared to soluble Y2 + CpG (Figure S4). The inferior protection afforded by Y2/ZnCar is likely due to the lower neutralizing titers produced in Y2/ZnCar treated mice (Figure 1E), and even though Y2/ZnCar had a strong cellular response (Figure 2) this was not enough to make up for the non-existent neutralizing response.
Figure 3.
On day 56, mice (n = 13) were challenged with 100k pfu/mouse of A/California/07/2009 (H1N1). (A-B) Mice were weighed daily and mice that lost more than 20% of their initial body weight were humanely euthanized. (C) On day 59, mice (n = 3) were humanely euthanized, and their lung viral titers were assessed via a plaque assay. (A) # denotes significance between ZnCar-Y2 + ZnCar-CpG and Y2/ZnCar + ZnCar-CpG on day 5. (A) x denotes significance between Y2 + Addavax and Y2/ZnCar + ZnCar-CpG on day 5. (A) * denotes significance between ZnCar-Y2 + ZnCar-CpG and Y2 + CpG on day 5. Data is represented as mean ± SD. * = p ≤ 0.05, **** = p ≤0.0001, xxxx = p ≤0.0001, and #### = p ≤0.0001.
Overall, these data reveal that ZnCar-Y2 is superior to Y2/ZnCar in vivo. ZnCar-Y2 produced significantly higher HAI titers and ADCD activity (Figure 1D–E), significantly less weight loss after a lethal H1N1 challenge (Figure 3A), and significantly lower viral titers in the lungs after a lethal H1N1 challenge (Figure S4). ZnCar-Y2 also showed superiority over Addavax in inducing a potent cellular immune response as after antigen recall splenocytes from mice treated with ZnCar-Y2 produced significantly more IFN-γ when compared to Addavax treated mice (Figure 2B). These results have also been summarized in terms of different types of vaccine induced immune responses (Figure S5). This makes ZnCar an attractive vehicle for a broadly active influenza vaccine; however, to take it one step further it is important to consider the thermal stability of this platform to increase its translatability, particularly to resource limited settings.
3.4. In Vitro Thermal Stability of Y2/ZnCar, ZnCar-Y2, and ZnCar-CpG
In order to assess the in vitro thermal stability of the vaccine platform Y2/ZnCar, ZnCar-Y2, and ZnCar-CpG were stored at either 40°C for 3 months or 24°C for 6 months. These temperatures were chosen because they are similar to the FDA guidelines for long-term and accelerated storage studies although with a shortened timeline 41. After storage the morphology of the coordination polymers was determined via SEM and there were no observable changes in the morphology of any of the ZnCar samples stored at 40°C for 3 months or 24°C for 6 months, compared to ZnCar before storage (Figure 4A–I). Furthermore, as a control the ZnCar samples were also stored at −20°C for 3 and 6 months. These samples also had no obvious morphological change when compared to the ZnCar samples before storage (Figure S6A–F).
Figure 4.
SEM micrograph of (A-C) ZnCar-CpG, (D-F) ZnCar-Y2, and (G-I) Y2/ZnCar before storage or stored at either 40°C for 3 months or 24°C for 6 months. Scale bar represents 2 μm.
