Abstract
Rhodococcus equi is a facultative intracellular pathogen of macrophages and a cause of pneumonia in young horses (foals) and immunocompromised people. Isolates of R. equi from pneumonic foals typically contain large, 85- or 90-kb plasmids encoding a highly immunogenic virulence-associated protein (VapA). The objective of this study was to determine the role of the 85-kb plasmid and VapA in the intracellular survival and virulence of R. equi. Clinical isolates containing the plasmid and expressing VapA efficiently replicated within mouse macrophages in vitro, while plasmid-cured derivatives of these organisms did not multiply intracellularly. An isolate harboring the large plasmid also replicated in the tissues of experimentally infected mice, whereas its plasmid-cured derivative was rapidly cleared. All foals experimentally infected with a plasmid-containing clinical isolate developed severe bronchopneumonia, whereas the foals infected with its plasmid-cured derivative remained asymptomatic and free of visible lung lesions. By day 14 postinfection, lung bacterial burdens had increased considerably in foals challenged with the plasmid-containing clinical isolate. In contrast, bacteria could no longer be cultured from the lungs of foals challenged with the isogenic plasmid-cured derivative. A recombinant, plasmid-cured derivative expressing wild-type levels of VapA failed to replicate in macrophages and remained avirulent for both mice and foals. These results show that the 85-kb plasmid of R. equi is essential for intracellular replication within macrophages and for development of disease in the native host, the foal. However, expression of VapA alone is not sufficient to restore the virulence phenotype.
Rhodococcus equi, a gram-positive facultative intracellular pathogen of macrophages, is one of the most important causes of disease in foals between 1 and 5 months of age and has emerged as a significant opportunistic pathogen of immunosuppressed people, especially those infected with the human immunodeficiency virus (1, 5, 11, 17). Infection in either species is most commonly characterized by a life-threatening pyogranulomatous pneumonia. Other, less-common clinical manifestations of R. equi infections in foals include ulcerative enterocolitis, colonic or mesenteric lymphadenopathy, immune-mediated synovitis and uveitis, osteomyelitis, and septic arthritis (7). R. equi is widespread in the environment of horse-breeding farms. Unlike most environmental isolates of R. equi, strains isolated from pneumonic foals typically contain 85- or 90-kb plasmids encoding a highly immunogenic, lipid-modified, virulence-associated protein (VapA) (23, 25, 29, 32, 33, 34). VapA is located on the bacterial surface, and its expression is thermoregulated, occurring between 34 and 41°C, temperatures encountered in vivo (26, 27). Because vapA has no significant homology with other known bacterial genes, one can only speculate on the function of the protein.
Study of the virulence of R. equi has been complicated by the fact that typical granulomatous lung lesions have not been consistently reproduced in any immunocompetent animal species other than young horses. The normal murine lung can progressively clear an inoculum of R. equi sufficient to induce severe pneumonia in foals (36). Nevertheless, a murine intravenous 50% lethal dose infection model has demonstrated that plasmid-cured derivatives of R. equi show a dramatic decrease in lethality (32). However, the role of the 85-kb plasmid in the pathogenesis of R. equi infections in foals has not been definitively addressed. Furthermore, it remains to be established whether VapA is a true virulence determinant or merely a marker for virulence plasmid possession.
As the basis for the work described here, we hypothesized that the 85-kb plasmid of R. equi is essential for virulence for both mice and foals. Moreover, we questioned whether expression of VapA alone was sufficient for virulence. We addressed the necessity of the 85-kb plasmid by infection of macrophages, mice, and foals with either a virulent strain of R. equi containing an 85-kb plasmid and expressing VapA or infection with its plasmid-cured derivative. Electroporation of the plasmid-cured derivative with a shuttle plasmid in which vapA was subcloned and expressed and infection studies with the recombinant strain allowed us to evaluate whether VapA expression was sufficient for virulence.
MATERIALS AND METHODS
Bacteria.
