Abstract
Background
This study aimed to investigate the associations between noninsulin-dependent insulin resistance indices (NI-IRIs), including the triglyceride-glucose (TyG) index, TyG-BMI, triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-C), and metabolic score for insulin resistance (METS-IR), as well as the occurrence of restenosis in patients with lower extremity atherosclerotic occlusive disease after drug-coated balloon (DCB) treatment.
Methods
The primary endpoint was restenosis within one year after the procedure, which was defined as ≥ 50% stenosis of the treated artery segment. The association between NI-IRIs and restenosis was assessed via multivariable logistic regression analysis. Restricted cubic spline (RCS) analysis was performed to quantify nonlinearity. The consistency of these associations was confirmed through subgroup and interaction analyses. Additionally, the additional predictive value of NI-IRIs beyond established risk factors for restenosis was evaluated via receiver operating characteristic (ROC) curves, the net reclassification improvement (NRI), and integrated discrimination improvement (IDI) indices.
Results
Except for the TyG index, the other three NI-IRIs demonstrated nonlinear relationships with the probability of postoperative restenosis. Specifically, TG/HDL-C (inflection point: 1.48, P for nonlinearity: 0.003) exhibited a saturating effect, whereas METS-IR (inflection point: 49.30, P for nonlinearity: 0.017) and TyG-BMI (inflection point: 221.53, P for nonlinearity: 0.039) showed threshold effects. Subgroup analysis revealed that the interactions among the subgroups were not statistically significant. Furthermore, among the four NI-IRIs, the addition of the TG/HDL-C index significantly enhanced the predictive power of the base model for restenosis in ASO patients following DCB angioplasty (AUC values: 0.726 vs. 0.760, P = 0.042). The P values for the NRI and IDI were 0.001 and 0.002, respectively.
Conclusion
TG/HDL-C showed a saturating effect on restenosis within one year after DCB treatment in ASO patients, and METS-IR and TyG-BMI showed threshold effects. The addition of the TG/HDL-C index significantly improved the predictive ability of the base model for restenosis in ASO patients who underwent DCB angioplasty.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02394-5.
Keywords: Insulin resistance, Atherosclerotic occlusive disease, Drug-coated balloon, Restenosis, Retrospective study
Background
Atherosclerosis encompasses a wide range of cardiovascular diseases and is the leading cause of death and disability worldwide [1, 2]. Arterial sclerosis obliterans (ASO), a common form of atherosclerotic vascular disease, is characterized by thickening of the intima-media in the arteries that supply blood to the lower limbs, leading to lumen narrowing or even occlusion. This results in inadequate blood supply, causing symptoms such as intermittent claudication, numbness, reduced skin temperature, and pain. In severe cases, ulceration or necrosis may develop, significantly impairing patients’ quality of life and prognosis [3–5]. Current treatment strategies for ASO include medication, interventional therapies, and surgery. Interventional therapy, which involves procedures such as stent implantation and balloon dilation, alleviates ischaemic symptoms by restoring blood flow. However, prior studies have reported restenosis rates as high as 33% within six months of treating femoropopliteal artery disease via standard balloon dilation [6]. Drug-coated balloons (DCBs) were developed to reduce restenosis by delivering antiproliferative agents directly to the vessel wall. Despite this advancement, some patients still experience restenosis after DCB treatment [7], highlighting the need for further research into the long-term effects of DCB therapy and the underlying mechanisms of postoperative restenosis.
Insulin resistance (IR), characterized by reduced sensitivity to insulin and diminished glucose uptake and utilization, plays a central role in various metabolic disorders, including diabetes, obesity, and metabolic syndrome [8]. IR is strongly linked to the onset and progression of peripheral artery disease (PAD), likely through mechanisms such as increased inflammation, oxidative stress, and impaired endothelial function, all of which exacerbate atherosclerosis and increase the risk of PAD [9, 10]. Although the hyperinsulinaemic-normoglycaemic clamp and intravenous glucose tolerance test are considered the gold standards for assessing IR, these methods are limited by their complexity and cost [11]. In contrast, noninsulin-based insulin resistance indices (NI-IRIs), including the triglyceride-glucose index (TyG), TyG-BMI, triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-C), and metabolic score for insulin resistance (METS-IR), provide simpler, more cost-effective alternatives that have shown accuracy and feasibility in assessing IR [12].
Although a relationship between IR and PAD has been established, few studies have explored the association between IR and restenosis in ASO patients treated with DCBs. This study aimed to investigate the association between NI-IRIs and restenosis in lower limb arteries after DCB treatment. These findings could help identify high-risk individuals for restenosis after DCB treatment, guiding the development of personalized treatment strategies to minimize restenosis risk and improve patient outcomes.
Methods
Study population
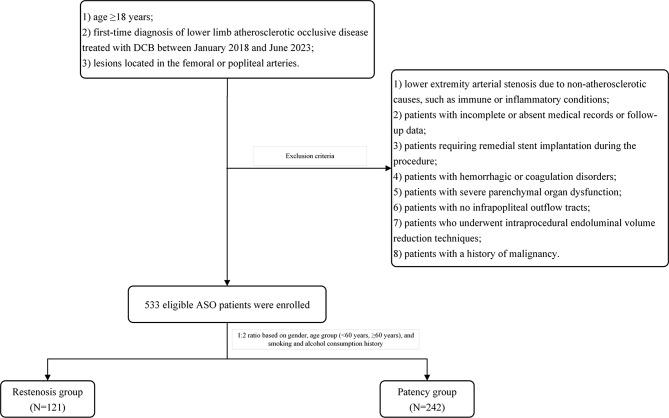
This study is an observational, single-centre, retrospective cohort study. The inclusion criteria were as follows: (1) patients aged 18 years or older; (2) patients with first-time diagnosis of lower limb atherosclerotic occlusive disease treated with DCBs between January 2018 and June 2023 at the First Affiliated Hospital of Zhengzhou University; and (3) patients with lesions located in the femoral or popliteal arteries. The exclusion criteria were as follows: (1) patients with lower extremity arterial stenosis resulting from nonatherosclerotic causes, such as immune or inflammatory conditions; (2) patients with incomplete or missing medical records or follow-up data; (3) patients requiring remedial stent implantation during the procedure; (4) patients with haemorrhagic or coagulation disorders; (5) patients with severe parenchymal organ dysfunction; (6) patients with no infrapopliteal outflow tracts; (7) patients who underwent endoluminal volume reduction techniques during the procedure; and (8) a history of malignancy. A total of 533 eligible ASO patients were enrolled in this study. The restenosis group included patients who developed restenosis or occlusion within one year after DCB treatment (N = 121), whereas the patency group consisted of 242 patients without restenosis or occlusion, selected at a 1:2 ratio on the basis of sex, age group (< 60 years or ≥ 60 years), and smoking and alcohol consumption history. The patient selection process is shown in Fig. 1. More information about patient selection can be found in the supplementary materials.
Fig. 1.
Flowchart for the selection of patients
Data collection
Data for all patients were retrospectively collected from electronic medical records, including (1) demographic information such as age, sex, height, weight, body mass index (BMI), and smoking and alcohol consumption history; (2) medical history, including hypertension, diabetes, coronary heart disease, and stroke; (3) lesion characteristics, including transatlantic intersociety consensus (TASC) II classification and distal outflow assessment; (4) imaging data, specifically pre- and post-balloon dilatation computed tomography angiography (CTA) or digital subtraction angiography (DSA) of the lower limb arteries; (5) postoperative medication regimens; and (6) laboratory test results, including complete blood count, fasting blood glucose, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), creatinine, uric acid, triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C). All biochemical parameters were measured via standardized methods at the Central Laboratory of the First Affiliated Hospital of Zhengzhou University based on fasting blood samples collected before the DCB procedure.
