Abstract
Introduction
Delirium is a frequent complication in critically ill patients and is associated with adverse outcomes such as long-term cognitive impairment and increased mortality. It is unknown whether there are sex-related differences in intensive care unit (ICU) delirium and associated outcomes. We aimed to assess sex-specific differences in short-term mortality following ICU-delirium.
Methods
We conducted a retrospective cohort study using the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database. Adult ICU patients who were diagnosed with delirium using the Confusion Assessment Method for the ICU (CAM-ICU) were included. The primary outcome was 30-day mortality following delirium onset. To control for baseline differences in demographics, illness severity, and comorbidities, we applied 1:1 propensity score matching. Cox proportional hazards regression models were used to evaluate the association between sex and mortality.
Results
A total of 8950 ICU patients with delirium were analyzed, of whom 42.6% were women. In univariable analysis, women had higher crude mortality (26.0% vs. 23.4%; HR 1.16, 95% CI 1.071–1.267, p < 0.001). After propensity score matching, the cohort included 3811 women and 3811 men. In adjusted analysis, risk for thirty-day mortality remained higher in women (HR 1.16, 95% CI 1.064–1.273, p < 0.001).
Conclusion
Our study suggests that women with ICU-delirium have a significantly higher risk of short-term mortality than men. Acknowledging the limitations inherent to observational studies with potential for residual confounding, further research is needed to understand the biological and clinical factors driving this disparity and to inform sex-specific interventions for ICU-delirium.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05204-7.
Keywords: ICU-delirium, Sex differences, Mortality, Personalized ICU-care
Introduction
Delirium is a heterogeneous syndrome of acute brain dysfunction, characterized by acute and fluctuating disturbance of consciousness, cognition and attention [1]. It affects up to half of critically ill patients and is associated with adverse outcomes, including prolonged ICU stays and long-term cognitive impairment [2]. ICU-delirium results from a complex interplay of risk factors and precipitants, and currently there is no pharmacologic intervention with substantial evidence for benefit [3, 4].
Emerging research has identified significant differences in how women and men experience, respond to, and recover from other entities in critical care such as, cardiogenic shock [5], sepsis [6] or acute kidney injury [7]. To ensure a nuanced interpretation of current evidence, it is crucial to differentiate between gender and sex: gender involves socially constructed roles and behaviors considered appropriate by a society, while sex refers to biological attributes [8]. However, the impact of sex-specific differences on ICU delirium and related outcomes remains poorly understood, with existing data on sex-related influences—such as delirium duration, subtypes, treatment approaches, and patient outcomes—being conflicting, inconclusive, and limited [9, 10].
Understanding and addressing sex and gender differences regarding ICU-delirium is essential for improving patient care and outcomes, enabling personalized management that fosters equitable, patient-centered care [11].
The aim of this study was to explore whether critically ill patients with ICU-delirium exhibit sex-specific differences in short-term mortality.
Methods
Data source and study design
To ensure transparency and reproducibility, we utilized data from the openly accessible Medical Information Mart for Intensive Care-IV (MIMIC-IV) database [12].
The MIMIC-IV database was accessed through PostgreSQL, with variables extracted using SQL queries provided by the official MIMIC GitHub repository. All subsequent data preparation and analyses were conducted using Python version 3.12.4.
Our study adhered to the REporting of studies Conducted using Observational Routinely-collected Data (RECORD) guideline and has been registered on the Open Science Framework (https://osf.io/g6fr8). The code to fully reproduce our analysis is available (https://github.com/schrnik/sex_specific_differences_delirium).
Study population and screening for delirium
Patients aged 18 years or older who were admitted to the ICU and screened positive for delirium during their stay at the ICU using the Confusion Assessment Method for the ICU (CAM-ICU) [13] were eligible for analysis.
For a diagnosis of delirium, patients were required to have a Richmond Agitation-Sedation Scale (RASS) of − 3 or higher, along with an acute change or fluctuation in mental status (feature 1), inattention (feature 2), and either disorganized thinking (feature 3) or an altered level of consciousness (feature 4).