R. equi 238, 2+, and 103+, originally isolated from pneumonic foals, were used. All three strains contain an 85-kb plasmid and produce VapA (3, 12). The plasmid-cured VapA-negative isogenic derivatives of strains 103+ and 2+ (designated strains 103− and 2−, respectively) were also used (3). R. equi 103−/415-VapA was created by the electroporation of strain 103− with pMH1. pMH1 was created by subcloning a 1.6-kb vapA-containing fragment of the R. equi virulence plasmid into pYUB415 which had been previously digested with EcoRV and BamHI. R. equi 103−/415 was created by the electroporation of strain 103− with pYUB415 (without an insert), and this strain was used as a control in infection experiments. pYUB415 is a 9,301-bp Mycobacterium-Escherichia coli shuttle plasmid kindly provided by William Jacobs (Albert Einstein College of Medicine, Bronx, N.Y.). This vector contains an origin of replication derived from the pAL5000 plasmid of Mycobacterium fortuitum subsp. fortuitum (14). It also contains an E. coli origin of replication, along with ampicillin and hygromycin resistance genes for selection in E. coli and Mycobacterium spp., respectively. Hygromycin (150 to 175 μg/ml) is also used for selection in R. equi. VapA expression by strain 103−/415-VapA was confirmed by immunoblotting and flow cytometry with a monoclonal antibody (MAb) specific for the VapA protein. Aliquots of the three strains were stored at −70°C.
Plasmid isolation.
Plasmid DNA was isolated from R. equi by using the Qiagen plasmid isolation system (Qiagen, Inc., Chatsworth, Calif.). Samples were run on 0.7% agarose gels, and plasmids were visualized by ethidium bromide staining.
Electroporation of R. equi.
Bacteria were grown in brain heart infusion (BHI) broth to an optical density at 600 nm of 0.6 of 0.8. Bacteria were pelleted and then washed twice with an equal volume of cold 10% glycerol in distilled water. The final resuspension of the bacterial cells (in cold 10% glycerol) was at a 1:20 dilution of the original culture volume. Then, 400 μl of the cells with 0.5 to 1.0 μg of DNA was placed in a prechilled 0.2-cm electroporation cuvette (Bio-Rad, Melville, N.Y.). Electroporation was performed by using a Gene Pulser (Bio-Rad) set at 2.5 kV, 25 μF, 1,000 Ω, and a single pulse. Immediately after electroporation, 1 ml of BHI broth supplemented with 0.5 M sucrose was added to the cuvette. Bacteria were then incubated for 1 to 2 h at 37°C and subsequently plated on BHI agar with 175 μg of hygromycin per ml.
Immunoblotting.
Immunoblotting for detection of VapA was done as previously described with a mouse MAb to VapA (MAb103) (34).
Flow cytometry.
After overnight culture at 37°C in BHI or Mueller-Hinton broth, bacteria were washed twice and resuspended in cation-free Dulbecco phosphate-buffered saline (PD) containing 1% bovine serum albumin (BSA). Approximately 108 CFU of bacteria were incubated at 4°C with a 1:2 dilution of hybridoma culture supernatant containing MAb103. After being washed with PD containing 1% BSA, bacteria were stained by a 45-min incubation at 4°C with a 1:100 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG; Jackson ImmunoResearch, West Grove, Pa.). Bacteria were then washed three times with PD supplemented with 1% BSA, fixed in 1% paraformaldehyde, and analyzed on an Epics Elite Flow Cytometer (Coulter Diagnostics, Hialeah, Fla.). Negative controls included the addition of secondary antibody without prior incubation in primary antibody and the use of an isotype-matched, irrelevant primary antibody (MAb29.1, murine anti-human Mac-1).
Assessment of shuttle vector stability during in vitro culture.
Flow cytometry was used to assess the stability of VapA expression by a recombinant strain of R. equi in the presence or absence of antibiotic pressure. R. equi 103−/415-VapA was cultured in 10 ml of BHI broth with 150 μg of hygromycin per ml at 37°C. After a 24-h incubation, 200 μl of culture was transferred into 10 ml of fresh BHI broth with or without hygromycin and grown for another 24 h. The remainder of the culture was centrifuged, and the bacterial pellet was frozen for later flow cytometric analysis. This process was repeated every 24 h for 5 days. To detect the presence or absence of VapA on the surface of the bacteria, frozen bacterial pellets were thawed and washed once with PD. Pellets were then stained as described above in the description of methods for flow cytometry.
Macrophages and macrophage cell lines.
