Abstract
Overexpression of dynein fragments in Dictyostelium induces the movement of the entire interphase microtubule array. Centrosomes in these cells circulate through the cytoplasm at rates between 0.4 and 2.5 μm/s and are trailed by a comet-tail like arrangement of the microtubule array. Previous work suggested that these cells use a dynein-mediated pulling mechanism to generate this dramatic movement and that similar forces are at work to maintain the interphase MTOC position in wild-type cells. In the present study, we address the nature of the forces used to produce microtubule movement. We have used a laser microbeam to sever the connection between the motile centrosomes and trailing microtubules, demonstrating that the major force for such motility results from a pushing on the microtubules. We eliminate the possibility that microtubule assembly/disassembly reactions are significant contributors to this motility and suggest that the cell cortex figures prominently in locating force-producing molecules. Our findings indicate that interphase microtubules in Dictyostelium are subject to both dynein- and kinesin-like forces and that these act in concert to maintain centrosome position in the cell and to support the radial character of the microtubule network.
INTRODUCTION
Microtubules are dynamic polymers that are essential for eukaryotic cell viability. They provide general support for overall cell shape and can be modeled into a number of functional configurations for use in cell-motility events. For example, microtubules form the structural core of cilia and flagella and the spindle apparatus during cell division. Microtubules also form extended arrays that support highly asymmetric cell shapes, such as those commonly seen in neurons. These distinctive architectures are actively built and maintained; that is, individual microtubules are acted upon by accessory proteins to alter their dynamics, to cross-link with adjacent microtubules or other structures, and to produce various movements. In most interphase cells, the “default” microtubule arrangement consists of a clustering of minus ends at or near the cell center, with the plus ends extended in a radial manner out to, or near the cell periphery. This arrangement, too, is actively maintained, and it can be altered depending on cell-cycle progression or cell stimulation.
In Dictyostelium, overexpression of the dynein motor domain produces a phenotype in which the microtubule array loses its radial character and becomes motile through the cytoplasm (Koonce et al., 1999). Microtubules in these cells (380K cells) remain firmly attached to the centrosome and, in large part, are dramatically rearranged to form a trailing “comet-tail”. The same phenotype can be generated in multiple ways: by expressing small fragments of the dynein motor that do not have catalytic or microtubule-binding activity (Koonce et al., 1999), by expression of the Dictyostelium Lis-1 protein (Rehberg et al., 2005), by expression of an N-terminal fragment of the CP-224 MAP (Hestermann and Gräf, 2004), or expression of an N-terminal fragment of the Dictyostelium NudE-like homolog (Koonce and Tikhonenko, unpublished results). Thus, the effect appears to involve both the Lis-1/Ndel-1/dynein regulatory pathway (Smith et al., 2000, Dujardin et al., 2003, Liang et al., 2004, Shu et al., 2004) and linkages to the cell cortex. Disruption of dyneincargo linkages by dominant-negative expression of dynein intermediate-chain fragments also produces such a phenotype (Ma et al., 1999), but with far greater effects on the microtubule array and on cell division. This more severe dynein disruption is lethal, arguing that the Lis-l/Ndel-1 governs only a subset of dynein activities. We are actively pursuing the connections between this regulatory pathway and dynein motor activity.
In addition to the loss of cortical attachments, this novel centrosome motility must be based on a pulling or pushing force applied directly to the centrosome or along the microtubules. A priori, this force can be generated by microtubule polymerization or by a motor protein(s). In this respect, a number of dynein perturbations in other organisms have similar consequences (Smith et al., 2000, Palazzo et al., 2001, Burakov et al., 2003, Dujardin et al., 2003). Taken together, these results strongly indicate a general role for dynein in stabilizing and/or positioning interphase microtubule arrays. Our previous work supported a model in which cortically anchored dynein motors exert transient pulling forces on microtubules. A balance of these force transients over the entire cortical surface would thus provide a means by which the cell continually reinforces the central position of the centrosome and radial character of the microtubule array, whether the cell is stationary or actively moving. One possibility for the aberrant movement in the mutant cells is that this balance of dynein activity is disturbed (activity on some motors is either reduced or stimulated), such that a cortical dynein-microtubule engagement becomes dominant and provides an extended pulling force to displace the centrosome. In the current work, we directly test this idea by using a laser microbeam to sever the connection between the centrosome and the trailing microtubule array. We present evidence that the bulk of the centrosome movement in the mutant cells results from kinesin-like pushing forces acting on the trailing microtubules. The work suggests that both kinesin and dynein-like motor activities can displace interphase microtubules and that a balance between these activities is important for maintaining the character of the microtubule array.
