Abstract
The chemokine CXCL12 promotes T lymphocyte adhesion mediated by the integrin α4β1. CXCL12 activates the GTPase Rac, as well as Vav1, a guanine-nucleotide exchange factor for Rac, concomitant with up-regulation of α4β1-dependent adhesion. Inhibition of CXCL12-promoted Rac and Vav1 activation by transfection of dominant negative Rac or Vav1 forms, or by transfection of their siRNA, remarkably impaired the increase in T lymphocyte attachment to α4β1 ligands in response to this chemokine. Importantly, inhibition of Vav1 expression by RNA interference resulted in a blockade of Rac activation in response to CXCL12. Adhesions in flow chambers and soluble binding assays using these transfectants indicated that initial ligand binding and adhesion strengthening mediated by α4β1 were dependent on Vav1 and Rac activation by CXCL12. Finally, CXCL12-promoted T-cell transendothelial migration involving α4β1-mediated adhesion was notably inhibited by expression of dominant negative Vav1 and Rac. These results indicate that activation of Vav1-Rac signaling pathway by CXCL12 represents an important inside-out event controlling efficient up-regulation of α4β1-dependent T lymphocyte adhesion.
INTRODUCTION
Extravasation of T lymphocytes into tissues depends on their integrin-mediated interactions with the endothelium to achieve firm adhesion and allow resistance to detachment from blood shear stress. The integrin α4β1 is a main player in lymphocyte transendothelial migration by mediating adhesion to its ligands VCAM-1 and fibronectin that are expressed by endothelial cells (Springer, 1994; Butcher and Picker, 1996; Ebnet et al., 1996; von Andrian and Mackay, 2000). Although it can support lymphocyte arrest on high-density VCAM-1, adhesion dependent on α4β1 is highly regulated by the activity of chemokines, which bind to their G protein-coupled receptors and rapidly promote increased lymphocyte attachment mediated by this integrin (Springer, 1994; Butcher and Picker, 1996; von Andrian and Mackay, 2000; Rose et al., 2002). Previous reports clearly established that interaction of the chemokine CXCL12 (SDF-1) with its receptor CXCR4 triggers rapid up-regulation of α4β1-dependent lymphocyte adhesion without altering α4β1 cell surface expression (Grabovsky et al., 2000). Therefore, signaling initiated upon CXCL12/CXCR4 interaction should finally impinge on α4β1, triggering its activation.
Associated to the development of firm adhesion, lymphocytes adapt their morphology to a migratory phenotype that is promoted by reorganization of their actin cytoskeleton (Sanchez-Madrid and del Pozo, 1999; Worthylake and Burridge, 2001). Key players in the regulation of the dynamics of actin cytoskeleton are the small GTPases of the Rho subfamily, such as Rho, Rac, and Cdc42 proteins, which cycle between inactive GDP-bound and active GTP-bound forms (Etienne-Manneville and Hall, 2002; Burridge and Wennerberg, 2004). Guanine-nucleotide exchange factors (GEF) stimulate the exchange of GDP for GTP to generate active Rho GTPases that can interact with downstream targets to produce different biological responses, whereas GTPase activating proteins accelerate the intrinsic GTPase activity of Rho GTPases resulting in their inactivation (Schmidt and Hall, 2002). Activation of Rho regulates the assembly of actin filaments, whereas Rac and Cdc42 regulate actin polymerization to generate lamellipodia and filopodia protrusions, respectively (Etienne-Manneville and Hall, 2002). Although Rac1 is widespread expressed, Rac2 expression is restricted to cells of the hematopoietic lineage (Burridge and Wennerberg, 2004)
Vav1 is a 95-kDa protein mainly expressed on hematopoietic cells that functions as a GEF predominantly for Rac (Bustelo, 2000; Turner and Billadeau, 2002; Tybulewicz et al., 2003). Importantly, T-cells from vav1–/– mice have decreased Rac activation (Reynolds et al., 2002), and it was demonstrated that constitutively active Rac rescues developmental defects in vav1–/– pre-T-cells (Gomez et al., 2000). Vav proteins contain distinct domains including CH (calponin-homology), Ac (acidic), DH (Dbl-homology), PH (pleckstrin-homology), ZF (zinc-finger), PR (proline-rich), SH3 (Src-homology 3), and SH2 (Src-homology 2), which have the potential to participate in different interactions (Bustelo, 2000; Turner and Billadeau, 2002). Activation of Vav GEF activity requires phosphorylation at tyrosine residues located in the Ac domain (Crespo et al., 1997; Aghazadeh et al., 2000). The DH domain binds to Rho GTPases and is the responsible for GEF activity, whereas deletion of domains CH and Ac generates an active Vav displaying constitutive GEF activity for Rac (Schuebel et al., 1998; Aghazadeh et al., 2000; Bustelo, 2000). On the other hand, the SH2 and SH3 domains interact with autophosphorylated tyrosine kinases and with several adaptor proteins (Bustelo, 2000; Turner and Billadeau, 2002; Tybulewicz et al., 2003). Hematopoietic cells from vav1–/–, as well as from rac–/– knockout mice display deficient adhesive properties, evidencing their important roles in this process (Yang et al., 2001; Krawczyk et al., 2002; Gu et al., 2003; Gakidis et al., 2004).
CXCL12 rapidly activates Rho GTPases and triggers actin polymerization in lymphocytes (Nishita et al., 2002; Wright et al., 2002). This activation takes place concomitant with the rapid enhancement in α4 integrin-dependent adhesion in response to this chemokine, raising the possibility that activation of Rho GTPases by CXCL12 could be functionally associated with efficient increased adhesion.
Characterization of the role that CXCL12-promoted Rho GTPase activation has on the regulation of integrin α4β1-dependent lymphocyte adhesion is of key importance for a better knowledge of mechanisms governing integrin activation during T lymphocyte transendothelial migration. In the present study we have expressed mutant forms of Rac and Vav1, as well as knocked-down their expression by transfecting siRNA, to study their involvement on CXCL12-promoted α4β1-dependent lymphocyte adhesion and migration.
MATERIALS AND METHODS
Cells and Antibodies
The human T-cell lines Peer and Molt-4 were cultured in RPMI 1640 medium (Life Technologies Invitrogen, Paisley, Scotland) supplemented with 10% fetal bovine serum (FBS; Biowhittaker, Verviers, Belgium; growth medium). Human peripheral blood mononuclear cells were prepared from buffy coats using a ficoll density gradient. T lymphocytes were isolated by negative selection using anti-CD14 –and anti-CD19–conjugated beads (Dynal ASA, Oslo, Norway) and purity was >95% for each sample as analyzed by flow cytometry (Coulter Epics XL, Hialeah, FL) using anti-CD3 T3b monoclonal antibody (mAb). Human umbilical vein endothelial cells (HUVEC) were obtained and cultured as described (Bartolome et al., 2003). Anti-CXCR4 mAb was purchased from R&D Systems (Minneapolis, MN), anti-Rho and antiphosphotyrosine PY20 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), anti-Rac and anti-Cdc42 from BD Biosciences PharMingen (San Diego, CA), and anti-GFP from Molecular Probes (Eugene, OR). Control mAb P3X63 and function-blocking integrin anti-α4 HP1/2 were gifts from Dr. Francisco Sánchez-Madrid (Hospital de la Princesa, Madrid, Spain), whereas nonblocking B-5G10 was from Dr. Martin E. Hemler (Dana-Farber Cancer Institute, Boston, MA). Anti-Vav mAb was from Santa Cruz Biotechnology, whereas polyclonal anti-DH and anti-SH2 Vav1 antibodies were generated and described in a previous work (Zugaza et al., 2002). The anti-α4β7 Act-1 mAb was a gift from Millenium Pharmaceuticals (Cambridge, MA).
Transfections
Peer and Molt-4 cells were transiently transfected with expression vectors (pEGFP-C1) coding for GFP alone, GFP-fused wild-type Rac and Cdc42, or their dominant negative counterparts (N17-Rac and N17-Cdc42). For Vav1, we used GFP-fused Vav1 wild-type, and the mutant forms Vav1 SH3-SH2-SH3 and ΔCH+Ac (Lopez-Lago et al., 2000). Expression vectors (2 μg per million of cells) were incubated for 5 min at 4°C with cells in RPMI 1640 medium and mixtures in electroporation cuvettes (Bio-Rad, Hercules, CA) single-pulsed at 230 V and 960 μF. Cuvettes were left in ice for 10 min, and subsequently cells were cultured in growth medium for 16 h and finally analyzed by flow cytometry to determine GFP-expression. For nucleofection, human T lymphocytes were washed with phosphate-buffered saline (PBS)/EDTA 0.3 mM and resuspended in T-cell Nucleofector solution at 5 × 106 cells/100 μl. Plasmids (5 μg) were mixed with cellular suspensions and nucleofected using an Amaxa Nucleofector device (Amaxa, Cologne, Germany). After transfection, cells were transferred to growth medium, cultured for 6 h, and analyzed by flow cytometry to determine GFP-expression. siRNA duplexes were transfected using nucleofection protocols (Amaxa) and transfectants assayed 16 h posttransfection (T-cell lines) or 6 h (T lymphocytes). Transfection of wild-type and mutant GTPases or Vav1, or transfection of their siRNA, did not affect cell viability as assessed in cell cycle analyses by flow cytometry and by trypan blue exclusion (unpublished data).