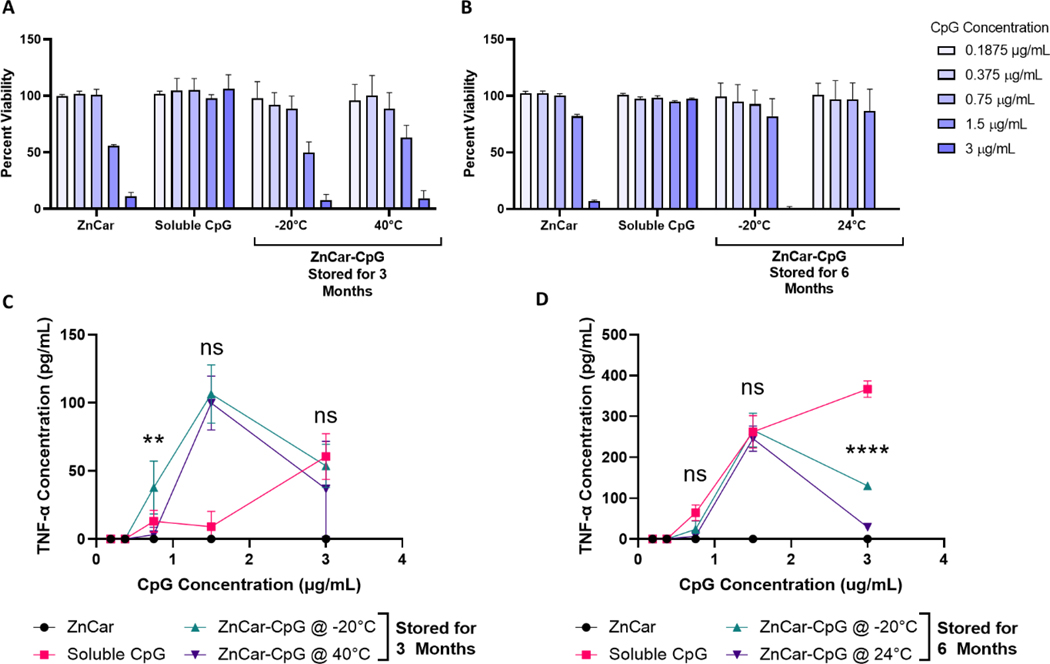
Next, to determine whether the adjuvanticity of ZnCar-CpG was intact after the storage conditions innate immune activity was measured when formulations were cultured with DC2.4 cells. The viability of cells treated with ZnCar-CpG stored at 40°C for 3 months was the same as those treated with ZnCar-CpG stored at −20°C for 3 months (Figure 5A), similarly the viability of cells treated with ZnCar-CpG stored at either 24°C or −20°C for 6 months was the same (Figure 5B). Cytokines expression from DC2.4 was also measured, with TNF-α being reported as a indicator of TLR9 activity, particularly from CpG 42. ZnCar-CpG stored for 3 months at −20°C or 40°C stimulated similar levels of TNF-α production except for the 0.75 μg/mL CpG concentration where ZnCar-CpG at −20°C slightly outperformed ZnCar-CpG at 40°C (p ≤ 0.01) (Figure 5C). After 6 months storage at −20°C or 24°C, the TNF-α production was also the same except for at the 3 μg/mL CpG concentration where the ZnCar-CpG stored at −20°C for 6 months outperformed the ZnCar-CpG stored at 24°C (p ≤0.0001); however, the peak of the TNF-α response at 1.5 μg/mL was still the same between the two storage conditions (Figure 5D). Overall, this demonstrates that ZnCar-CpG stored at 40°C for 3 months and 24°C for 6 months both maintain immunostimulatory properties which makes ZnCar-CpG a promising adjuvant for storage outside of the cold chain. This is a significant improvement over Addavax (similar to the only FDA approved influenza adjuvant MF59) which is only able to be stored at 4°C.
Figure 5.
ZnCar-CpG stored at various conditions along with CpG and ZnCar stored at −20°C were used to stimulate DC2.4 cells to determine whether the in vitro activity of CpG was maintained. After 24 h, the (A-B) percent viability and (C-D) TNF-α production were assessed. (A-B) Percent viability is measured by normalizing the experimental samples to an untreated control. (C) Statistical significance shown is for the comparison of ZnCar-CpG @ −20°C and ZnCar-CpG @ 40°C. (D) Statistical significance shown is for the comparison of ZnCar-CpG @ −20°C and ZnCar-CpG @ 24°C. Data is represented as mean ± SD. ns = p > 0.05, ** = p ≤ 0.01, and **** = p ≤0.0001.