MATERIALS AND METHODS
Cell Culture
Dictyostelium AX-2 cell lines expressing α-tubulin GFP alone (control cells; Neujahr et al., 1998) or with the 380-kDa motor domain of cytoplasmic dynein (380K cells; Koonce and Samsó, 1996) were prepared and maintained as described (Koonce et al., 1999).
For use, cells were plated on glass coverslips and allowed to adhere for 1–2 h in 17 mM K/Na phosphate buffer, pH 6.0, to reduce background fluorescence; they were then overlaid and flattened with small agarose sheets (Yumura et al., 1984). These coverslips were either inverted over slides and sealed with VALAP or assembled into Rose chambers, containing a small piece of moistened Kimwipe to maintain humidity. Latrunculin A was purchased from Sigma Chemical Co. (St. Louis, MO) and used at a final concentration of 5 μM in both phosphate buffer and the agarose sheets.
Laser Microsurgery and Imaging
Laser microsurgery was conducted on a custom-assembled workstation centered around an inverted fluorescence microscope (model TE2000E; Nikon Instruments, Melville, NY). We used an independent second (lower) epi-port that is available on this model, to direct a collimated laser beam into the back aperture of a 60× 1.4-NA PlanApo lens. A 532-nm beam was used for laser cutting (Nd:YAG laser; 7-ns pulses, 10 Hz). This system is described in more detail in Khodjakov et al. (2004). The same optical system was also used to record nonlaser sequences.
All light sources in our system were shuttered with fast UniBlitz shutters (Vincent Associates, Rochester, NY), so that each cell was exposed to light only during laser operations and/or image acquisition. Wide-field images were collected under low light level conditions with a Coolsnap HQ camera (Photometrics, Tucson, AZ). The whole system was driven by IP Lab software (Scanalytics, Billerica, MA) run on a PC. Image sequences were collected at 3-s intervals, and were analyzed using Image J (v1.31, NIH). The panels shown in the figures were compiled using Photoshop 6.0 (Adobe Systems, San Jose, CA). For the latrunculin work, centrosome position was followed automatically using the cross-correlation tracking algorithm in ISEE Software (ISEE Imaging, Durham, NC).
RESULTS
Individual interphase microtubules in control Dictyostelium cells are exceptionally motile, displaying a variety of rapid movements that appear to be driven by both plus end– and minus end–directed motors (Figure 1; Supplementary Movie 1; Kimble et al., 2000, Koonce and Khodjakov, 2002). Such movements include simple bending and straightening, flailing back and forth in a whip-like manner, and arcing along the cell cortex. Forces acting on the microtubules appear able to originate at any point along the entire microtubule length. Overexpression of the dynein motor domain introduces an additional motile component: the entire microtubule array appears to circulate through the cytoplasm and along the inner surface of the cell cortex, at rates up to 2.5 μm/s (Koonce et al., 1999; see also Supplementary Movies 3a and 5c). We used a laser microbeam to sever the connection between the centrosome and the trailing comet tail of microtubules in these 380K cells (Figure 2; Supplementary Movies 2a–d). In 15 of 18 cells, centrosomes immediately cease their movement, suggesting that much of the motility is driven by force exerted on the trailing microtubules. The microtubule arrays in the three cells whose centrosomes continued moving were less focused, and we could not be certain that most of the microtubules had actually been severed. Control irradiations in the cytoplasm, adjacent to or in front of the centrosome had no effect on this movement. In some instances after cutting (Figure 2D), microtubules were reorganized on the other side of the centrosome and began to drive motion in the direction opposite the preirradiation movement.
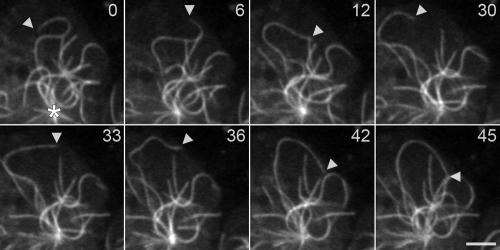
Figure 1.
Interphase microtubule movements in a control cell. An arrowhead marks the plus end of a single GFP-labeled microtubule in Dictyostelium. In the first four frames (0–30 s), the plus end follows a whip-like bending trajectory through the cytoplasm, in a direction consistent with the application of kinesin-like forces to the microtubule. In the last four frames (33–45 s), the same microtubule end now leads, in a direction consistent with the application of dynein force. The microtubule itself maintains a constant length (13.3 μm) throughout the sequence. The position of the centrosome is marked with an asterisk in the first panel (t = 0). Time indicates seconds of observation; scale bar, 2.5 μm.