Study endpoints
The primary endpoint of this study was restenosis within one year after the procedure, defined as ≥ 50% stenosis of the treated vessel segment as detected by computed tomography angiography (CTA) or digital subtraction angiography (DSA) during the one-year follow-up period.
Related definitions
The definitions for the NI-IRIs are shown in Table 1.
Details of procedure of percutaneous drug-coated balloons angioplasty can be found in the supplementary materials.
Chronic lower extremity ischemia-femoral popliteal arteriopathy TASC II subtypes are defined in Table S1.
Table 1.
The definition of the four NI-IRIs
| NI-IRI | Definition |
|---|---|
| TyG index | TyG = Ln (TG × FBG/2) |
| METS-IR index | METS-IR = Ln (2 × FPG + TG) × BMI / Ln (HDL-C) |
| TG/HDL-C ratio | TG/HDL-C = TG / HDL-C |
| TyG-BMI index | TyG-BMI = Ln (TG × FBG/2) × BMI |
(BMI (kg/m²): Body Mass Index; FBG (mg/dl): Fasting Blood Glucose; HDL-C (mg/dl): High-Density Lipoprotein Cholesterol; TG (mg/dl): Triglycerides; TyG index: Triglycerides and Glucose Index; TyG-BMI index: Triglycerides and Glucose Body Mass Index; TG/HDL-C ratio: Ratio of Triglycerides to High-Density Lipoprotein Cholesterol; METS-IR index: Metabolic Insulin Resistance Score)
Statistical analysis
All the statistical analyses were performed via R software (version 4.3.1). Continuous variables following a normal distribution are expressed as the means and standard deviations, and independent samples t tests were used for group comparisons. Nonnormally distributed data are presented as medians and interquartile ranges, with group comparisons made via the Mann‒Whitney U test. Categorical variables are reported as frequencies (%) and were compared via chi-square tests. Box plots were generated via the ggplot2 package (version 3.5.1) to visualize the distributions of different NI-IRIs between the two groups. To enhance comparability, the four NI-IRIs were standardized via Z scores. Variables with P values less than 0.1 from univariable logistic regression models were considered to correspond to covariable groups, and variance inflation factor (VIF) values were calculated to assess for multicollinearity among independent variables. Different covariables were adjusted sequentially to explore the associations between NI-IRIs and restenosis within one year after DCB treatment.
Furthermore, restricted cubic spline (RCS) plots were generated via the rms package (version 6.8-2) to explore potential linear or nonlinear associations between the four NI-IRIs and the probability of restenosis. Segmented logistic regression models were employed to evaluate the threshold effect between NI-IRIs and restenosis after DCB treatment. Subgroup analyses were conducted on the basis of sex, age, BMI, hypertension, diabetes, coronary artery disease, and stroke status, with interaction P values calculated. Receiver operating characteristic (ROC) curves were plotted via the pROC package (version 1.18.4), and the area under the curve (AUC) was calculated to assess the model’s predictive accuracy. Additionally, the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated via the PredictABEL package (version 1.2-4) to evaluate the incremental predictive value beyond established risk factors. All the statistical tests were two-tailed, with a P value < 0.05 considered statistically significant.
Research ethics
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. As the study was retrospective, the Ethics Committee waived the requirement for written informed consent (Ethics Approval Number: 2024-KY-1169-001). All procedures involving human participants adhered to the ethical standards of the Declaration of Helsinki.
Results
Demographic and laboratory data of the enrolled patients
Table 2 presents a detailed comparison of the demographic and laboratory characteristics between the restenosis and patency groups. The results revealed that compared with those in the patency group, patients who developed restenosis had significantly greater BMIs, blood glucose levels, and LDL-C (P < 0.05). Conversely, ALT and AST levels were significantly lower in the restenosis group (P < 0.05). In addition, the restenosis group presented a greater prevalence of coronary heart disease, diabetes, and stroke (P < 0.05). Among the four NI-IRIs, METS-IR, TG/HDL-C, and TyG-BMI were significantly elevated in the restenosis group (P < 0.05), whereas no significant difference in TyG index distribution was observed between the two groups (P > 0.05). Regarding the distribution of NI-IRIs in the two groups, please refer to Fig. S1 in the supplementary materials. Other variables, including leukocytes, erythrocytes, platelets, albumin, total bilirubin, direct bilirubin, TC, TG, uric acid, HDL-C, eGFR, use of antihypertensive medications, use of diabetes medication, and TASC II classification of lesions, did not significantly differ between the groups (P > 0.05).
Table 2.
Demographic and laboratory test data results of enrolled patients
| Characteristic | Overall (n = 363) |
Patency group (n = 242) |
Restenosis group (n = 121) |
P value |
|---|---|---|---|---|
| Sex (%) | 1.000 | |||
| Male | 219 (60.3) | 146 (60.3) | 73 (60.3) | |
| Female | 144 (39.7) | 96 (39.7) | 48 (39.7) | |
| Age (year) | 62.6 ± 10.5 | 62.3 ± 10.6 | 63.3 ± 10.2 | 0.373 |
| BMI (Kg/m2) | 23.6 ± 4.2 | 23.3 ± 3.8 | 24.2 ± 4.9 | 0.028 |
| Smoking (%) | 1.000 | |||
| No | 210 (57.9) | 140 (57.9) | 70 (57.9) | |
| Yes | 153 (42.1) | 102 (42.1) | 51 (42.1) | |
| Alcohol (%) | 1.000 | |||
| No | 219 (60.3) | 146 (60.3) | 73 (60.3) | |
| Yes | 144 (39.7) | 96 (39.7) | 48 (39.7) | |
| Leukocyte (109/L) | 6.4 (5.4, 7.7) | 6.3 (5.4, 7.7) | 6.4 (5.6, 7.7) | 0.509 |
| Erythrocyte (109/L) | 4.3 (4.0, 4.6) | 4.3 (4.0, 4.6) | 4.3 (4.0, 4.7) | 0.310 |
