Abstract
Background
MUC1 and UMOD pathogenic variants cause autosomal dominant tubulointerstitial kidney disease (ADTKD). MUC1 is expressed in kidney, nasal mucosa and respiratory tract, while UMOD is expressed only in kidney. Due to haplo-insufficiency ADTKD-MUC1 patients produce approximately 50% of normal mucin-1.
Methods
To determine whether decreased mucin-1 production was associated with an increased COVID-19 risk, we sent a survey to members of an ADTKD registry in September 2021, after the initial, severe wave of COVID-19. We linked results to previously obtained ADTKD genotype and plasma CA15-3 (mucin-1) levels and created a longitudinal registry of COVID-19 related deaths.
Results
Surveys were emailed to 637 individuals, with responses from 89 ADTKD-MUC1 and 132 ADTKD-UMOD individuals. 19/83 (23%) ADTKD-MUC1 survey respondents reported a prior COVID-19 infection vs. 14/125 (11%) ADTKD-UMOD respondents (odds ratio (OR) 2.35 (95%CI 1.60–3.11, P = 0.0260). Including additional familial cases reported from survey respondents, 10/41 (24%) ADTKD-MUC1 individuals died of COVID-19 vs. 1/30 (3%) with ADTKD-UMOD, with OR 9.21 (95%CI 1.22–69.32), P = 0.03. The mean plasma mucin-1 level prior to infection in 14 infected and 27 uninfected ADTKD-MUC1 individuals was 7.06 ± 4.12 vs. 10.21 ± 4.02 U/mL (P = 0.035). Over three years duration, our longitudinal registry identified 19 COVID-19 deaths in 360 ADTKD-MUC1 individuals (5%) vs. 3 deaths in 478 ADTKD-UMOD individuals (0.6%) (P = 0.0007). Multivariate logistic regression revealed the following odds ratios (95% confidence interval) for COVID-19 deaths: ADTKD-MUC1 8.4 (2.9–29.5), kidney transplant 5.5 (1.6–9.1), body mass index (kg/m2) 1.1 (1.0-1.2), age (y) 1.04 (1.0-1.1).
Conclusions
Individuals with ADTKD-MUC1 are at an eight-fold increased risk of COVID-19 mortality vs. ADTKD-UMOD individuals. Haplo-insufficient production of mucin-1 may be responsible.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03896-1.
Keywords: Autosomal Dominant Tubulointerstitial kidney disease, MUC1, UMOD, COVID-19, Mucin-1, CA15-3
Background
Mucin-1 is a membrane-anchored glycoprotein that provides physical protection to the epithelial surface of many tissues [1] as well as performing other functions, including preventing infection [2], mediating inflammation [3], and modulating apoptosis [4]. In autosomal dominant tubulointerstitial kidney disease (ADTKD) due to heterozygous frameshift variants in the MUC1 gene (ADTKD-MUC1) (OMIM 174000) [5], the wild-type allele synthesizes normal mucin-1, and the allele with the pathogenic MUC1 variant produces a frameshift mucin-1 protein that deposits in the endoplasmic reticulum Golgi intermediate compartment (ERGIC) [6]. While abnormal protein deposition occurs within all epithelial cells expressing mucin-17, clinical sequelae of the disease were thought to be limited to the kidney, with no respiratory, gastrointestinal, or skin manifestations previously identified [8, 9]. Affected individuals develop slowly progressive chronic kidney disease (CKD) that leads to kidney failure at a mean age of 45 years [8]. ADTKD-UMOD (OMIM 162000, 603860) is phenotypically similar and often clinically indistinguishable from ADTKD-MUC1 (OMIM 17400) [10]. Unlike mucin-1, uromodulin is expressed exclusively in the thick ascending limb of Henle [11]. The median age of kidney failure in ADTKD-UMOD is 49 years [12]. Both groups of individuals have excellent outcomes with kidney transplantation [13], as the diseases do not recur in the transplanted kidneys and individuals usually have few other comorbid conditions.
Mucin-1 has been implicated as a protective factor in COVID-19, though this relationship has not been firmly established in human studies. Mucin-1 is produced in both the nares and the lungs, primary sites of COVID-19 infection [14, 15]. Mucin-1 in breast milk has been shown to inhibit SARS-CoV-2 infection [16]. In in vitro studies, mucin-1 was highly expressed on the surface of ACE2-positive respiratory epithelial cells, and the glycosylated extracellular domain of mucin-1 restricted SARS-CoV-2 binding and entry [17].
Patients with ADTKD-MUC1 may be at increased risk of COVID-19 due to haploinsufficiency of mucin-1. In ADTKD-MUC1, there is one wild-type allele that produces mucin-1 and one mutated allele that produces a truncated version of mucin-1 that deposits intracellularly and does not function as a normal mucin-1 protein. In a recent investigation, the mean plasma CA15-3 level, which measures mucin-1 levels with an immunoassay using the DF3 antibody [18], was 8.6 ± 4.3 U/mL in individuals with ADTKD-MUC1 vs. 14.6 ± 5.6 U/mL in controls (P < 0.001).19 The mucin-1 content of plasma is derived primarily from the lungs [20]. Thus, ADTKD-MUC1 patients produce less mucin-1, which may protect against COVID-19.
To determine if individuals with ADTKD-MUC1 were at increased risk of COVID-19, we decided to compare the rates of COVID-19 infections between patients with ADTKD-MUC1 and patients with ADTKD-UMOD in the Wake Forest Rare Inherited Kidney Disease registry. The registry attempts to recruit all individuals with ADTKD-UMOD and ADTKD-MUC1 and includes 360 adults from 119 families affected with ADTKD-MUC1 and 478 individuals from 171 families affected with ADTKD-UMOD. The registry has grown over the last two decades, with approximately 25% of individuals making contact with the Wake Forest Rare Inherited Kidney Disease Team independently without physician referral [21]. The registry keeps in contact with family members through webinars, in-person meetings, email, and a private Facebook© support page. Thus, there is a large registry containing individuals with ADTKD-MUC1 and ADTKD-UMOD, without any perceived bias in interactions based on ADTKD type. The two diseases have a bland urinary sediment, slowly progressive chronic kidney disease leading to kidney failure at a mean age of approximately 45 years, and the absence of non-renal clinical manifestations.
