Abstract
Anthrax is an acute infectious disease caused by Bacillus anthracis, which can infect various animals and humans. Cutaneous anthrax primarily presents as infiltrative, edematous erythema, surface vesicles, hemorrhagic vesicles, and necrotic eschar; some patients may also experience systemic symptoms such as fever and leukocytosis. With economic development and improvements in public health conditions, naturally occurring cases of cutaneous anthrax have significantly decreased, leading to limited reports on the pathological manifestations of this disease. This article reports a case of a patient with cutaneous anthrax diagnosed and treated in our hospital, aiming to explore the laboratory examinations, imaging, pathological features, and clinical treatment methods of the disease. The goal is to enhance understanding of anthrax and increase vigilance in clinical practice to avoid misdiagnosis and missed diagnosis. The described diagnostic and therapeutic processes are accurate and reliable, with no modifications or exaggerations. It is important to note that the patient’s treatment may have been influenced by local social, economic, health, and epidemiological conditions, which introduces certain limitations. We hope that our colleagues will approach this study with an objective mindset for learning and reference.
Keywords: Cutaneous anthrax, Treatment, Pathological manifestations, Case report
Background
Anthrax is a zoonotic disease caused by Bacillus anthracis. Humans typically become infected through contact with infected animals (such as cattle, sheep, and other herbivores) or contaminated animal products (such as meat or hides). Although secondary infections due to injection drug use are relatively rare, the general population is considered susceptible to the disease. Anthrax is predominantly endemic in agricultural regions of the Americas, sub-Saharan Africa, Central Asia, Southwestern Asia, and Southern and Eastern Europe [1]. In China, anthrax predominantly occurs in the central and northern regions, including Ningxia, Gansu, Inner Mongolia, and Sichuan. Young and middle-aged men engaged in animal husbandry face a higher risk of infection, with all reported outbreaks linked to contact with infected livestock [2].
The causative agent of anthrax is Bacillus anthracis, which belongs to the genus Bacillus. This bacterium is characterized as an aerobic or facultatively anaerobic, Gram-positive, rod-shaped organism. It grows readily on agar media or blood agar at 37 °C [3]. Bacillus anthracis exists in two forms: the biologically active encapsulated form and the biologically inert spore form. Spores exhibit remarkable resistance to harsh environmental conditions and can remain dormant in the environment for decades. High temperatures, high pressure, and strong oxidizing agents (such as chlorine- and iodine-containing disinfectants, potassium permanganate, peracetic acid, and formaldehyde) can effectively kill spores, while alcohol, quaternary ammonium compounds, and carbolic acid demonstrate relatively weaker efficacy against them. Anthrax spores are infectious, whereas the encapsulated form is not commonly pathogenic within the host [4].
Anthrax can be classified into different clinical types, including cutaneous, gastrointestinal, and inhalational anthrax. Cutaneous anthrax is primarily observed in exposed areas such as the face, neck, forearms, hands, and feet. The pathogenesis involves the entry of Bacillus anthracis through damaged skin, leading to localized replication in the skin and mucosal tissues, followed by the release of anthrax toxins that cause tissue edema, necrosis, and hemorrhage, ultimately resulting in primary cutaneous anthrax. Cutaneous anthrax cases account for approximately 95% of all anthrax cases and typically present as a solitary skin lesion, although multiple foci can also occur; it mainly affects the skin exposed to the external environment (such as the face, neck, forearms, hands, and feet), with hand cases being the most prevalent. The incubation period for cutaneous anthrax is generally 1 to 5 days, with initial symptoms manifesting as a painless red papule, which can develop into a vesicular lesion within 48 to 72 h, gradually evolving into a painless hemorrhagic vesicle surrounded by edema. Upon rupture of the vesicle, an ulcerative wound may form, which then transforms into a brownish surface before ultimately developing a black necrotic eschar, often with significant surrounding edema and satellite lesions. Additionally, another characteristic of this condition is the initial absence of significant pain at the wound site; some patients may present with systemic symptoms such as fever, localized lymphadenopathy, and general fatigue [5]. Clinical manifestations, laboratory findings, and imaging results for cutaneous anthrax lack specificity, and reports on pathological features are scarce [6]. This article reports a case of cutaneous anthrax treated at our institution, aiming to explore its laboratory examinations, imaging, pathological manifestations, and clinical management methods, thereby enhancing awareness and vigilance regarding anthrax and preventing misdiagnosis and missed diagnosis.