| Platelet (109/L) | 212.5 (173.0, 258.2) | 213.0 (171.8, 259.0) | 212.0 (174.5, 245.8) | 0.743 |
| ALT (U/L) | 18.0 (12.0, 41.0) | 21.0 (12.0, 41.0) | 17.0 (12.0, 30.0) | 0.032 |
| AST (U/L) | 20.0 (16.0, 31.0) | 20.0 (16.0, 31.0) | 19.0 (13.5, 31.0) | 0.037 |
| ALB (g/L) | 42.1 (39.5, 44.2) | 42.0 (39.0, 44.2) | 42.1 (40.0, 44.2) | 0.703 |
| Total bilirubin (µmol/L) | 8.8 ± 4.1 | 8.9 ± 3.6 | 8.6 ± 4.9 | 0.487 |
| Direct bilirubin (µmol/L) | 4.1 ± 2.1 | 4.2 ± 1.9 | 4.0 ± 2.4 | 0.336 |
| TC (mmol/L) | 3.6 (2.9, 4.4) | 3.6 (2.9, 4.4) | 3.6 (2.8, 4.3) | 0.397 |
| TG (mmol/L) | 1.5 (1.0, 2.1) | 1.5 (1.0, 2.1) | 1.5 (1.0, 2.0) | 0.654 |
| Glucose(mmol/L) | 7.1 (5.7, 9.1) | 7.0 (5.7, 8.8) | 7.6 (6.0, 10.2) | 0.013 |
| UA (mmol/L) | 285.0 (237.0, 346.0) | 279.0 (227.8, 343.0) | 292.0 (253.0, 351.0) | 0.062 |
| HDL-C (mmol/L) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.1) | 0.210 |
| LDL-C (mmol/L) | 2.6 (1.7, 3.0) | 2.3 (1.6, 3.0) | 2.9 (1.9, 3.1) | 0.003 |
| eGFR (ml/min/1.73m2) | 96.0 (85.9, 103.7) | 97.1 (86.2, 104.4) | 95.0 (84.4, 103.0) | 0.151 |
| TyG | 9.1 (8.7, 9.5) | 9.0 (8.6, 9.5) | 9.1 (8.7, 9.5) | 0.143 |
| METS-IR | 47.2 (41.7, 55.1) | 46.8 (41.5, 53.7) | 49.3 (42.2, 59.3) | 0.008 |
| TG/HDL | 1.6 (1.0, 2.5) | 1.4 (0.9, 2.4) | 1.8 (1.3, 2.7) | < 0.001 |
| TyG-BMI | 210.9 (193.1, 246.5) | 204.4 (185.5, 232.4) | 215.5 (202.0, 268.6) | 0.004 |
| Hypertension (%) | 0.546 | |||
| No | 149 (41.0) | 102 (42.1) | 47 (38.8) | |
| Yes | 214 (59.0) | 140 (57.9) | 74 (61.2) | |
| Diabetes (%) | 0.001 | |||
| No | 170 (46.8) | 128 (52.9) | 42 (34.7) | |
| Yes | 193 (53.2) | 114 (47.1) | 79 (65.3) | |
| Coronary heart disease (%) | < 0.001 | |||
| No | 287 (79.1) | 208 (86) | 79 (65.3) | |
| Yes | 76 (20.9) | 34 (14) | 42 (34.7) | |
| Stroke (%) | < 0.001 | |||
| No | 256 (70.5) | 187 (77.3) | 69 (57) | |
| Yes | 107 (29.5) | 55 (22.7) | 52 (43) | |
| Antihypertensive medications (%) | 0.546 | |||
| No | 149 (41.0) | 102 (42.1) | 47 (38.8) | |
| Yes | 214 (59.0) | 140 (57.9) | 74 (61.2) | |
| Diabetes medication (%) | 0.603 | |||
| No | 181 (49.9) | 123 (50.8) | 58 (47.9) | |
| Yes | 182 (50.1) | 119 (49.2) | 63 (52.1) | |
| TASCII classification | 0.444 | |||
| A | 90 (24.8) | 58 (24) | 32 (26.4) | |
| B | 141 (38.8) | 93 (38.4) | 48 (39.7) | |
| C | 102 (28.1) | 67 (27.7) | 35 (28.9) | |
| D | 30 (8.3) | 24 (9.9) | 6 (5) |
ALT: alanine aminotransferase, AST: aspartate aminotransferase, ALB: albumin, TC: total cholesterol, TG: triglycerides, UA: uric acid, eGFR: estimated glomerular filtration rate, TASC II: the TransAtlantic Inter-Society Consensus II
Associations between NI-IRIs and restenosis after DCB angioplasty
Univariable logistic regression analysis was performed to assess the associations between each variable and restenosis following DCB angioplasty, and the results are presented in Table S2. Variables with a P value < 0.1 and those identified as potential clinical confounders were included in the adjusted models. These covariables were scrutinized for multicollinearity. All the VIF values were less than 2, indicating that there was no multicollinearity. These findings are presented in Table S3.
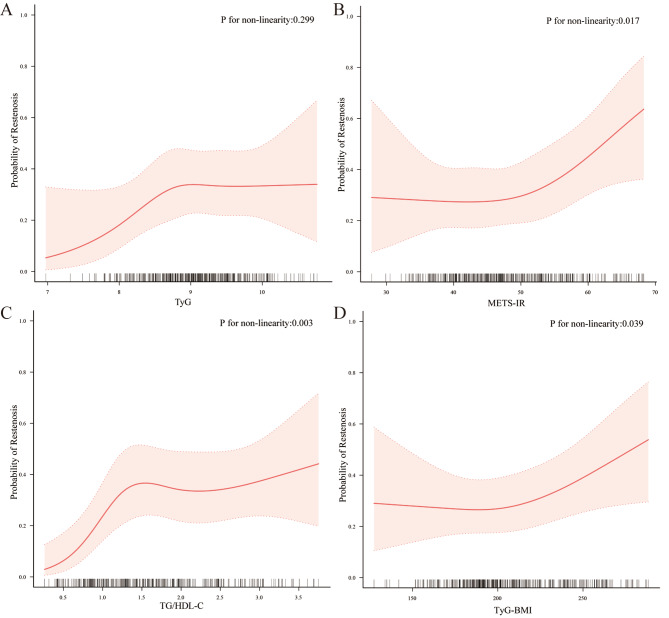
RCS analysis was used to further explore the relationship between NI-IRIs and restenosis risk. As presented in Fig. 2, the results demonstrated that, with the exception of the TyG index, the other NI-IRIs exhibited a nonlinear association with the likelihood of postoperative restenosis (P for nonlinearity < 0.05).
Fig. 2.
Restricted cubic spline curves for the four IR Indices. (The curves have been adjusted for age, hypertension, diabetes, coronary artery disease, stroke, leukocyte, erythrocyte, platelet, uric acid, AST, albumin, total bilirubin, eGFR, and TASC II classification. The solid red line represents the probability of restenosis occurrence, while the red shaded area indicates the 95% confidence interval for restenosis probability)
Multivariable logistic regression models were employed to investigate the association between TyG index and restenosis after DCB treatment, as detailed in Table 3. After adjusting for all covariables (Model 3), we found that TyG index (OR: 1.17, 95% CI: 0.93–1.49, P = 0.186) were no significantly associated with restenosis after DCB angioplasty.
Table 3.
Results of multivariable logistic regression
| Characteristic | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| TyG | 1.14 (0.94–1.39) | 0.185 | 1.16 (0.94–1.43) | 0.169 | 1.17 (0.93–1.49) | 0.186 |
Model 1: no adjustment; Model 2: adjusted for age, hypertension, diabetes, coronary artery disease, and stroke; Model 3: further adjusted for leukocytes, erythrocyte, platelet, uric acid, aspartate aminotransferase, albumin, total bilirubin, estimated glomerular filtration rate, and TASC II fractionation, on the basis of Model 2
Segmented logistic regression models were used to assess the differential effects before and after the inflection points of the RCS curves, as shown in Table 4. A significant threshold effect was observed for the METS-IR and TyG-BMI, indicating that when the METS-IR was below 49.30 or the TyG-BMI was below 221.53, no significant association with restenosis was found. However, when the METS-IR reached 49.30 or higher or the TyG-BMI reached 221.53 or higher, the risk of postoperative restenosis increased by 74% (OR: 1.74, 95% CI: 1.18–2.91, P = 0.012) and 68% (OR: 1.68, 95% CI: 1.22–1.94, P = 0.024), respectively, per increase in the standard deviation. In contrast, TG/HDL-C showed a saturation effect. When the TG/HDL-C ratio was less than 1.48, the risk of postoperative restenosis increased by 113% per 1 standard deviation increase (OR: 2.13, 95% CI: 1.24–3.02; P = 0.014). However, at TG/HDL-C levels of 1.48 or above, its association with restenosis after DCB angioplasty in ASO patients was no longer significant.
Table 4.