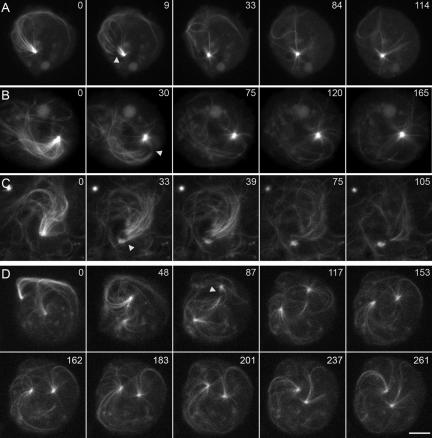
Figure 2.
Laser-mediated cutting of motile microtubule comet tails. (A–D). Four examples of 380K cells in which the trailing GFP-labeled microtubule bundle is separated from the leading centrosome. The first panel in the A–C sequence shows a “before” view; the second panel shows the cell immediately after (or during as in C) laser cutting of the microtubule tail. The two rows in D show an extended sequence of a binucleate cell, containing two microtubule arrays. The arrowhead marks the site of laser ablation. In all cases shown here, the centrosome stopped movement upon severing of the microtubule bundle. Note in D that the second microtubule array movement is unaffected by the ablation and that in the last few panels (153 s and beyond), the unaffected microtubules on the side opposite to the cut have begun to organize and push the centrosome toward the bottom of the frame. Time is displayed in seconds of observation; scale bar, 5 μm.
In nearly every case, the laser-mediated liberation of minus microtubule ends resulted in the disassembly of the trailing arrays. However, in a few cells (e.g., Figure 3), the microtubule array appeared tightly bundled and did not immediately disassemble. In these cells, we found that the microtubule bundles themselves were capable of motion, independent of the centrosome. This result indicates that the centrosome itself is not required; instead, the force responsible for the motility acts along the microtubule bundle (Supplementary Movie 3b).
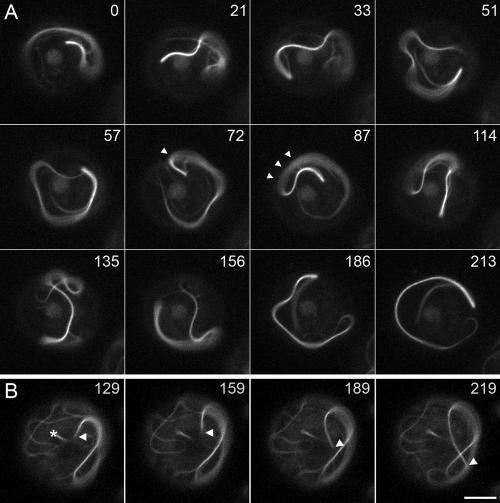
Figure 3.
Movements of the microtubule bundle. These panels show examples of tightly packed bundles of microtubules in 380K cells that exhibit movement independent of the centrosome. In the uncut example shown in A, the centrosome leads the movement over a 3.5-min time period. In frames 72–87, the centrosome pauses briefly, but the trailing microtubule bundle continues its movement (marked by arrowheads). The comet tail shown in B required two attempts to fully sever with the laser microbeam. A partial cut is shown after 129 s, and the fully cut bundle is shown in 159 s. The centrosome is marked with an asterisk. On complete cutting, the bundle itself began moving, independent of the centrosome, with the new minus ends leading. The peak rate of movement here is 0.5 μm/s. Time is displayed in seconds of observation; scale bar, 5 μm.
To distinguish whether the force is due to molecular motors or microtubule-end dynamics, we measured assembly/disassembly rates for Dictyostelium microtubules. The goal was to determine whether this organism's tubulins have any unusual kinetic properties that could contribute both to the lateral movements in control cells and to the centrosome movements in the 380K cells. We restricted our analysis to those microtubules whose entire length remained in focus throughout the observation period. Our records revealed that while transitions between growing and shrinking are extremely rare, Dictyostelium microtubules do undergo dynamic instability (Figure 4A). Using the laser microbeam, we also created free plus and minus ends of single microtubules. Except for those in tightly bundled microtubule arrangements (as described above), free minus ends of microtubules in Dictyostelium are unstable (Figure 4B). In a number of cases, we could readily follow this depolymerization and thus generate rate measurements for minus end depolymerization (Figure 4C). We saw no evidence for tubulin assembly at free minus ends of microtubules. Plus ends were more stable: only half of the newly created plus ends depolymerized to varying degrees, whereas the other half remained stable throughout the observation period (Figure 4D).