We classified patients into delirium subtypes as follows: Hyperactive delirium was defined by RASS scores between + 1 and + 4 at the time of delirium diagnosis, while hypoactive delirium was defined by RASS scores between 0 and − 3 at the time of delirium diagnosis [14].
Patients were excluded if they screened negative for delirium, lacked documentation of delirium screening, or had incomplete data required for time-to-event analysis.
Outcome
The censoring date of the study was set the latest at 30 days from delirium onset. The primary outcome of interest was 30-day mortality following the onset of delirium and was defined as the time interval from delirium onset to death-from-any-cause or the censoring date when being still alive 30 days after delirium onset.
Statistical analysis
Continuous variables were reported as medians with interquartile ranges (IQRs) and compared between sexes using the Wilcoxon rank-sum test. Categorical variables were summarized as counts and percentages and compared using the Chi-Square test. The magnitude of differences between groups was quantified using standardized mean differences (SMDs).
To examine the association between sex and 30-day mortality, we performed Cox proportional hazards regression. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). The proportional hazards assumption was assessed using Schoenfeld residuals.
Survival probabilities were visualized with Kaplan–Meier curves, and differences between sexes were assessed using the log-rank test.
To account for potential imbalances in baseline demographics, illness severity, treatment modalities and comorbidities between sexes, we applied propensity score matching (PSM). We derived the propensity score from a multivariable logistic regression model with sex as the dependent variable. Conditional on the propensity score, the distribution of baseline covariates was expected to be similar between women and men [15, 16].
The 24 covariates for the logistic regression model were selected based on existing literature [17] and are listed in the supplementary material.
We performed 1:1 nearest-neighbor matching with a caliper width of 0.1. After matching, balance between groups was assessed by re-estimating SMDs within the matched cohort to ensure that baseline covariates were well balanced between sexes.
After PSM, we estimated the HRs and their standard errors by using Cox models with a robust variance estimator to account for the matched pairs. The model was adjusted for delirium subtype.
To assess the robustness of our findings, we performed sensitivity analyses, the details of which are provided in the Additional Files.
Results
A total of 8950 ICU patients developed delirium during their stay and were eligible for analysis (Study Flow Chart, Additional Figure A1). Of these, 42.6% were women. Women were significantly older than men (median age: 71 [58–81] vs. 66 [54–77], p < 0.001) and had higher illness severity (SAPS II: 39.0 [31.0–50.0] vs. 38.0 [30.0–49.0], p < 0.001), though they were less likely to receive invasive ventilation (48.4% vs. 53.5%, p < 0.001) and vasoactive medication (43.7% vs. 46.2%, p = 0.020) before delirium onset. The baseline demographics, ICU-admission types, ICU-treatments, and comorbidities of the entire cohort, stratified by sex, are detailed in Table 1.
Table 1.
Demographics, illness severity and comorbidities stratified by sex
| Variable | Overall n = 8950 | Women n = 3811 | Men n = 5139 |
|---|---|---|---|
| Age | 68.0 [56.0–79.0] | 71.0 [58.0–81.0] | 66.0 [54.0–77.0] |
| Comorbidity and Illness severity scores | |||
| Charlson Comorbidity Index | 5.0 [3.0–7.0] | 5.0 [3.0–7.0] | 5.0 [3.0–7.0] |
| SAPS II | 39.0 [31.0–49.0] | 39.0 [31.0–50.0] | 38.0 [30.0–49.0] |