Threshold analysis results
| Characteristic | OR (95%CI) | P Value | P for LRT |
|---|---|---|---|
| METS-IR | 0.015 | ||
| METS-IR < 49.30 | 0.91 (0.27–3.08) | 0.789 | |
| METS-IR ≥ 49.30 | 1.74 (1.18–2.91) | 0.012 | |
| TG/HDL-C | 0.039 | ||
| TG/HDL-C < 1.48 | 2.13 (1.24–3.02) | 0.014 | |
| TG/HDL-C ≥ 1.48 | 1.04 (0.67–1.61) | 0.858 | |
| TyG-BMI | 0.044 | ||
| TyG-BMI < 221.53 | 1.18 (0.78–1.76) | 0.871 | |
| TyG-BMI ≥ 221.53 | 1.68 (1.22–1.94) | 0.024 |
The segmented regression model was also adjusted for age, hypertension, diabetes, coronary artery disease, stroke, leukocyte, erythrocyte, platelet, uric acid, AST, albumin, total bilirubin, eGFR, and TASC II classification. P for LRT refers to the likelihood ratio test
Subgroup analyses
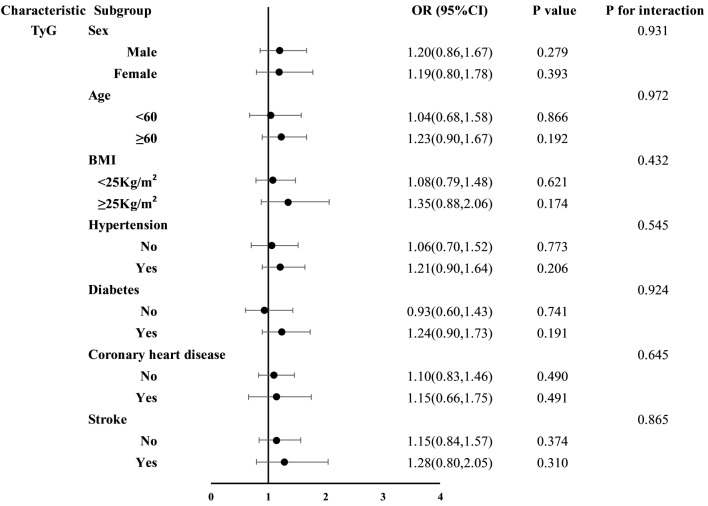
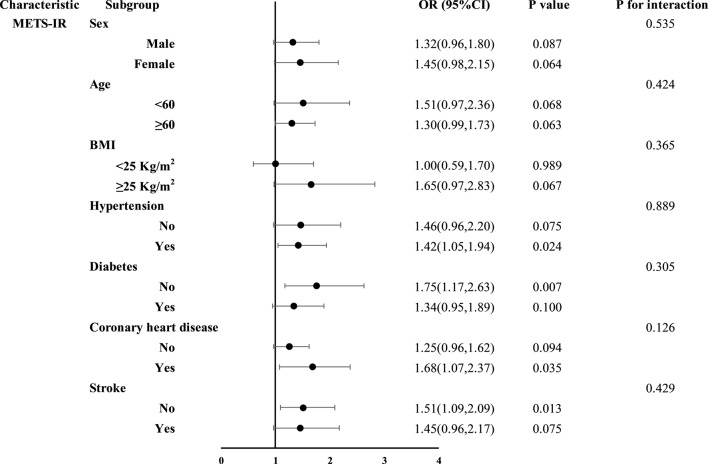
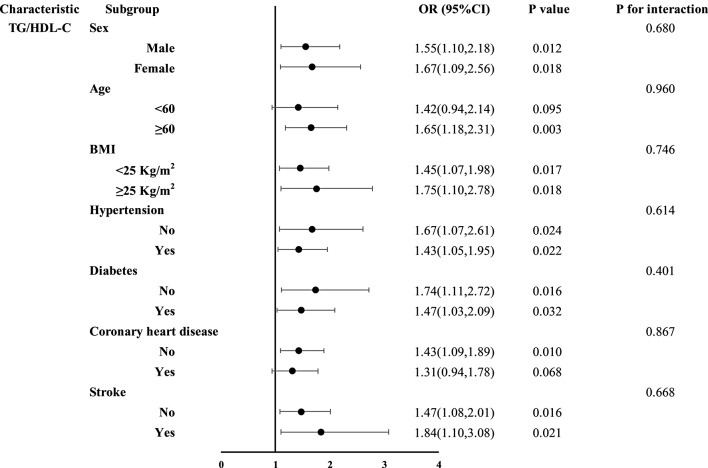
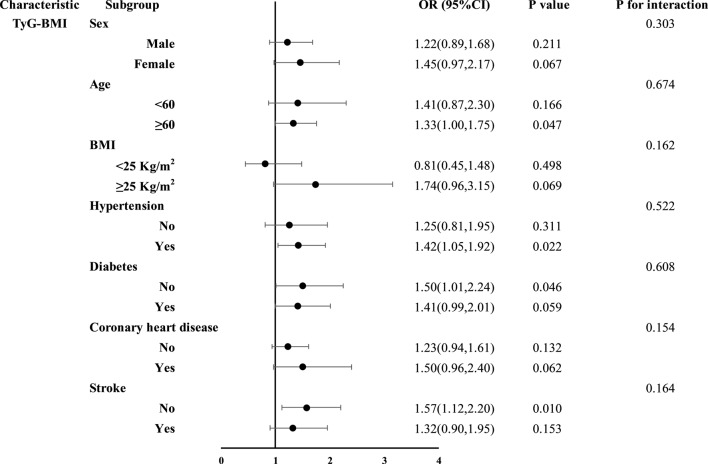
Subgroup analyses of the four NI-IRIs were stratified by sex, age, BMI, hypertension, diabetes, coronary artery disease, and stroke (Figs. 3, 4, 5 and 6). No significant association between the TyG index and restenosis was observed in any subgroup. In contrast, METS-IR was significantly associated with restenosis in patients with hypertension, coronary artery disease, no diabetes, and no history of stroke. TG/HDL-C was significantly associated with restenosis across all subgroups except those aged < 60 years and patients with coronary artery disease. The TyG-BMI was significantly associated with restenosis in patients aged ≥ 60 years, those with hypertension, nondiabetic patients, and those without stroke. Importantly, no significant interaction was found between any of the insulin resistance indices and restenosis across subgroups.
Fig. 3.
Subgroup analysis results of the TyG index
Fig. 4.
Subgroup analysis results of the METS-IR
Fig. 5.
Subgroup analysis results of the TG/HDL-C
Fig. 6.
Subgroup analysis results of the TyG-BMI index
Incremental effect of NI-IRIs on the predictive value of base risk factors
To evaluate the incremental impact of the four IR-related indices on the predictive performance of classical restenosis risk models, we constructed a base model incorporating variables such as sex, smoking, hypertension, diabetes mellitus, coronary artery disease, stroke, age, and lesion site, based on previous literature [6, 7, 13, 14]. The results of the ROC analysis revealed that the AUC of the base model was 0.726 (95% CI: 0.673–0.775).
We subsequently introduced different IR parameters to construct the composite model and observed its ability to predict postoperative restenosis. Figure 7; Table 5 display the ROC curves and related statistics of the different models. Among the four indices, the TG/HDL-C index significantly enhanced the predictive ability of the base model for restenosis following DCB angioplasty in ASO patients (AUC: 0.726 vs. 0.760, P = 0.042). The P values for the NDI and IDI were 0.001 and 0.002, respectively, which indicated that the addition of the TG/HDL-C index significantly improved the base model after the addition of the TG/HDL-C index.
Fig. 7.