Static Cell Adhesion Assays
Soluble recombinant human VCAM-1 (sVCAM-1) consisting of domains 1–4 fused to the Fc portion of IgG1 (Munoz et al., 1996), and the recombinant FN-H89 fragment of fibronectin, which contains the CS-1 site (Mould et al., 1994), were coated on 96-well plates (High binding, Costar, Cambridge, MA). Before addition to wells, nontransfected cells or siRNA transfectants were labeled with 2′,7′-bis(carboxyethyl)-5(6′)-carboxyfluorescein-acetoxymethyl ester (BCECF-AM; Molecular Probes, Leiden, The Netherlands). Cells were resuspended in adhesion medium (RPMI 1640/bovine serum albumin 0.5%) and added to wells (7.5 × 104 in 100 μl) containing either sVCAM-1 or FN-H89 alone or coinmobilized with CXCL12 (R&D Systems). After a 15-s spin, plates were incubated for 2 min at 37°C, and subsequently nonbound cells were removed by washing with RPMI 1640 medium. Adhered cells were lysed and extent of adhesion was quantified using a fluorescence analyzer (BMG Labtechnologies, Offenburg, Germany). For GFP transfectants, after the 2-min adhesion step and washes, bound cells were detached, counted, and analyzed by flow cytometry to determine GFP expression. The inhibitor AG490 (Calbiochem, Darmstadt, Germany) was used at concentrations that were not cytotoxic, as measured in cell cycle analysis (unpublished data). To detect Vav1 phosphorylation after cell attachment to α4β1 ligands, Molt-4 cells (2 × 107) were placed in wells containing FN-H89, sVCAM-1 or bovine serum albumin (BSA; Medium), and plates were centrifuged for 15 s followed by incubation for 2 min at 37°C. Cells attached to FN-H89 or sVCAM-1, or the same cell number from wells with BSA, were solubilized and lysates subjected to immunoprecipitation and Western blotting using anti-Vav1 antibodies.
Flow Chamber Adhesion Assays
We followed the procedure already described (Stein et al., 2003). Briefly, a 15 μl-drop in PBS containing sVCAM-1 (2 μg/ml) and 0.5 μg of CXCL12 was spotted on Petri dishes, which were later incorporated as lower walls of a parallel flow chamber. Wild-type Rac or Vav1 Molt-4 transfectants were first infused at 1 ml/min before flow rate was adjusted to 1 dyn/cm2 and observed with a 10× objective. Events were recorded for 3–4 min for subsequent off-line analysis. Adherent cells were washed off and DN Rac or Vav1 transfectants were subsequently infused and filmed in the same field. Both wt and DN Rac and Vav1 were infused in an alternating order to control for an eventual washout effect of CXCL12 caused by prolonged perfusion during the experiment. Rolling cells that became firmly adherent (stationary for at least 20 s) were expressed as sticking cells (percent of rollers becoming adherent related to total number of interacting cells during 2-min analysis). Otherwise interacting cells that did not firmly attach were expressed as rolling cells (percent of rollers related to total number of interacting cells). To evaluate shear resistance, cells were allowed to adhere for 8 min and then flow was increased at 1 dyn/cm2 every 30 s. The number of cells remaining bound was determined as percent of total adhered cells after 8-min adhesion stage.
Soluble Binding Assays
We followed the method described (Chan et al., 2001) with some modifications. Molt-4 cells suspended in 50 μl of binding buffer (Hanks' balanced salt solution containing 2% FBS) at 6 × 106 cells/ml were preincubated at 37°C in the presence or absence of antibodies (10 μg/ml). For binding assays involving chemokine stimulation, cells were incubated for 45 s at 37°C with CXCL12 (150 ng/ml) or binding buffer alone, and then sVCAM-1/Fc at saturating concentrations (20 μg/ml) was added and incubated with cells for 75 s at 37°C. For assays involving exposure to Mn2+, after preincubation with antibodies cells were incubated directly with sVCAM-1/Fc in binding buffer supplemented with 1 mM MnCl2. Binding was stopped by washing with ice-cold binding buffer (with 0.5 mM MnCl2 for samples exposed to Mn2+) and bound sVCAM-1/Fc was detected by flow cytometry using phycoerythrin-conjugated AffiniPure F(ab′)2 fragment goat anti-human IgG, Fc-gamma fragment specific (Jackson ImmunoResearch Laboratories, West Grove, PA).
RNA Interference and RT-PCR
We designed a siRNA duplex corresponding to a common sequence for human Rac1 and Rac2 (Rac1/Rac2; targeted to bases 382–402 on rac1 gene sequence), sense strand: ACUGAAGGAGAAGAAGCUGdTdT. siRNA duplexes specific for Rac1 were designed according to the sequences described (Deroanne et al., 2003, 2005; Rac1.1, bases 229–249; Rac1.3, bases 305–325). We also designed an siRNA for Rac1 (Rac1.M) containing a point mutation in position 242 of Rac1.1 sequence (U to A). Four siRNA duplexes were designed corresponding to human Vav1 (Vav1.1, bases 1393–1413; Vav1.2, bases 307–327; Vav1.3, bases 2134–2154; Vav1.4, bases 2512–2532 from gene sequence). Sense strands were as follows: GAAGUGGAGCCACAUGUUCdTdT for Vav1.1; GGAUUUUGGCAAGGUCAUCdTdT for Vav1.2; CGUCGAGGUCAAGCACAUUdTdT for Vav1.3; and GGAAGAUUAUUCUGAAUACdTdT for Vav1.4. We designed a siRNA for Vav1 (Vav1.M) containing a point mutation in position 2145 of Vav1.3 sequence (U to A). The siRNA sequence sense strand used as negative control for siRNA activity was AUUGUAUGCGAUCGCAGACdTdT. Control, Rac and Vav1 siRNA duplexes were purchased from Dharmacon (Lafayette, CO) and Ambion (Austin, TX). All 21-nucleotide siRNA duplexes were verified to be specific for their targets by Blast search against the human genome. siRNA transfectants were lysed in TriReagent (Sigma-Aldrich, St. Louis, MO), and RNA was extracted and reverse transcribed using M-MLV reverse transcriptase (Promega, Madison, WI). Amplification of Vav1 was performed by PCR using primers 5′-TGCCTATGCAGCGAGTTCTC-3′ and 5′-CCCTGCGATGTAGTTTGTCC-3′, and TaqDNA polymerase (Invitrogen). The PCR profile consisted of 1-min initial denaturation at 94°C followed by 35 cycles of 30-s denaturation at 94°C, 30-s annealing at 58°C, and 1-min and 30-s polymerization at 72°C, and finally by 10-min extension at 72°C. Aliquots of each sample were amplified using the same conditions with human GAPDH primers 5′-GGCTGAGAACGGGAAGCTTGTCA-3′ and 5′-CGGCCATCACGCCACAGTTTC-3′ as cDNA loading control.
Confocal Microscopy
Cells attached to poly-l-lysine were incubated for 5 min at 37°C with CXCL12 (150 ng/ml) or adhesion medium alone, fixed with paraformaldehyde 4% in PBS, and mounted with mowiol. Images were captured using a Leica TCS-SP2-AOBS-UV confocal microscope (Mannheim, Germany) with 100× oil immersion objective. For staining of F-actin, after adhesion cells were fixed as above and permeabilized with 0.5% Triton-X in PBS. Cells were subsequently incubated with Alexa 633-Phalloidin (Molecular Probes) and observed with the confocal microscope. Images displayed were captured at the same section in the different samples.
Transendothelial Migration Assays
HUVEC (7.5 × 104) were plated on FN-coated upper chambers of 5-μm-poresize Transwells (Costar), and confluent monolayers were incubated for 12 h before the assay with TNF-α (R&D Systems, Abingdon, United Kingdom) to induce cell surface VCAM-1 expression. The lower chambers contained 600 μl of adhesion medium alone or with CXCL12 (100 ng/ml). Transfectants (3 × 105) in adhesion medium were added to Transwells in the presence or absence of antibodies (10 μg/ml), followed by incubation for 3.5 h at 37°C. Extent of transfectant migration was analyzed in a flow cytometer by passing each sample in the same predetermined time and flow conditions. After migration, HUVECs were stained with violet crystal to exclude any alteration in the integrity of the monolayer.
Immunoprecipitation, Western Blotting, and GTPase Assays
For immunoprecipitation, Molt-4 cells (1.5 × 107) preincubated with or without CXCL12 were washed in ice-cold stop buffer and lysed as described (Ticchioni et al., 2002). Lysates were precleared with protein A-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden), and supernatants were incubated with antibodies, followed by specific coupling to protein A-Sepharose beads. Proteins were eluted in Laemmli buffer, resolved by SDS-PAGE, and transferred to polyvinylidene fluoride (PVDF) membranes (Amersham Pharmacia Biotech). Membranes were incubated with antibodies, followed by washing and incubation with horseradish peroxidase–conjugated secondary antibodies. Proteins were visualized using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL). After stripping and blocking, the blots were reprobed with control antibodies to test for total protein content or with anti-GFP antibodies. For GTPase assays we followed essentially the method described (Bartolome et al., 2003). Briefly, cells were exposed to CXCL12 (150 ng/ml) followed by washing with ice-cold PBS and lysis. Aliquots of extracts were kept aside for total lysate controls, and the remaining volume was mixed with GST-PAK-CD fusion protein (Sander et al., 1998) in the presence of glutathione-agarose beads. The mixtures were incubated at 4°C and bound proteins eluted in electrophoresis buffer. Proteins were separated by SDS-PAGE and transferred to PVDF membranes that were incubated with antibodies against Rac or Cdc42. Protein detection was performed as above.
Statistical Analyses
Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey-Kramer multiple comparisons. In both analyses, the minimum acceptable level of significance was p < 0.05.
RESULTS
Inhibition of CXCL12-promoted Up-regulation of Integrin α4β1–dependent T-cell Adhesion by Dominant Negative Rac or siRNA for Rac
GTPase assays performed with lysates from nonstimulated Peer T-cells displayed minimal Rac and Cdc42 activation (Figure 1A). Incubation with CXCL12 in solution rapidly (≤1 min) promoted the activation of both GTPases in these cells, as well as in Molt-4 T-cells (unpublished data; see also Figure 3B), and this activation persisted up to 15 min incubation. Furthermore, short-term adhesion assays (2 min at 37°C after a short spin) revealed that CXCL12 triggered an increase in cell attachment to sVCAM-1 and FN-H89, both when the chemokine was coimmobilized with ligands or used in solution (Supplementary Figure 1), whereas anti-α4 mAb inhibited these adhesions (unpublished data). Flow cytometry analyses revealed that these cells did not express the α4β7 integrin (unpublished data), indicating that up-regulation of cell attachment was mediated by α4β1.