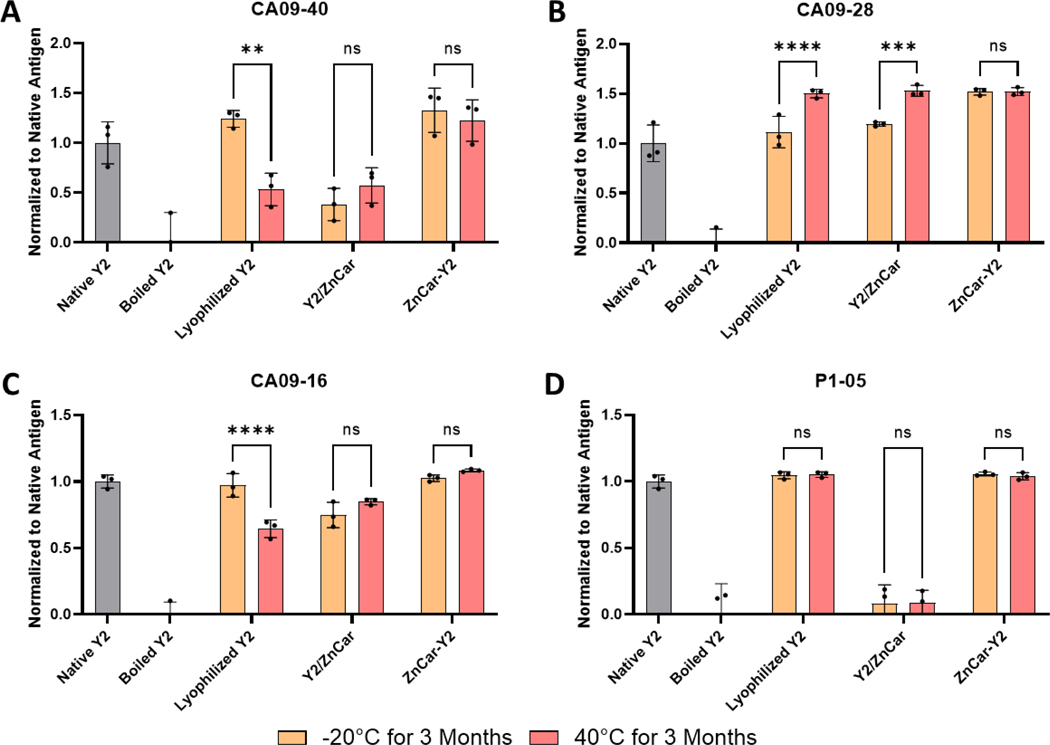
Given that ZnCar-CpG remained stable outside of cold chain storage, we were also interested in investigating the stability of Y2/ZnCar and ZnCar-Y2. The structural stability of Y2 was evaluated by using pre-existing human antibodies that bind to distinct epitopes on Y2 32. Lyophilized Y2 appeared to be denatured after storage at 40°C for 3 months as shown by the reduced binding of CA09–40 and CA09–16 (Figure 6AC). Y2/ZnCar was also not stable at these storage conditions. Interestingly, Y2/ZnCar appeared to be denatured whether stored at −20°C or 40°C for 3 months which indicates that the fabrication process itself denatured the antigen. ZnCar-Y2 on the other hand remained stable at both storage temperatures for 3 months (Figure 6A–D). These results could also explain why Y2/ZnCar produced significantly lower HAI titers than ZnCar-Y2 (Figure 1E).
Figure 6.
To assess the in vitro stability of Y2 in either ZnCar-Y2 or Y2/ZnCar stored at −20°C or 40°C for 3 months, a competitive ELISA was employed where the ability of the formulated Y2 to outcompete native Y2 for the binding to a Y2 specific antibody was measured. A library of four distinct Y2 specific antibodies consisting of (A) CA09–40, (B) CA09–28, (C) CA09–16, and (D) P1–05 was used to ensure that the structural integrity of Y2 after storage was maintained in diverse epitopes. Data is reported as the ELISA signal normalized to native Y2 as 100%. Data is represented as mean ± SD. ns = p > 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, and **** = p ≤0.0001.