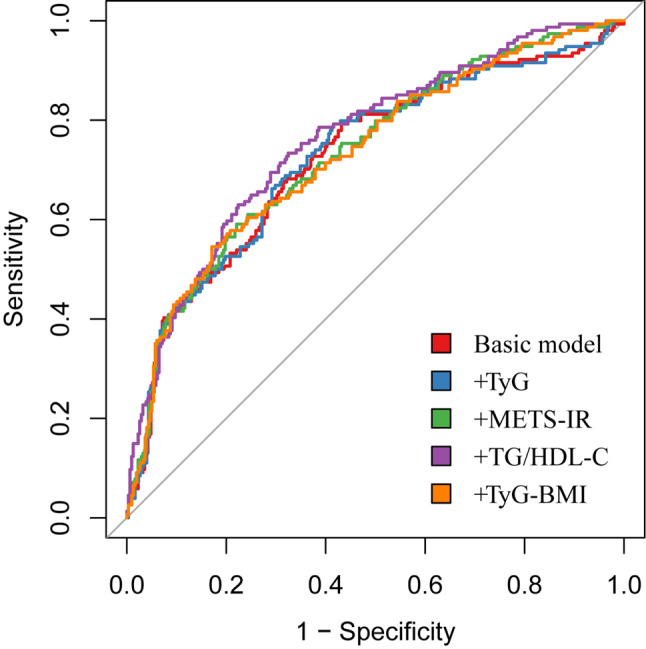
ROC analysis of the basic model and IR indices. The basic model includes age, sex, smoking, hypertension, diabetes, coronary artery disease, stroke, and lesion site
Table 5.
Postoperative restenosis risk prediction model performance analysis
| Model | AUC (95%CI) | P value | NDI (95%CI) | P value | IDI (95%CI) | P value |
|---|---|---|---|---|---|---|
| Basic model | 0.726(0.673–0.775) | Ref. | Ref. | Ref. | Ref. | Ref. |
| + TyG | 0.728(0.677–0.779) | 0.453 | 0.151(-0.041-0.343) | 0.124 | 0.024(-0.008-0.039) | 0.063 |
| +METS-IR | 0.734(0.682–0.782) | 0.476 | 0.182(-0.009-0.373) | 0.062 | 0.027(0.010–0.043) | 0.002 |
| +TG/HDL-C | 0.760(0.712–0.806) | 0.042 | 0.422 (0.233–0.612) | 0.001 | 0.035(0.016–0.054) | 0.002 |
| +TyG-BMI | 0.731(0.683–0.780) | 0.554 | 0.164(-0.028-0.356) | 0.094 | 0.024(0.008–0.040) | 0.003 |
Discussion
Despite the widespread impact of atherosclerosis, PAD often receives less attention than coronary heart disease and stroke do. Specific vascular interventions, such as DCB angioplasty, and their impact on restenosis in lower limb arteries are under-researched. This study was designed to address these gaps by comprehensively assessing the relationship between four NI-IRIs and restenosis after DCB angioplasty in patients with ASO. The findings of this study provide valuable evidence for risk stratification in postoperative restenosis, potentially shaping follow-up strategies for ASO patients undergoing DCB treatment.
The application of NI-IRIs in vascular diseases
In insulin-resistant patients, normal plasma insulin levels fail to adequately suppress glucose production, reduce lipolysis, promote glucose uptake, and increase glycogen synthesis [15]. In the early stages of IR, triglycerides stored in adipose tissue are released as free fatty acids into the bloodstream through lipolysis before an increase in plasma glucose levels occurs [16]. As early as thirty years ago, Reaven and others demonstrated that IR promotes de novo lipogenesis, leading to elevated triglyceride levels even in nonhypertriglyceridemic and nonobese populations [17]. On the basis of these mechanisms, the TyG index was proposed as a simple marker of IR. Numerous large-scale prospective cohort studies have confirmed a strong association between the TyG index and hypertension [18–20]. A meta-analysis suggested that the TyG index could be a reliable predictor of hypertension in the general population [21]. Additionally, studies by Ding and colleagues revealed that a higher TyG index is associated with an increased risk of ASCVD in individuals without a prior history of ASCVD [22]. Liu et al. reported that the TyG index is an independent predictor of peripheral artery disease complexity [23]. Park and Lee also reported associations between the TyG index, arterial stiffness, and coronary artery calcification in Korean adults [24, 25]. Gökalp et al. confirmed that a high TyG index is an independent risk factor for mitral annular calcification [26].
The TyG-BMI index, a combination of the TyG index and BMI, was recently introduced as a novel surrogate for insulin resistance. In an early study, Er and colleagues demonstrated that the TyG-BMI was superior to lipid parameters, glucose measures, and obesity-related metrics in reflecting insulin resistance [27]. Research has further confirmed its association with various metabolic disorders, reinforcing its association with insulin resistance [28–30]. Several studies have explored the relationship between the TyG-BMI and vascular diseases. For example, Zeng et al. reported a strong positive association between the TyG-BMI and blood pressure levels in a cross-sectional study of Chinese adults [31]. Similarly, Du and colleagues reported that the TyG-BMI is linearly and independently related to the incidence of ischaemic stroke in the general population [32].
METS-IR, introduced by Bello et al. in 2018, combines fasting blood glucose, triglycerides, HDL-C, and BMI, and has demonstrated high accuracy in detecting insulin resistance, with an AUC of 0.84 (95% CI: 0.78–0.90) [33]. Previous large studies have established strong associations between METS-IR and hypertension, coronary artery calcification, and ischaemic heart disease [34–37]. A retrospective study of 15,453 Japanese subjects revealed a significant positive correlation between METS-IR and the prevalence of prehypertension or hypertension [34]. Wang et al. further noted that in asymptomatic adults without a history of cardiovascular disease, METS-IR was a better predictor of coronary artery calcification than other insulin resistance indices [37]. METS-IR has also been shown to reliably predict the severity of coronary heart disease, and in a large longitudinal cohort study of nondiabetic Korean adults, higher METS-IR levels were significantly associated with an increased risk of ischaemic heart disease [38].
The TG/HDL-C ratio is a well-established marker of IR, and its elevation has been linked to an increased risk of PAD. In a large cohort of hypertensive patients, Ding et al. reported that each one-standard-deviation increase in the TG/HDL-C ratio was associated with a 14% increase in PAD risk [39].In the Atherosclerosis Risk in Communities (ARIC) study, higher TG levels combined with lower HDL-C levels were independently associated with PAD incidence [40].Other cross-sectional and longitudinal studies further support the strong association between the TG/HDL-C ratio and PAD risk [41]. Among the diabetic patients, PAD patients had significantly higher TG/HDL-C ratios than the controls did, suggesting that this ratio could aid in early PAD identification [42]. Moreover, the TG/HDL-C ratio has been proven to predict the complexity of PAD, offering new insights into clinical assessment and management [43].
Mechanism of IR in vascular restenosis
Previous research has demonstrated that IR significantly increases the risk of atherosclerotic cardiovascular diseases [44, 45]. The hyperglycaemic state induced by IR impairs vascular endothelial function, reduces vascular dilation capacity, and increases the risk of restenosis [46–48]. Intravascular ultrasound studies in nondiabetic coronary heart disease patients revealed that those with impaired glucose tolerance exhibited greater intimal hyperplasia after stent implantation than did patients with normal glucose tolerance [49]. Additionally, Piatti and colleagues reported that IR was prominent in coronary artery disease patients who experienced restenosis after stent surgery [50]. Babalik and others measured insulin levels one day before coronary stent implantation, and the results revealed that in nondiabetic patients, hyperinsulinaemia was a strong predictor of restenosis at the six-month follow-up after stent surgery [51]. However, research on the role of insulin resistance in restenosis following DCB treatment remains scarce. Some previous studies have confirmed that DCBs are noninferior to first-generation drug-eluting stents (DESs) in treating coronary small vessel disease [52, 53]. A meta-analysis of DCBs in de novo coronary lesions in diabetic patients revealed that DCBs did not demonstrate a potential benefit over DESs in reducing vascular restenosis (OR: 0.42, 95% CI: 0.09–1.92; p = 0.26) [54]. Regarding arterial type, insulin resistance also plays a significant role in restenosis of the carotid artery. Mark et al. reported that patients with elevated perioperative fasting glucose had significantly less resolution of restenosis at five years compared with those with normal fasting glucose [55]. Zhao et al. reported that an elevated preoperative TyG index was associated with a significantly increased risk of restenosis after carotid stent implantation [56].
