Abstract
Background
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment by enhancing the immune system’s ability to target cancer cells. However, ICIs can lead to immune-related adverse events (irAEs), including dermatologic manifestations such as bullous pemphigoid (BP).
Objective
To evaluate the efficacy and safety of omalizumab and other biologics in the treatment of ICI-induced refractory bullous pemphigoid and to derive a strategy for selecting biologic treatments for this condition.
Methods
A 48-year-old female with pulmonary squamous cell carcinoma developed erythema and blisters following tislelizumab treatment. Despite initial steroid therapy (1.8 mg/kg/day), new blisters formed. Laboratory tests revealed elevated BP180/230 levels, confirming BP diagnosis. Treatments with intravenous corticosteroids, cyclosporine, and dapsone were ineffective. Omalizumab 300 mg every four weeks was initiated based on elevated serum IgE levels. The patient’s response was monitored over four weeks. A comprehensive literature review was conducted, including 4 relevant articles.
Results
Omalizumab treatment resulted in the cessation of blister formation and significant symptom alleviation within one week. The overall treatment duration was four weeks, with stable improvement observed. Follow-up for 4 months with no recurrence.
Conclusion
This case illustrates the challenges of managing ICI-induced BP and highlights omalizumab as a potentially effective treatment option. The study proposes a personalized therapeutic strategy for refractory ICI-induced BP, emphasizing the selection of biologic agents based on specific immune profiles, including serum markers like IgE, eosinophils, and cytokine levels.
Keywords: immune checkpoint inhibitors, bullous pemphigoid, omalizumab, adverse events, case report
Introduction
Immune checkpoint inhibitors (ICIs) are drugs that enhance the immune system’s ability to attack cancer cells by inhibiting checkpoint proteins such as programmed cell death receptor 1 (PD-1) and CTLA-4. These proteins normally maintain immune balance and prevent tissue damage from excessive immune responses.1 Tislelizumab, an anti-PD-1 monoclonal antibody, targets PD-1 with high specificity to inhibit its interaction with ligand programmed cell death ligand 1(PD-L1). This blockade reactivates T cells that have been suppressed by tumor mechanisms, bolstering their anticancer activity. Notably, Tislelizumab’s Fc region has been engineered to minimize effector function, potentially enhancing its selectivity and reducing off-target effects compared to other ICIs. This distinct feature may contribute to a more focused immune response against tumors with less interference from the patient’s own immune regulatory processes.2 However, this immune activation can also target normal tissues, leading to immune-related adverse events (irAEs). These irAEs can affect multiple systems, including the skin, cardiovascular, and respiratory systems. As the use of ICIs increases, so does the incidence of irAEs. Bullous pemphigoid (BP) has been identified in some patients undergoing anti–PD-1/PD-L1 therapy, though its pathogenesis and prognostic implications remain unclear.2–4 Understanding and managing BP in the context of ICI therapy is crucial due to its potential impact on treatment and oncological outcomes. Treating skin toxicities could accelerate tumor progression, necessitating a comprehensive assessment and individualized treatment plan to ensure a satisfactory prognosis. Here, we report a case of successful treatment of grade 3 BP induced by tislelizumab with omalizumab and summarize our diagnostic and therapeutic experience.
Presentation of the Case and Intervention
A 48-year-old female with a history of advanced pulmonary squamous cell carcinoma developed erythema and blistering after receiving a combination treatment of tislelizumab and albumin-bound paclitaxel. Notably, the patient had a history of blister formation in 2022, which was successfully treated with corticosteroids, leading to complete resolution without recurrence. In November 2023, following the diagnosis of her lung tumor, she initiated chemotherapy. After completing the fifth cycle, she experienced a sudden onset of widespread blistering. The patient’s medical history includes autoimmune thyroid disease, which was identified during this episode of blister formation, as well as a prior diagnosis of cervical cancer in 2020. Additionally, she has a known history of thalassemia, although no other significant comorbidities or autoimmune conditions were present at baseline. There was no family history of autoimmune diseases, and her psychosocial background was unremarkable, with no history of psychological disorders or significant stressors that could have influenced her condition. During her current presentation, the patient developed erythema and tense blisters predominantly on her face, chest, abdomen, and limbs (shown in Figure 1). These were most prominent in the axillary, groin, and genital areas, some of which had progressed to form erosive crusts. Laboratory tests confirmed elevated BP180 (197 u/mL) and BP230 (6 u/mL) levels, along with high thyroid antibody levels, indicating multiorgan autoimmune involvement. Direct immunofluorescence exhibited weakly positive staining for IgG and C3 at the basement membrane (shown in Figures 2 and 3). Initial treatments, including intravenous corticosteroids, doxycycline, nicotinamide, and topical applications, were ineffective, and the patient’s condition deteriorated. Subsequent administration of cyclosporine, increased corticosteroid dosages, and intravenous immunoglobulin (IVIG) also failed to yield significant improvement. Persistent disease activity necessitated the addition of dapsone and further escalation of corticosteroid doses (shown in Figure 4). Given elevated serum IgE levels (516.0 IU/mL), omalizumab 300 mg every 4 weeks was introduced, resulting in the cessation of new blister formation and stabilization within a week. The treatment spanned 4 weeks, highlighting omalizumab’s potential efficacy in managing refractory BP induced by ICIs.