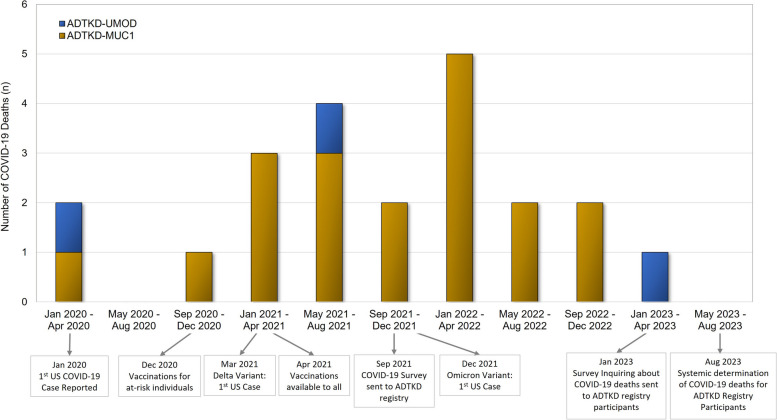
To determine if individuals with ADTKD-MUC1 were at increased risk of COVID-19, we first conducted a systematic survey of individuals with these two conditions in September 2021 (see Fig. 1), with a specific goal of avoiding any ascertainment bias between the ADTKD-UMOD and ADTKD-MUC1 groups. At that time, most individuals had had the opportunity to obtain COVID-19 vaccination, and the worst wave of COVID-19 deaths in the US had occurred. We informed patients in our registry of our interest in COVID-19 deaths in ADTKD-UMOD and ADKTD-MUC1 patients and began tracking these occurrences.
Fig. 1.
Study timeline and COVID-19 related deaths in ADTKD patients
Materials and methods
This study was approved by the Wake Forest University Health Sciences Institutional Review Board (IRB00000352, Sub-study: COVID-19 Effects in Inherited Kidney Disease, approved September 8, 2021). We developed a survey and distributed it electronically to all individuals with ADTKD within our registry (see Fig. 1 and Supplementary Material). Survey data were collected between September 24, 2021 and November 1, 2021 and managed using REDCap electronic data capture tools hosted at Wake Forest School of Medicine. REDCap is a secure, web-based, National Institutes of Health (NIH)–sponsored application [22] that supports confidential data capture for research studies. Information collected included: COVID-19 vaccination type and number of vaccines administered; COVID-19 infection characteristics including the date of first clinical symptoms, need for hospitalization, need for intensive care unit admission, and death or recovery from infection. Survey respondents provided information about their personal COVID-19 infections as well as those of family members, as family members may have died or become too sick to respond to our survey. For reported COVID-19 deaths, we spoke personally with first-degree family members to ascertain that the death was related to COVID-19. We only included individuals from our registry who had died of COVID-19 and did not include other family members who may have died of COVID-19 but were not part of our registry. Data from the survey respondents were linked to other data in our registry that had been obtained prior to COVID-19 infections. This data included the ADTKD mutation (UMOD vs. MUC1) and plasma CA15-3 (mucin-1) levels. CA15-3 levels were measured as previously reported [19]. We collected available information from our prior surveys about the presence of cancer and smoking. As we had not asked on prior surveys about chronic obstructive pulmonary disease, diabetes, or hyperlipidemia, we reviewed the medication lists to see if study participants were taking medications indicated for these conditions.
We continued to ascertain COVID-19 deaths in our survey. In January 2023, an additional survey was sent to individuals with ADTKD that requested information about COVID-19 deaths. In August 2023, we reviewed all family trees of ADTKD-UMOD and ADTKD-MUC1 families to identify new deaths by searching each name on-line for potential obituaries and then contacting family members about causes of death. We then performed a case-control study with cases including adults affected with ADTKD-UMOD and ADTKD-MUC1 who died of COVID-19 and controls including adults affected with ADTKD-UMOD and ADTKD-MUC1 who did not die from COVID-19.
Statistical analysis
Survey questions were summarized with the addition of descriptive statistics and additional descriptive data were added from registry data when available. Associations were assessed using a generalized estimating equation to account for the family structure. We assumed exchangeable correlation within family and computed a robust sandwich estimator of variance [23]. A compound-symmetry covariance structure was used to account for the correlation between family members for continuous outcomes.
Genotyping
Individuals were genotyped for UMOD pathogenic variants using standard genetic techniques [12]. For MUC1 sequencing, a CLIA-approved mass spectrometry-based assay was performed the Broad Institute of MIT and Harvard, Cambridge, MA [24] or Illumina and/or PacBio® sequencing of MUC1 PCR amplicon was performed by the Kmoch laboratory, First Faculty of Medicine, Prague, Czech Republic [7].
Results
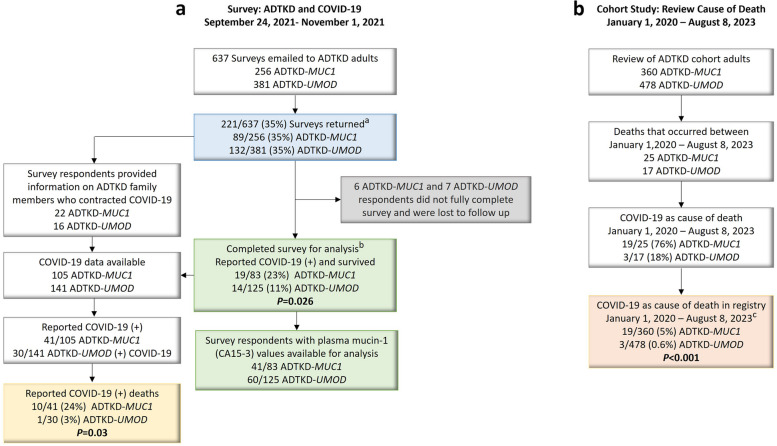
The initial survey was emailed to 637 individuals (see Fig. 2a), including 256 patients with ADTKD-MUC1 and 381 patients with ADTKD-UMOD. There were 221 respondents (35% response rate). The response rate for individuals with ADTKD-MUC1 was 89/256(35%), and the response rate for individuals with ADTKD-UMOD was 132/381(35%). Compared to non-respondents, respondents were more likely to be White (97% vs. 90%, P = 0.02) and older (50.2 ± 15.2 years vs. 47.0 ± 16.0 years, P = 0.02) (see Supplementary Table S1). Of the respondents, six individuals with ADTKD-MUC1 and seven with ADTKD-UMOD did not complete the survey about personal COVID-19 infection and were removed. Clinical characteristics were similar between the remaining 83 respondents with ADTKD-MUC1 and 125 individuals with ADTKD-UMOD (see Supplementary Table S2). The respondents provided information on an additional 16 family members with ADTKD-MUC1, resulting in 105 individuals with ADTKD-MUC1 and an additional nine family members with ADTKD-UMOD, resulting in 141 individuals (see Fig. 2a).