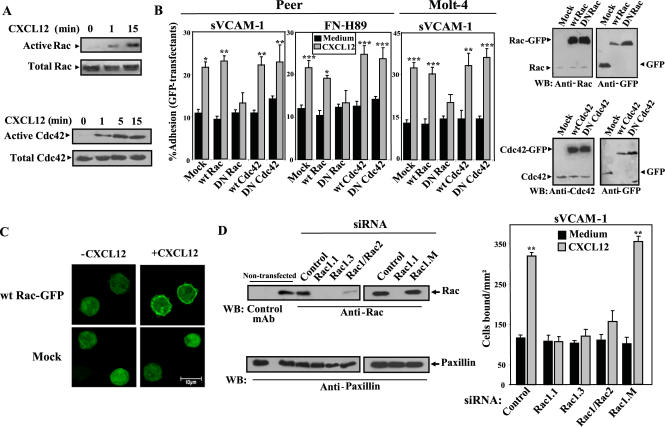
Figure 1.
Role of Rac on CXCL12-promoted α4β1-dependent T-cell adhesion. (A) Peer T-cells were incubated for the indicated times with or without CXCL12, followed by cell solubilization and incubation of extracts with glutathione-agarose–bound GST-PAK-CD to detect active Rac and Cdc42. Bound Rac and Cdc42 were determined by Western blot using anti-Rac or anti-Cdc42 mAb. Aliquots from each lysate were kept aside for detecting total protein. (B, left) Peer or Molt-4 cells transfected with expression vectors coding for wild-type (wt) or dominant negative (DN) Rac- or Cdc42-GFP-fusion proteins, or GFP alone (Mock), were subjected to adhesions for 2 min at 37°C to sVCAM-1 or FN-H89 coimmobilized with or without (Medium) CXCL12. Nonbound cells were washed and extent of adhesion quantified in a flow cytometer as specified in Materials and Methods. Percentage data of adhered GFP transfectants represent the mean ± SD of at least three independent experiments done for each panel. Right: Molt-4 transfectants were subjected to immunoblotting using the indicated antibodies. (C) Wild-type Rac-GFP or mock Molt-4 transfectants attached to poly-Lys were incubated with or without CXCL12 and analyzed by confocal microscopy. Equivalent cell sections corresponding to representative fields are showed. (D, top) Molt-4 cells were transfected with control or the indicated Rac siRNA, and after solubilization, lysates were analyzed by Western blotting using anti-Rac or control mAb, followed by sequential membrane reprobing with antipaxillin and anti-Rho antibodies. Also shown is Western blotting from nontransfected Molt-4 cells (bottom). BCECF-AM–labeled siRNA transfectants were subjected to adhesion assays to sVCAM-1 coimmobilized with or without CXCL12 (2 min, 37°C). Extent of adhesion was quantified in a fluorescence analyzer, and data represent the mean ± SD of triplicate samples from a representative result of three independent experiments. Adhesions were significantly up-regulated, ***p < 0.001, **p < 0.01, or *p < 0.05, according to one-way ANOVA test.
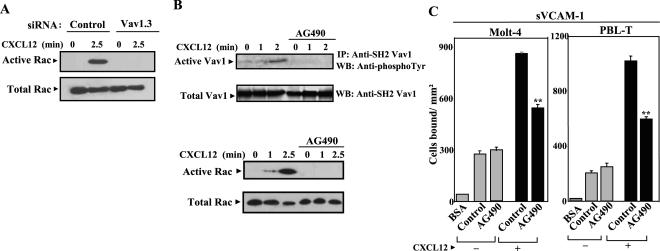
Figure 3.
CXCL12-promoted activation of Rac requires Vav1. Role of Vav1-Rac signaling axis in up-regulation by CXCL12 of α4β1-mediated T-cell adhesion. (A) Molt-4 cells were transfected with control or Vav1.3 siRNA. Transfectants were subsequently incubated for the indicated times with CXCL12 and subjected to GTPase assays to detect active Rac. (B) Molt-4 cells preincubated with or without AG490 (100 μM) were exposed to CXCL12 for the indicated times, and after cell solubilization, extracts were subjected to analysis of Vav1 phosphorylation (top) as described in legend for Figure 2, or to GTPase assays to detect active Rac (bottom), as in Figure 1. (C) Molt-4 and PBL-T-cells incubated with AG490 or RPMI medium alone (Control), were labeled with BCECF-AM and subjected to adhesions for 2 min at 37°C to sVCAM-1 coimmobilized with (+) or without (–) CXCL12. Nonbound cells were washed and extent of adhesion quantified in a fluorescence analyzer. Data represent the mean ± SD of triplicate samples from representative results of two independent experiments per each panel. Adhesion was significantly inhibited, **p < 0.01, according to one-way ANOVA test.
To investigate the potential involvement of CXCL12-promoted GTPase activation on the increase of T-cell adhesion mediated by α4β1, we used two different approaches. First, we transfected Peer and Molt-4 cells with GFP-fused wild-type (wt) or dominant negative (DN) forms of Rac or Cdc42, as well as GFP alone (mock), and measured transfectant attachment to α4β1 ligands in response to CXCL12. Dominant negative GTPases compete with wt counterparts for binding to GEFs and are unable to activate GTPase downstream effectors, translating into inhibition in the activation of the endogenous GTPase pool (Feig, 1999). Western blot analyses revealed that GFP-fused wt and DN Rac and Cdc42 were overexpressed in Molt-4 cells compared with endogenous Rac and Cdc42 (Figure 1B, right). Control experiments indicated that α4β1 and CXCR4 expression was unaltered upon expression of DN Rac compared with wt Rac transfectants (Supplementary Figure 2A). Cell adhesions were performed as indicated above, and extent of transfectant attachment was measured by flow cytometry. Overexpression of GFP-fused wt Rac or Cdc42 did not significantly alter the enhancement by CXCL12 of α4β1-mediated transfectant adhesion in comparison to GFP mock transfectants, and expression of DN forms of these GTPases did not affect adhesion of nonstimulated transfectants (Figure 1B, left). In contrast, up-regulation by CXCL12 of α4β1-dependent attachment of DN Rac transfectants was substantially reduced, whereas DN Cdc42 transfectants did not display alterations in their increased adhesion (Figure 1B, left).
To investigate Rac localization on CXCL12-stimulated T-cells, we performed confocal microscopy on Molt-4 cells transfected with wt Rac-GFP or on mock transfectants. Images showed that CXCL12 induced in most cells a rapid translocation of wt Rac-GFP from a diffuse cytosolic staining in nonstimulated cells to a predominant pattern of plasma membrane localization that was not observed in mock transfectants (Figure 1C). Membrane localization of Rac-GFP upon exposure to CXCL12 is consistent with proper cell trafficking of active Rac-GTP (del Pozo et al., 2000).
Second, to more directly determine Rac involvement in CXCL12-promoted enhancement of cell adhesion mediated by α4β1, we transfected Molt-4 cells with two siRNA covering different Rac1 sequences (siRNA Rac1.1 and Rac1.3), as well as with an additional siRNA recognizing a common sequence on both Rac1 and Rac2 (siRNA Rac1/Rac2). Western blotting on Rac siRNA transfectant lysates revealed a dramatic decrease in Rac1 and Rac1/2, whereas Rac expression levels similar to those found in nontransfected cells were detected using control siRNA (Figure 1D, top). Furthermore, transfection of a siRNA differing from the siRNA Rac1.1 sequence just in a single-base mutation (siRNA Rac1.M) resulted in expression of normal Rac levels. In addition, expression of another small GTP-ase, Rho, was unaltered by transfection of siRNA for Rac. Control experiments indicated that α4β1 and CXCR4 expression was unaffected by transfection of Rac siRNA (Supplementary Figure 2B). Decreased Rac expression in Rac1.1, Rac1.3, and Rac1/Rac2 siRNA transfectants correlated with a remarkable impairment in their up-regulation of attachment to sVCAM-1 in response to CXCL12, as compared with enhancement in adhesion exhibited by control or Rac1.M siRNA transfectants (Figure 1D, bottom), thus confirming the adhesion results obtained with DN Rac transfectants. These data also suggest that inhibition of Rac1 expression alone is sufficient to impair CXCL12-triggered, α4β1-dependent adhesion.
Role of Vav1 on CXCL12-promoted Up-regulation in α4β1-mediated T-cell Adhesion
Phosphorylated Vav1 catalyzes GDP release from Rac and transition to a GTP-bound active form. CXCL12 rapidly (≤1 min) and transiently activated Vav1 on Molt-4 cells, as detected by its increase in tyrosine phosphorylation (5–10-fold at 1 min; Figure 2A). To investigate the role of Vav1 activation by CXCL12 on α4β1-dependent adhesion, we compared the attachment to sVCAM-1 of Molt-4 transfectants expressing GFP-fused mutant and wt Vav1. For mutant Vav1 we used a truncated form that only contains the C-terminal SH3-SH2-SH3 region (Vav 1 SH3-SH2-SH3; Figure 2B, top; Zugaza et al., 2002), a domain that interacts with tyrosine kinases responsible for Vav1 phosphorylation. Thus, this mutant should interfere with the activation of endogenous Vav1 by sequestering kinases important for its phosphorylation, therefore acting as a putative dominant negative. In addition, we used a mutant Vav1 lacking the CH and acidic regions (Vav1 ΔCH+Ac) that displays constitutive GEF activity toward Rac (Schuebel et al., 1998). Endogenous Vav1 was easily detected by anti-DH Vav1 mAb in GFP mock transfectants, as well as in wt and ΔCH+Ac Vav1 transfectants (Figure 2B, middle). However, these GFP-fused Vav1 forms were hardly detected unless we used anti-GFP antibodies. Analysis of SH3-SH2-SH3 Vav1 transfectants revealed that this form was overexpressed in comparison to endogenous Vav1, as determined using anti-SH2 Vav1 antibodies (Figure 2B, bottom).