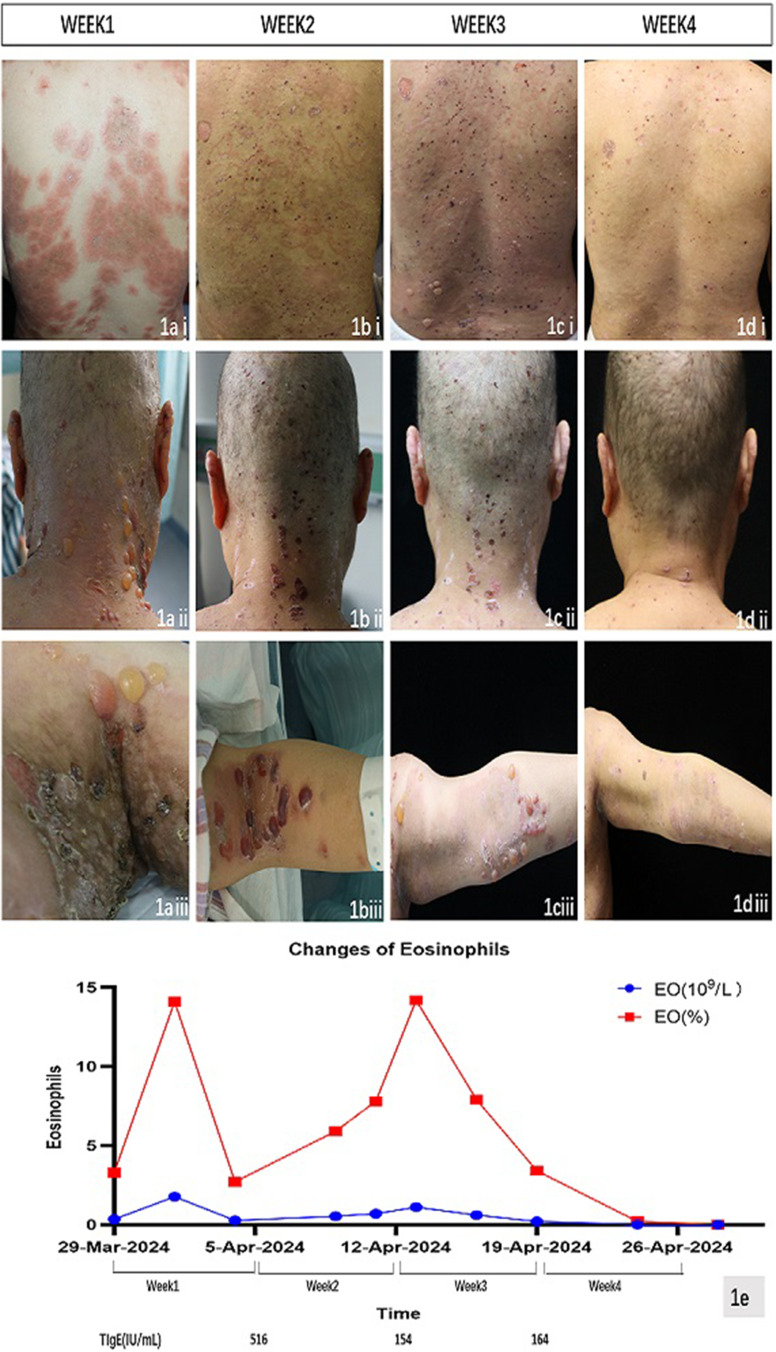
Figure 1.
Clinical manifestations from week 1 to week 4. (ai)–(aiii) New urticarial-like erythema, blisters, localized ulceration, and erosion appeared on the trunk, back of the neck, groin, and axillae during week 1. (bi)–(biii) New erythema, blisters, localized ulceration, and erosion appeared on the upper limbs. The blisters on the back of the neck resolved, and the urticarial-like erythema slightly diminished during week 2. (ci)–(ciii) New blisters, localized ulceration, and erosion appeared on the trunk, popliteal fossae, and cubital fossae during week 3. (di)–(diii) The blisters in the popliteal fossae and cubital fossae resolved. There were no instances of new erythema or blisters on the body, with residual ulcers and pigmentation during week 4. (e) The red line represents the absolute eosinophil count, while the blue line represents the eosinophil percentage.