Results
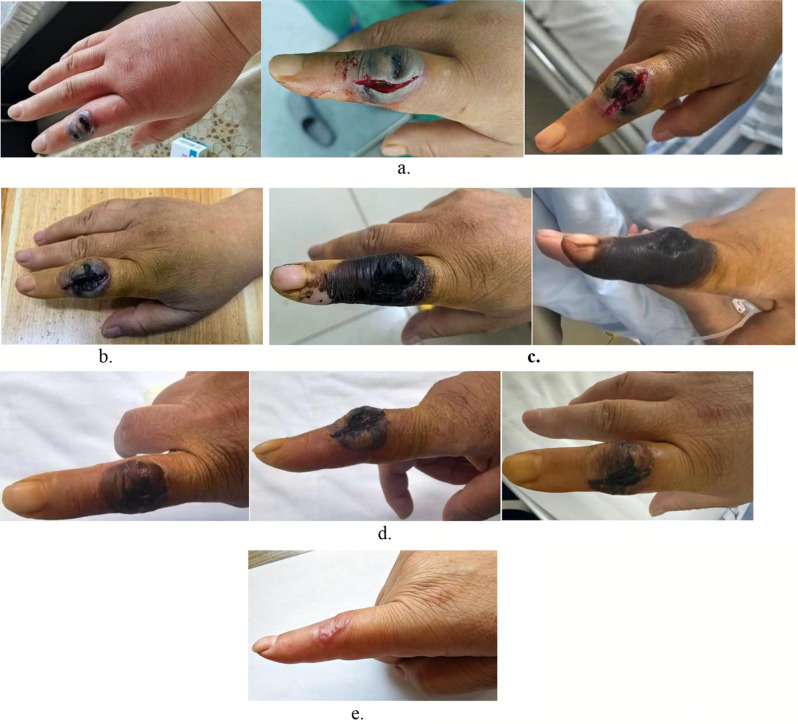
We report a case of a patient who contracted cutaneous anthrax through contact with livestock. The patient developed skin lesions on the right hand following an insect bite one week prior, and later came into contact with and consumed contaminated beef in an endemic area. This resulted in pain and swelling of the right index finger, with the surrounding skin gradually turning black (Fig. 1a). The affected area felt fluctuant upon palpation and was tender to touch. Additionally, the temperature of the affected skin was higher than that of the unaffected side, and there was limited range of motion in the index finger.
Fig. 1.
a. The patient’s right index finger exhibits redness and swelling, with local skin gradually turning black. b. The patient’s right index finger shows an enlarged area of charred tissue, with a dry wound and partial scabbing. c. The patient’s right index finger shows a dry wound with scabbing and charred tissue on the wound surface. d. The patient’s right index finger shows a well-dried wound with scabbing. e. The patient’s right index finger had completely scabbed over, with no signs of char; only minimal scarring remained
The patient presented to our hospital on August 6, 2024. Laboratory tests revealed a white blood cell count of 10.98 × 10^9/L, neutrophil count of 8.18 × 10^9/L, C-reactive protein level of 67.45 mg/L, and an erythrocyte sedimentation rate of 57 mm/h, all of which were significantly elevated. Chest CT showed multiple enlarged lymph nodes in the affected axilla with surrounding inflammation. During debridement of the right hand, inflammatory exudate was noted in the subcutaneous muscle, and local muscle necrosis was observed. For this patient, we sent specimens of the exudate smear, culture, and other relevant samples of the cutaneous anthrax case to the Ningxia Hui Autonomous Region Center for Disease Control and Prevention for microscopic examination, isolation identification, and nucleic acid testing. The results indicated that no gram-positive bacilli were detected, nor was anthrax spore bacillus isolated; however, the nucleic acid test for anthrax spore bacillus returned a positive result. Based on the aforementioned clinical evidence, the patient presented with an acute onset that aligns with the diagnostic criteria set forth in the “Diagnosis and Treatment Plan for Anthrax (2023 Edition.” A definitive diagnosis of cutaneous anthrax was established. Additionally, differential diagnoses, including cellulitis, boils, carbuncles, and scrub typhus, were considered and subsequently ruled out. Considering the open lesions of the patient, there is a heightened awareness of the potential for systemic dissemination. Therefore, meropenem 1 g intravenous infusion every 8 h and doxycycline 0.1 g intravenous infusion every 12 h were administered to intensify the antimicrobial treatment.