| Diagnosis and admission type | |||
| Sepsis at admission | 6477 (72.4%) | 2699 (70.8%) | 3778 (73.5%) |
| Type of admission | |||
| Cardiac Vascular Intensive Care Unit (CVICU) | 1077 (12.0%) | 378 (9.9%) | 699 (13.6%) |
| Coronary Care Unit (CCU) | 846 (9.5%) | 322 (8.4%) | 524 (10.2%) |
| Medical Intensive Care Unit (MICU) | 2320 (25.9%) | 1003 (26.3%) | 1317 (25.6%) |
| Medical/Surgical Intensive Care Unit (MICU/SICU) | 1202 (13.4%) | 570 (15.0%) | 632 (12.3%) |
| Neuro Surgical Intensive Care Unit (Neuro SICU) | 420 (4.7%) | 183 (4.8%) | 237 (4.6%) |
| Neurology | 702 (7.8%) | 335 (8.8%) | 367 (7.1%) |
| Surgical Intensive Care Unit (SICU) | 1239 (13.8%) | 578 (15.2%) | 661 (12.9%) |
| Trauma SICU (TSICU) | 1144 (12.8%) | 442 (11.6%) | 702 (13.7%) |
| ICU—treatment administered before delirium onset | |||
| Invasive ventilation before onset of delirium | 4593 (51.3%) | 1843 (48.4%) | 2750 (53.5%) |
| Renal replacement therapy before onset of delirium | 730 (8.2%) | 306 (8.0%) | 424 (8.3) |
| Vasoactive medication* before onset of delirium | 4039 (45.1%) | 1665 (43.7%) | 2374 (46.2%) |
| Sedation with benzodiazepines before onset of delirium | 2426 (27.1%) | 993 (26.1%) | 1433 (27.9%) |
| Transfusion of pRBCs before onset of delirium | 2576 (28.8%) | 1159 (30.4%) | 1417 (27.6%) |
| Comorbidities | |||
| Peripheral vascular disease | 1008 (11.3%) | 382 (10.0%) | 626 (12.2%) |
| Coronary artery disease | 1507 (16.8%) | 533 (14.0%) | 974 (19.0%) |
| Cerebrovascular disease | 1961 (21.9%) | 923 (24.2%) | 1038 (20.2%) |
| Congestive heart failure | 2478 (27.7%) | 1057 (27.7%) | 1421 (27.7%) |
| Renal disease | 1750 (19.6%) | 645 (16.9%) | 1105 (21.5%) |
| Dementia | 671 (7.5%) | 346 (9.1%) | 325 (6.3%) |
| Chronic pulmonary disease | 2226 (24.9%) | 1104 (29.0%) | 1122 (21.8%) |
| Malignant cancer | 1004 (11.2%) | 386 (10.1%) | 618 (12.0%) |
| Rheumatic disease | 276 (3.1%) | 185 (4.9%) | 91 (1.8%) |
| Peptic ulcer disease | 252 (2.8%) | 107 (2.8%) | 145 (2.8%) |
| Mild liver disease | 1201 (13.4%) | 424 (11.1%) | 777 (15.1%) |
| Severe liver disease | 630 (7.0%) | 225 (5.9%) | 405 (7.9%) |
| Diabetes without complications | 1981 (22.1%) | 836 (21.9%) | 1145 (22.3%) |
| Diabetes with complications | 916 (10.2%) | 330 (8.7%) | 586 (11.4%) |
| Paraplegia | 838 (9.4%) | 388 (10.2%) | 450 (8.8%) |
| Metastatic solid tumor | 449 (5.0%) | 189 (5.0%) | 260 (5.1%) |
| Acquired immune deficiency syndrome (AIDS) | 35 (0.4%) | 10 (0.3%) | 25 (0.5%) |
For continuous variables medians with 25th–75th percentile in brackets are depicted, whereas for categorical variables absolute values and percent in brackets are presented
*Vasoactive medication was defined as infusion of dopamine, epinephrine, norepinephrine, phenylephrine, vasopressin, dobutamine or milrinone for at least 10 consecutive minutes or longer
AIDS Acquired immune deficiency syndrome; CCU Coronary Care Unit; CVICU Cardiac Vascular Intensive Care Unit; MICU Medical Intensive Care Unit; MICU/SICU Medical/Surgical Intensive Care Unit; Neuro SICU Neuro Surgical Intensive Care Unit; pRBCs Packed red blood cells; SAPS II Simplified Acute Physiology Score II; SICU Surgical Intensive Care Unit; TSICU Trauma SICU
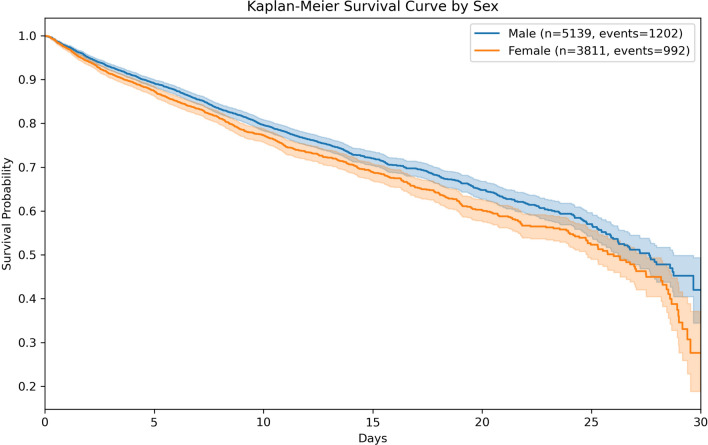
At 30 days, 992 of 3811 women and 1202 of 5139 men (26.0% vs. 23.4%, p = 0.004) had died, resulting in a crude HR of 1.16 (95% CI 1.071–1.267, p < 0.001; Fig. 1). After propensity score matching, the cohort included 3811 women and 3811 men. In the matched cohort, 909 men and 992 women had died after 30 days. After adjustment for delirium subtype, this corresponded to a HR of 1.16 (95% CI 1.064–1.273, p < 0.001) for female sex (Additional Table T1).