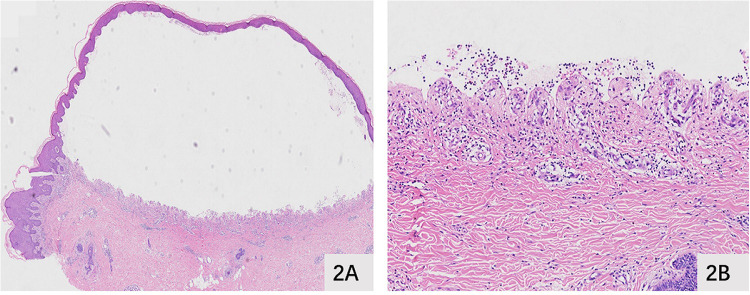
Figure 2.
Histopathological examination. (A and B) Histopathological examination demonstrated subepidermal blister formation with localized spongiosis and perivascular inflammatory infiltrate. The superficial dermal blood vessels were dilated and congested, with diffuse lymphocytic infiltration and a few eosinophils.
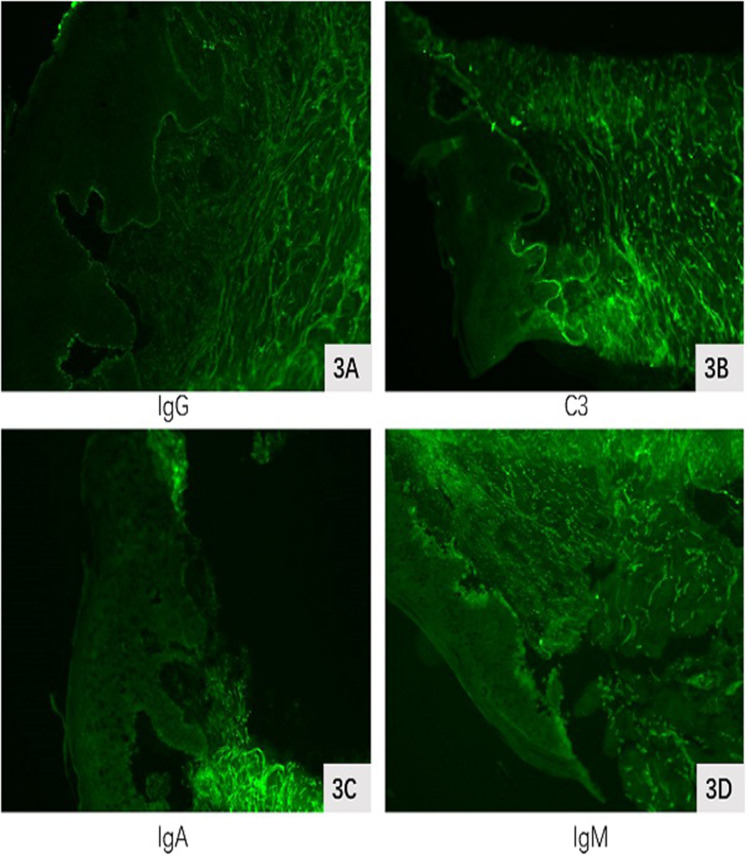
Figure 3.
Direct immunofluorescence. (A and B) Direct immunofluorescence showed weak positivity for IgG and C3 at the basement membrane, while IgA and IgM (C and D)were negative.
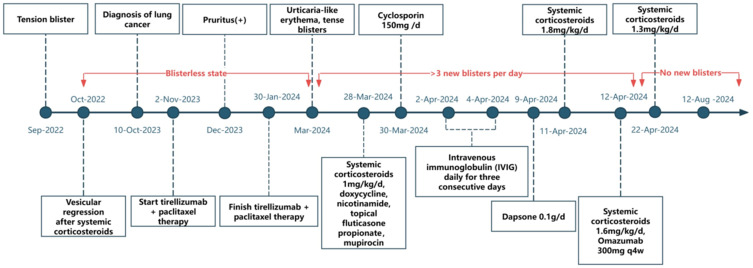
Figure 4.
Timeline of clinical events and treatments for the patient with PD-1 inhibitor–induced bullous pemphigoid. The patient developed tense blisters, urticarial-like erythema, and pruritus during treatment with tislelizumab plus paclitaxel for squamous cell lung cancer. Systemic corticosteroids, cyclosporine, intravenous immunoglobulin (IVIG), and dapsone were administered sequentially to manage the symptoms. The final regimen, which includes systemic corticosteroids and omalizumab, led to stabilization without new blister formation.