On the ward round on August 9, 2024, the patient reported relief from pain in the right index finger, decreased swelling, and an enlarged area of eschar, with the wound drying out and some scabbing present. (Fig. 1b). Follow-up CT showed a reduction in the size of the affected axillary lymph nodes, indicating a positive treatment response. Consequently, intravenous meropenem was discontinued, and the patient was switched to intravenous penicillin 3.2 million units every 8 h and oral doxycycline 0.1 g twice a day for ongoing anti-infective treatment. By the ward round on August 12, 2024, the wound on the right index finger was dry and scabbed (Fig. 1c), with the axillary lymph nodes no longer palpable. Laboratory tests indicated a white blood cell count of 7.73 × 10^9/L, neutrophil count of 4.67 × 10^9/L, C-reactive protein < 10 mg/L, and a normalized erythrocyte sedimentation rate. Despite multiple follow-up tests of the exudate swab from August 15 to August 18, 2024, still showing positive anthrax nucleic acid, anti-infective treatment continued. During the ward round on August 20, 2024, the right index finger wound was completely dry and scabbed (Fig. 1d), meeting the discharge criteria outlined in the “Anthrax Diagnosis and Treatment Protocol (2023 Edition),” and the patient was discharged. The patient will continue oral anti-infective medication (doxycycline 0.1 g, twice daily) after discharge and was advised to seek immediate medical attention if any discomfort arises. After the patient was discharged, we conducted a follow-up visit. The wound on the patient’s right index finger had completely scabbed over, with no signs of char; only minimal scarring remained (Fig. 1e).
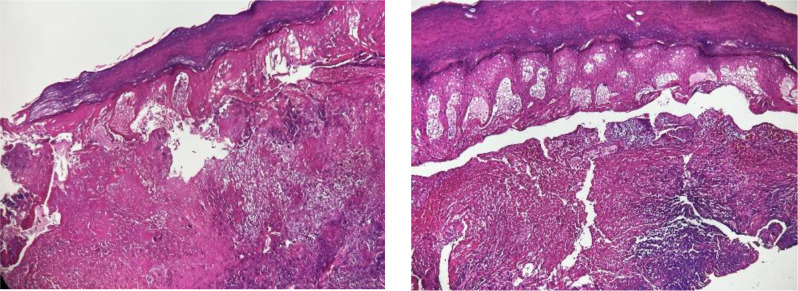
The skin pathology results showed hyperkeratosis of the epidermis, with pronounced edema, necrosis, collagen fiber proliferation, hemorrhage, and neutrophilic infiltration in the superficial dermis. Additionally, the adnexal structures within the dermis were absent, with residual nerve fibers observed. Localized granulation tissue and a multinucleated giant cell response were noted in the surrounding stroma (Fig. 2).
Fig. 2.