Fig. 2.
Flow diagram for the study. A Initial ADTKD Registry COVID-19 Survey. aSurvey respondents and non-respondents described in Supplementary Table S1.bSurvey respondent results are described in Table 1 and Supplemetary Table S2. B Cohort study reviewing the ADTKD registry for COVID-19 related deaths. cThis includes the 11 COVID-19 deaths reported in the 2021 survey and used for analysis described in Table 2, Table 3, and Supplementary Table S3
COVID-19 outcomes during the early phase of the pandemic
We compared COVID-19 outcomes in survey respondents with ADTKD-MUC1 vs. ADTKD-UMOD in Sep 2021, after the Delta variant had peaked in the US (see Fig. 2a). Of 83 ADTKD-MUC1 individuals, 19 (22%) developed COVID-19 infection vs. 14/125 (11%) of ADTKD-UMOD individuals (odds ratio (OR) 2.35 (95%CI 1.60–3.11, P = 0.026). Table 1 shows a comparison of the individuals who developed COVID-19 by disease type. There was no statistical difference between individuals with regards to age, gender, or body mass index, though numbers were small in each group. At the time of COVID-19 infection, 16% of ADTKD-MUC1 and 21% of ADTKD-UMOD individuals were unvaccinated. ADTKD-MUC1 individuals who did not develop COVID-19 were more likely to be vaccinated, but this is likely related to bias, as ADTKD-MUC1 individuals who developed COVID-19 may have developed it several months prior to the survey and often did so before the vaccine was available, while individuals who did not develop COVID-19 could have received the vaccine up until the time of the survey. As stated above, in a univariate model the odds ratio for developing COVID-19 infection was 2.35 for ADTKD-MUC1 vs. ADTKD-UMOD. Kidney transplantation status, body mass index, age, and gender were not significant in univariate models or when added to ADTKD type in a multivariate model.
Table 1.
Characteristics of individuals developing COVID-19 by disease type
| ADTKD-MUC1 | ADTKD-UMOD | |||||
|---|---|---|---|---|---|---|
| COVID-19 infection | Yes | No | P -value | Yes | No | P-value |
| Individuals (n(%))a | 19 (22%) | 64 (88%) | -- | 14 (11%) | 111 (89%) | -- |
| Gender (n male (%)) | 11(58%) | 24 (38%) | 0.079 | 2 (14%) | 44 (40%) | 0.063 |
| Age, years (mean ± sd (median) | 49.6 ± 19.9 (50.6) | 51.6 ± 16.8 (55.6) | 0.63 | 47.0 ± 13.3 (49.5) | 51.6 ± 14.0 (52.8) | 0.14 |
| Age > 60 (n (%)) | 6 (32%) | 22 (34%) | 0.99 | 1 (7%) | 37 (33%) | 0.029 |
| Body mass index, kg/m2 (mean ± sd (median)) | 25.5 ± 5.6 (24) | 25.1 ± 5.0 (24.5) | 0.76 | 24.9 ± 4.5 (24.5) | 25.9 ± 5.1 (25) | 0.47 |
| Active Cancer Diagnosis b | 0 | 0 | -- | 0 | 1 (1%)b | 0.72 d |
| Diabetesc | 0 | 0 | -- | 0 | 1 (1%)c | 0.72 d |
| Chronic obstructive pulmonary disease/asthma | 0 | 1 (2%) | 0.58 d | 0 | 1 (1%) | 0.72 d |
| Hyperlipdemia at risk for cardiovascular disease | 0 | 4 (6%) | 0.26 d | 2 (14%) | 11 (10%) | 0.61 |
| Current Smoker | 0 | 1 (2%) | 0.26 d | 0 | 8 (7%) | 0.30 d |
| eGFRe (mean ± sd (median)) for pre-kidney failure, non-kidney transplanted | 49.7 ± 32.5 (42.6) | 49.4 ± 37.5 (37.6) | 0.98 | 43.9 ± 22.4 (37.5) | 46.2 ± 29.8 (41.7) | 0.77 |
| eGFR > 60 ml/min/1.73m2 (n, column percent) | 2 (11%) | 8 (13%) | 0.52 | 3 (21%) | 15 (14%) | 0.33 |
| eGFR 30 to ≤ 60 ml/min/1.73m2 (n, column percent) | 5 (28%) | 8 (13%) | 4 (29%) | 29 (26%) | ||
| eGFR < 30 ml/min/1.73m2 (n, column percent) and not kidney failure | 2 (11%) | 11 (17%) | 3 (21%) | 21 (19%) | ||
| Transplanted | 9 (50%) | 36 (56%) | 4 (29%) | 44 (40%) | ||
| Receiving dialysis | 0 | 1 (2%) | 0 | 1(1%) | ||
| Duration kidney failure to COVID or time of survey | 14.2 ± 14.5 (11.5) | 17.1 ± 13.0 (17) | 0.57 | 4.4 ± 4.9 (2.6) | 10.8 ± 8.3 (10) | 0.014 |
| Pre-kidney failure | 10 (53%) | 27 (42%) | 0.30 | 10 (71%) | 66 (60%) | 0.44 |
| Duration kidney failure < 10 years | 4 (21%) | 14 (22%) | 3 (21%) | 21 (19%) | ||
| Duration kidney failure 10 to < 15 years | 2 (11%) | 2 (3%) | 1 (7%) | 11 (10%) | ||
| Duration kidney failure 15 to < 20 years | 0 | 5 (8%) | 0 | 6 (5%) | ||
| Duration kidney failure > 20 years | 3 (16%) | 16 (25%) | 0 | 7 (11%) | ||
| No vaccination (n (percent of those reporting vaccination status)) | 3 (16%) | 1 (2%) | 0.040d | 3 (21%) | 12 (11%) | 0.24e |
| Partial vaccination (n (percent of those reporting vaccination status)) | 1 (5%) | 2 (3%) | 1 (7%) | 0 | ||
| Full vaccination (n (percent of those reporting vaccination status)) | 15 (79%) | 59 (95%) | 10 (71%) | 95 (89%) | ||
| Full vaccination and 3rd dose (n (percent of those with full vaccination)) | 5 (33%) | 26 (44%) | 0.49 | 2 (20%) | 40 (42%) | 0.18 |
aPercentages in the first row represent row percent. For other rows in the table, column percentages are given
bNon-Hodgkins Lymphoma
CType 1 Diabetes Mellitus
dP-values calculated using permutation test
eEstimated glomerular filtration rate
At this stage of the analysis, we started to include data that respondents had provided regarding affected family members with COVID-19 (see Fig. 2a), including 16 individuals with ADTKD-MUC1 and nine individuals with ADTKD-UMOD, all of whom were previously in our registry. Of the total number of cases of COVID-19 identified, 10/41 (24%) of ADTKD-MUC1 COVID-19 individuals died vs. 1/30 (3%) ADTKD UMOD COVID-19 individuals. Using a generalized estimating equation to account for the family structure, we found an odds ratio of mortality in ADTKD-MUC1 vs. ADTKD-UMOD of 9.21, 95% CI 1.22–69.32, P = 0.03.