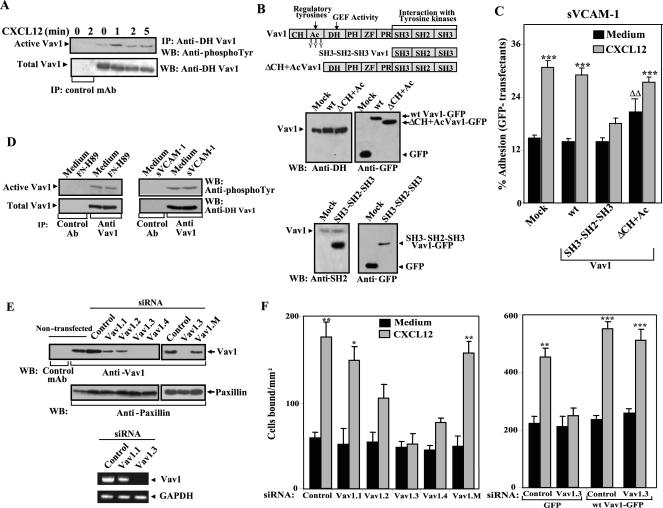
Figure 2.
Role of Vav1 on CXCL12-promoted α4β1-dependent T-cell adhesion. (A) Serum-starved Molt-4 cells were incubated for the indicated times in the presence or absence of CXCL12. Cells were solubilized, and extracts were immunoprecipitated with anti-DH Vav1 or control antibodies, followed by SDS-PAGE and immunoblotting using antiphosphotyrosine antibodies to detect active Vav1 or anti-DH Vav1 to control for total Vav1. (B, top) Schematic representation of Vav1 mutants used in these experiments. The distribution of Vav1 structural domains together with known assigned functions is shown. (B, middle and bottom) Molt-4 cells transfected with expression vectors coding for GFP-fused wt Vav1, SH3-SH2-SH3 Vav1, ΔCH+Ac Vav1, or GFP alone (Mock) were solubilized and extracts assayed by immunoblotting using the indicated antibodies. (C) The same transfectants were subjected to adhesions for 2 min at 37°C to sVCAM-1 coimmobilized with or without (Medium) CXCL12. Nonbound cells were washed and extent of adhesion quantified in a flow cytometer. Percentage data of adhered GFP transfectants represent the mean ± SD of three independent experiments. Adhesion was significantly up-regulated, ***p < 0.001, according to one-way ANOVA test. Adhesion was significantly increased, ΔΔp < 0.01, in comparison with adhesion values displayed by Vav1 wt transfectants incubated without CXCL12. (D) Molt-4 cells were placed in wells containing FN-H89, sVCAM-1 or BSA (Medium) and subjected to adhesion for 2 min at 37°C. Cells attached to α4β1 ligands or the same cell number from BSA wells were solubilized, and lysates immunoprecipitated with anti-Vav1 or control antibodies, followed by immunoblotting using antiphosphotyrosine antibodies to detect active Vav1. (E) Molt-4 cells were transfected with control or the indicated Vav1 siRNA, and following solubilization, lysates were analyzed by Western blotting (left) or RT-PCR (right) using Vav1 specific reagents. Results were compared with loading controls GAPDH and paxillin, respectively. Also shown is Western blotting from nontransfected Molt-4 cells. (F) Same transfectants (left), or cells cotransfected with the indicated siRNA and GFP or wt Vav1-GFP expression vectors (right) were subjected to adhesion assays to sVCAM-1 (2 min, 37°C) coimmobilized with or without CXCL12. Adhesion data represent the mean ± SD of triplicate samples from representative results of three independent experiments for each panel. Adhesion was significantly up-regulated, ***p < 0.001, **p < 0.01, or *p < 0.05, according to one-way ANOVA test.
Mock and wt Vav1 (Figure 2C), as well as Cdc42 transfectants (unpublished data), showed similar levels of upregulation by CXCL12 of adhesion to sVCAM-1. Instead, the extent of increase in α4β1-mediated adhesion was remarkably reduced on Vav1 SH3-SH2-SH3 transfectants. In support for an involvement of Vav1 activation in α4β1-dependent T-cell adhesion, we observed that Vav1 ΔCH+Ac transfectants displayed a modest but significant increase (p < 0.01) in attachment without CXCL12 stimulation, and this chemokine further enhanced the adhesion, likely reflecting activation of the endogenous Vav1 pool (Figure 2C). Under the conditions used in our adhesion assays (2 min at 37°C after a short spin), α4β1-dependent adhesion per se did not induce Vav1 phosphorylation, as analyzed from cells attached to FN-H89 or sVCAM-1 in the absence of CXCL12 (Figure 2D), indicating that Vav1 phosphorylation was mostly CXCL12-dependent.
To more directly assess the involvement of Vav1 in CXCL12-promoted T-cell adhesion, we transfected siRNA for Vav1 in Molt-4 cells and measured transfectant attachment to sVCAM-1 coimmobilized with or without CXCL12. Transfection of two siRNA (Vav1.3 and Vav1.4) covering different Vav1 sequences resulted in a large inhibition of Vav1 expression, whereas transfection of two additional Vav1 siRNA (Vav1.1 and Vav1.2) produced a partial impairment in expression, as compared with cells transfected with control siRNA (Figure 2E). Furthermore, transfection of a siRNA differing from the Vav1.3 siRNA sequence just in a single-base mutation (siRNA Vav1.M) resulted in expression of normal Vav1 levels. Flow cytometry experiments indicated that α4β1 and CXCR4 expression was unaffected by transfection with Vav1 siRNA (Supplementary Figure 2B). Adhesion assays revealed that enhancement by CXCL12 of attachment to sVCAM-1 was greatly impaired in cells transfected with Vav1.3 and Vav1.4 siRNA, and to a lesser extent in Vav1.2 transfectants, compared with the adhesion of control or Vav1.M siRNA transfectants (Figure 2F, left). Increase by CXCL12 of attachment to sVCAM-1 was not affected in cells transfected with Vav1.1 siRNA. These results were further substantiated by rescuing the Vav1 siRNA inhibitory effects on adhesion by overexpression of mouse Vav1 wt vector. The mouse Vav1 mRNA sequence is not affected by the human specific siRNA, because it contains four mismatches in the same sequence when compared with human Vav1 mRNA. These data indicate that activation of Vav1 by CXCL12 is an important signaling event for the up-regulation of α4β1-mediated T-cell adhesion.
Altogether, the results point to a key role of activation of Vav1-Rac signaling pathway by CXCL12 for an efficient enhancement in α4β1-dependent T-cell adhesion. To investigate whether Rac activation by CXCL12 requires Vav1 activity, we performed GTPase assays for Rac with Molt-4 cells transfected with Vav1.3 siRNA. The data showed that inhibition of Vav1 expression by siRNA resulted in a subsequent blockade of Rac activation by CXCL12 (Figure 3A), therefore indicating that CXCL12-promoted up-regulation of T-cell attachment mediated by α4β1 depends on activation of Vav1-Rac signaling pathway by this chemokine.
Further support for an involvement of CXCL12-triggered Vav1-Rac activation in α4β1-dependent cell adhesion came from experiments using the Jak inhibitor AG490. Previous reports established that Jak2 stimulates Vav1 (Matsuguchi et al., 1995) and that CXCL12 activates the Jak/Stat pathway, whereas inhibition of Jak with AG490 impaired CXCL12-triggered cell responses (Vila-Coro et al., 1999). Treatment of Molt-4 cells with AG490 prevented subsequent CXCL12-induced Vav1 phosphorylation, concomitant with inhibition of Rac activation in response to this chemokine (Figure 3B). Moreover, up-regulation by CXCL12 of Molt-4 and PBL-T-cell attachment to sVCAM-1 was substantially reduced by this inhibitor (Figure 3C). These results indicate that simultaneous pharmacological inhibition of CXCL12-promoted Vav1 and Rac activation impairs the increase in α4β1-mediated T-cell adhesion, further pointing to the implication of the signaling axis Vav1-Rac in the regulation of this adhesion. In addition, these data identify Jak activation as an early activation event controlling posterior increase in T-cell attachment to α4β1 ligands, similarly to what was reported for CCL21 (Stein et al., 2003).
Inhibition of Rac and Vav1 in PBL T-lymphocytes Impairs Up-regulation by CXCL12 of α4β1-dependent Adhesion
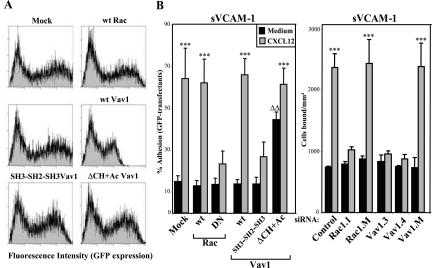
Peripheral blood T lymphocytes were transfected with mutant forms of Rac and Vav1 and subjected to adhesions to sVCAM-1 in response to CXCL12. The different GFP-fused expression vectors were transfected by nucleofection, which gave transfection efficiencies ranging from 40 to 70% (Figure 4A). As with Peer and Molt-4 cells, expression of wt Rac and Vav1 in T lymphocytes did not significantly alter the degree of increase in CXCL12-dependent adhesion to sVCAM-1 in comparison to mock transfectants. In contrast, enhancement of attachment to sVCAM-1 triggered by CXCL12 was notably reduced in DN Rac or Vav1 SH3-SH2-SH3 T lymphocyte transfectants (Figure 4B, left). Moreover, Vav1 (ΔCH+Ac) transfectants displayed significantly higher adhesion without CXCL12 stimulation, whereas CXCL12 further promoted an up-regulation in this attachment (Figure 4B). These results were further confirmed in adhesion assays to sVCAM-1 using T lymphocytes transfected with siRNA for Rac and Vav1, which showed a large inhibition on their CXCL12-promoted attachment mediated by α4β1 in comparison with control siRNA transfectants (Figure 4B, right). Therefore, data obtained with freshly isolated T lymphocytes support the participation of Rac and Vav1 on the enhancement by CXCL12 of α4β1-mediated adhesion that we observed on T-cell lines.
Figure 4.
Inhibition of Rac and Vav1 in PBL T lymphocytes impairs up-regulation of α4β1-dependent adhesion in response to CXCL12. Peripheral blood T lymphocytes were transfected by nucleofection with expression vectors coding for the indicated GFP-fused forms of Rac, Vav1 or with GFP alone (Mock), or with different siRNA for Rac and Vav1. The different GFP transfectants were analyzed by flow cytometry to determine GFP expression levels (A). GFP transfectants or BCECF-AM–labeled siRNA transfectants were subjected to adhesions to sVCAM-1 coimmobilized with or without (Medium) CXCL12 for 2 min at 37°C (B). Nonbound cells were washed and extent of adhesion quantified in a flow cytometer. Adhesion data represent the mean ± SD of 3 independent experiments. Adhesion was significantly up-regulated, ***p < 0.001, according to one-way ANOVA test. Adhesion was significantly increased, ΔΔp < 0.01, in comparison with adhesion values displayed by Vav1 wt transfectants incubated without CXCL12.