Discussion
PD-1 is an inhibitory receptor on the surface of T and B cells. By binding to PD-L1, it inhibits the activity of these immune cells and helps maintain immune tolerance to normal tissues. Blocking this pathway with ICIs like tislelizumab can unblock T and B cells, thereby enhancing their ability to attack tumor cells. However, the use of PD-1 or PD-L1 inhibitors has been identified as a risk factor for the induction of bullous pemphigoid (BP).5–7
ICIs have been shown to enhance the diversity of T-cell clones in the circulating blood, potentially leading to the development of irAEs by activating T cells that may recognize cross-reactive antigens between tumors and normal tissues. Berner et al collected peripheral blood mononuclear cells from patients receiving anti–PD-1 therapy, as well as tumor biopsy specimens and biopsy samples from sites of autoimmune skin toxicity, and identified nine shared T-cell antigens between tumor tissue and skin.8 This discovery enhanced our understanding of the specificity of antigen cross-presentation in circulating antigens and its effect on irAEs. Patients with preexisting or de novo autoantibodies may also be potentially susceptible to irAEs. In a retrospective study of 137 patients with lung cancer treated with anti–PD-1 therapy, a significant association was found between the presence of preexisting antibodies, such as antinuclear antibodies and anti-thyroglobulin antibodies, and the incidence of irAEs.9 Our reported patient had a history of blisters 2 years ago, which resolved completely with steroid treatment, and had no new blisters within the past 2 years. After 3 months of PD-1 inhibitor therapy, the patient developed pruritus, erythema, and tense blisters. The PD-1 inhibitor likely stimulated and amplified preexisting epidermal antibodies. It could also be that the immune system recognized cross-reactive antigens between the tumor and epidermal tissues, leading to the production of new antigens.10 Furthermore, ICIs may disrupt T follicular helper (TFH) cells, which are crucial for B-cell maturation and antibody production, suggesting that TFH cells contribute to autoantibody production. Thus, ICIs may promote autoantibody production by directly affecting B cells and indirectly altering TFH cell function through T-cell modulation.11 Elevated levels of IL-10 were observed in cytokine assays of our patient, and despite treatment with corticosteroids at 1.8 mg/kg/day, the condition remained uncontrolled, indicating refractory irAEs, consistent with findings by Patel et al. Cytokines may play a role in predicting disease severity, underscoring their significant potential in guiding clinical corticosteroid dosing.
There is evidence suggesting a prominent type II inflammatory response in BP, involving IgE, eosinophils, and Th2 cytokines and chemokines. Eosinophil infiltration is a prominent feature of pemphigoid diseases, with around 50% of patients showing peripheral eosinophilia.12,13 Eosinophils may contribute to the pathogenesis of BP through the following mechanisms: (1) as effector cells, directly acting on dermal–epidermal separation; (2) producing cytokines and chemokines to amplify and sustain local immune reactions; and (3) playing a role in BP-associated pruritus. Inflammatory cells release matrix metalloproteinase-9, neutrophil elastase, thrombin, and eosinophil cationic protein, which degrade BP180,14 thereby leading to dermal–epidermal separation and blister formation.
According to Chu et al, IgE antibodies can bind to keratinocyte adhesion proteins, specifically BP180 (type XVII collagen), which compromises the connection between the epidermis and the dermis, resulting in blister formation.15 Van Beek et al further demonstrated that elevated serum levels of anti-BP180 NC16A IgE correlate with disease activity in BP, underscoring the pathogenic significance of anti-BP180 IgE.16 This relationship suggests that IgE contributes to the disruption of skin integrity, leading to BP lesions.
Omalizumab, an anti-IgE monoclonal antibody, functions by neutralizing circulating IgE and preventing its interaction with mast cells and basophils. This action reduces IgE-mediated inflammation, which is particularly relevant in BP cases characterized by high IgE levels and urticarial plaques. Omalizumab’s efficacy in BP is thought to stem from its ability to inhibit IgE binding to BP180, thereby mitigating the immune response and preventing further skin damage.15,16 Thus, omalizumab offers a targeted approach to managing BP, particularly in cases associated with elevated IgE levels and a prominent urticarial component. Our patient exhibited elevated serum IgE levels, eosinophilia, and increased levels of IL-5, IL-6, IL-8, and IL-10. As suggested by Phillips et al, the elevation of eosinophils, IL-6, IL-10, and IgE is associated with irAEs, suggesting that they may represent actionable therapeutic targets for immune-related skin toxicity.13
During the treatment course of this patient, the doses of systemic corticosteroids were increased to 1.8 mg/kg/day, along with dapsone, cyclosporine, and IVIG, which failed to control the emergence of new blisters. The patient exhibited pronounced pruritus and urticarial-like manifestations of edematous erythema on the skin, accompanied by elevated serum IgE levels and eosinophilia. Treatment with omalizumab at a dose of 300 mg every 4 weeks was initiated, resulting in no new blister formation and significant alleviation of itching over the overall 4-week course. Successful treatment of refractory ICI–induced pemphigoid was achieved within 1 month, reducing the adverse effects of high-dose corticosteroids through the use of biologic agents. Based on this case experience, individualized biologic agent selection for treating refractory ICI-induced pemphigoid can be guided by the patient’s rash characteristics, subjective symptoms, and serum markers of hypersensitivity (such as IgE, eosinophils, and cytokine levels). Considering the patient’s status as a lung cancer patient who has already undergone PD-1 inhibitor therapy to modulate T-cell and B-cell balance for tumor control, the potential effect of using rituximab, targeting B cells, on T-cell and B-cell re-balancing, tumor recurrence, and susceptibility to infections should be carefully assessed.17 Existing data may be limited due to publication bias. Some studies have suggested similar safety profiles between rituximab and omalizumab,18 while Lamberts et al reported five serious adverse events and three deaths. One death could be related to rituximab, while another death was disease-related.17 Cao et al found a higher recurrence rate, adverse events, and mortality associated with rituximab.19 Therefore, when considering rituximab, overall immune status factors such as age, hematologic parameters, lymphocyte proportions, and infection risks should be evaluated to prevent infection outbreaks or tumor progression. When there is no evidence to support the use of omalizumab or dupilumab, and rituximab is necessary to control the emergence of blisters, considering IVIG therapy, antibiotics, or antiviral treatment to prevent infection outbreaks is advisable, ensuring comprehensive management of the patient’s diagnosis and treatment.