Murine resident peritoneal macrophages were washed from the peritoneal cavity of adult female BALB/c mice with cold PD. Cells were resuspended in Dulbecco modified Eagle medium (DMEM) (Gibco-BRL, Grand Island, N.Y.) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and penicillin G-streptomycin (100 U and 100 μg per ml, respectively) (D-10). A total of 5 × 105 peritoneal cells were placed on 13-mm-diameter glass coverslips in 24-well plates. The cells were allowed to adhere for 1 h at 37°C, washed with warm DMEM, and incubated an additional 2 h at 37°C in D-10 medium. At that time the cells were washed again and then cultured overnight in antibiotic-free DMEM supplemented with 10% FCS and 2 mM glutamine. After washing and overnight incubation, approximately 105 cells remained per coverslip.
J774A.1 murine monocyte-macrophage-like cells (TIB 67) were obtained from the American Type Tissue Culture Collection (Rockville, Md.) and were maintained in D-10 medium. To prepare them for use in bacterial intracellular growth assays, the cells were removed from tissue culture plates by washing in phosphate-buffered saline (PBS) containing 5 mM EDTA, along with gentle scraping. The cells were resuspended in antibiotic-free D-10 medium and irradiated with 5,000 rads. It was necessary to irradiate the cells to inhibit their replication because continued cell division resulted in an artificial decrease in the number of bacteria per cell. After irradiation, cells were washed once with DMEM and then resuspended in D-10 medium. Cells (105) were cultured overnight on glass coverslips in 24-well plates and infected the following day.
Bacterial intracellular growth assay.
Overnight broth cultures of bacteria at a density of ca. 108 CFU/ml were washed twice with PD and resuspended in phagocytosis buffer (PB), which consisted of 0.1% gelatin in equal parts of Medium 199 (Gibco) and DMEM, containing 12.5 mM HEPES. Macrophage monolayers were washed once with warm DMEM, and the medium was replaced with PB. Fresh normal mouse serum providing a source of complement components was added to the wells at a final concentration of 5%. Bacteria were added at a multiplicity of infection of 5 to 20 bacteria per macrophage. Bacterial incubation with macrophages proceeded for 30 min at 37°C, and then the monolayers were washed with PB to remove unbound bacteria. After an additional 30-min incubation period to allow bound bacteria to be internalized, the monolayers were washed again, and the medium was replaced with DMEM supplemented with 10% FCS, 2 mM glutamine, and 1 to 10 μg of gentamicin sulfate per ml. In preliminary experiments, these concentrations of gentamicin killed extracellular bacteria while minimally affecting intracellular organisms. At various times postinfection, parallel monolayers were fixed with 100% methanol (20 min at 4°C) and then repeatedly washed with PD containing 5% FCS. The fixed monolayers were incubated for 45 min with a 1:200 dilution of rabbit polyclonal anti-R. equi antiserum in PD supplemented with 5% FCS. The coverslips were then washed four times with PD with 5% FCS. The bacteria associated with the macrophage monolayers were stained by a 45-min incubation with FITC-conjugated goat anti-rabbit IgG (heavy- and light-chain specific; Jackson ImmunoResearch). The cells were then washed four more times with PD supplemented with 5% FCS and examined by fluorescence microscopy. Two hundred macrophages per coverslip were counted, and the number of bacteria associated with those cells was determined. Because of the difficulty in reliably quantifying large bacterial numbers within an individual macrophage, any cell containing more than 10 R. equi bacteria was simply scored as having 10 bacteria. The number of macrophages containing 10 or more bacteria was also determined at each time point.
Infection of mice.
Female BALB/c mice were obtained from either the National Cancer Institute (Frederick, Md.) or Jackson Laboratories (Bar Harbor, Maine). Mice were received at 6 weeks of age and were used when they were between the ages of 7 and 16 weeks. In preparation for the infection of mice, frozen aliquots of the bacterial strains were thawed and grown for 3 h at 37°C in Mueller-Hinton broth. Bacteria were pelleted and resuspended in PBS. Groups of mice were infected intravenously through the tail vein with either strain 103+, 103−/415, or 103−/415-VapA. The total number of bacteria injected was confirmed retrospectively by dilution plating of the injection stock. The inocula actually administered were 5 × 106 CFU for strain 103+ and 2 × 106 CFU for strains 103−/415 and 103−/415-VapA. At various times postinfection, five mice from each group were euthanized, and their spleens and livers were removed. Each organ was placed in 10 ml of sterile H2O and homogenized with a tissue probe homogenizer (Tekmar, Cincinnati, Ohio). Serial 10-fold dilutions of the homogenate were plated onto chocolate agar without antibiotic or onto BHI agar with or without hygromycin. CFU counts were determined after 36 h of incubation at 37°C.