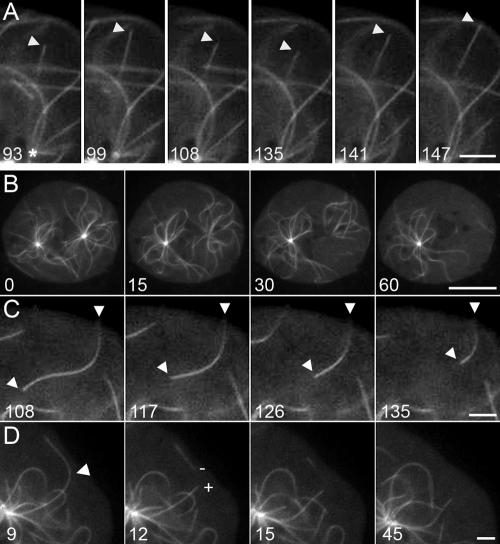
Figure 4.
Microtubule end dynamics in Dictyostelium control cells. (A) Example of dynamic instability. Arrowhead marks the growing and shrinking plus end of a single GFP-microtubule; the minus end is anchored at the centrosome at the bottom of the field (asterisk in first panel). (B) Binucleate cell with two independent microtubule arrays. A laser microbeam was used to ablate the centrosome anchoring the right-hand network (t = 0 before, t = 15 just after irradiation), resulting in the generation of free minus ends and depolymerization of the right-hand microtubule network. As a control, the left-hand network is unaffected. (C) Panel follows minus-end depolymerization of a single microtubule. (D) Panel shows laser-mediated cutting of a single microtubule (t = 9 before, t = 12 just after). Note here that the newly created minus end (–) is unstable, but the new plus end (+) remains a constant length (9.5 μm). Time in seconds of observation; scale bar: (A, C, and D) 2.5 μm; (B) 10 μm.
Our measurements of microtubule end dynamics (assembly and disassembly) are shown in Table 1; these averages are comparable to kinetics seen in GFP-labeled mammalian microtubules (also included in Table 1; Rusan et al., 2001). In contrast, the rapid movements of individual microtubules in Dictyostelium occur at rates averaging 1.1 μm/s, approximately four times faster than could be explained solely on the basis of assembly or disassembly. There were no rate differences between what we interpreted as plus end– or minus end–directed force production, a finding consistent with other measurements of organelle trafficking in Dictyostelium (Table 1). These results strongly imply that the rapid microtubule and centrosome movements in control and 380K cells are largely due to molecular motor activities, rather than to unusual kinetics of assembly.
Table 1.
Average rates and comparisons of microtubule assembly, disassembly, and motor-driven movements in control cells
| Microtubule end kinetics | Dictyostelium | Mammaliana |
|---|---|---|
| Plus end polymerization | 14.4 ± 1.5 μm/min (n = 6) | 11.5 ± 7 μm/min |
| Plus end depolymerization | 16.8 ± 5.1 μm/min (n = 12) | 13.1 ± 8 μm/min |
| Minus end depolymerization | 18.1 ± 3.3 μm/min (n = 12) | |
| Microtubule end movements | 1.14 ± 0.1 μm/s (69.0 ± 6.5 μm/min; n = 12) | |
| Organelle movements | ||
| Centrosomes | 0.4-2.5 μm/sb | |
| Minus end—directed GFP-dynein | 1.84 ± 0.34 μm/sc | |
| Plus end—directed GFP-dynein | 1.77 ± 0.40 μm/sc | |
| Aggregate organelles | 1.4-2.8 μm/sd |
Finally, to address potential contributions of the actin-rich cell cortex to the microtubule motions, we examined the effects of latrunculin in both control and 380K cells. Cells treated with 5 μM latrunculin showed the rapid, characteristic effects of actin depolymerization: cells halted their motility, rounded up, and detached from the coverslip. We applied an agar overlay (presoaked in latrunculin) to hold cells in place so as to enable their live observation, and we also examined fixed cells to confirm the efficacy of the drug in destabilizing the actin arrays (unpublished data). The individual bending motions of microtubules occurred in control, latrunculin-treated control, and latrunculin-treated 380K cells (Supplementary Movies 5a–c). However, there were differences in the overall microtubule patterns in control cells and in the motility of the comet-tail arrangements in the 380K cells. In control cells, latrunculin-treated microtubules appeared to be more curved and fewer microtubules appeared to engage in tension-generating events on cell cortex (Figure 5). Although these differences are readily seen in the Supplementary Movies, we also include a plot of centrosome position over time (Figure 5). Note that the lateral back and forth motions of the centrosome are much reduced in the latrunculin treatment. A significant difference was also seen in the 380K cells. Comet tail-like microtubule arrangements could be found (Figure 5), and occasional short centrosome movements (1–2 μm) were observed. However, none of the latrunculin-treated 380K cells displayed the persistent directional motions that were seen in untreated 380K cells. Thus, actin filament disruption does not appear to affect individual microtubule motions, but it does alter the overall architecture in control cells and significantly inhibits movement of the entire array in the 380K cells.