The skin pathology results showed hyperkeratosis of the epidermis, with pronounced edema, necrosis, collagen fiber proliferation, hemorrhage, and neutrophilic infiltration in the superficial dermis. Additionally, the adnexal structures within the dermis were absent, with residual nerve fibers observed. Localized granulation tissue and a multinucleated giant cell response were noted in the surrounding stroma (100 ×)
Discussion
The capsule and exotoxins of Bacillus anthracis are important virulence factors. The capsule helps the bacterium evade phagocytosis by the immune system, leading to rapid dissemination and proliferation within the host [7, 8]. The exotoxins of Bacillus anthracis are encoded by specific plasmids, namely pX01 (182 kb) and pX02 (95 kb), and include protective antigen (PA), edema factor (EF), and lethal factor (LF). The absence of these plasmids significantly reduces the virulence of the bacteria. PA serves to deliver EF and LF into host cells, activating essential cellular signaling pathways (such as the MAPK pathway and Caspase-8 pathway), which results in cytotoxic effects characterized by tissue edema, inflammation and necrosis, as well as damage to endothelial cells [9].
Laboratory examination results typically exhibit an elevated white blood cell count ranging from (10–20) × 10^9/L and can even reach (60–80) × 10^9/L, predominantly consisting of neutrophils. This may be accompanied by hypoalbuminemia and elevated liver function markers (such as ALT and AST). In cases progressing to severe toxemia, leukopenia, thrombocytopenia, and even coagulopathy (DIC) may occur. For suspected anthrax cases, pathogenic and serological tests should be performed on specimens from skin lesions, including vesicular fluid, blood, sputum, oral secretions, pleural effusion, bronchial biopsy specimens, ascitic fluid, vomitus, feces, and cerebrospinal fluid. Microscopic examination of bacterial smears may reveal Gram-positive large rods arranged in chains. Culture of the bacteria can yield Bacillus anthracis, and detection of anthrax-specific nucleic acids can be performed using PCR or real-time fluorescent PCR. Immunochromatographic methods can detect anthrax antigens, while enzyme-linked immunosorbent assay (ELISA) and immunochromatography can measure antibodies against anthrax toxin in the blood [10–12]. According to the “Anthrax Diagnosis and Treatment Protocol (2023 Edition),” patients should be classified as suspected cases, clinically diagnosed cases, or confirmed cases based on clinical presentation, laboratory investigations, and epidemiological history [2].
Suspected cases present with typical skin lesions and an epidemiological history, meeting any one of these criteria for diagnosis. Clinically diagnosed cases are characterized by the observation of Gram-positive rods with square ends under microscopy; positivity for anthrax antigens in specimens; detection of anthrax antibodies in blood samples; or diagnosis of anthrax in animals contacted by the patient. Additionally, clinical diagnosis can be made based on the presence of any four criteria on top of the suspected case. Confirmed cases are those that meet any of the following criteria based on suspected or clinically diagnosed cases: (1) isolation of Bacillus anthracis from bacterial culture; (2) positive detection of anthrax nucleic acid; (3) significant change in the titre of specific antibodies against anthrax toxin in serum, or an increase of at least fourfold in titre during the convalescent phase compared to the acute phase; (4) any two of the following: (a) discovery of large bacilli arranged in chains under microscopy; (b) positivity for anthrax antigens; © positivity for Bacillus anthracis antibodies; (d) isolation of Bacillus anthracis from specimens of exposed animals or environmental samples [2].
Patients with anthrax should be strictly isolated, and appropriate fluid replacement should be provided to maintain water and electrolyte balance. For localized cutaneous anthrax (excluding severe edema, head and neck wounds, or those caused by biological terrorism), oral antibiotic treatment with a single agent is recommended. First-line treatment regimens include fluoroquinolone antibiotics (such as ciprofloxacin, moxifloxacin, or levofloxacin) or doxycycline; alternative regimens may include clindamycin, amoxicillin, and penicillin V potassium (only for penicillin-sensitive strains). Dosages should be based on the recommended doses for systemic anthrax oral antibiotics, with a treatment course of 7 to 10 days [13–15].
Cutaneous anthrax is commonly seen in agricultural and pastoral communities, often leading to outbreaks. Therefore, enhancing education and awareness of anthrax is essential to guide farmers on proper handling of infected livestock. Moreover, hospitals should collaborate closely with veterinary departments to conduct surveillance and treatment for workers in farming and slaughterhouse areas to reduce the incidence of outbreaks.
Acknowledgements
The authors thank this patient for her cooperation in this study.