Inhibition of Rac and Vav1 Impairs CXCL12-promoted Adhesion to sVCAM-1 under Flow Conditions
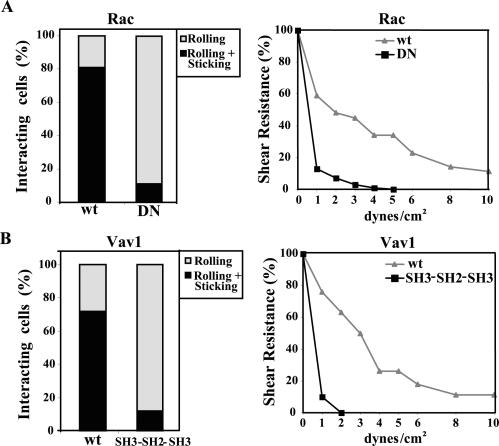
As integrins on lymphocytes become activated by chemokines under shear conditions during attachment to endothelium, we used a flow chamber to study adhesion of wt or DN Rac or Vav1 transfectants to sVCAM-1 coimmobilized with CXCL12. Wild-type Rac or Vav1 transfectants were perfused at an initial shear rate of 1 dyn/cm2 and their behavior analyzed for rolling, firm adhesion and later for detachment at increasing shear rates. Preliminary experiments indicated that in the absence of CXCL12, transfectants predominantly rolled (unpublished data), whereas CXCL12 triggered a very rapid development of firm adhesion (<1 s) in a cell population higher than 70% in both wt Rac and Vav1 transfectants (Figure 5, A and B, left), that was abolished by pretreatment with pertussis toxin (unpublished data). Instead, DN Rac and Vav1 SH3-SH2-SH3 transfectants mainly rolled, with <15% of cells firmly sticking after some rolling (p <0.01). The means of the actual cell numbers of total tethering (rolling+sticking) were 29 and 4 for wt and SH3-SH2-SH3 Vav1 transfectants, respectively (per field and unit of time analyzed), whereas for Rac wt and DN transfectants these numbers were 24 and 4, respectively.
Figure 5.
CXCL12-promoted adhesion to sVCAM-1 of Rac and Vav1 transfectants under flow conditions. Wild-type or DN Rac (A), or wild-type or SH3-SH2-SH3 Vav1 (B) Molt-4 transfectants were perfused at 1 dyn/cm2 in a flow chamber coated with sVCAM-1 coimmobilized with CXCL12 and analyzed for rolling and firmly adherent cells (left), or cell detachment after increasing shear rates (right), as specified in Materials and Methods. Shown are representative results of at least three independent experiments for each panel.
To evaluate shear resistance, after transfectants were allowed to adhere, then flow was increased from 1 to 10 dyn/cm2, measuring cells remaining bound. Wild-type Rac and Vav1 transfectants developed a substantial higher resistance to detachment than their mutant counterparts, with ∼50% of wt transfectants remaining bound at 3 dyn/cm2, a shear rate that resulted in detachment of more than 95% of DN Rac and Vav1 SH3-SH2-SH3 transfectants (Figure 5, A and B, right). About 15% of wt transfectants still remained bound at 10 dyn/cm2, whereas basically no mutant cells were attached at higher rates than 3 dyn/cm2. These results confirm data obtained with static adhesion assays and indicate that expression of DN mutant Rac and Vav1 results in impairment in the step of cell rolling toward development of firm adhesion triggered by CXCL12, leading to inefficient strengthening of adhesion.
Knocking-down Rac and Vav1 Expression Impairs CXCL12-promoted Soluble Binding of sVCAM-1-Fc
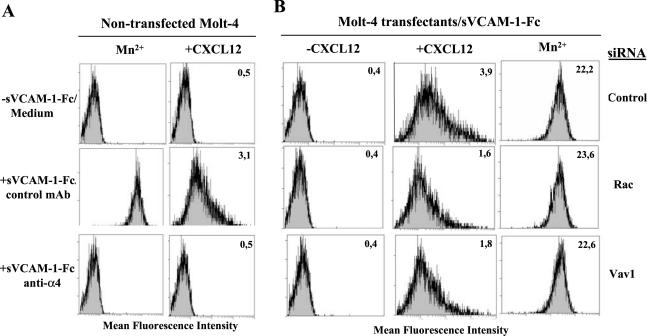
To analyze the role of Rac and Vav1 on the initial steps of CXCL12-triggered α4β1-dependent adhesion, we subjected Molt-4 Rac and Vav1 siRNA transfectants to binding assays to sVCAM-1-Fc in solution, therefore excluding adhesion events subsequent to initial integrin/ligand interactions. To set up the binding assay, we established the best conditions using nontransfected Molt-4 cells. A short incubation (75 s, 37°C) in the presence of Mn2+, a commonly used potent positive control for affinity regulation, triggered a robust binding of sVCAM-1-Fc that was abolished by preincubation with anti-α4 mAb (Figure 6A), whereas no binding was observed without Mn2+ and sVCAM-1-Fc (unpublished data) or when this cation was present but no soluble ligand was added. Likewise, a 45-s cell exposure to CXCL12 followed by the 75-s binding assay promoted sVCAM-1-Fc binding though to a lower extent, which was fully blocked by anti-α4 mAb. Again, no binding was detected without sVCAM-1-Fc (Figure 6A) or when no CXCL12 was added (unpublished data). Molt-4 cells transfected with control siRNA achieved soluble sVCAM-1-Fc binding to levels similar to nontransfected cells, whereas Rac or Vav1 siRNA transfectants showed a 50–60% reduction in sVCAM-1-Fc binding in comparison to control siRNA transfectants (Figure 6B). Control binding experiments revealed that Rac, Vav1, or control siRNA transfectants retained similar levels of sVCAM-1-Fc binding upon exposure to Mn2+. These results suggest that triggering of α4β1-dependent T-cell adhesion by CXCL12 depends on Rac and Vav1 activity already at the initial promotion of α4β1/VCAM-1 interactions.
Figure 6.
Soluble binding of sVCAM-1-Fc to Molt-4 Rac or Vav1 siRNA transfectants. (A) Molt-4 cells preincubated with control (P3X63) or anti-α4 mAb, or in binding medium alone, were incubated directly with or without sVCAM-1-Fc in the presence of Mn2+ (left), or with CXCL12 before addition of sVCAM-1-Fc (right). (B) Molt-4 cells transfected with control, Rac or Vav1 siRNA, were preincubated in the presence or absence of CXCL12, or with Mn2+, followed by binding assay to sVCAM-1-Fc. Cell bound ligand was detected by PE-conjugated goat anti-human IgG using flow cytometry. Insert numbers represent mean fluorescence intensity units.
Mathematical modeling of the interaction between α4β1 and bivalent VCAM-1-Fc revealed that both monovalent and bivalent binding may occur, resulting in α4β1/VCAM-1-Fc and (α4β1)2/VCAM-1-Fc complexes, respectively (Jakubowski et al., 1995). However, at high ligand concentrations bivalent binding begins to decrease because of an excess of competing monovalent binding that has low affinity and results in overall decrease in detectable binding. Because our assays are performed at saturating concentrations of sVCAM-1-Fc, monovalent binding should indeed take place. We used monomeric VCAM-1κ instead of bivalent sVCAM-1-Fc in an attempt to further characterize the binding of soluble VCAM-1 to the different siRNA transfectants. Preliminary control experiments indicated that VCAM-1κ supported static adhesion of Molt-4 cells that was fully blocked by anti-α4 mAb (Supplementary Figure 3A). Mn2+ triggered α4β1-dependent binding of VCAM-1κ to Molt-4 cells but to substantially lower levels compared with sVCAM-1-Fc binding (Supplementary Figure 3B), in agreement with previous results on the low affinity of α4β1/monovalent VCAM-1 interaction (Jakubowski et al., 1995). In addition, untransfected Molt-4 cells, or cells transfected with Rac, Vav1, or control siRNA incubated with CXCL12, were unable to bind VCAM-1κ, whereas binding was preserved with transfectants exposed to Mn2+ (Supplementary Figure 3C). Therefore, we were unable to test whether a possible Rac-induced α4β1 clustering dependent on bivalent sVCAM-1-Fc could account for a certain level of binding independent of CXCL12-promoted Vav1-Rac activation. However, our data together with the abovementioned observations on the characterization of α4β1/VCAM-1-Fc interactions strongly points to the importance of the activation of Vav1-Rac by CXCL12 on the subsequent stimulation of α4β1-mediated T-cell interaction with VCAM-1.
T-cell Morphology on CXCL12-promoted Adhesion to α4β1 Ligands
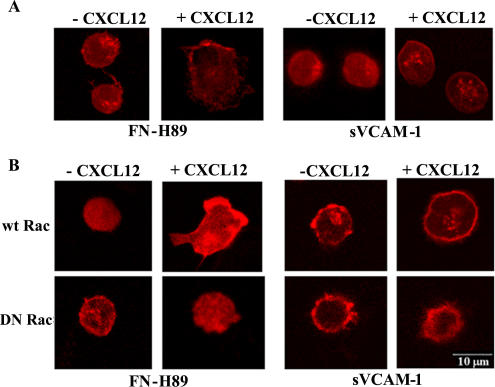
To obtain morphological insights into the modulation by CXCL12 of α4β1-dependent adhesion, we performed confocal microscopy on Molt-4 cells subjected to attachment to FN-H89 or sVCAM-1 coimmobilized with or without CXCL12. Cells attached to α4β1 ligands in the presence of CXCL12 displayed substantially higher degree of spreading, as indicated by enlargement of cell diameter and reduction of thickness, than cells adhered in the absence of the chemokine (Figure 7A). Although spreading was clearly detected on both ligands, it was generally more evident on FN-H89, and cells attached on this ligand coimmobilized with CXCL12 showed a polarized morphology, whereas those adhered to sVCAM-1 displayed a predominant round phenotype with lamelipodia protrusions. CXCL12 promoted the spreading of wt Rac transfectants on FN-H89 and to a lesser extent on sVCAM-1, whereas it failed to induce spreading of DN Rac transfectants (Figure 7B), indicating that triggering of α4β1-mediated T-cell adhesion results in development of a remarkable spread phenotype that is dependent on Rac activity.