Thus, in the early and more virulent phase of the COVID-19 epidemic, patients with ADTKD-MUC1 were more likely to develop COVID-19 and were much more likely to die from COVID-19 compared to patients with ADTKD-UMOD.
International assessment of mortality in COVID-19 according to ADTKD type
We then queried collaborators (CS, JAS, PJC, LR) from three other academic centers in November 2021 and found 3/24 (13%) deaths in individuals with ADTKD-MUC1 who developed COVID-19 vs. 0 deaths in 11 individuals with ADTKD-UMOD who developed COVID-19. Six of the 24 individuals with ADTKD-MUC1 were kidney transplanted vs. 4/11 in the ADTKD-UMOD group. The mean age of the ADTKD-MUC1 group was 50.8 ± 14.4 years vs. 53.7 ± 12.3 years in the ADTKD-UMOD group.
Plasma mucin-1 levels in ADTKD- MUC1 in infected vs. uninfected ADTKD- MUC1 patients
To evaluate stability of plasma CA 15 − 3 levels over time, we used a paired t-test to compare baseline plasma CA15-3 levels with levels measured at least a year later in 11 patients. Initial levels were similar to follow-up levels (13.24 ± 5.63 U/ml vs. 11.01 ± 7.44 U/ml (p = 0.18)).
To determine whether the increased infection rate was associated with decreased mucin-1 production, we compared plasma CA15-3 (mucin-1) levels obtained as part of a prior study [19] on many of the survey individuals. Plasma CA 15 − 3 concentrations were measured on average 18.6 ± 17.7 months (range 8 to 52 months) prior to COVID-19. The mean CA15-3 level for 14 COVD-19 infected ADTKD-MUC1 individuals was 7.06 ± 4.12 U/mL vs. 10.21 ± 4.02 U/mL for 27 uninfected ADTKD-MUC1 individuals (P = 0.035). For 11 COVID-19 infected ADTKD-UMOD individuals, the mean CA15-3 level was 14.18 ± 2.35 U/mL vs. 13.28 ± 5.53 U/mL for 49 uninfected individuals (P = 0.60).
Observational cohort study of COVID-19 deaths in ADTKD-MUC1
We then continued an observational cohort study of COVID-19 deaths (see Figs. 1 and 2b). Table 4 shows the characteristics of adults in our registry according to ADTKD type. 19/360 (5%) individuals with ADTKD-MUC1 died from COVID-19 vs. 3/478 (0.6%) individuals with ADTKD-UMOD (P = 0.0007). The 19 deaths in ADTKD-MUC1 individuals occurred in 16 families. The three deaths in ADTKD-UMOD individuals occurred in three families. Table 2 shows the characteristics of patients who died of COVID-19 according to ADTKD type. Univariate and multivariate logistic models were then created with death from COVID-19 as the binary outcome (see Table 3). Patients with ADTKD-MUC1 had an eight-fold increased risk of death (8.4 (29–29.5%, P = 0.009)) vs. ADTKD-UMOD after adjustment for body mass index, kidney transplant status, and age. Of the patients with ADTKD-MUC1 who died (see Supplemental Table S3), 84% had undergone kidney transplant vs. 53% in ADTKD-MUC1 patients who had not died (P = 0.008). 43% of the ADTKD-MUC1 patients who died had reached kidney failure > 10 years prior to death vs. 26% of ADTKD-MUC1 patients in our registry who did not die of ADTKD-MUC1 (P = 0.001). Table S3 shows a comparison of characteristics for patients with ADTKD-MUC1 who died vs. those who did not.
Table 4.