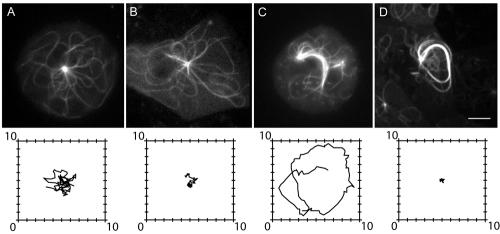
Figure 5.
Effects of latrunculin on centrosome movement. (A-D) Still frames of GFP-labeled microtubules in a control cell (A), a control cell treated with 5 μM latrunculin (B), a 380K cell (C), and a 380K cell treated with 5 μM latrunculin (D). Below each panel is a tracing of that centrosome's position throughout the 5-min recording period. Note that for both control and 380K cells, latrunculin treatment significantly decreases the range of centrosome movement. Latrunculin had no obvious effect on individual microtubule bending movements in control or 380K cells (see Supplementary Movies for the entire sequences). Scale bar for light micrographs, 5 μm; Tracings are outlined with 10-μm2 box.
Together, these results support the idea that a balance of plus end– and minus end–directed forces acting on the microtubules are involved with maintaining the organization of the radial interphase microtubule array in eukaryotic cells. In addition, they also suggest that an intact actin-filament cortex is instrumental in supporting tension-generating microtubule interactions. These ideas are summarized in Figure 6 and are further elaborated in the Discussion.
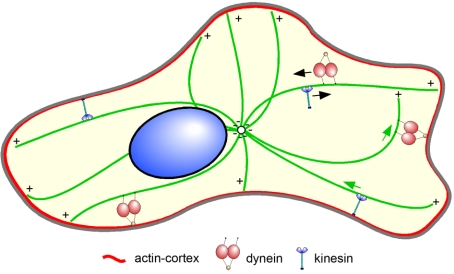
Figure 6.
Summary of motor function in interphase cells. In this model, motor molecules exert two types of forces along microtubules. Free motors in the cytoplasm, or ones bound to simple organelles, will move in the direction specified by their mechanochemistry (toward minus ends for dynein, toward plus ends for kinesin, directions indicated here by black arrows). However, if the motors are anchored at more elaborate sites, for example, enmeshed in the cortex (red), attached to a complex cargo with significant resistance, or anchored at adhesion sites, these motors appear able to exert enough force to move the microtubule substrate. Thus, anchored dyneins will pull on a microtubule and anchored kinesins will push microtubules (green arrows). The combined activity of both anchored motors is likely to be instrumental in maintaining centrosome position and supporting the radial character of the interphase microtubule array.
DISCUSSION
In the studies described, we sought to address the general question of how centrosome position is determined and maintained in eukaryotic cells. The dramatic motility of the microtubule array in the 380K cells, combined with laser-microbeam ablation, provides an opportunity to address the nature of the force-generating machinery that can actively displace both microtubules and centrosomes. There are two types of microtubule motility described in this study: the bending, straightening, sweeping motions of individual microtubules, and a larger scale motion of the entire microtubule array. The individual microtubule motions occur in both control and 380K cells, whether or not the actin filament meshwork has been disrupted by latrunculin. The larger scale comet-like movements of the entire array are induced by dominant-negative expression of dynein motor fragments, and ceases if actin filaments are disrupted.
Single Microtubule Motility
The individual microtubule movements appear to result from direct interactions with motor proteins. Because each microtubule behaves independently, the motions are clearly not due to any bulk cytoplasmic rearrangements or membrane flow (e.g., Kaverina et al., 1998, Ligon et al., 2001, Salmon et al., 2002). The rate of movement is much faster than could be accounted for solely by polymerization or depolymerization and is consistent with the microtubule motor-based activity seen in this organism. Microtubule bending and translocation would occur when the resistance encountered by a motor-driven cargo exceeds the stiffness provided by its microtubule track. Because of their inherent polarity, dyneins would pull on a microtubule and thus straighten the microtubule between the motor and the minus-end anchorage at the centrosome. In the other direction, kinesins would push between the motor attachment and the centrosome, causing a bending of the polymer. As revealed in Figure 1, microtubules frequently undergo both types of movement, indicating that both kinesin- and dynein-like motor activities affect microtubule position in Dictyostelium, under normal interphase conditions.