There is evidence suggesting that omalizumab may be particularly effective for BP associated with ICIs in cases where there is an elevated eosinophil count. In BP, eosinophils are often involved in the inflammatory process, and their levels can correlate with disease activity.20 For example, Alexandre et al21 reported that omalizumab led to significant improvements in BP symptoms, including pruritus and blister count, particularly in patients with elevated eosinophil levels at baseline. This correlation indicates that eosinophilic activity may be a driver of disease severity in these cases, making omalizumab an appropriate therapeutic choice due to its mechanism of action targeting IgE, which is implicated in eosinophil activation and survival.21 Furthermore, the ability of omalizumab to reduce eosinophil counts and the associated inflammatory response offers a rationale for its use in BP cases with pronounced eosinophilia, especially when traditional treatments have failed or are contraindicated. This approach aligns with the immunopathological findings that link eosinophilia with the pathogenesis of BP, suggesting that a targeted intervention like omalizumab could yield better disease control and improve patient outcomes. There is evidence suggesting that ICI-induced BP with elevated IL-6 and IL-10 levels could benefit from interventions targeting these cytokines. For example, Guan et al reported a case of a patient with BP induced by ICIs who exhibited elevated levels of IL-6 and IL-10.22 Standard therapies were insufficient in that case, highlighting the complexity and refractoriness of such cases to conventional treatments. Interventions that reduce IL-6 and IL-10 levels have shown promise in ameliorating symptoms and potentially improving the prognosis of patients with ICI-induced BP. The rationale for using omalizumab in treating ICI-induced BP, particularly with elevated IL-6 and IL-10 levels, lies in its mechanism of action, which indirectly modulates the inflammatory process. Omalizumab binds to IgE, thereby reducing its interaction with FcεRI receptors on inflammatory cells such as mast cells and basophils. This interaction downregulates the inflammatory cascade typically exacerbated by elevated IgE levels, potentially reducing the synthesis and release of proinflammatory cytokines, including IL-6 and IL-10. Previous studies have shown that omalizumab can decrease the secretion of IL-6 in response to IgE-mediated stimuli in patients with asthma, demonstrating a direct link between IgE modulation and reduced proinflammatory cytokine release.23 This evidence suggests that omalizumab could be a promising therapeutic option for managing ICI-induced BP with elevated IL-6 and IL-10 levels. Omalizumab has shown effectiveness in treating dermatologic conditions linked to immune responses, particularly those manifesting with urticaria-like erythema and wheals. These symptoms often suggest an IgE-mediated process, which is targeted by omalizumab. Clinical studies highlight omalizumab’s efficacy in mitigating pruritus-associated cutaneous adverse events related to ICIs.20 In this study, a significant proportion of patients experienced symptom relief, illustrating omalizumab’s advantage, especially when traditional therapies such as corticosteroids are ineffective or inappropriate due to their adverse effects. Furthermore, omalizumab has been effectively used in cases of BP triggered by ICIs. Notably, omalizumab has helped achieve remission in patients who did not respond to conventional treatments. This includes instances where BP initially presented with urticaria-like symptoms before progressing to blister formation, indicating a potential IgE-mediated component in the pathogenesis of ICI-induced BP.24 Omalizumab shows promising long-term outcomes in BP, particularly in reducing disease severity and the need for corticosteroids. Several studies have reported sustained symptom relief with minimal side effects, alongside reduced IgE production and lower inflammatory markers such as eosinophils and FcεRI expression.18,25,26 However, larger clinical trials are necessary to fully validate its long-term efficacy, particularly in cases induced by ICIs. This evidence supports the strategic use of omalizumab in treating complex skin conditions induced by immunotherapies, providing a rationale for its application in managing ICI-related BP with urticarial features.