Characteristics ADTKD-MUC1 and ADTKD-UMOD adults in registry
| Characteristic | ADTKD-MUC1 | ADTKD-UMOD |
P -value
Odd Ratio (OR) given with 95% Confidence Interval (CI) where applicable |
P-value grouping for Kidney Failure Modality
(No Kidney Failure, Dialysis, Kidney Transplant) To consider a change to the table |
|---|---|---|---|---|
| n | 360 | 478 | ||
| Gender (% male) | 41% | 45% | 0.19 | |
| Age, y (mean ± s.d. (median)) | 47.40 ± 15.8 (47.74) | 47.38 ± 14.89 (48.07) | 0.97 | |
| Age > 60 (n,%) | 86 (24%) | 118 (25%) | 0.68 | |
| Race (% White) | 86% | 95% | ||
| Body mass index, kg/m2 (mean ± sd (median)) | 26.22 ± 5.58 (25.5) | 26.86 ± 5.75 (25.8) | 0.47 | |
| Body mass index, greater than 25 kg/m2 (n,%) | 161 (55%) | 211 (56%) | 0.89 | |
| On dialysis | 34 (9%) | 26 (5%) | 0.0027 | 0.0011 |
| Living with kidney transplant | 166 (46%) | 164 (34%) | 0.0037 | |
| Not on dialysis or kidney transplanted (n, column percent) | 159 (44%) | 287 (60%) | < 0.0001 | |
| Duration kidney failurea < 10 years | 104 (29%) | 110 (38%) | ||
| Duration kidney failure 10 to ≤ 15 years | 25 (7%) | 33 (11%) | ||
| Duration kidney failure 15 to ≤ 20 years | 31 (9%) | 18 (6%) | ||
| Duration kidney failure > 20 years | 41 (11%) | 25 (9%) |
aDuration of kidney failure until the start of 2020
Table 2.
Characteristics of individuals who died of COVID-19 related infection
| Characteristic | ADTKD-MUC1 | ADTKD-UMOD | P-value |
|---|---|---|---|
| Individuals (n) | 19 | 3 | |
| Gender (n male (%)) | 11 (58%) | 2 (67%) | 0.89 |
| Age, years (mean ± sd (median)) | 56.49 ± 8.45 (57.62) | 71.44 ± 7.25 (74.80) | 0.0002 |
| Age > 60 years (n (%)) | 7 (37%) | 3 (100%) | < 0.0001b |
| Body mass index, kg/m2 (mean ± sd (median)) | 30.78 ± 6.46 (30.74) | 25.56 ± 2.02 (25.11) | 0.02 |
| Kidney transplanted (n (%)) | 16 (84%) | 3 (100%) | 0.46b |
| Pre-kidney failure (n (%)) | 2 (11%) | 0 | 0.01b |
| No vaccination (n (percent of those reporting vaccination status) | 11/16 (69%) | 1 (33%) | 0.23 |
| Heart failure | 0/18 | 0/3 | --- |
| Chronic obstructive pulmonary disease or asthma | 1/18 | 0/3 | 0.14b |
| Diabetes mellitus | 4/18 | 0/3 | 0.14b |
| Active malignancy | 0/18 | 0/3 | --- |
| Past malignancyc | 3/18 | 1/3 | 0.15b |
| Average length of hospital stay (mean ± sd (median)) | 18.67 ± 10.80 (21) | 12 ± 5 (12) | 0.15 |
| Required mechanical ventilation | 18/18 (100%) | 2 (67%) | 0.01b |
| Years of kidney failurea (mean ± sd (median)) | 12.86 ± 10.84 (9) | 21.93 ± 14.96 (13.76) | 0.45 |
| > 10 years kidney failurea | 9/17 (53%) | 3 (100%) | 0.003b |
aYears of kidney failure are years from start of kidney failure until contracting COVID-19
bP-values calculated using permutation test
CPast malignancies included Burkitts lymphoma, renal low grade papillary carcinoma, and cervical cancer in ADTKD-MUC1 and breast cancer in ADTKD-UMOD
Table 3.
Univariate and multivariate logistic regression models with death from COVID-19 as the outcome variable for 291 MUC1 patients (18 deaths) and 379 UMOD patients (3 deaths)
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameter | Reference | Risk group | Odds Ratio | P-value | Odds Ratio | P-value |
| ADTKD | UMOD | MUC1 | 8.26 (2.42–28.14) | 0.0007 | 8.68 (2.46–30.58) | 0.0008 |
| Body mass index (kg/m2) | Increase by 1 kg/m2 | 1.09 (1.03–1.15) | 0.003 | 1.11 (1.04–1.18) | 0.002 | |
|
Kidney Transplant Status |
No | Yes | 9.72 (2.79–33.86) | 0.0004 | 5.49 (1.58–19.05) | 0.009 |
| Age (years) | Increase by 1 year | 1.06 (1.03–1.09) | 0.0002 | 1.04 (1.01–1.07) | 0.002 | |
Discussion
This investigation found that patients with ADTKD-MUC1 are at a markedly increased risk of death from COVID-19, with 5% of adult ADTKD-MUC1 patients dying of COVID-19 during the three years of the pandemic vs. 0.6% of ADTKD-UMOD adults (P = 0.0007). Most, but not all, of the deaths occurred in patients who had undergone kidney transplantation, though the death rate was not similarly high in transplanted patients with ADTKD-UMOD. Patients died of respiratory complications, and many had prolonged hospitalizations. While most of the deaths occurred early in the pandemic, deaths have continued to occur. Patients with ADTKD-MUC1 who died of COVID-19 had a low prevalence of comorbidities (Table 2) except for kidney transplantation (84%).
While patient numbers were small due to the rarity of ADTKD, we showed consistent results with several different analyses: (1) We identified a 2.35 (95% confidence interval 1.60–3.11) increased odds of COVID-19 infection in ADTKD-MUC1 individuals early in the pandemic, with 23% of ADTKD-MUC1 individuals developing COVID-19 vs. 11% of ADTKD-UMOD individuals (P = 0.026). (2) There was an early increased risk of death, with 24% of individuals with ADTKD-MUC1 who developed a clinical COVID-19 infection dying from COVID-19 vs. 3% in individuals with ADTKD-UMOD (P = 0.03). (3) We found that the mean steady state CA15-3 level at least 30 days prior to COVID-19 infection [19] for 14 COVD-19 infected ADTKD-MUC1 individuals was 7.06 ± 4.12 U/mL vs. 10.21 ± 4.02 U/mL for 27 uninfected individuals (P = 0.035). (4) Our longitudinal study then revealed an 8-fold risk of death from COVID-19 in patients with ADTKD-MUC1 vs. ADTKD-UMOD (P = 0.0009). Thus, compared to ADTKD-UMOD individuals, ADTKD-MUC1 individuals had a higher COVID-19 infection rate and were more likely to die from COVID-19. ADTKD-MUC1 individuals were more likely to contract COVID-19 if their plasma CA15-3 levels were low.