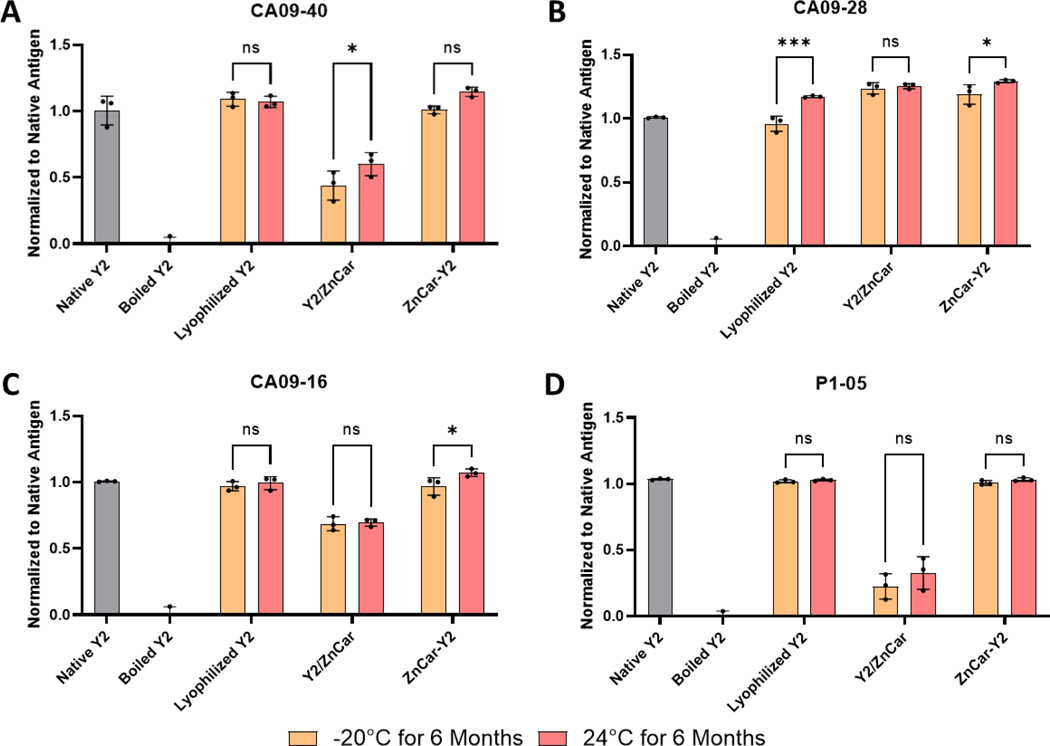
The stability of Y2/ZnCar and ZnCar-Y2 was also measured after 6 months storage at −20°C and 24°C (Figure 7). Interestingly, lyophilized Y2 did not appear to be denatured when stored at 24°C for 6 months (Figure 7) whereas it did appear to be denatured when stored at 40°C for 3 months (Figure 6). This data shows that unencapsulated Y2 is sensitive to increases in temperature during storage and more work will have to be conducted to determine the exact threshold where the protein is denatured. ZnCar-Y2 did not show any signs of denaturation when stored at both temperatures for 6 months. Y2/ZnCar on the other hand was denatured when stored at both −20°C and 24°C for 6 months as evidenced by CA09–40 and P1–05 binding (Figure 7AD) which corroborates the stability of Y2/ZnCar for 3 months. Interestingly, there appeared to be some improvement in the binding when compared to native antigen for some samples such as in lyophilized Y2 stored at 24°C for 6 months (Figure 7B). This could be due to changes in the HA structure that increased antibody affinity, but confirming this will require further investigation. Together these data support that ZnCar-Y2 has in vitro stability outside of cold chain storage for 3 and 6 months at 40°C and 24°C, respectively, whereas Y2 is not as stable when stored as a lyophilized powder or in Y2/ZnCar.
Figure 7.
To assess the in vitro stability of Y2 in either ZnCar-Y2 or Y2/ZnCar stored at −20°C or 24°C for 6 months, a competitive ELISA was employed where the ability of the formulated Y2 to outcompete with native Y2 for the binding to a Y2 specific antibody was measured. A library of four distinct Y2 specific antibodies consisting of (A) CA09–40, (B) CA09–28, (C) CA09–16, and (D) P1–05 was used to ensure that the structural integrity of Y2 after storage was maintained in diverse epitopes. Data is represented as mean ± SD. ns = p > 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, and **** = p ≤0.0001.