Microtubule Array Motility
The comet-tail phenotype of the 380K cells can be generated by multiple perturbations that target dynein motor regulation or anchorage of microtubules at their plus ends. (Koonce et al., 1999, Rehberg et al., 2005; Hestermann and Gräf, 2004). Measured in vitro, dynein motor domain overexpression in the mutant 380K cells causes a ∼40% decrease in the frequency of minus end organelle movements, but has no effect on the plus end–directed motility (Pollock et al., 1998). The distribution of organelles in these cells appears no different from in controls. Therefore, the remaining ∼60% of minus end–directed organelle movements must be sufficient for most trafficking requirements and could be used to produce the straightening motions of individual microtubules in the 380K cells. A striking difference between control and 380K cells lies in the rapid comet like movements of the microtubule array. If pushing and pulling forces acting on the microtubule array are balanced in normal cells, then a 40% decrease in dynein activity may be significant to allow a pushing force to dominate. The laser work presented here is consistent with this idea, indicating pushing-type forces act on and move the centrosomes and microtubules in the mutant 380K cells. The obvious candidate for such a pushing action is a plus end–directed kinesin-type motor. It remains unclear how a pushing force directed to one or perhaps only a few flexible microtubules can support movement of the entire array (and the attached nucleus), but this mechanism has been shown to work in yeast (Tran et al., 2001).
Subpopulations of Motor Activity
Although motor activity bound to organelles could be sufficient to move individual microtubules, moving the entire array would require a far more sturdy anchorage. The most obvious location that has such structural rigidity is the peripheral actin-rich cortical meshwork. There are a number of examples that support both the targeting of motors to the cortex (Holleran et al., 1996, Iwai et al., 2004) and force production at this site (reviewed in Allan and Näthke, 2001, Bloom, 2001, Dujardin and Vallee, 2002). Thus, we surmise that there are two distinct populations of motors that engage microtubules: one on actively moving organelles and the other enmeshed into the actin-rich cortex. Our observation that latrunculin stops the comet-like microtubule movements is particularly important since it directly tests this idea. Disrupting the cortical meshwork eliminates motor anchorage, and also the force contributions of both pushing and pulling motors at this site. In control cells, eliminating contributions of both motors only mildly affects the radial component of the array. In the mutant 380K cells, comet tails no longer move after latrunculin treatment, a result consistent with displacement of the now dominant plus end–directed forces. Given that there are no differences in vesicle motion or in the bending movements of single microtubules after latrunculin treatment (Figure 5), we infer that motor-organelle linkages and motor activities per se are not sensitive to actin-filament disruption.
A Role For The Cortex
There are numerous reports that describe a general integration of actin filament and microtubule polymer systems, including effects on organelle motility (Rogers and Gelfand, 1998, Rodionov et al., 1998), centrosome position (Euteneuer and Schliwa, 1985, Whitehead et al., 1996, Piel et al., 2000, Burakov et al., 2003), and actin-microtubule based motor interactions (Huang et al., 1999, Weber et al., 2004). To couple these two filament systems in the comet-tail motion reported here, either myosin motors are stably attached to the microtubules and pull along actin filaments, or a set of microtubule-based motors must be anchored in the actin-rich cortex that push on microtubules. Given rates that are consistent with the microtubule-based motility in Dictyostelium and contrasting the relatively short lengths of actin filaments (avg. 200 nm; Podolski and Steck, 1990) with persistence of the directional comet-like movements (Figure 3), the results presented in this study are most consistent with the latter mechanism. Though we cannot conclusively rule out a myosin role, we favor the idea that the actin-rich cortical meshwork serves as a platform to anchor microtubule-based motors (or linker proteins).
Summary
Our data are summarized in the model presented in Figure 6. Here, both dynein and kinesin function in mediating organelle transport and provide support for the interphase microtubule array. Indeed, we believe that a balance of multiple forces and nonforce linkers (push, pull, and static) engage microtubules at or near their ends, so as to maintain the radial character of the interphase array. The cell cortex figures prominently in this model, by offering a relatively firm platform on which to push or pull against. Cortically linked dynein has long been implicated in the positioning of mitotic and meiotic spindles (e.g., Carminati and Stearns, 1997, Bloom, 2001, Gönczy, 2002), and in the engagement of cytoplasmic microtubules (Allan and Näthke, 2001, Schuyler and Pellman, 2001, Dujardin and Vallee, 2002, Hestermann et al., 2002). Recently, a role for a kinesin-like pushing force has been described in orienting microtubules along the cortex of Xenopus embryos (Marrari et al., 2004). Our work further indicates that along with dynein, kinesin-like motors may play much more active roles in organizing microtubule-based structures than previously considered, especially in interphase cells.