In the early stages of COVID-19, eight (36%) of twenty-two ADTKD-MUC1 kidney transplanted patients with COVID-19 infection died, compared to one (25%) of four ADTKD-UMOD kidney transplanted patients. In a meta-analysis of COVID-19 outcomes in patients with kidney transplants during the Delta strain of SARS-CoV-225, the mortality rate was approximately 21%. While it is difficult to compare patient characteristics between studies, ADTKD-MUC1 patients are in general quite healthy with few comorbidities except kidney disease (see Table 2). In the meta-analysis of COVID-19 in transplant patients [25], there was an approximate 10% prevalence of diabetes and 8% prevalence of cardiovascular disease.
There were several weaknesses in our study. Most importantly, the sample size was small. Fortunately, we have the largest registry of ADTKD, with over 280 families and 800 individuals, and the response rate to our survey was good. However, by definition, any study of rare diseases will be associated with a small patient population. Another potential weakness was ascertainment and selection bias. We attempted to eliminate as many sources of bias as possible. Our registry has been historically designed to study all individuals with ADTKD-UMOD and ADTKD-MUC1 in a similar manner. The surveys were sent out in an un-biased fashion, and the characteristics of respondents was similar for ADTKD-UMOD and ADKTD-MUC1. However, there could be unanticipated forms of bias that we did not identify. For ADTKD-MUC1 patients who developed COVID-19 early in the pandemic, we calculated a 24% mortality rate. This death rate from COVID-19 may have been lower due to mild cases of COVID-19 that were undetected. While we could adjust for transplant status, we could not adjust for eGFR because we did not collect these values in the transplant patients in our registry. We also did not have more specific information about the COVID-19 hospitalizations leading to death. Another weakness of our study is that we did not examine the prevalence of other respiratory infections according to disease type. We plan to do this in the future.
The results of our investigation provide unique clinical information regarding the role of mucin-1 in COVID-19 infection. Our investigation shows that ADTKD-MUC1 individuals are at increased risk from COVID-19, based on lower mucin-1 levels prior to infection. There is emerging information that mucin-1 in the nares and lungs serves as an initial protective barrier against infection. Lai et al. showed that mucin-1 inhibited SARS-CoV-2 viral attachment, entry, and post-entry replication [16]. Biering et al. performed genome-wide bidirectional CRISPR screens to define host-pathogen interactions required for facilitating or restricting SARS-CoV-2 in a human lung cell line and identified mucin-1 as an important host defense factor [14]. The investigators then showed an antiviral role for membrane-anchored mucins (including mucin-1) in vitro and in mouse models of SARS-CoV-2 infection. Membrane-bound mucins specifically inhibited spike-mediated viral entry into lung cells. Chatterjee et al. showed that mucin-1 is highly expressed on the ACE2-positive respiratory epithelial cells, and that removal of the mucin domains enhances spike binding [17]. Our investigation provides complementary evidence in humans. Patients with ADTKD-MUC1 have mutations that prevent expression of the mucin domain, and these patients are at markedly increased risk of infection and death from COVID-19.
While there is evidence that decreased production of mucin-1 is associated with an increased risk from COVID-19, there is also evidence that increased production of mucin-1 may be harmful. Mucin-1 participates in the cytokine storm that occurs with COVID-19 and leads to an inflammatory response that is highly damaging. In studies of individuals who do not have ADTKD-MUC1, elevated mucin-1 content of bronchoalveolar fluid [26], airway mucus [27], and blood [28–31] at the time of infection is associated with worse and more severe COVID-19 outcomes. A recent genome-wide association study [32] identified the rs41264915 intronic variant THBS3 (associated with increased mucin-1 expression) as being associated with critical COVID-19. Another GWAS study suggested that increased mucin-1 expression is associated with more severe COVID-19, while other GWAS studies have not found this association [33]. Thus, lowering mucin-1 production during infection has been postulated as a potential therapy for COVID-1934.
Compounds increasing mucin-1 production could be prophylactic for individuals with low mucin-1 levels, whereas compounds reducing mucin-1 production could be therapeutic during acute infection. The finding that both pathologically low and elevated clinical characteristics are associated with increased mortality is well known and has also been noted with body mass index [35], sleep time [36], and many laboratory parameters [37].
Given the increased risk associated with ADTKD-MUC1 individuals from COVID-19, it would be prudent for families with an unknown cause of ADTKD (chronic kidney disease, autosomal dominant transmission, bland urinary sediment) to be tested for a MUC1 mutation. As these mutations are not identified by standard Sanger sequencing [38], commercial laboratories in the US do not currently provide genetic testing for MUC1 mutations. However, clinical testing is currently available at no cost through the Broad Institute of Harvard and MIT (contact ableyer@wakehealth.edu for information). Mucin-1 plays an important role in other respiratory tract infections [14], and we need to extend our studies in patients with ADTKD-MUC1 to other pulmonary infections.
Conclusions
This investigation shows that individuals with ADTKD-MUC1 are at increased risk of COVID-19 infection and mortality, especially for immunosuppressed and kidney transplanted patients exposed to more virulent COVID-19 variants. ADTKD-MUC1 patients with low plasma mucin-1 levels may be especially at increased risk. We be lieve that clinicians should consider more aggressive preventive care and treatment for COVID-19 in patients with ADTKD-MUC1.
Supplementary Information
Acknowledgements
The authors thank all patients and families who participated in this study.
Clinical Trial Number
Not applicable.
Authors’ contributions
Conceptualization: AJB; Data curation: KOK, AT, CS, GP, HRM, REH; Formal analysis: AJB, KOK, AHW; Funding acquisition: AJB, SK; Investigation: KOK, AT, LM, VR, APR-C, JAS, EO, HRM, GP, CD, CS, PJC, REH, EE, PV, KH, MZ; Methodology: AJB, KOK, DS, AV, LP, PV, KH, MZ, SK; Project Administration: KOK, AT; Resources: KOK, AHW, AT, LM, VR, JAS, EO, HRM, GP, CD, CS, PJC, REH, EE, CI, MP, MR, PV, KH, MZ, SK, AJB; Software: KOK, AHW, AT; Supervision: AJB, SK, TZ, CS, PJC, JAS; Validation: KOK, AHW; Visualization: KOK, AHW; Writing-original draft: AJB, KOK, AHW; Writing-review & editing: KOK, AHW, AT, LM, VR, JAS, EO, HRM, GP, CD, CS, PJC, REH, EE, CI, PV, KH, MZ, SK, and AJB.