4. Conclusion
In conclusion, we demonstrated the delivery of a broadly active influenza antigen Y2 and the adjuvant CpG via ZnCar. Mice vaccinated with ZnCar-Y2 and ZnCar-CpG produced a substantial neutralizing antibody response against antigenically distinct influenza viruses while mice vaccinated with Y2/ZnCar did not have this response, highlighting the importance of incorporating antigens to the coordination polymer after synthesis to increase immunogenicity (Figure 1E). Outside of a humoral response, ZnCar-Y2 treated mice also produced a stronger antigen specific cellular response when compared to Addavax (Figure 2A–B), which indicates it elicits a more balanced Th1/Th2 immune response to better protect against influenza. As an illustration of the ability of this broad response to better protect, mice vaccinated with ZnCar-Y2 were protected from a lethal influenza challenge with no weight loss or lung viral titers (Figure 3A–C), in contrast to the other groups which did not illustrate the same strong outcomes. ZnCar was also able to protect CpG and Y2 from thermal degradation outside the cold chain as demonstrated by in vitro analysis. After 3-month storage at 40°C or 6-month storage at 24°C, ZnCar-CpG was still able to stimulate dendritic cells to the same degree as ZnCar-CpG stored at −20°C. After these same storage conditions, ZnCar-Y2 also protected Y2 from denaturation which we observed by using a library of antibodies that bind to distinct epitopes on Y2. Together, these results indicate that CpG and Y2 formulated with ZnCar are a promising broadly active influenza vaccine platform as demonstrated by its superior in vivo potency and stability outside of cold-chain storage. While these results do not prove that this platform maintains in vivo activity during these storage conditions the results here are promising and future studies can focus on this question. Future studies could also include the investigation of other adjuvants and antigens with this platform to see whether what was demonstrated here applies to other systems. Further, other metals and organic linkers that possess immunostimulatory properties could be employed to further increase the potency of this platform.
Supplementary Material
Acknowledgements
Part of this work was conducted at the Chapel Hill Analytical and Nanofabrication Laboratory (CHANL). We thank Dr. Amar Kumbhar for his assistance in obtaining SEM micrographs of the formulations. This work was supported, in part, by National Institutes of Health (NIH) NIAID Collaborative Influenza Vaccine Innovation Centers (CIVICs) Contract #75N93019C00052 (PI: Ross) and NIH R01AI147497 (PI: Ainslie).
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
References
- 1.CDC Disease Burden of Flu. https://www.cdc.gov/flu/about/burden/index.html
- 2.de Courville C; Cadarette SM; Wissinger E; Alvarez FP The economic burden of influenza among adults aged 18 to 64: A systematic literature review. Influenza Other Respir Viruses 2022, 16, (3), 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding AT; Heaton NS Efforts to Improve the Seasonal Influenza Vaccine. Vaccines (Basel) 2018, 6, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng L; Cho KJ; Fiers W; Saelens X. M2e-Based Universal Influenza A Vaccines. Vaccines (Basel) 2015, 3, (1), 105–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valkenburg SA; Mallajosyula VV; Li OT; Chin AW; Carnell G; Temperton N; Varadarajan R; Poon LL Stalking influenza by vaccination with pre-fusion headless HA mini-stem. Sci Rep 2016, 6, 22666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khurana S; Loving CL; Manischewitz J; King LR; Gauger PC; Henningson J; Vincent AL; Golding H. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 2013, 5, (200), 200ra114. [DOI] [PubMed] [Google Scholar]