The underlying mechanisms of these phenomena may involve IR stimulating the proliferation and migration of vascular smooth muscle cells through hyperinsulinaemia, enhancing lipoprotein metabolism, and promoting vascular disease [57]. Furthermore, elevated insulin levels increase sympathetic nerve activity and cardiac output, leading to hypertension and vascular damage [58]. The body’s reduced response to insulin also hampers fat metabolism, increasing blood lipid concentrations. Hyperlipidaemia damages arterial endothelial function, increases platelet aggregation, and enhances inflammation, all of which contribute to the occurrence of vascular restenosis after surgery [15].
Nonlinear associations between IR indices and vascular restenosis
This study revealed a significant nonlinear association between insulin resistance indices and vascular restenosis. The threshold effect observed with METS-IR indicated that when the METS-IR value was below 48.10, the association with restenosis risk was not significant. However, when the value exceeded this threshold, the risk increased significantly. Based on the calculation of METS-IR, individuals below the threshold are generally lean, where insulin resistance plays a weaker role, resulting in a lower risk of restenosis [59]. In contrast, individuals above the threshold are more likely to be obese, and insulin resistance becomes more pronounced, thereby increasing the risk of restenosis. The TyG-BMI index, which is determined by a combination of the TyG score and BMI, offers a comprehensive assessment of insulin resistance. The threshold effect of the TyG-BMI may be due to the amplifying impact of obesity on insulin resistance. Obesity, an inflammatory state, exacerbates insulin resistance and accelerates the development of vascular disease [59]. When the TyG-BMI is less than 219.32, the adverse effects of obesity may not be sufficient to significantly increase the risk of restenosis. However, when it exceeds this threshold, the combined impact of obesity and insulin resistance results in substantial vascular damage and an elevated risk of restenosis. The saturation effect observed with the TG/HDL-C ratio may be due to the role of dyslipidaemia in vascular endothelial dysfunction [60]. At lower TG/HDL-C levels, dyslipidaemia promotes atherosclerosis by increasing oxidative stress, inflammation, and endothelial dysfunction. However, when TG/HDL-C exceeds 1.42, these effects may reach a biological saturation point, beyond which further increases in dyslipidaemia contribute minimally to restenosis risk.
The TG/HDL-C ratio is a strong predictor of restenosis
In this study, compared with the other three IR indices, the inclusion of the TG/HDL-C index in the basic vascular restenosis model yielded the greatest incremental predictive value for restenosis events after DCB angioplasty. Several factors may explain this finding: (1) The TG/HDL-C ratio is closely related to insulin resistance, which is itself associated with the development and progression of PAD, increasing the risk of restenosis. (2) Previous studies have shown that a high TG/HDL-C ratio reflects a relatively high load of atherogenic particles, closely linked to the total number of LDL particles and small LDL particles. Differences in LDL subcategories may explain the residual cardiovascular risk and unique lipoprotein characteristics of PAD patients [61–63]. (3) Small LDL particles possess greater atherogenic properties, are more likely to penetrate the arterial intima, and exhibit longer circulation times with lower LDL receptor affinity, promoting atherosclerosis and restenosis formation [62, 64]. (4) HDL-C is considered antiatherosclerotic. A higher TG/HDL-C ratio may indicate a decrease in large, mature HDL particles, which are crucial for reversing cholesterol transport and reducing atherosclerosis [65]. (5) The TG/HDL-C ratio is also associated with non-HDL cholesterol, a collective marker for all atherogenic lipoproteins and a strong predictor of atherosclerotic vascular diseases [66].
In subgroup analyses, the TG/HDL-C ratio showed a meaningful association with most subgroups, although the association was not statistically significant in patients under 60 years of age or those with CHD. However, in these subgroups, the trend remained evident (P < 0.1). Among younger patients, the lack of a significant association may be due to confounding biological or lifestyle factors. Younger patients generally have higher metabolic rates, greater physical activity, and better disease management adaptability, which may influence their relationship with restenosis [67]. In patients with coronary heart disease, various factors, such as myocardial infarction history, coronary artery disease severity, and medication use, could mask the direct association between TG/HDL-C and restenosis risk [68]. Nevertheless, the observed trend suggests that even in patients with coronary heart disease, TG/HDL-C may still have predictive value. Compared with other IR indices, TG/HDL-C consistently showed a significant association with restenosis in most subgroups, indicating its broader clinical applicability. The simplicity, cost-effectiveness, and direct relationship with lipid metabolism make TG/HDL-C an ideal indicator for use in diverse clinical settings.
Conclusion
The TG/HDL-C showed a saturating effect on restenosis within one year after DCB treatment in ASO patients, and METS-IR and TyG-BMI showed threshold effects. Compared with other IR indices, the TG/HDL-C index demonstrated a stable association with postoperative restenosis. The inclusion of the TG/HDL-C index significantly enhanced the predictive performance of the base model for restenosis in ASO patients who underwent DCB angioplasty.
Strengths and limitations
This study has several notable strengths. First, to our knowledge, this is the first study to investigate the relationship between noninsulin-dependent insulin resistance indices and restenosis following DCB angioplasty in lower limb arteries. These findings may have significant implications for the secondary prevention and postoperative management of restenosis in ASO patients. Second, the inclusion of the TG/HDL-C ratio significantly improved the predictive performance of traditional restenosis risk models. Given the ease of obtaining TG and HDL-C levels and their cost-effectiveness, the results of this study have the potential to enhance the scoring systems used by specialists for routine preoperative assessments of postoperative restenosis risk.
However, there are several limitations of this study that should not be overlooked. First, we acknowledge limitations due to the small sample size and the relatively short follow-up period. Future studies with larger sample sizes and longer follow-up durations are warranted to thoroughly elucidate the association between IR and restenosis following DCB procedures. Second, as a retrospective study, we were unable to control for the type of antiproliferative drugs carried by the DCBs or the technical proficiency of the surgeons, both of which may influence long-term outcomes and the occurrence of restenosis. Third, we only measured the values of various indices at a single time point, leaving the relationship between the longitudinal changes in these parameters and clinical outcomes unknown. Additionally, the study was conducted at a single centre, and given the potential differences in ethnicity, diet, and physical activity, these results should be validated in multicentre studies. Finally, as an observational study, there may be potential confounding factors that were not fully accounted for, which could affect the interpretation of the findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ASO
Arterial sclerosis obliterans
- DCB
Drug-coated balloon
- IR
Insulin resistance
- PAD
Peripheral artery disease
- NI-IRIs
Non-insulin dependent insulin resistance indices
- TyG
Triglyceride-glucose index
- BMI
Body mass index
- TG/HDL-C
Triglyceride-to-high-density lipoprotein cholesterol ratio
- METS-IR
Metabolic score for insulin resistance
- TASC
Transatlantic inter-society consensus
- CTA
Computed tomography angiography
- DSA
Digital subtraction angiography
- TC
Total cholesterol
- TG
Triglycerides
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- FBG
Fasting blood glucose
- VIF
Variance inflation factor
- RCS
Restricted cubic spline
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- NRI
Net reclassification improvement
- IDI
Integrated discrimination improvement
- ARIC
Atherosclerosis risk in communities
Author contributions
Z.Q. and Y.Z. defined the study theme and methods, performed the data collection, data analyses and wrote the original manuscript. Z.W. checked the data. Z.Q., Y.Z. and Z.W. contributed to interpreted the results. All authors made critical revision of the manuscript for important intellectual content and approved the final manuscript.