Fig. 1.
Kaplan–Meier survival curve by sex. 30-day survival probability after delirium onset compared between men (in blue) and women (in orange). 95%-Confidence Intervals are depicted as shaded areas. Log-Rank Test p-value: 0.0004
Baseline characteristics in the matched cohort were well-balanced, with all SMDs < 0.1 (Additional Figure A2). The distribution of the propensity score before and after matching is shown in Additional Figure A3, potential differences for delirium subtypes in Additional Figure A4.
Discussion
This study identified a significantly higher risk of short-term mortality for women with ICU-delirium compared to men.
The existing literature on sex-specific differences in delirium is limited and somewhat contradictory [11]. While some studies identify male sex as a risk factor for ICU-delirium, others have found an increased risk among women [17]. However, the impact of sex on short-term mortality in ICU patients in general remains uncertain, with research suggesting higher risk-adjusted ICU mortality in women [18], whereas other studies suggest no difference [16]. Despite these findings, the relationship between sex and outcomes specifically in ICU-delirium has not been thoroughly explored.
Hence, current guidelines do not explicitly address sex-specific differences in the prevention or management of ICU-delirium, despite the growing recognition of sex differences in critical care literature [19]. To the best of our knowledge, our study is the first to specifically assess sex-related differences in short-term mortality among ICU-delirium patients.
This study provides new insights but also raises important questions:
-
(i)
Why do women with ICU-delirium experience a higher risk of mortality?
-
(ii)
What are the implications of identifying a mortality difference between men and women with delirium?
-
(iii)
Men and women may follow different trajectories of recovery or deterioration after ICU-delirium due to differences in genetic predisposition, hormonal factors, and immunological responses to acute brain dysfunction, as sex hormones, including estrogens, progesterone, and androgens, regulate immune responses differently in each sex [20].
-
(iv)
Within the context of personalized medicine, our findings reinforce the need for special focus on women with ICU-delirium, both in everyday clinical practice and in future interventional trials, where women recently were underrepresented [3]. Moreover, emerging evidence suggests that women are undertreated in the ICU despite experiencing higher illness severity [11]. Although this hypothesis was not directly tested in our study, our findings may indirectly reflect this disparity, as, for example, women in our cohort were also less likely to receive vasoactive medication.
The critical care literature has shown significant progress in understanding sex-specific differences in other conditions, such as sepsis and cardiogenic shock [11], whereas our findings underline that further research into ICU-delirium is essential.
Considering the clinical implications of our findings, a critical starting point is recognizing the potential for unconscious bias when designing new interventions or preventive strategies for ICU-delirium. The higher mortality observed in women in our study may serve as an indicator of such bias, suggesting that current approaches might inadvertently overlook sex-specific needs. Future interventions should consider the possibility of these biases, promoting more tailored and equitable care that proactively addresses the unique risks and treatment responses associated with each sex.
The strengths of our study are the use of a large, openly available dataset and availability of detailed methodology and code to facilitate the reproduction and extension of our findings. Additionally, the substantial sample size enabled us to adjust for a comprehensive range of covariates within a propensity score matching framework, thereby enhancing the robustness and reliability of our results.