In addition to omalizumab, dupilumab has also shown great potential in mass BP. Multiple studies have confirmed significantly elevated serum levels of IL-4 and IL-13 in patients with BP, particularly in those with PD-1 inhibitor–induced BP, mirroring the profile observed in patients with traditional autoimmune BP.27 These Th2 cytokines play a critical role in the pathogenesis of BP, exacerbating the disease by promoting inflammatory responses and autoimmune attacks.27 Dupilumab, which is an IL-4 receptor alpha antagonist, has shown substantial therapeutic efficacy by inhibiting IL-4 and IL-13 signaling. Numerous case reports and studies have demonstrated that dupilumab effectively reduces the levels of IL-4 and IL-13, thereby improving symptoms and disease conditions in patients with BP.28 In patients with PD-1 inhibitor–induced BP, the application of dupilumab has also proven effective, with partial or complete remission observed after treatment, supporting its potential as a novel therapeutic option.22 Mechanistically, dupilumab mitigates the pathological processes of BP by inhibiting the expression of Th2 cytokines. The suppression of IL-4 and IL-13 contributes to the reduction of inflammatory responses and autoimmune attacks, thereby improving the symptoms and disease condition of BP.27 Furthermore, previous studies have shown that dupilumab exhibits rapid and significant efficacy across various clinical forms of BP, with good tolerability in elderly patients, which is particularly important given the prevalence of BP in this demographic category.29 Table 1 presents the safety and efficacy of different biologics in treating immune checkpoint inhibitor-induced bullous pemphigoid.
Table 1.
Safety and Efficacy of Biologics for Immune Checkpoint Inhibitor-Induced Bullous Pemphigoid
| Author | Cancer Type | Age | Gender | Dosage | Clinical Efficacy | Tumor Progression | Follow-up Duration | Study Type |
|---|---|---|---|---|---|---|---|---|
| Klepper et al28 | Lung Cancer | 68 | F | Dupilumab | Rapid disease control, symptom resolution | Not applicable | 5 months | Case Report |
| Schauer et al30 | Various cancers | 50–80 | M/F | Rituximab, | Clinical improvement | Not applicable | 12 months | Retrospective Study |
| Fournier et al31 | Various cancers | 60–75 | M/F | Dupilumab | Successful treatment of BP | Remission or Death from cancer progression | 3–6 months | Case Series |
| Yun et al32 | Various cancers | 55–78 | M/F | Rituximab, Omalizumab | Adequate control of BP | Increased tumor progression in some patients |
6–18 months | Case Series |
Conclusion
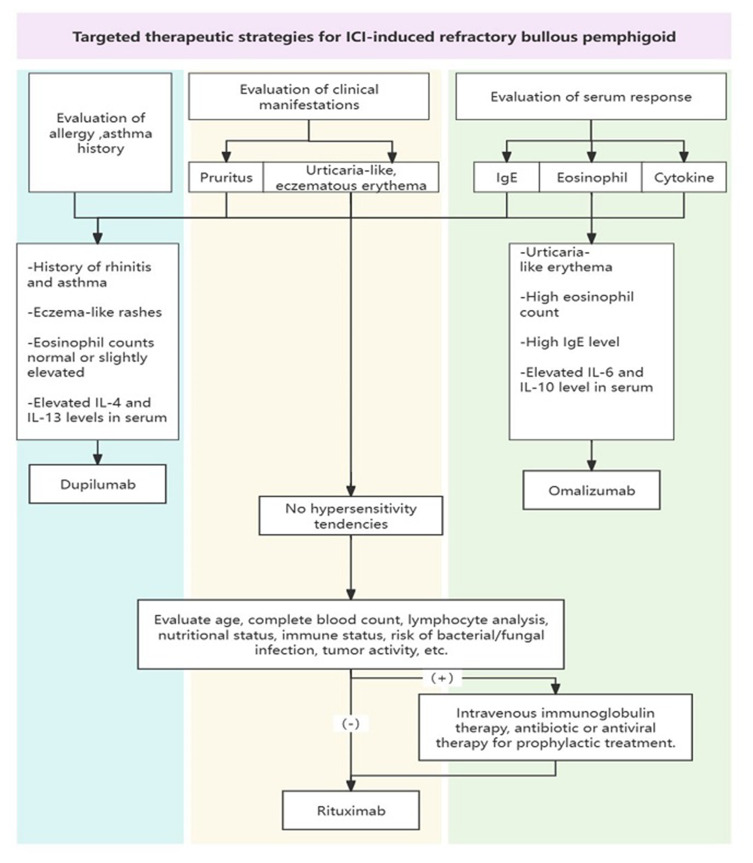
This study outlines a personalized therapeutic strategy for managing PD-1 inhibitor–induced refractory BP, emphasizing a detailed evaluation of clinical manifestations and serum markers (shown in Figure 5). Based on symptoms such as pruritus and urticarial-like erythema, and serum levels of IgE, eosinophils, and cytokines, treatments with biologic agents such as dupilumab and omalizumab are recommended. For patients without hypersensitivity, comprehensive assessments guide the use of rituximab under specific protocols. IVIG therapy, combined with antibiotics or antiviral drugs, is suggested to prevent infections. This approach ensures effective management of refractory BP, providing a systematic framework for future clinical practice in addressing ICI-related adverse events.