Funding
This study was supported by grants from the Joint Construction Project of Henan Medical Science and Technology Research Plan (grant number: LHGJ20240265).
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Ethics Approval Number: 2024-KY-1169-001).
Consent for publication
All authors have consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhentao Qiao and Yuansong Zhuang contributed equally to this work.
References
- 1.Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, DePalma SM, et al. 2017 focused update of the 2016 ACC Expert Consensus decision pathway on the role of Non-statin therapies for LDL-Cholesterol lowering in the management of atherosclerotic Cardiovascular Disease Risk: a report of the American College of Cardiology Task Force on Expert Consensus decision pathways. J Am Coll Cardiol. 2017;70(14):1785–822. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT, AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA. /ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. J Am Coll Cardiol. 2019;73(24):3210–27. [DOI] [PubMed] [Google Scholar]
- 3.Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL et al. Society for vascular surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61(3 Suppl). [DOI] [PubMed]
- 4.Ozbeyaz NB, Gokalp G, Algul E, Sahan HF, Aydinyilmaz F, Guliyev I, et al. Platelet-hemoglobin ratio predicts amputation in patients with below-knee peripheral arterial disease. BMC Cardiovasc Disord. 2022;22(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ÖZbeyaz NB, AlgÜL E, Erken Pamukcu H. Whole blood viscosity predicts amputation in patients with lower extremity peripheral arterial disease. Firat Universitesi Sağlik Bilimleri Tip Dergisi. 2023;37(3%@ 1308–9315).
- 6.Beckman JA, Schneider PA, Conte MS. Advances in revascularization for peripheral artery disease: revascularization in PAD. Circ Res. 2021;128(12):1885–912. [DOI] [PubMed] [Google Scholar]
- 7.Shanmugasundaram M, Murugapandian S, Truong HT, Lotun K, Banerjee S. Drug-coated balloon in peripheral artery disease. Cardiovasc Revasc Med. 2019;20(4):338–43. [DOI] [PubMed] [Google Scholar]
- 8.Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. [DOI] [PubMed] [Google Scholar]
- 9.Fu J, Yu MG, Li Q, Park K, King GL. Insulin’s actions on vascular tissues: physiological effects and pathophysiological contributions to vascular complications of diabetes. Mol Metab. 2021;52:101236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozbeyaz NB, Gokalp G, Algul E, Kilic P, Saricam O, Aydinyilmaz F, et al. Could systemic inflammation in healthy individuals with obesity indicate subclinical atherosclerosis? Angiology. 2023;74(1):62–9. [DOI] [PubMed] [Google Scholar]
- 11.Love KM, Liu Z. DPP4 activity, Hyperinsulinemia, and atherosclerosis. J Clin Endocrinol Metab. 2021;106(6):1553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rattanatham R, Tangpong J, Chatatikun M, Sun D, Kawakami F, Imai M, et al. Assessment of eight insulin resistance surrogate indexes for predicting metabolic syndrome and hypertension in Thai law enforcement officers. PeerJ. 2023;11:e15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biscetti F, Nardella E, Rando MM, Cecchini AL, Gasbarrini A, Massetti M et al. Outcomes of lower extremity endovascular revascularization: potential predictors and prevention strategies. Int J Mol Sci. 2021;22(4). [DOI] [PMC free article] [PubMed]
- 14.Yoshioka N, Tokuda T, Koyama A, Yamada T, Nishikawa R, Shimamura K, et al. Clinical outcomes and predictors of restenosis in patients with femoropopliteal artery disease treated using polymer-coated paclitaxel-eluting stents or drug-coated balloons. Heart Vessels. 2022;37(4):555–66. [DOI] [PubMed] [Google Scholar]
- 15.Lee S-H, Park S-Y, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norton L, Shannon C, Gastaldelli A, DeFronzo RA. Insulin: the master regulator of glucose metabolism. Metabolism. 2022;129:155142. [DOI] [PubMed] [Google Scholar]
- 17.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. [DOI] [PubMed]
- 18.Tan L, Liu Y, Liu J, Zhang G, Liu Z, Shi R. Association between insulin resistance and uncontrolled hypertension and arterial stiffness among US adults: a population-based study. Cardiovasc Diabetol. 2023;22(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X, Han B, Huang W, Song Z, Huang N, Zhao Y, et al. Association of TyG index with hypertension in Chinese adults: the China Health Examination Collaborative Study (CHEC Study). Asia Pac J Clin Nutr. 2023;32(3):362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Hu P, He Y, Qin H, Hu L, Yang R. Association of TyG index and central obesity with hypertension in middle-aged and elderly Chinese adults: a prospective cohort study. Sci Rep. 2024;14(1):2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Yang W, Jiang X. Association between triglyceride-glucose index and hypertension: a Meta-analysis. Front Cardiovasc Med. 2021;8:644035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Chang L, Wu M, Xu B, Kang L. Triglyceride glucose index was Associated with the risk of Peripheral Artery Disease. Angiology. 2022;73(7):655–9. [DOI] [PubMed] [Google Scholar]
- 24.Lee D-H, Park JE, Kim SY, Jeon HJ, Park J-H. Association between the triglyceride-glucose (TyG) index and increased blood pressure in normotensive subjects: a population-based study. Diabetol Metab Syndr. 2022;14(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–73. [DOI] [PubMed] [Google Scholar]
- 26.GÖKalp G, Özbeyaz N, Ozilhan M. Evaluation of the relationship between mitral annular calcification and triglyceride-glucose index. J Health Sci Med. 2023;6:1114–8. [Google Scholar]
- 27.Er L-K, Wu S, Chou H-H, Hsu L-A, Teng M-S, Sun Y-C, et al. Triglyceride glucose-body Mass Index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3):e0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. 2024;23(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Sun M, Yang Y, Yao N, Yan S, Wang L, et al. Predictive effect of triglyceride glucose-related parameters, obesity indices, and lipid ratios for diabetes in a Chinese Population: a prospective cohort study. Front Endocrinol (Lausanne). 2022;13:862919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuang Y, Qiu L, Han D, Qiao Z, Wang F, Jiang Q, et al. The association between triglyceride-glucose index and related parameters and risk of cardiovascular disease in American adults under different glucose metabolic states. Diabetol Metab Syndr. 2024;16(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng ZY, Liu SX, Xu H, Xu X, Liu XZ, Zhao XX. Association of triglyceride glucose index and its combination of obesity indices with prehypertension in lean individuals: a cross-sectional study of Chinese adults. J Clin Hypertens (Greenwich). 2020;22(6):1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Z, Xing L, Lin M, Sun Y. Estimate of prevalent ischemic stroke from triglyceride glucose-body mass index in the general population. BMC Cardiovasc Disord. 2020;20(1):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–44. [DOI] [PubMed] [Google Scholar]