Author contributions
Yongjun Du, Jizhou Ma, Lan Yang, Li Jing, Fangjing Xu and Yucheng Fan were actively involved in the conception and design of the study, the acquisition of data, and the analysis and interpretation of the results. G.L., Z.C., H.H. and T.C. contributed to the drafting of the manuscript and participated in the critical revision of the manuscript, ensuring its intellectual rigor. All authors had full access to the study data, collaborated throughout the research process, approved the final version of the manuscript for publication, and assume responsibility for the accuracy and integrity of the work.
Funding
This study was supported by Ningxia Medical University Key Research Project (grant no. XZ2024040 to Y.F.), Ningxia Medical University General Research Project (grant no. XY2024138 to F.X.) and Ningxia Key R&D Project (Talent Introduction Special Project) (grant no. 2021BEB04035 to L.Y).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Consent for publication
The patient provided written informed consent for the publication of this case, including the publication of images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yongjun Du, Jizhou Ma, Guozhen Liu, Zhi Chai, Hang Han, Fangjing Xu and Yucheng Fan contributed equally to this work.
Contributor Information
Li Jing, Email: 1203220205@qq.com.
Fangjing Xu, Email: xufangjing1204@163.com.
Yucheng Fan, Email: fan1993919@hotmail.com.
References
- 1.World Health Organization. Anthrax in humans and animals. 4th ed. Geneva: World Health Organization; 2008. [PubMed] [Google Scholar]
- 2.He D, Zhou LY. Interpretation of the Anthrax Diagnosis and treatment protocol (2023 Edition). China Antibiot. 2024;49:749–54. [Google Scholar]
- 3.Swartz MN. Recognition and management of anthrax—an update. N Engl J Med. 2001;345:1621–6. [DOI] [PubMed] [Google Scholar]
- 4.Lu HZ, Weng XH. Basic knowledge Q&A on anthrax. Chin J Infect Dis. 2001;19:57–8. [Google Scholar]
- 5.Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax N Engl J Med. 1999;341:815–26. [DOI] [PubMed] [Google Scholar]
- 6.Lin R. Z. advances in imaging of anthrax. Int J Med Radiol. 2002;25:328–9. [Google Scholar]
- 7.Wang YC, Tao HX, Yuan SL et al. Research progress on the infection mechanism of Bacillus anthracis. In Proceedings of the 6th Forum on Infectious Disease Prevention and Control Technology Research and Application 1–9. (2015).
- 8.Liu YC, Yang ZF, Gao F. Introduction to virulence factors and infection processes of Bacillus anthracis. Heilongjiang Anim Vet. 2017;6:85–7. [Google Scholar]
- 9.Doganay M, Dinc G, Kutmanova A, et al. Human anthrax: update of the diagnosis and treatment. Diagnostics. 2023;13:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander AM. Clinical aspects, diagnosis, and treatment of anthrax. J Appl Microbiol. 1999;87:303–6. [DOI] [PubMed] [Google Scholar]
- 11.Sirisanthana T, Navachareon N, Tharavichitkul P, Sirisanthana V, Brown AE. Outbreak of oral-oropharyngeal anthrax: an unusual manifestation of human infection with Bacillus anthracis. Am J Trop Med Hyg. 1984;33:144–50. [DOI] [PubMed] [Google Scholar]
- 12.Yakupogullari Y, Kabakus N, Durukan M, Kizirgil A, Bulut Y, Yilmaz M. Anthrax meningoencephalitis secondary to oral infection. Pediatr Infect Dis J. 2006;25:572–3. [DOI] [PubMed] [Google Scholar]
- 13.Hendricks KA, Wright ME, Shadomy AV, et al. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis. 2014;20:e130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower WA, Yu Y, Person MK, et al. CDC guidelines for the Prevention and Treatment of Anthrax, 2023. MMWR Recomm Rep. 2023;72:1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern SJ, Uhde KB, Shadomy SV et al. Conference report on public health and clinical guidelines for anthrax. Emerg. Infect. Dis. 14, e1 (2008). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.