- 7.Hoa LNM; Mai LQ; Bryant JE; Thai PQ; Hang NLK; Yen NTT; Duong TN; Thoang DD; Horby P; Werheim HFL; Fox A. Association between Hemagglutinin Stem-Reactive Antibodies and Influenza A/H1N1 Virus Infection during the 2009 Pandemic. J Virol 2016, 90, (14), 6549–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajic G; van der Poel CE; Kuraoka M; Schmidt AG; Carroll MC; Kelsoe G; Harrison SC Autoreactivity profiles of influenza hemagglutinin broadly neutralizing antibodies. Sci Rep 2019, 9, (1), 3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khurana S; Hahn M; Klenow L; Golding H. Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins. Viruses 2020, 12, (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labombarde JG; Pillai MR; Wehenkel M; Lin CY; Keating R; Brown SA; Crawford JC; Brice DC; Castellaw AH; Mandarano AH; Guy CS; Mejia JR; Lewis CD; Chang TC; Oshansky CM; Wong SS; Webby RJ; Yan M; Li QZ; Marion TN; Thomas PG; McGargill MA Induction of broadly reactive influenza antibodies increases susceptibility to autoimmunity. Cell Rep 2022, 38, (10), 110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yassine HM; Boyington JC; McTamney PM; Wei CJ; Kanekiyo M; Kong WP; Gallagher JR; Wang L; Zhang Y; Joyce MG; Lingwood D; Moin SM; Andersen H; Okuno Y; Rao SS; Harris AK; Kwong PD; Mascola JR; Nabel GJ; Graham BS Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 2015, 21, (9), 1065–70. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y; Franca MS; Allen JD; Shi H; Ross TM Next Generation of Computationally Optimized Broadly Reactive HA Vaccines Elicited Cross-Reactive Immune Responses and Provided Protection against H1N1 Virus Infection. Vaccines (Basel) 2021, 9, (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalaj-Hedayati A; Chua CLL; Smooker P; Lee KW Nanoparticles in influenza subunit vaccine development: Immunogenicity enhancement. Influenza Other Respir Viruses 2020, 14, (1), 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coenen F; Tolboom JT; Frijlink HW Stability of influenza sub-unit vaccine. Does a couple of days outside the refrigerator matter? Vaccine 2006, 24, (4), 525–31. [DOI] [PubMed] [Google Scholar]
- 15.Flood A; Estrada M; McAdams D; Ji Y; Chen D. Development of a Freeze-Dried, Heat-Stable Influenza Subunit Vaccine Formulation. PLoS One 2016, 11, (11), e0164692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luykx DM; Casteleijn MG; Jiskoot W; Westdijk J; Jongen PM Physicochemical studies on the stability of influenza haemagglutinin in vaccine bulk material. Eur J Pharm Sci 2004, 23, (1), 65–75. [DOI] [PubMed] [Google Scholar]
- 17.Ott G; Radhakrishnan R; Fang J-H; Hora M. Methods in Molecular Medicine. Vaccine Adjuvants Preparation Methods and Research Protocols 2000. [Google Scholar]
- 18.Pena ES; Lifshits LM; Eckshtain-Levi M; Bachelder EM; Ainslie KM Metal–organic coordination polymers for delivery of immunomodulatory agents, and infectious disease and cancer vaccines. WIREs Nanomedicine and Nanobiotechnology 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh R; White JF; de Vries M; Beddome G; Dai M; Bean AG; Mulet X; Layton D; Doherty CM Biomimetic metal-organic frameworks as protective scaffolds for live-virus encapsulation and vaccine stabilization. Acta Biomater 2022, 142, 320–331. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H; Chen W; Gong K; Chen J. Nanoscale Zeolitic Imidazolate Framework-8 as Efficient Vehicles for Enhanced Delivery of CpG Oligodeoxynucleotides. ACS Applied Materials & Interfaces 2017, 9, (37), 31519–31525. [DOI] [PubMed] [Google Scholar]
- 21.Teng Z; Hou F; Bai M; Li J; Wang J; Wu J; Ru J; Ren M; Sun S; Guo H. Bio-mineralization of virus-like particles by metal-organic framework nanoparticles enhances the thermostability and immune responses of the vaccines. J Mater Chem B 2022, 10, (15), 2853–2864. [DOI] [PubMed] [Google Scholar]
- 22.Luzuriaga MA; Herbert FC; Brohlin OR; Gadhvi J; Howlett T; Shahrivarkevishahi A; Wijesundara YH; Venkitapathi S; Veera K; Ehrman R; Benjamin CE; Popal S; Burton MD; Ingersoll MA; De Nisco NJ; Gassensmith JJ Metal–Organic Framework Encapsulated Whole-Cell Vaccines Enhance Humoral Immunity against Bacterial Infection. ACS Nano 2021, 15, (11), 17426–17438. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y; Zhong L; Bilal M; Tan Z; Hou Y; Jia S; Cui J. Enzymes@ZIF-8 Nanocomposites with Protection Nanocoating: Stability and Acid-Resistant Evaluation. Polymers (Basel) 2018, 11, (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil PD; Yadav GD Rapid In Situ Encapsulation of Laccase into Metal-Organic Framework Support (ZIF-8) under Biocompatible Conditions. ChemistrySelect 2018, 3, (17), 4669–4675. [Google Scholar]