Supplementary Material
Acknowledgments
Our work is supported in part by the National Institutes of Health (GM51532 to M.P.K., GM59363 to A.K.) and the Foundation for Science and Technology (Portugal; FCT grant SFRH/BD/13663/2003 to D.A.B.). We also want to express our gratitude to Dr. Adriana Verschoor for editorial assistance and to Dr. Günther Gerisch for providing the GFP-α-tubulin plasmid.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–01–0057) on April 27, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Allan, V., and Näthke, I. S. (2001). Catch and pull a microtubule: getting a grasp on the cortex. Nat. Cell Biol. 3, E226–E228. [DOI] [PubMed] [Google Scholar]
- Bloom, K. (2001). Nuclear migration: cortical anchors for cytoplasmic dynein. Curr. Biol. 11, R326–R329. [DOI] [PubMed] [Google Scholar]
- Burakov, A., Nadezhdina, E., Slepchenko, B., and Rodionov, V. (2003). Centrosome positioning in interphase cells. J. Cell Biol. 162, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati, J. L., and Stearns, T. (1997). Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 138, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin, D. L., Barnhart, L. E., Stehman, S. A., Gomes, E. R., Gundersen, G. G., and Vallee, R. B. (2003). A role for cytoplasmic dynein and LIS1 in directed cell movement. J. Cell Biol. 163, 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin, D. L., and Vallee, R. B. (2002). Dynein at the cortex. Curr. Opin. Cell Biol. 14, 44–49. [DOI] [PubMed] [Google Scholar]
- Euteneuer, U., and Schliwa, M. (1985). Evidence for an involvement of actin in the positioning and motility of centrosomes. J. Cell Biol. 101, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy, P. (2002). Mechanisms of spindle positioning: focus on flies and worms. Trends Cell Biol. 12, 332–339. [DOI] [PubMed] [Google Scholar]
- Hestermann, A., and Gräf, R. (2004). The XMAP215-family protein DdCP224 is required for cortical interactions of microtubules. BMC Cell Biol. 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestermann, A., Rehberg, M., and Gräf, R. (2002). Centrosomal microtubule plus end tracking proteins and their role in Dictyostelium cell dynamics. J. Muscle Res. Cell Motil. 23, 621–630. [DOI] [PubMed] [Google Scholar]
- Holleran, E. A., Tokito, M. K., Karki, S., and Holzbaur, E. L. (1996). Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J. Cell Biol. 135, 1815–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. D., Brady, S. T., Richards, B. W., Stenolen, D., Resau, J. H., Copeland, N. G., and Jenkins, N. A. (1999). Direct interaction of microtubule- and actin-based transport motors. Nature 397, 267–270. [DOI] [PubMed] [Google Scholar]
- Iwai, S., Ishiji, A., Mabuchi, I., and Sutoh, K. (2004). A novel actin-bundling kinesin-related protein from Dictyostelium discoideum. J. Biol. Chem. 279, 4696–4704. [DOI] [PubMed] [Google Scholar]
- Kaverina, I., Rottner, K., and Small, J. V. (1998). Targeting, capture, and stabilization of microtubules at early focal adhesions. J. Cell Biol. 142, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., La Terra, S., and Chang, F. (2004). Laser microsurgery in fission yeast; role of the mitotic spindle midzone in anaphase B. Curr. Biol. 14, 1330–1334. [DOI] [PubMed] [Google Scholar]
- Kimble, M., Kuzmiak, C., McGovern, K. N., and de Hostos, E. L. (2000). Microtubule organization and the effects of GFP-tubulin expression in Dictyostelium discoideum. Cell Motil. Cytoskelet. 47, 48–62. [DOI] [PubMed] [Google Scholar]
- Koonce, M. P., and Khodjakov, A. (2002). Dynamic microtubules in Dictyostelium. J. Muscle Res. Cell Motil. 23, 613–619. [DOI] [PubMed] [Google Scholar]
- Koonce, M. P., Köhler, J., Neujahr, R., Schwartz, J. M., Tikhonenko, I., and Gerisch, G. (1999). Dynein motor regulation stabilizes interphase microtubule arrays and determines centrosome position. EMBO J. 18, 6786–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce, M. P., and Samsó, M. (1996). Overexpression of cytoplasmic dynein's globular head causes a collapse of the interphase microtubule network in Dictyostelium. Mol. Biol. Cell 7, 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y., Yu, W., Li, Y., Yang, Z., Yan, X., Huang, Q., and Zhu, X. (2004). Nudel functions in membrane traffic mainly through association with Lis1 and cytoplasmic dynein. J. Cell Biol. 164, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon, L. A., Karki, S., Tokito, M., and Holzbaur, E. L. (2001). Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat. Cell Biol. 3, 913–917. [DOI] [PubMed] [Google Scholar]