Figure 7.
Alterations in cell morphology during CXCL12-promoted T-cell attachment to α4β1 ligands.(A) Molt-4 cells were subjected to adhesions (2 min at 37°C) to FN-H89 or sVCAM-1 coimmobilized with or without CXCL12 on coverslips. Nonbound cells were removed and attached cells were fixed, stained with Alexa 633-Phalloidin, and analyzed by confocal microscopy. Shown are equivalent cell sections corresponding to representative fields. (B) Molt-4 cells were transfected with GFP-fused wt or DN Rac forms and subjected to adhesion assays to α4β1 ligands and confocal microscopy as above. Only GFP-positive transfectants are displayed.
Inhibition of Vav1 and Rac Impairs CXCL12-promoted Induction of Integrin α4β1-dependent T-cell Transendothelial Migration
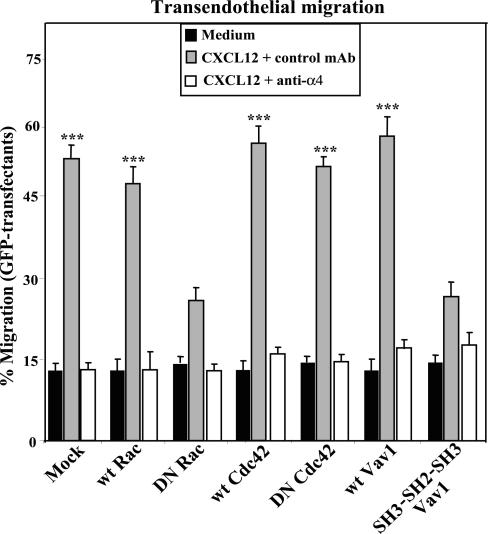
To determine the role of Vav1 and Rac on CXCL12-triggered T-cell migration involving α4β1 activity, we subjected wt and DN Vav1, Rac, and Cdc42 Peer cell transfectants to migration assays across confluent layers of TNF-α-activated HUVEC (to induce VCAM-1 expression; unpublished data). The results revealed that CXCL12-promoted migration of DN Rac or Vav1 SH3-SH2-SH3 transfectants was substantially lower than that displayed by transfectants expressing wt Rac, Vav1, or Cdc42, or DN Cdc42 (Figure 8). This migration was inhibited by anti-CXCR4 mAb (unpublished data), and by anti-α4 (Figure 8), indicating that was mediated by α4β1. Together, these data indicate that activation of Rac and Vav1 by CXCL12 is a key signaling event that leads to efficient up-regulation of α4β1-dependent T-cell adhesion and transendothelial migration.
Figure 8.
Expression of DN Rac or Vav1 impairs CXCL12-promoted, α4β1-mediated, transendothelial T-cell migration. Peer cells transfected with the indicated GFP-fused forms of Vav1, Rac, or Cdc42, or with GFP alone (Mock), were placed on top of TNF-α-treated HUVEC confluent monolayers in the presence of control (P3X63) or anti-α4 mAb, or adhesion medium alone (Medium) and subjected to transendothelial migration assays in response to CXCL12. Extent of cell migration was analyzed in a flow cytometer, and data represent the mean ± SD of duplicate samples from one representative result from three independent experiments. Migration was significantly stimulated, ***p < 0.001, according to one-way ANOVA test.
DISCUSSION
CXCL12 promotes T lymphocyte adhesion involving a rapid and transient up-regulation of the integrin α4β1 activity (Grabovsky et al., 2000), a dynamic process controlling lymphocyte migration. CXCL12 rapidly activates Rac and Cdc42, which takes place concomitant with increase in α4β1-dependent lymphocyte adhesion, as reported here. Using Molt-4 and Peer T-cells transfected with dominant negative Rac, or knocking-down its expression by transfection of siRNA for Rac, we show that activation of Rac by CXCL12 represents an important signaling event for efficient enhancement of α4β1-mediated T-cell adhesion. In contrast, DN Cdc42 transfectants displayed no alterations on their increase in attachment to α4β1 ligands upon stimulation with CXCL12.
Phosphorylation of Vav1 is a required step for the stimulation of its GEF activity predominantly on Rac (Crespo et al., 1997; Aghazadeh et al., 2000). Our data indicated that CXCL12 rapidly phosphorylated Vav1 and that expression of a putative DN Vav1 form that should interfere with its phosphorylation (Vav1 SH3-SH2-SH3; Zugaza et al., 2002), results in a notable reduction in up-regulation of α4β1-dependent Molt-4 cell adhesion triggered by CXCL12. The relevance of Vav1 activation on CXCL12-promoted increase in attachment to α4β1 ligands was further substantiated by transfection of siRNA specific for Vav1. Moreover, transfectants expressing a mutant Vav1 form that displays constitutive GEF activity toward Rac (Vav1 ΔCH+Ac; Schuebel et al., 1998) showed an increase in basal (no CXCL12 stimulation) cell attachment to sVCAM-1, an additional indication of the functional link between Vav1 and Rac on the upregulated T-cell adhesion. Importantly, inhibition of Vav1 expression by transfection of siRNA for Vav1 resulted in a blockade of Rac activation in response to CXCL12, a result that strongly suggests that the activation of Vav1-Rac signaling axis by CXCL12 represents an important inside-out event leading to stimulation of α4β1-mediated T-cell adhesion. This direct key observation is supported by indirect experimental evidence pointing to a linear Vav1-Rac signaling activated by CXCL12 and leading to increased adhesion. First, expression of DN Rac or transfection with siRNA for Rac, although theoretically preserving Vav1 phosphorylation by CXCL12, resulted in inhibition of CXCL12-promoted up-regulation of α4β1-dependent adhesion to levels similar to those achieved by DN Vav1 transfectants or by cells transfected with Vav1 siRNA. Second, inhibition of Jak2, a signaling molecule activated by CXCL12 (Vila-Coro et al., 1999) that activates Vav (Matsuguchi et al., 1995), provoked the blockade of Vav1, as well as Rac activation, in addition to inhibiting T-cell adhesion to α4β1 ligands in response to CXCL12. More definitive support for Vav1-Rac axis comes from the demonstration that T-cells from vav1–/– mice have substantially decreased Rac activation (Reynolds et al., 2002). At present we cannot however completely exclude the participation of an additional Vav1-dependent, Rac-independent, signaling pathway modulating CXCL12-triggered increase in α4β1-mediated T-cell adhesion. Thus, a previous work reported GEF-dependent, as well as independent, activity of Vav1 on T-cell spreading on fibronectin mediated by β1 integrins (del Pozo et al., 2003).
Importantly, the functional association of Vav1 and Rac activation with an efficient enhancement in α4β1-mediated T-cell line adhesion in response to CXCL12 was also observed with fresh T lymphocytes isolated from human PBL and transfected with DN forms or siRNA for these proteins. The possibility that alterations in cell surface expression of α4β1 and CXCR4 due to transfection of DN Rac or siRNA for Rac or Vav1, or induction of cell death, that could be responsible for cell adhesion changes mentioned above were excluded upon flow cytometry analyses.
Dynamic regulation of adhesion is a prerequisite for efficient cell migration (Ridley et al., 2003). As α4β1 plays key roles during T-cell transendothelial migration, we approached this process by using migration chambers to assay the capability of DN Rac or Vav1 transfectants to migrate toward CXCL12 across TNF-α activated HUVEC. The results indicated that triggering by CXCL12 of efficient T-cell transendothelial migration mediated by α4β1 was dependent on Vav1 and Rac activation. The data on cell migration likely reflects the defects on α4β1-dependent adhesion to VCAM-1 and fibronectin of DN Vav1 and Rac transfectants in response to CXCL12 as shown above. Therefore, these results confirm in a more complex adhesion scenario the importance of Vav1 and Rac activation during CXCL12-promoted, α4β1-mediated T-cell attachment.
Identification of adhesion events regulated by Vav1 and Rac activation during α4β1-dependent attachment induced by CXCL12 came from soluble ligand binding, adhesion in flow chambers, and confocal microscopy experiments. α4β1 on cells transfected with siRNA for Vav1 or Rac displayed a substantial, but not total, impairment in CXCL12-promoted binding to soluble VCAM-1. The degree of inhibition of binding was similar between Vav1 and Rac siRNA transfectants, again pointing to the involvement of a Vav1-Rac signaling axis activated by CXCL12 during adhesion. On the other hand, adhesion assays under shear stress carried out on sVCAM-1 coimmobilized with CXCL12 indicated that activation of Vav1 and Rac was necessary to develop and maintain T-cell firm adhesion against detachment from increased shear rates. Finally, expression of DN Rac interfered with CXCL12-promoted transfectant spreading on α4β1 ligands that otherwise was observed with wt Rac transfectants, as well as with nontransfected cells. Together, these results indicate that activation by CXCL12 of Vav1 and Rac leads to increased binding to its ligands and that this activation is required to maintain a sufficient level of adhesion strengthening to allow resistance of cell detachment from shear stress. CXCL12-promoted, Rac-dependent, spread cell phenotype could represent an important mechanism for adhesion strengthening mediated by α4β1, as it should more efficiently resist shear stress.