- 25.Eckshtain-Levi M; Batty CJ; Lifshits LM; McCammitt B; Moore KM; Amouzougan EA; Stiepel RT; Duggan E; Ross TM; Bachelder EM; Ainslie KM Metal–Organic Coordination Polymer for Delivery of a Subunit Broadly Acting Influenza Vaccine. ACS Applied Materials & Interfaces 2022, 14, (25), 28548–28558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudreau CM; Yu WH; Suscovich TJ; Talbot HK; Edwards KM; Alter G. Selective induction of antibody effector functional responses using MF59-adjuvanted vaccination. J Clin Invest 2020, 130, (2), 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batty CJ; Lifshits LM; Hendy DA; Eckshtain-Levi M; Ontiveros-Padilla LA; Carlock MA; Ross TM; Bachelder EM; Ainslie KM Vinyl Sulfone-functionalized Acetalated Dextran Microparticles as a Subunit Broadly Acting Influenza Vaccine. AAPS J 2023, 25, (1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunez IA; Huang Y; Ross TM Next-Generation Computationally Designed Influenza Hemagglutinin Vaccines Protect against H5Nx Virus Infections. Pathogens 2021, 10, (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baer A; Kehn-Hall K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. J Vis Exp 2014, (93), e52065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flano E; Jewell NA; Durbin RK; Durbin JE Methods used to study respiratory virus infection. Curr Protoc Cell Biol 2009, Chapter 26, Unit 26 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anamur C; Winter G; Engert J. Stability of collapse lyophilized influenza vaccine formulations. Int J Pharm 2015, 483, (1–2), 131–41. [DOI] [PubMed] [Google Scholar]
- 32.Nagashima K; Dzimianski JV; Han J; Abbadi N; Gingerich AD; Royer F; O’Rourke S; Sautto GA; Ross TM; Ward AB; DuBois RM; Mousa JJ The Pre-Existing Human Antibody Repertoire to Computationally Optimized Influenza H1 Hemagglutinin Vaccines. J Immunol 2022, 209, (1), 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins AM IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol Cell Biol 2016, 94, (10), 949–954. [DOI] [PubMed] [Google Scholar]
- 34.Huber VC; McKeon RM; Brackin MN; Miller LA; Keating R; Brown SA; Makarova N; Perez DR; Macdonald GH; McCullers JA Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol 2006, 13, (9), 981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu LL; Suscovich TJ; Fortune SM; Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol 2018, 18, (1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boudreau CM; Alter G. Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine. Front Immunol 2019, 10, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmann MF; Jennings GT Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010, 10, (11), 787–96. [DOI] [PubMed] [Google Scholar]
- 38.Sia ZR; He X; Zhang A; Ang JC; Shao S; Seffouh A; Huang WC; D’Agostino MR; Teimouri Dereshgi A; Suryaprakash S; Ortega J; Andersen H; Miller MS; Davidson BA; Lovell JF A liposome-displayed hemagglutinin vaccine platform protects mice and ferrets from heterologous influenza virus challenge. Proc Natl Acad Sci U S A 2021, 118, (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahrs C; Harrison N. Vaccine Response in the Immunocompromised Patient with Focus on Cellular Immunity. Vaccines (Basel) 2022, 10, (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruthrich MM; Giesen N; Mellinghoff SC; Rieger CT; von Lilienfeld-Toal M. Cellular Immune Response after Vaccination in Patients with Cancer-Review on Past and Present Experiences. Vaccines (Basel) 2022, 10, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products. Administration, F. a. D., Ed. 2003. [Google Scholar]
- 42.Wagner H. The immunobiology of the TLR9 subfamily. Trends in Immunology 2004, 25, (7), 381–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.