Funding
SK and colleagues were supported by the OP Integrated Infrastructure, the project: Research on COVID-19 progressive diagnostic methods and biomarkers useful in early detection of individuals at increased risk of severe disease, ITMS: 313011ATA2, co-financed by the European Regional Development Fund and by the Slovak Research and Development Agency under the Contract no. PP-COVID-20-0056, by the Ministry of Health of the Czech Republic (grants NU21-07-00033, NU22-A-123), the Ministry of Education of the Czech Republic (grant LTAUSA19068) and by institutional programs of Charles University in Prague (UNCE/MED/007). JAS and HRM are funded by the Medical Research Council. The National Center for Medical Genomics (LM2023067) kindly provided sequencing and genotyping. AJB was funded by NIH-NIDDK R21 DK106584, CKD Biomarkers Consortium Pilot and Feasibility Studies Program funded by the NIH-NIDDK (U01 DK103225), the Slim Health Foundation, the Black-Brogan Foundation, Soli Deo Gloria. EE reports funds from the Royal College of Surgeons in Ireland StAR PhD. CD received funding from The Slim Health Foundation and the European Union’s Horizon 2020 Program, the Government of Cyprus and the University of Cyprus, under grant agreement number 857122.
Data availability
The datasets generated and analysed during the current study are not publicly available to protect patient confidentiality. We are very happy to work with any groups interested in studying this condition and providing genetic information that can satisfy their research requests. An anonymized dataset analyzed in the study will be available from the European Genome-Phenome Archive (EGA-archive.org), with request of data access.
Declarations
Ethics approval and consent to participate
This study was approved by the Wake Forest University Health Sciences Institutional Review Board, Winston-Salem, NC, United States, in accordance with the Declaration of Helsinki. All individuals presented in the study provided voluntary, autonomous consent to participate.
Consent for publication
Not applicable.
Competing interests
AJB has also received funding from the Black Brogan Foundation, The Slim Health Foundation, Soli Deo Gloria. He has received compensation as follows: advisory board, Horizon Pharma; speaker, Natera; author, UpToDate; advisor, First Faculty of Medicine, Charles University; royalty; patent for UMOD genetic diagnosis. PJC has received funding from Astra Zeneca, Bohringher, Hansa, and medical advisory board fees. KOK, AHW, AT, AK, LM, VR, APR-C, JAS, EO, HRM, GP, CD, CS, REH, EE, DS, CI, AV, LP, TZ, MP, MR, PV, KH, MZ, and SK have nothing to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Apostolopoulos V, Stojanovska L, Gargosky SE. MUC1 (CD227): a multi-tasked molecule. Cell Mol Life Sci. 2015;72(23):4475–4500. Not in File. 10.1007/s00018-015-2014-z. [DOI] [PMC free article] [PubMed]
- 2.Verceles AC, Bhat P, Nagaria Z, et al. MUC1 ectodomain is a flagellin-targeting decoy receptor and biomarker operative during Pseudomonas aeruginosa lung infection. Sci Rep Nov. 2021;22(1):22725. 10.1038/s41598-021-02242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballester B, Milara J, Cortijo J. The role of mucin 1 in respiratory diseases. Eur Respir Rev Mar. 2021;31(159). 10.1183/16000617.0149-2020. [DOI] [PMC free article] [PubMed]
- 4.Supruniuk K, Radziejewska I. MUC1 is an oncoprotein with a significant role in apoptosis (review). Int J Oncol Sep. 2021;59(3). 10.3892/ijo.2021.5248. [DOI] [PMC free article] [PubMed]
- 5.Kirby A, Gnirke A, Jaffe DB, et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet. 2013;45:288–393 Not in File. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvela-Levitt M, Kost-Alimova M, Emani M, et al. Small molecule targets TMED9 and promotes lysosomal degradation to reverse proteinopathy. Cell. 2019;178(3):521-535.e23. 10.1016/j.cell.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Zivna M, Kidd K, Pristoupilova A et al. Noninvasive immunohistochemical diagnosis and novel MUC1 mutations causing autosomal Dominant Tubulointerstitial kidney disease. J Am Soc Nephrol. 2018; Not in File. 10.1681/ASN.2018020180. [DOI] [PMC free article] [PubMed]
- 8.Bleyer AJ, Kmoch S, Antignac C, et al. Variable clinical presentation of an MUC1 mutation causing medullary cystic kidney disease type 1. Clin J Am Soc Nephrol. 2014(3):527–35. Not in File. 10.2215/CJN.06380613. [DOI] [PMC free article] [PubMed]
- 9.Devuyst O, Olinger E, Weber S, et al. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers Sep. 2019;5(1):60. 10.1038/s41572-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 10.Olinger E, Hofmann P, Kidd K et al. Clinical and genetic spectra of autosomal dominant tubulointerstitial kidney disease due to mutations in UMOD and MUC1. Kidney Int. 2020; 10.1016/j.kint.2020.04.038 [DOI] [PubMed]
- 11.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 2011;80(4):338–47. Not in File. 10.1038/ki.2011.134. [DOI] [PubMed]
- 12.Kidd K, Vylet’al P, Schaeffer C, et al. Genetic and clinical predictors of age of ESKD in individuals with autosomal Dominant Tubulointerstitial kidney Disease due to UMOD mutations. Kidney Int Rep Sep. 2020;5(9):1472–85. 10.1016/j.ekir.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cormican S, Kennedy C, Connaughton DM, et al. Renal transplant outcomes in patients with autosomal dominant tubulointerstitial kidney disease. Clin Transpl Feb. 2020;34(2):e13783. 10.1111/ctr.13783. [DOI] [PubMed] [Google Scholar]
- 14.Biering SB, Sarnik SA, Wang E, et al. Genome-wide bidirectional CRISPR screens identify mucins as host factors modulating SARS-CoV-2 infection. Nat Genet Jul. 2022;25. 10.1038/s41588-022-01131-x. [DOI] [PMC free article] [PubMed]