- 34.Han K-Y, Gu J, Wang Z, Liu J, Zou S, Yang C-X, et al. Association between METS-IR and prehypertension or hypertension among normoglycemia subjects in Japan: a retrospective study. Front Endocrinol (Lausanne). 2022;13:851338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Wang R, Fu X, Song H. Non-insulin-based insulin resistance indexes in predicting severity for coronary artery disease. Diabetol Metab Syndr. 2022;14(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta R, Antonio-Villa NE, Bello-Chavolla OY, Martagón AJ, Elias-López D, Vargas-Vázquez A, et al. Association between insulin resistance and arterial stiffness in Mexican patients without type 2 diabetes. Gac Med Mex. 2021;157(5):522–30. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Hui X, Huang X, Li J, Liu N. Relationship between a novel non-insulin-based metabolic score for insulin resistance (METS-IR) and coronary artery calcification. BMC Endocr Disord. 2022;22(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon J, Jung D, Lee Y, Park B. The metabolic score for insulin resistance (METS-IR) as a predictor of incident ischemic heart disease: a longitudinal study among Korean without diabetes. J Pers Med. 2021;11(8). [DOI] [PMC free article] [PubMed]
- 39.Ding C, Chen Y, Shi Y, Li M, Hu L, Zhou W, et al. Association between nontraditional lipid profiles and peripheral arterial disease in Chinese adults with hypertension. Lipids Health Dis. 2020;19(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nettleton JA, Volcik KA, Hoogeveen RC, Boerwinkle E. Carbohydrate intake modifies associations between ANGPTL4[E40K] genotype and HDL-cholesterol concentrations in white men from the atherosclerosis risk in communities (ARIC) study. Atherosclerosis. 2009;203(1):214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kou M, Ding N, Ballew SH, Salameh MJ, Martin SS, Selvin E, et al. Conventional and novel lipid measures and risk of Peripheral Artery Disease. Arterioscler Thromb Vasc Biol. 2021;41(3):1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertrand C, Saulnier P-J, Potier L, Croyal M, Blanchard V, Gand E, et al. Plasma concentrations of lipoproteins and risk of lower-limb peripheral artery disease in people with type 2 diabetes: the SURDIAGENE study. Diabetologia. 2021;64(3):668–80. [DOI] [PubMed] [Google Scholar]
- 43.Evsen A, Aktan A, Altunova M, Özbek M. Is plasma atherogenic index or LDL/HDL ratio more predictive of peripheral arterial disease complexity? Vascular. 2024:17085381241260203. [DOI] [PubMed]
- 44.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. [DOI] [PubMed] [Google Scholar]
- 45.Gökalp G, Özbeyaz NB. The effect of SGLT2 inhibitors on cardio-electrophysiological balance index in diabetic patients with preserved ejection heart failure. Archives Curr Med Res. 2023;4(3):192–7. [Google Scholar]
- 46.Milicevic Z, Raz I, Beattie SD, Campaigne BN, Sarwat S, Gromniak E, et al. Natural history of cardiovascular disease in patients with diabetes: role of hyperglycemia. Diabetes Care. 2008;31(Suppl 2):S155–60. [DOI] [PubMed] [Google Scholar]
- 47.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bigornia SJ, Farb MG, Tiwari S, Karki S, Hamburg NM, Vita JA, et al. Insulin status and vascular responses to weight loss in obesity. J Am Coll Cardiol. 2013;62(24):2297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takagi T, Akasaka T, Yamamuro A, Honda Y, Hozumi T, Morioka S, et al. Impact of insulin resistance on neointimal tissue proliferation after coronary stent implantation. Intravascular ultrasound studies. J Diabetes Complications. 2002;16(1):50–5. [DOI] [PubMed] [Google Scholar]
- 50.Piatti P, Monti LD. Insulin resistance, hyperleptinemia and endothelial dysfunction in coronary restenosis. Curr Opin Pharmacol. 2005;5(2):160–4. [DOI] [PubMed] [Google Scholar]
- 51.Babalik E, Gürmen T, Orhan L, Bulur H, Gülbaran M, Ersanli M, et al. Increased secretion of insulin during oral glucose tolerance test can be a predictor of stent restenosis in nondiabetic patients. Catheter Cardiovasc Interv. 2003;58(3):306–12. [DOI] [PubMed] [Google Scholar]
- 52.Alfonso F, Cuesta J, Pérez-Vizcayno MJ, García D, Blanco B, Rumoroso JR, Bosa F, et al. Bioresorbable vascular scaffolds for patients with In-Stent restenosis: the RIBS VI study. JACC Cardiovasc Interv. 2017;10(18):1841–51. [DOI] [PubMed] [Google Scholar]
- 53.Jeger RV, Farah A, Ohlow M-A, Mangner N, Möbius-Winkler S, Weilenmann D, et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396(10261):1504–10. [DOI] [PubMed] [Google Scholar]
- 54.Megaly M, Ali A, Abraham B, Khalil C, Zordok M, Shaker M, et al. Outcomes with drug-coated balloons in percutaneous coronary intervention in Diabetic patients. Cardiovasc Revasc Med. 2020;21(1):78–85. [DOI] [PubMed] [Google Scholar]
- 55.Davies MG, Saad WE. Impact of elevated perioperative fasting blood glucose on carotid artery stenting outcomes. Ann Vasc Surg. 2014;28(8):1885–91. [DOI] [PubMed] [Google Scholar]
- 56.Zhao S-S, Jiang Z-Z, Wei B, Zhu J-B, Liu X-T. The preoperative triglyceride-glucose index has a positive effect on predicting the risk of short-term restenosis after carotid artery stenting: a retrospective cohort study. Front Neurol. 2023;14:1159601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dannecker C, Wagner R, Peter A, Hummel J, Vosseler A, Häring H-U, et al. Low-density lipoprotein cholesterol is Associated with insulin secretion. J Clin Endocrinol Metab. 2021;106(6):1576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Limberg JK, Soares RN, Padilla J. Role of the autonomic nervous system in the hemodynamic response to hyperinsulinemia-implications for obesity and insulin resistance. Curr Diab Rep. 2022;22(4):169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruze R, Liu T, Zou X, Song J, Chen Y, Xu R, et al. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne). 2023;14:1161521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tejera CH, Minnier J, Fazio S, Safford MM, Colantonio LD, Irvin MR, et al. High triglyceride to HDL cholesterol ratio is associated with increased coronary heart disease among White but not black adults. Am J Prev Cardiol. 2021;7:100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, Standard Lipids, and Peripheral Artery Disease incidence. Circulation. 2018;138(21):2330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia L, Long S, Fu M, Yan B, Tian Y, Xu Y, et al. Relationship between total cholesterol/high-density lipoprotein cholesterol ratio, triglyceride/high-density lipoprotein cholesterol ratio, and high-density lipoprotein subclasses. Metabolism. 2006;55(9):1141–8. [DOI] [PubMed] [Google Scholar]
- 63.Mathews SC, Mallidi J, Kulkarni K, Toth PP, Jones SR. Achieving secondary prevention low-density lipoprotein particle concentration goals using lipoprotein cholesterol-based data. PLoS ONE. 2012;7(3):e33692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maeda S, Nakanishi S, Yoneda M, Awaya T, Yamane K, Hirano T, et al. Associations between small dense LDL, HDL subfractions (HDL2, HDL3) and risk of atherosclerosis in japanese-americans. J Atheroscler Thromb. 2012;19(5):444–52. [DOI] [PubMed] [Google Scholar]
- 65.Sviridov D, Nestel P. Dynamics of reverse cholesterol transport: protection against atherosclerosis. Atherosclerosis. 2002;161(2):245–54. [DOI] [PubMed] [Google Scholar]
- 66.Zvintzou E, Xepapadaki E, Skroubis G, Mparnia V, Giannatou K, Benabdellah K et al. High-density lipoprotein in metabolic disorders and beyond: an exciting new world full of challenges and opportunities. Pharmaceuticals (Basel). 2023;16(6). [DOI] [PMC free article] [PubMed]
- 67.Whytock KL, Shepherd SO, Cocks M, Wagenmakers AJM, Strauss JA. Young, healthy males and females present cardiometabolic protection against the detrimental effects of a 7-day high-fat high-calorie diet. Eur J Nutr. 2021;60(3):1605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia C, Zeng Y, Huang X, Yang H, Qu Y, Hu Y, et al. Lifestyle patterns, genetic susceptibility, and risk of valvular heart disease: a prospective cohort study based on the UK Biobank. Eur J Prev Cardiol. 2023;30(15):1665–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.