Figure 5.
Targeted therapeutic strategies for ICI–induced refractory bullous pemphigoid. The flowchart outlines the evaluation and treatment strategies based on clinical manifestations and serum response. It categorizes therapeutic options such as dupilumab, omalizumab, and rituximab, considering factors such as allergy/asthma history, serum IgE, eosinophil count, and cytokine levels. It also provides guidance on the use of intravenous immunoglobulin therapy, antibiotics, or antiviral prophylaxis.
Acknowledgments
The authors would like to express their sincere gratitude to the patient for her cooperation and consent to publish this case report, which has greatly contributed to advancing our understanding of this condition. We also extend our thanks to the medical and nursing staff involved in the patient’s care for their invaluable support.
Funding Statement
This study was financially supported by the Scientific Research Plan (2019) from the Guangzhou Science and Technology Bureau (No. 201904010352) and The School & Institute Joint Fund Project in Basic and Applied Research Areas from the Guangzhou Science and Technology Bureau 2023 (NO.2023A03J0946).
Consent for Publication Statement
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. The patient was fully informed about the nature and purpose of the case report, including the understanding that their medical information would be anonymized and published in a peer-reviewed journal. The patient has expressly consented to the use of their clinical data for research and educational purposes, with the assurance that all efforts would be made to protect their identity. Institutional approval was obtained for the publication of the case details. The ethics approval was granted by Guangzhou Dermatology Hospital, with the approval number gzsp202483.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Chen ST, Semenov YR, Alloo A. et al. Defining D-irAEs: consensus-based disease definitions for the diagnosis of dermatologic adverse events from immune checkpoint inhibitor therapy. J Immunother Cancer. 2024;12(4):e007675. doi: 10.1136/jitc-2023-007675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsiogka A, Bauer J, Patsatsi A. Bullous Pemphigoid Associated with Anti-programmed Cell Death Protein 1 and Anti-programmed Cell Death Ligand 1 Therapy: a Review of the Literature. Acta Derm Venereol. 2021;101(1):adv00377. doi: 10.2340/00015555-3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor–related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255–1268. doi: 10.1016/j.jaad.2020.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Yu S, Zhang G, Xie Y, Wei JCC. Interleukin-17 Monoclonal Antibody Successfully Treated Psoriasiform Drug Eruption Induced by Immune Checkpoint Inhibitors: a Case Report and Review of Literature. Dermatitis. 2024;35(4):410–412. doi: 10.1089/derm.2023.0208 [DOI] [PubMed] [Google Scholar]
- 5.Verheyden M, Bilgic A, Murrell D. A Systematic Review of Drug-Induced Pemphigoid. Acta Derm Venereol. 2020;100(15):adv00224. doi: 10.2340/00015555-3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merli M, Accorinti M, Romagnuolo M, et al. Autoimmune bullous dermatoses in cancer patients treated by immunotherapy: a literature review and Italian multicentric experience. Front Med. 2023;10:1208418. doi: 10.3389/fmed.2023.1208418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panariello L, Fattore D, Annunziata MC, Piantedosi F, Gilli M, Fabbrocini G. Bullous pemphigoid and nivolumab: dermatologic management to support and continue oncologic therapy. Eur J Cancer. 2018;103:284–286. doi: 10.1016/j.ejca.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 8.Berner F, Bomze D, Diem S, et al. Association of Checkpoint Inhibitor–Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non–Small Cell Lung Cancer. JAMA Oncol. 2019;5(7):1043. doi: 10.1001/jamaoncol.2019.0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toi Y, Sugawara S, Sugisaka J, et al. Profiling Preexisting Antibodies in Patients Treated With Anti–PD-1 Therapy for Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2019;5(3):376. doi: 10.1001/jamaoncol.2018.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFalco J, Harbell M, Manning-Bog A, et al. Non-progressing cancer patients have persistent B cell responses expressing shared antibody paratopes that target public tumor antigens. Clin Immunol. 2018;187:37–45. doi: 10.1016/j.clim.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Patel AJ, Willsmore ZN, Khan N, et al. Regulatory B cell repertoire defects predispose lung cancer patients to immune-related toxicity following checkpoint blockade. Nat Commun. 2022;13(1):3148. doi: 10.1038/s41467-022-30863-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kridin K. Peripheral eosinophilia in bullous pemphigoid: prevalence and influence on the clinical manifestation. Br J Dermatol. 2018;179(5):1141–1147. doi: 10.1111/bjd.16679 [DOI] [PubMed] [Google Scholar]