- 15.Sungnak W, Huang N, Becavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med May. 2020;26(5):681–7. 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai X, Yu Y, Xian W, et al. Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication. iScience Apr. 2022;15(4):104136. 10.1016/j.isci.2022.104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee MHL, Mykytyn AZ, Wang C, Lamers MM, Westendorp B, Wubbolts RW, van Putten JPM, Bosch BJ, Haagmans BL, Strijbis K. Glycosylated extracellular mucin domains protect against SARS-CoV-2 infection at the respiratory surface. PLoS Pathog. 2023;19(8). 10.1371/journal.ppat.1011571. [DOI] [PMC free article] [PubMed]
- 18.Klee GG, Schreiber WE. MUC1 gene-derived glycoprotein assays for monitoring breast cancer (CA 15 – 3, CA 27.29, BR): are they measuring the same antigen? Arch Pathol Lab Med Oct. 2004;128(10):1131–5. 10.1043/1543-2165(2004)128<1131:MGGAFM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Vylet’al P, Kidd K, Ainsworth HC, et al. Plasma Mucin-1 (CA15-3) levels in autosomal Dominant Tubulointerstitial kidney disease due to MUC1 mutations. Am J Nephrol. 2021;52(5):378–87. 10.1159/000515810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakuma T, Takahashi K, Ohya N, Usuda K, Handa M, Serum. KL-6, a novel mucin-like glycoprotein, as an indicator of interstitial pneumonitis following lobectomy. Surg Today. 1999;29(2):121–8. 10.1007/BF02482236. [DOI] [PubMed] [Google Scholar]
- 21.Bleyer AJ, Kidd K, Robins V, et al. Outcomes of patient self-referral for the diagnosis of several rare inherited kidney diseases. Genet Med Jul. 2019;24. 10.1038/s41436-019-0617-8. [DOI] [PMC free article] [PubMed]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. Not in File. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed]
- 23.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics Dec. 1988;44(4):1049–60. [PubMed] [Google Scholar]
- 24.Blumenstiel B, Defelice M, Birsoy O et al. Development and Validation of a Mass Spectrometry-Based Assay for the Molecular Diagnosis of Mucin-1 Kidney Disease. J Mol Diagn. 2016;18(4):566–571. Not in File. 10.1016/j.jmoldx.2016.03.003. [DOI] [PubMed]
- 25.Jayant K, Reccia I, Bachul PJ, et al. The impact of COVID-19 on kidney transplant recipients in Pre-vaccination and Delta strain era: a systematic review and Meta-analysis. J Clin Med Sep. 2021;30(19). 10.3390/jcm10194533. [DOI] [PMC free article] [PubMed]
- 26.Wu D, Yang XO. Dysregulation of pulmonary responses in severe COVID-19. Viruses. May. 2021;21(6). 10.3390/v13060957. [DOI] [PMC free article] [PubMed]
- 27.Lu W, Liu X, Wang T, et al. Elevated MUC1 and MUC5AC mucin protein levels in airway mucus of critical ill COVID-19 patients. J Med Virol Feb. 2021;93(2):582–4. 10.1002/jmv.26406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold DT, Donald C, Lyon M, et al. Krebs Von Den Lungen 6 (KL-6) as a marker for disease severity and persistent radiological abnormalities following COVID-19 infection at 12 weeks. PLoS ONE. 2021;16(4):e0249607. 10.1371/journal.pone.0249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.d’Alessandro M, Bergantini L, Cameli P, et al. Serial KL-6 measurements in COVID-19 patients. Intern Emerg Med. Sep 2021;16(6):1541–5. 10.1007/s11739-020-02614-7. [DOI] [PMC free article] [PubMed]
- 30.d’Alessandro M, Cameli P, Bergantini L, Franchi F, Scolletta S, Bargagli E. Serum concentrations of Krebs von den Lungen-6 in different COVID-19 phenotypes. J Med Virol Aug. 2020;14. 10.1002/jmv.26431. [DOI] [PMC free article] [PubMed]
- 31.Deng K, Fan Q, Yang Y, et al. Prognostic roles of KL-6 in disease severity and lung injury in COVID-19 patients: a longitudinal retrospective analysis. J Med Virol Apr. 2021;93(4):2505–12. 10.1002/jmv.26793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kousathanas A, Pairo-Castineira E, Rawlik K, et al. Whole genome sequencing reveals host factors underlying critical Covid-19. Nat Mar. 2022;7. 10.1038/s41586-022-04576-6. [DOI] [PMC free article] [PubMed]
- 33.Initiative C-HG. Mapping the human genetic architecture of COVID-19. Nat Dec. 2021;600(7889):472–7. 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alimova M, Sidhom EH, Satyam A et al. A High Content Screen for Mucin-1-Reducing Compounds Identifies Fostamatinib as a Candidate for Rapid Repurposing for Acute Lung Injury during the COVID-19 pandemic. bioRxiv. Jun 30. 2020;10.1101/2020.06.30.180380 [DOI] [PMC free article] [PubMed]
- 35.Yoo HJ. Body Mass Index and Mortality. J Obes Metab Syndr Mar. 2017;26(1):3–9. 10.7570/jomes.2017.26.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hossin MZ. From habitual sleep hours to morbidity and mortality: existing evidence, potential mechanisms, and future agenda. Sleep Health Jun. 2016;2(2):146–53. 10.1016/j.sleh.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Seay NW, Lehrich RW, Greenberg A. Diagnosis and management of disorders of Body Tonicity-Hyponatremia and Hypernatremia: Core Curriculum 2020. Am J Kidney Dis Feb. 2020;75(2):272–86. 10.1053/j.ajkd.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Zivna M, Kidd KO, Baresova V, Hulkova H, Kmoch S, Bleyer AJ. Sr. Autosomal dominant tubulointerstitial kidney disease: a review. Am J Med Genet C Semin Med Genet Sep. 2022;190(3):309–24. 10.1002/ajmg.c.32008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available to protect patient confidentiality. We are very happy to work with any groups interested in studying this condition and providing genetic information that can satisfy their research requests. An anonymized dataset analyzed in the study will be available from the European Genome-Phenome Archive (EGA-archive.org), with request of data access.