Limitations
Several limitations warrant consideration. Firstly, this study was a post hoc analysis of single-center observational data, which inherently limits the generalizability of our findings. Additionally, the data originates from a North American population, which may differ from European populations, potentially further limiting the applicability of our results across different geographic regions. Despite using stringent propensity score matching, residual confounding cannot be excluded and hence causality cannot be established. To address unmeasured confounding, we calculated E-values to provide an estimate of the strength that unmeasured confounding would need to have to explain away our observed associations. Nevertheless, differences in ICU-care practices or the use of specific delirium treatment protocols between sexes might not be fully accounted for. Moreover, we were not able to investigate potential sex-related biases in the assessment of delirium, which might have played a role. Therefore, replication and validation of our findings in different cohorts are needed.
Furthermore, the MIMIC database lacks information about treatment limitations and long-term follow-up data on outcomes like neurological impairment, quality of life, and anxiety, which limits our ability to fully assess the extended impact of ICU-delirium, particularly in women. Another limitation is that our study only included patients with documented CAM-ICU assessments for delirium, thereby excluding those without documented CAM-ICU assessments and those who died before any screening was conducted. This exclusion may further impact the generalizability of our findings.
These data limitations highlight how unprepared current large databases are to comprehensively study sex-specific outcomes in ICU delirium.
Conclusion
Our findings indicate a higher risk of short-term mortality in for women with ICU-delirium, highlighting the need for sex-specific considerations in delirium management. These results suggest that immediate clinical applications could include heightened awareness of potential treatment disparities and closer monitoring of delirium in women. Future research should focus on replicating our findings in different cohorts and directly investigating the biological and clinical mechanisms that may underlie sex differences in ICU delirium outcomes.
Supplementary Information
Acknowledgements
The authors thank Prof. Andrea Kurz and Prof. Alexander Rosenkranz for their invaluable support.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- BIDMC
Beth Israel Deaconess Medical Center
- CAM-ICU
Confusion Assessment Method for the ICU (CAM-ICU)
- CI
Confidence interval
- HR
Hazard ratio
- ICU
Intensive Care Unit
- IQR
Interquartile range
- MIMIC-IV
Medical Information Mart for Intensive Care-IV
- MIT
Massachusetts Institute of Technology
- PSM
Propensity score matching
- RASS
Richmond Agitation-Sedation Scale
- SAPS II
Simplified Acute Physiology Score II
- SMD
Standardized mean difference
- AIDS
Acquired immune deficiency syndrome
- BIDMC
Beth Israel Deaconess Medical Center
- CAM-ICU
Confusion Assessment Method for the ICU (CAM-ICU)
- CCU
Coronary Care Unit
- CI
Confidence interval
- CVICU
Cardiac Vascular Intensive Care Unit
- HR
Hazard ratio
- ICU
Intensive Care Unit
- IQR
Interquartile range
- MICU
Medical Intensive Care Unit
- MICU/SICU
Medical/Surgical Intensive Care Unit
- MIMIC-IV
Medical Information Mart for Intensive Care-IV
- MIT
Massachusetts Institute of Technology
- Neuro SICU
Neuro Surgical Intensive Care Unit
- pRBCs
Packed red blood cells
- PSM
Propensity score matching
- RASS
Richmond Agitation-Sedation Scale
- RECORD
REporting of studies Conducted using Observational Routinely-collected Data
- SAPS II
Simplified Acute Physiology Score II
- SAPS II
Simplified Acute Physiology Score II
- SICU
Surgical Intensive Care Unit
- SMD
Standardized mean difference
- TSICU
Trauma SICU
Author contributions
NS and PE designed the study and drafted the first manuscript. NS, SO, SFH, CK and PE analyzed the data. LS, SFH, PZ, ME, AP and JB gave conceptual input and revised the manuscript significantly. All authors read final manuscript, and approved the final version submitted for publication.
Funding
None.