Efficient soluble binding of ligands to integrins is considered to reflect integrin high-affinity conformations (Liddington and Ginsberg, 2002; Carman and Springer, 2003). In this regard, CXCL12-triggered binding of VCAM-1 to α4β1 in solution has been proposed to be the result of high-affinity α4β1, a finding that was supported by increased binding on CXCL12-treated cells of anti-β1 mAb that detect high-affinity β1 integrins (Chan et al., 2001). Therefore, our results raise the possibility that activation of Vav1-Rac by CXCL12 might constitute one of the mechanisms contributing to a switch from low to higher affinity α4β1 conformations that are efficient for initial ligand binding, although failure to detect monovalent binding does not allow at present to conclusively demonstrate this point. In addition, the data also suggest that Vav1-Rac activation could contribute to the sustenance of the high-affinity state to mediate cell arrest. However, it is important to keep in mind that Vav1 and Rac siRNA transfectants retained a portion (<45%) of CXCL12-promoted soluble VCAM-1 binding, indicating that some efficient binding was still achieved, as discussed below. As impairment in Vav1 and Rac activation lead to a predominant rolling behavior on VCAM-1, these results suggest that Vav1 and Rac activation by CXCL12 appears to be more critical in the adhesion strengthening step. The importance of Vav1 in sustained adhesion and spreading has been also recently reported for β2 integrin-dependent neutrophil adhesion (Gakidis et al., 2004).
Solid evidence indicates the existence of a variety of αβ integrin heterodimer conformations displaying from low to high-affinity for their ligands. The latest is generated upon unclasping of αβ cytoplasmic and transmembrane domains leading to an extended extracellular domain from the bent, low-affinity conformation, representing an important mechanism for integrin activation in inside-out signaling (Emsley et al., 2000; Xiong et al., 2001; Liddington and Ginsberg, 2002; Takagi et al., 2002; Vinogradova et al., 2002; Carman and Springer, 2003; Shimaoka et al., 2003; Vinogradova et al., 2004). Talin is an actin-binding protein that activate integrins upon interaction with the β subunit cytoplasmic domains (Liu et al., 2000; Tadokoro et al., 2003). Importantly, FRET analyses showed that αLβ2 binding to the talin head domain or stimulation with CXCL12 caused spatial separation of the subunit cytoplasmic domains (Kim et al., 2003), confirming that this structural change is a key event in inside-out signaling. Therefore, it is tempting to speculate that CXCL12-promoted stimulation of Vav1 and/or Rac might induce talin binding to the β1 subunit and activation of the α4β1 heterodimer resulting in stronger interaction with its ligands. A possible link with this hypothesis arises from the fact that Vav1 has been reported to interact with talin (Fischer et al., 1998), which opens the possibility that CXCL12 might signal via Vav1-talin. Whether part of this signaling represents Rac-independent events deserves further investigation.
Members of the Ras-family GTP-ases such as R-ras, H-ras, and Rap1, are involved in activation of α4β1-dependent adhesion of hematopoietic cells (Kinbara et al., 2003). Rap1 triggers β1- and β2-integrin dependent lymphocyte adhesion, and expression of active Rap1 promotes both increased affinity and avidity for β1- and β2-integrin ligands (Reedquist et al., 2000; Sebzda et al., 2002; Katagiri et al., 2004). In addition, CXCL12 and CCL21 up-regulate lymphocyte adhesion mediated by these integrins through Rap1 activation (Shimonaka et al., 2003; Katagiri et al., 2004). Therefore, Rap is a serious candidate to mediate the binding of VCAM-1 promoted by CXCL12 that is independent of Vav1 and Rac (see above). However, functional connections have been recently reported between Rap1 and Rac. Although not yet documented in lymphocytes, Rap1 activation by the GEF Epac leads to Rac activation (Maillet et al., 2003), and active Rap1 induces Vav2 membrane localization and Rac activation (Arthur et al., 2004). On the other hand, Vav1 and Rac control membrane translocation of RasGRP2, a GEF for Rap1, translating into Rap1 activation (Caloca et al., 2004). These data illustrate the potential cross-talks between Vav1-Rac and Rap1 and raise the possibility that they could contribute to the increase by CXCL12 of lymphocyte adhesion mediated by α4β1.
Previous data showed that binding of VCAM-1-Fc promoted by CXCL12 was not affected by cytochalasin D, suggesting that interaction of VCAM-1 with α4β1 does not require active actin polymerization (Chan et al., 2001). However, cytochalasin D did inhibit postligand binding events. These data together with the present results suggest that initial α4β1/VCAM-1 interactions promoted by CXCL12 and dependent on Vav1 and Rac activation precedes actin polymerization and that subsequent adhesion strengthening mediated by α4β1 does require reorganization of the actin cytoskeleton. Clustering of high-affinity α4β1 by diffusion mobility should increase the overall avidity for its ligands. Therefore, if CXCL12 generates α4β1 high-affinity conformations as well as α4β1 clustering, both mechanisms could cooperate at different steps during adhesion to mediate firm attachment. Using the nonblocking anti-α4 mAb B-5G10 in confocal microscopy, we have been unable to detect CXCL12-promoted changes in α4β1 distribution on Molt-4 cell surfaces, at least at the level of macroclusters (unpublished data). This does not rule out that microclusters not detected by confocal microscopy might form in response to CXCL12, which could account for increased avidity for α4β1 ligands and contribute to the increase in adhesion. Indeed, α4β1 clusters have been reported in T lymphocytes transfected with active Rac that lead to enhancement in α4β1-dependent adhesion (D'Souza-Schorey et al., 1998).
In conclusion, this study indicates that activation of Vav1-Rac pathway by CXCL12 constitutes an important inside-out signaling event for efficient up-regulation of α4β1-dependent T lymphocyte adhesion, both at initial ligand binding and specially during adhesion strengthening by sustaining firm attachment to mediate cell arrest. It is likely that Vav1-Rac rapid activation by chemokines precedes subsequent, ligand-induced activation of this signaling pathway in an outside-in manner (Yron et al., 1999; Moores et al., 2000; del Pozo et al., 2003). The latter could further contribute to maintaining or even increasing the adhesion strength necessary to resist cell detachment and to set lymphocytes ready for diapedesis. Further investigation is required to identify Vav1 and Rac upstream and downstream molecules that collaborate to increase in adhesion in response to CXCL12. One of the upstream events in CXCL12-stimulated signaling is the activation of Gαi-dependent pathway, as pertussis toxin blocks up-regulation of α4β1-mediated T-cell attachment, and therefore functional relationships should exist between this pathway and Vav1-Rac activation by CXCL12.
Supplementary Material
Acknowledgments
We thank Drs. Francisco Sánchez-Madrid, Angel Corbí, Gabriele Weitz-Schmidt, and Inmaculada Valle for reagents and Pedro Lastres and M. Teresa Seisdedos for their help in flow cytometry and confocal microscopy. Drs. Miguel A. del Pozo, José Alberto García-Sanz, and Rubén A. Bartolomé are acknowledged for helpful discussions. This work was supported by Grants SAF2002-00207 and SAF2003-00028 from Ministerio de Educación y Ciencia (to J.T. and X.R.B., respectively) and by National Cancer Institute Grant RO1CA7373503 (to X.R.B). J.V.S. was supported by a Human Frontiers Science Program and a Ramón y Cajal contract.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–12–1049) on May 4, 2005.
Abbreviations used: VCAM-1, vascular cell adhesion molecule-1; GTPase, guanosine triphosphate-binding proteins; GEF, guanine nucleotide exchange factor.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Aghazadeh, B., Lowry, W. E., Huang, X. Y., and Rosen, M. K. (2000). Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102, 625–633. [DOI] [PubMed] [Google Scholar]
- Arthur, W. T., Quilliam, L. A., and Cooper, J. A. (2004). Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 167, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome, R. A., Sanz-Rodriguez, F., Robledo, M. M., Hidalgo, A., and Teixido, J. (2003). Rapid up-regulation of alpha4 integrin-mediated leukocyte adhesion by transforming growth factor-beta1. Mol. Biol. Cell 14, 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge, K., and Wennerberg, K. (2004). Rho and Rac take center stage. Cell 116, 167–179. [DOI] [PubMed] [Google Scholar]
- Bustelo, X. R. (2000). Regulatory and signaling properties of the Vav family. Mol. Cell. Biol. 20, 1461–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher, E. C., and Picker, L. J. (1996). Lymphocyte homing and homeostasis. Science 272, 60–66. [DOI] [PubMed] [Google Scholar]
- Caloca, M. J., Zugaza, J. L., Vicente-Manzanares, M., Sanchez-Madrid, F., and Bustelo, X. R. (2004). F-Actin-dependent translocation of the Rap1 GDP/GTP exchange factor RasGRP2. J. Biol. Chem. 279, 20435–20446. [DOI] [PubMed] [Google Scholar]
- Carman, C. V., and Springer, T. A. (2003). Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 15, 547–556. [DOI] [PubMed] [Google Scholar]
- Chan, J. R., Hyduk, S. J., and Cybulsky, M. I. (2001). Chemoattractants induce a rapid and transient up-regulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J. Exp. Med. 193, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo, P., Schuebel, K. E., Ostrom, A. A., Gutkind, J. S., and Bustelo, X. R. (1997). Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385, 169–172. [DOI] [PubMed] [Google Scholar]
- del Pozo, M. A., Price, L. S., Alderson, N. B., Ren, X. D., and Schwartz, M. A. (2000). Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19, 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo, M. A., Schwartz, M. A., Hu, J., Kiosses, W. B., Altman, A., and Villalba, M. (2003). Guanine exchange-dependent and -independent effects of Vav1 on integrin-induced T cell spreading. J. Immunol. 170, 41–47. [DOI] [PubMed] [Google Scholar]
- Deroanne, C., Vouret-Craviari, V., Wang, B., and Pouyssegur, J. (2003). EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 116, 1367–1376. [DOI] [PubMed] [Google Scholar]