- Ma, S., and Chisholm, R. L. (2002). Cytoplasmic dynein-associated structures move bidirectionally in vivo. J. Cell Sci. 115, 1453–1460. [DOI] [PubMed] [Google Scholar]
- Ma, S., Triviños-Lagos, L., Gräf, R., and Chisholm, R. L. (1999). Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 147, 1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrari, Y., Rouviere, C., and Houliston, E. (2004). Complementary roles for dynein and kinesins in the Xenopus egg cortical rotation. Dev. Biol. 271, 38–48. [DOI] [PubMed] [Google Scholar]
- Neujahr, R., Albrecht, R., Köhler, J., Matzner, M., Schwartz, J. M., Westphal, M., and Gerisch, G. (1998). Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J. Cell Sci. 111, 1227–1240. [DOI] [PubMed] [Google Scholar]
- Palazzo, A. F., Joseph, H. L., Chen, Y. J., Dujardin, D. L., Alberts, A. S., Pfister, K. K., Vallee, R. B., and Gundersen, G. G. (2001). Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 11, 1536–1541. [DOI] [PubMed] [Google Scholar]
- Piel, M., Meyer, P., Khodjakov, A., Rieder, C. L., and Bornens, M. (2000). The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolski, J. L., and Steck, T. L. (1990). Length distribution of F-actin in Dictyostelium discoideum. J. Biol. Chem. 265, 1312–1318. [PubMed] [Google Scholar]
- Pollock, N., Koonce, M. P., de Hostos, E. L., and Vale, R. D. (1998). In vitro microtubule-based organelle transport in wild-type Dictyostelium and cells overexpressing a truncated dynein heavy chain. Cell Motil. Cytoskelet. 40, 304–314. [DOI] [PubMed] [Google Scholar]
- Rehberg, M., Kleyein-Sohn, J., Faix, J., Ho, T.-H., Schultz, I, and Gräf, R. (2005). Dictyostelium LIS1 is a centrosomal protein required for microtubule/cell cortex interactions, nucleus/centrosomal linkage, and actin dynamics. Mol. Biol. Cell 16, 2759–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov, V. I., Hope, A. J., Svitkina, T. M., and Borisy, G. G. (1998). Functional coordination of microtubule-based and actin-based motility in melanophores. Curr. Biol. 8, 165–168. [DOI] [PubMed] [Google Scholar]
- Rogers, S. L., and Gelfand, V. I. (1998). Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr. Biol. 8, 161–164. [DOI] [PubMed] [Google Scholar]
- Roos, U. P., De Brabander, M., and Nuydens, R. (1987). Movements of intracellular particles in undifferentiated amebae of Dictyostelium discoideum. Cell Motil. Cytoskelet. 7, 258–271. [Google Scholar]
- Rusan, N. M., Fagerstrom, C. J., Yvon, A. M., and Wadsworth, P. (2001). Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-tubulin. Mol. Biol. Cell 12, 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon, W. C., Adams, M. C., and Waterman-Storer, C. M. (2002). Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 158, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler, S. C., and Pellman, D. (2001). Microtubule “plus-end-tracking proteins”: the end is just the beginning. Cell 105, 421–424. [DOI] [PubMed] [Google Scholar]
- Shu, T., Ayala, R., Nguyen, M. D., Xie, Z., Gleeson, J. G., and Tsai, L. H. (2004). Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44, 263–277. [DOI] [PubMed] [Google Scholar]
- Smith, D. S., Niethammer, M., Ayala, R., Zhou, Y., Gambello, M. J., Wynshaw-Boris, A., and Tsai, L. H. (2000). Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2, 767–775. [DOI] [PubMed] [Google Scholar]
- Tran, P. T., Marsh, L., Doye, V., Inoué, S., and Chang, F. (2001). A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 153, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, K. L., Sokac, A. M., Berg, J. S., Cheney, R. E., and Bement, W. M. (2004). A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature 431, 325–329. [DOI] [PubMed] [Google Scholar]
- Whitehead, C. M., Winkfein, R. J., and Rattner, J. B. (1996). The relationship of HsEg5 and the actin cytoskeleton to centrosome separation. Cell Motil. Cytoskelet. 35, 298–308. [DOI] [PubMed] [Google Scholar]
- Yumura, S., Mori, H., and Fukui, Y. (1984). Localization of actin and myosin for the study of amoeboid movement in Dictyostelium using improved immunofluorescence. J. Cell Biol. 99, 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.