Availability of data and materials
To ensure transparency and reproducibility, we utilized data from the openly accessible Medical Information Mart for Intensive Care-IV (MIMIC-IV) database, available via the PhysioNet repository (https://physionet.org/content/mimiciv/3.0/). The code for reproduction of our analysis is available on Github (https://github.com/schrnik/sex_specific_differences_delirium). We used MIMIC-IV version 3.0, released on July 23, 2024.
Declarations
Ethics approval and consent to participate
The MIMIC database was approved by the institutional review boards of the Beth Israel Deaconess Medical Center (2001-P-001699/14) and the Massachusetts Institute of Technology (No. 0403000206), which waived the requirement for individual patient consent because the datasets contained deidentified information.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stollings JL, Kotfis K, Chanques G, Pun BT, Pandharipande PP, Ely EW. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 2021;47(10):1089–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotfis K, van Diem-Zaal I, Williams Roberson S, Sietnicki M, van den Boogaard M, Shehabi Y, et al. The future of intensive care: delirium should no longer be an issue. Crit Care Lond Engl. 2022;26(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smit L, Slooter AJC, Devlin JW, Trogrlic Z, Hunfeld NGM, Osse RJ, et al. Efficacy of haloperidol to decrease the burden of delirium in adult critically ill patients: the EuRIDICE randomized clinical trial. Crit Care Lond Engl. 2023;27(1):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carayannopoulos KL, Alshamsi F, Chaudhuri D, Spatafora L, Piticaru J, Campbell K, et al. Antipsychotics in the treatment of delirium in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2024;52(7):1087–96. [DOI] [PubMed] [Google Scholar]
- 5.Fisher T, Hill N, Kalakoutas A, Lahlou A, Rathod K, Proudfoot A, et al. Sex differences in treatments and outcomes of patients with cardiogenic shock: a systematic review and epidemiological meta-analysis. Crit Care Lond Engl. 2024;28(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antequera A, Lopez-Alcalde J, Stallings E, Muriel A, Fernández Félix B, Del Campo R, et al. Sex as a prognostic factor for mortality in critically ill adults with sepsis: a systematic review and meta-analysis. BMJ Open. 2021;11(9): e048982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neugarten J, Golestaneh L. Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol. 2018;19(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson E. Sex matters: Is it time for a SOFA makeover? Crit Care Lond Engl. 2024;28(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trzepacz PT, Franco JG, Meagher DJ, Lee Y, Kim JL, Kishi Y, et al. Delirium phenotype by age and sex in a pooled data set of adult patients. J Neuropsychiatry Clin Neurosci. 2018;30(4):294–301. [DOI] [PubMed] [Google Scholar]
- 10.Krewulak KD, Stelfox HT, Ely EW, Fiest KM. Risk factors and outcomes among delirium subtypes in adult ICUs: a systematic review. J Crit Care. 2020;56:257–64. [DOI] [PubMed] [Google Scholar]
- 11.Merdji H, Long MT, Ostermann M, Herridge M, Myatra SN, De Rosa S, et al. Sex and gender differences in intensive care medicine. Intensive Care Med. 2023;49(10):1155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–10. [DOI] [PubMed] [Google Scholar]
- 14.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman Pun B, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33(10):1726–31. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollinger A, Gayat E, Féliot E, Paugam-Burtz C, Fournier MC, Duranteau J, et al. Gender and survival of critically ill patients: results from the FROG-ICU study. Ann Intensive Care. 2019;9(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ormseth CH, LaHue SC, Oldham MA, Josephson SA, Whitaker E, Douglas VC. Predisposing and precipitating factors associated with delirium: a systematic review. JAMA Netw Open. 2023;6(1): e2249950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modra LJ, Higgins AM, Pilcher DV, Bailey MJ, Bellomo R. Sex differences in mortality of ICU patients according to diagnosis-related sex balance. Am J Respir Crit Care Med. 2022;206(11):1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–73. [DOI] [PubMed] [Google Scholar]
- 20.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure transparency and reproducibility, we utilized data from the openly accessible Medical Information Mart for Intensive Care-IV (MIMIC-IV) database, available via the PhysioNet repository (https://physionet.org/content/mimiciv/3.0/). The code for reproduction of our analysis is available on Github (https://github.com/schrnik/sex_specific_differences_delirium). We used MIMIC-IV version 3.0, released on July 23, 2024.