- Deroanne, C. F., Hamelryckx, D., Ho, T. T., Lambert, C. A., Catroux, P., Lapiere, C. M., and Nusgens, B. V. (2005). Cdc42 downregulates MMP-1 expression by inhibiting the ERK1/2 pathway. J. Cell Sci. 118, 1173–1183. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C., Boettner, B., and Van Aelst, L. (1998). Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol. Cell. Biol. 18, 3936–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet, K., Kaldjian, E. P., Anderson, A.O., and Shaw, S. (1996). Orchestrated information transfer underlying leukocyte endothelial interactions. Annu. Rev. Immunol. 14, 155–177. [DOI] [PubMed] [Google Scholar]
- Emsley, J., Knight, C. G., Farndale, R. W., Barnes, M. J., and Liddington, R. C. (2000). Structural basis of collagen recognition by integrin alpha2beta1. Cell 101, 47–56. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629–635. [DOI] [PubMed] [Google Scholar]
- Feig, L. A. (1999). Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1, E25–E27. [DOI] [PubMed] [Google Scholar]
- Fischer, K. D. et al. (1998). Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8, 554–562. [DOI] [PubMed] [Google Scholar]
- Gakidis, M. A., Cullere, X., Olson, T., Wilsbacher, J. L., Zhang, B., Moores, S. L., Ley, K., Swat, W., Mayadas, T., and Brugge, J. S. (2004). Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J. Cell Biol. 166, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, M., Tybulewicz, V., and Cantrell, D. A. (2000). Control of pre-T cell proliferation and differentiation by the GTPase Rac-I. Nat. Immunol. 1, 348–352. [DOI] [PubMed] [Google Scholar]
- Grabovsky, V. et al. (2000). Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J. Exp. Med. 192, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Y. et al. (2003). Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302, 445–449. [DOI] [PubMed] [Google Scholar]
- Jakubowski, A., Rosa, M. D., Bixler, S., Lobb, R., and Burkly, L. C. (1995). Vascular cell adhesion molecule (VCAM)-immunoglobulin fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes. Commun. 3, 131–142. [DOI] [PubMed] [Google Scholar]
- Katagiri, K., Shimonaka, M., and Kinashi, T. (2004). Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-gamma1. J. Biol. Chem. 279, 11875–11881. [DOI] [PubMed] [Google Scholar]
- Kim, M., Carman, C. V., and Springer, T. A. (2003). Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720–1725. [DOI] [PubMed] [Google Scholar]
- Kinbara, K., Goldfinger, L. E., Hansen, M., Chou, F. L., and Ginsberg, M. H. (2003). Ras GTPases: integrins' friends or foes? Nat. Rev. Mol. Cell. Biol. 4, 767–776. [DOI] [PubMed] [Google Scholar]
- Krawczyk, C., Oliveira-dos-Santos, A., Sasaki, T., Griffiths, E., Ohashi, P. S., Snapper, S., Alt, F., and Penninger, J. M. (2002). Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity 16, 331–343. [DOI] [PubMed] [Google Scholar]
- Liddington, R. C., and Ginsberg, M. H. (2002). Integrin activation takes shape. J. Cell Biol. 158, 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Calderwood, D. A., and Ginsberg, M. H. (2000). Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113(Pt 20), 3563–3571. [DOI] [PubMed] [Google Scholar]
- Lopez-Lago, M., Lee, H., Cruz, C., Movilla, N., and Bustelo, X. R. (2000). Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol. Cell. Biol. 20, 1678–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet, M., Robert, S. J., Cacquevel, M., Gastineau, M., Vivien, D., Bertoglio, J., Zugaza, J. L., Fischmeister, R., and Lezoualc'h, F. (2003). Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat. Cell Biol. 5, 633–639. [DOI] [PubMed] [Google Scholar]
- Matsuguchi, T., Inhorn, R. C., Carlesso, N., Xu, G., Druker, B., and Griffin, J. D. (1995). Tyrosine phosphorylation of p95Vav in myeloid cells is regulated by GM-CSF, IL-3 and steel factor and is constitutively increased by p210BCR/ABL. EMBO J. 14, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores, S. L., Selfors, L. M., Fredericks, J., Breit, T., Fujikawa, K., Alt, F. W., Brugge, J. S., and Swat, W. (2000). Vav family proteins couple to diverse cell surface receptors. Mol. Cell. Biol. 20, 6364–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould, A. P., Askari, J. A., Craig, S. E., Garratt, A. N., Clements, J., and Humphries, M. J. (1994). Integrin alpha 4 beta 1-mediated melanoma cell adhesion and migration on vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced IIICS region of fibronectin. J. Biol. Chem. 269, 27224–27230. [PubMed] [Google Scholar]
- Munoz, M., Serrador, J., Sanchez-Madrid, F., and Teixido, J. (1996). A region of the integrin VLA alpha 4 subunit involved in homotypic cell aggregation and in fibronectin but not vascular cell adhesion molecule-1 binding. J. Biol. Chem. 271, 2696–2702. [DOI] [PubMed] [Google Scholar]
- Nishita, M., Aizawa, H., and Mizuno, K. (2002). Stromal cell-derived factor 1alpha activates LIM kinase 1 and induces cofilin phosphorylation for T-cell chemotaxis. Mol. Cell. Biol. 22, 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedquist, K. A., Ross, E., Koop, E. A., Wolthuis, R. M., Zwartkruis, F. J., van Kooyk, Y., Salmon, M., Buckley, C. D., and Bos, J. L. (2000). The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell Biol. 148, 1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, L. F., Smyth, L. A., Norton, T., Freshney, N., Downward, J., Kioussis, D., and Tybulewicz, V. L. (2002). Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 195, 1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T., and Horwitz, A. R. (2003). Cell migration: integrating signals from front to back. Science 302, 1704–1709. [DOI] [PubMed] [Google Scholar]
- Rose, D. M., Han, J., and Ginsberg, M. H. (2002). Alpha4 integrins and the immune response. Immunol. Rev. 186, 118–124. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid, F., and del Pozo, M. A. (1999). Leukocyte polarization in cell migration and immune interactions. EMBO J. 18, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, E. E., van Delft, S., ten Klooster, J. P., Reid, T., van der Kammen, R. A., Michiels, F., and Collard, J. G. (1998). Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A., and Hall, A. (2002). Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587–1609. [DOI] [PubMed] [Google Scholar]
- Schuebel, K. E., Movilla, N., Rosa, J. L., and Bustelo, X. R. (1998). Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 17, 6608–6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebzda, E., Bracke, M., Tugal, T., Hogg, N., and Cantrell, D. A. (2002). Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat. Immunol. 3, 251–258. [DOI] [PubMed] [Google Scholar]
- Shimaoka, M. et al. (2003). Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonaka, M., Katagiri, K., Nakayama, T., Fujita, N., Tsuruo, T., Yoshie, O., and Kinashi, T. (2003). Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J. Cell Biol. 161, 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, T. A. (1994). Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314. [DOI] [PubMed] [Google Scholar]
- Stein, J. V., Soriano, S. F., M'Rini, C., Nombela-Arrieta, C., de Buitrago, G. G., Rodriguez-Frade, J. M., Mellado, M., Girard, J. P., and Martinez, A. C. (2003). CCR7-mediated physiological lymphocyte homing involves activation of a tyrosine kinase pathway. Blood 101, 38–44. [DOI] [PubMed] [Google Scholar]
- Tadokoro, S., Shattil, S. J., Eto, K., Tai, V., Liddington, R. C., de Pereda, J. M., Ginsberg, M. H., and Calderwood, D. A. (2003). Talin binding to integrin beta tails: a final common step in integrin activation. Science 302, 103–106. [DOI] [PubMed] [Google Scholar]
- Takagi, J., Petre, B. M., Walz, T., and Springer, T. A. (2002). Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611. [DOI] [PubMed] [Google Scholar]
- Ticchioni, M., Charvet, C., Noraz, N., Lamy, L., Steinberg, M., Bernard, A., and Deckert, M. (2002). Signaling through ZAP-70 is required for CXCL12-mediated T-cell transendothelial migration. Blood 99, 3111–3118. [DOI] [PubMed] [Google Scholar]
- Turner, M., and Billadeau, D. D. (2002). VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2, 476–486. [DOI] [PubMed] [Google Scholar]
- Tybulewicz, V. L., Ardouin, L., Prisco, A., and Reynolds, L. F. (2003). Vav1, a key signal transducer downstream of the TCR. Immunol. Rev. 192, 42–52. [DOI] [PubMed] [Google Scholar]
- Vila-Coro, A. J., Rodriguez-Frade, J. M., Martin De Ana, A., Moreno-Ortiz, M. C., Martinez, A. C., and Mellado, M. (1999). The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 13, 1699–1710. [PubMed] [Google Scholar]
- Vinogradova, O., Vaynberg, J., Kong, X., Haas, T. A., Plow, E. F., and Qin, J. (2004). Membrane-mediated structural transitions at the cytoplasmic face during integrin activation. Proc. Natl. Acad. Sci. USA 101, 4094–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova, O., Velyvis, A., Velyviene, A., Hu, B., Haas, T., Plow, E., and Qin, J. (2002). A structural mechanism of integrin alpha(IIb)beta(3) “insideout” activation as regulated by its cytoplasmic face. Cell 110, 587–597. [DOI] [PubMed] [Google Scholar]
- von Andrian, U. H., and Mackay, C. R. (2000). T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034. [DOI] [PubMed] [Google Scholar]
- Worthylake, R. A., and Burridge, K. (2001). Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr. Opin. Cell Biol. 13, 569–577. [DOI] [PubMed] [Google Scholar]
- Wright, N., Hidalgo, A., Rodriguez-Frade, J. M., Soriano, S. F., Mellado, M., Parmo-Cabanas, M., Briskin, M. J., and Teixido, J. (2002). The chemokine stromal cell-derived factor-1 alpha modulates alpha 4 beta 7 integrin-mediated lymphocyte adhesion to mucosal addressin cell adhesion molecule-1 and fibronectin. J. Immunol. 168, 5268–5277. [DOI] [PubMed] [Google Scholar]
- Xiong, J. P., Stehle, T., Diefenbach, B., Zhang, R., Dunker, R., Scott, D. L., Joachimiak, A., Goodman, S. L., and Arnaout, M. A. (2001). Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science 294, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. C., Atkinson, S. J., Gu, Y., Borneo, J. B., Roberts, A. W., Zheng, Y., Pennington, J., and Williams, D. A. (2001). Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc. Natl. Acad. Sci. USA 98, 5614–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yron, I., Deckert, M., Reff, M. E., Munshi, A., Schwartz, M. A., and Altman, A. (1999). Integrin-dependent tyrosine phosphorylation and growth regulation by Vav. Cell Adhes. Commun. 7, 1–11. [DOI] [PubMed] [Google Scholar]
- Zugaza, J. L., Lopez-Lago, M. A., Caloca, M. J., Dosil, M., Movilla, N., and Bustelo, X. R. (2002). Structural determinants for the biological activity of Vav proteins. J. Biol. Chem. 277, 45377–45392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.