- 13.Phillips GS, Wu J, Hellmann MD, et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. JCO. 2019;37(30):2746–2758. doi: 10.1200/JCO.18.02141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin L, Betsuyaku T, Heimbach L, et al. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biol. 2012;31(1):38–44. doi: 10.1016/j.matbio.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu KY, Yu HS, Yu S. Current and Innovated Managements for Autoimmune Bullous Skin Disorders: an Overview. JCM. 2022;11(12):3528. doi: 10.3390/jcm11123528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Beek N, Lüttmann N, Huebner F, et al. Correlation of Serum Levels of IgE Autoantibodies Against BP180 With Bullous Pemphigoid Disease Activity. JAMA Dermatol. 2017;153(1):30. doi: 10.1001/jamadermatol.2016.3357 [DOI] [PubMed] [Google Scholar]
- 17.Lamberts A, Euverman HI, Terra JB, Jonkman MF, Horváth B. Effectiveness and Safety of Rituximab in Recalcitrant Pemphigoid Diseases. Front Immunol. 2018;9:248. doi: 10.3389/fimmu.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer N, Snast I, Cohen ES, et al. Rituximab and Omalizumab for the Treatment of Bullous Pemphigoid: a Systematic Review of the Literature. Am J Clin Dermatol. 2019;20(2):209–216. doi: 10.1007/s40257-018-0401-6 [DOI] [PubMed] [Google Scholar]
- 19.Cao P, Xu W, Rituximab ZL. Omalizumab, and Dupilumab Treatment Outcomes in Bullous Pemphigoid: a Systematic Review. Front Immunol. 2022;13:928621. doi: 10.3389/fimmu.2022.928621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrios DM, Phillips GS, Geisler AN, et al. IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies. Ann Oncol. 2021;32(6):736–745. doi: 10.1016/j.annonc.2021.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexandre M, Bohelay G, Gille T, et al. Rapid Disease Control in First-Line Therapy-Resistant Mucous Membrane Pemphigoid and Bullous Pemphigoid with Omalizumab as Add-On Therapy: a Case Series Of 13 Patients. Front Immunol. 2022;13:874108. doi: 10.3389/fimmu.2022.874108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan S, Zhang L, Zhang J, Song W, Zhong D. A case report of steroid-refractory bullous pemphigoid induced by immune checkpoint inhibitor therapy. Front Immunol. 2023;13:1068978. doi: 10.3389/fimmu.2022.1068978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth M, Tamm M. The effects of omalizumab on IgE-induced cytokine synthesis by asthmatic airway smooth muscle cells. Ann Allergy Asthma Immunol. 2010;104(2):152–160. doi: 10.1016/j.anai.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 24.James T, Salman S, Stevenson B, et al. IgE blockade in autoimmunity: omalizumab induced remission of bullous pemphigoid. Clin Immunol. 2019;198:54–56. doi: 10.1016/j.clim.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 25.Fairley JA, Messingham KAN. Omalizumab therapy of bullous pemphigoid. Br J Dermatol. 2024;190(2):142–143. doi: 10.1093/bjd/ljad432 [DOI] [PubMed] [Google Scholar]
- 26.Granados-Betancort E, Sánchez-Díaz M, Muñoz-Barba D, Arias-Santiago S. Omalizumab and Dupilumab for the Treatment of Bullous Pemphigoid: a Systematic Review. JCM. 2024;13(16):4844. doi: 10.3390/jcm13164844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamura S, Teraki Y. Treatment of bullous pemphigoid with dupilumab: dupilumab exerts its effect by primarily suppressing T-helper 2 cytokines. J Dermatol. 2022;49(9):845–850. doi: 10.1111/1346-8138.16428 [DOI] [PubMed] [Google Scholar]
- 28.Klepper EM, Robinson HN. Dupilumab for the treatment of nivolumab-induced bullous pemphigoid: a case report and review of the literature. DOJ. 2021;27(9). doi: 10.5070/D327955136 [DOI] [PubMed] [Google Scholar]
- 29.Moghadam P, Tancrede E, Bouaziz JD, et al. Efficacy and safety of dupilumab in bullous pemphigoid: a retrospective multicentric study of 36 patients. Br J Dermatol. 2023;189(2):244–246. doi: 10.1093/bjd/ljad136 [DOI] [PubMed] [Google Scholar]
- 30.Schauer F, Rafei-Shamsabadi D, Mai S, et al. Hemidesmosomal Reactivity and Treatment Recommendations in Immune Checkpoint Inhibitor-Induced Bullous Pemphigoid—A Retrospective, Monocentric Study. Front Immunol. 2022;13:953546. doi: 10.3389/fimmu.2022.953546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fournier C, Hirsch I, Spreafico A, Butler MO, Dhani N, Sauder MB. Dupilumab as a treatment for cutaneous immune-related adverse events induced by immune checkpoint inhibitors: a case series and review of the literature. SAGE Open Medical Case Rep. 2023;11:2050313X231195462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yun JSW, Chan OB, Goh M, McCormack CJ. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a case series of 13 patients. Australas J Dermatol. 2023;64(1):131–137. doi: 10.1111/ajd.13960 [DOI] [PubMed] [Google Scholar]