Infection of foals.
Twenty-eight healthy mixed-breed pony foals were used in this study. These foals were used in conjunction with another study on cell-mediated immunity to R. equi. Adequate passive transfer of immunoglobulin was confirmed in foals 12 to 24 h after birth by using an enzyme-linked immunosorbent assay (ELISA) kit for semiquantitative measurement of total IgG (Cite Test; Idexx Laboratories, Westbrook, Maine). Foals were reared with their mothers on pasture and were monitored weekly for seroconversion to R. equi by ELISA as previously described (19). At 18 to 23 days of age, foals were moved with their dams to individual box stalls in an isolation facility. Criteria for inclusion in the study were normality in physical examination, lung sounds on auscultation, temperature, radiographs of the lungs, and lack of seroconversion to R. equi by ELISA. Foals meeting these criteria were randomly assigned to four experimental groups and infected 1 or 2 days after arrival in the isolation facility. There were no differences in IgG titers against R. equi between groups at the time of infection.
For experimental infection of foals, aliquots of the three bacterial strains were grown on Trypticase soy agar (TSA) plates alone (strains 103+ and 103−) or on TSA plates containing 150 μg of hygromycin per ml (strain 103−/415-VapA) for 48 h at 37°C. Bacteria were harvested with 4 ml of sterile PBS per plate, the optical density of the resulting suspension was read at 540 nm, and the bacterial concentration was estimated from a standard curve. The bacterial suspension was diluted with sterile PBS to a final concentration of 5 × 107 bacteria/ml. The concentration of the inoculum actually administered was confirmed retrospectively by counting the CFU. Foals were sedated with 0.5 mg of xylazine hydrochloride (Rompun; Bayer, Inc., Etobicoke, Ontario, Canada) and 0.07 mg of butorphanol tartrate (Torbugesic; Ayerst Laboratories, Montreal, Quebec, Canada) per kg of body weight given intravenously. A flexible fiberoptic endoscope was used to deliver 1.25 × 109 bacteria suspended in 25 ml of sterile PBS into both main bronchi (total dose, 2.5 × 109 bacteria in 50 ml of PBS). Eight foals were infected with strain 103+, eight foals were infected with strain 103−, six foals were infected with strain 103−/415-VapA, and six foals received only PBS and were used as controls. The day of infection was designated as day 0. Baseline values for heart rate, respiratory rate, temperature, fibrinogen concentration, and leukocyte count were obtained on day 0 prior to sedation. Foals were clinically assessed based on daily complete physical examinations and twice-daily heart rate, respiratory rate, and temperature recordings. Leukocyte counts and fibrinogen concentrations were assessed every second day. Half of the foals in each group (strains 103+, 103−, and 103−/415-VapA and controls) were euthanized at 3 days postinfection, and the remaining half were euthanized at 14 days postinfection. However, one foal infected with strain 103+ was euthanized for humane reasons at 12 days postinfection. Euthanasia was performed by intravenous administration of a lethal dose of pentobarbital sodium.
Both lungs from each animal were weighed, and the lung weight/body weight ratio was calculated. All organs were examined grossly, and representative samples of normal and diseased lungs, bronchial lymph nodes, trachea, heart, thymus, kidneys, adrenals, spleen, liver, thyroid, stomach, duodenum, ileum, jejunum, cecum, large colon, synovial membrane, and colonic and mesenteric lymph nodes were fixed in 10% buffered formalin. The fixed tissues were embedded in paraffin, sectioned at 10 μm, stained with hematoxylin and eosin, and examined histologically. The pathologist was blinded as to the source of the tissue sample. The number of viable R. equi in four dispersed and preselected loci of both lungs was enumerated by culturing serial dilutions of lung homogenates on TSA plates (strains 103+, 103−, and 103−/415-VapA and controls) or TSA plates containing 150 μg of hygromycin (103−/415-VapA) per ml and counting the CFU. The eight sites represented the craniodorsal, cranioventral, middle dorsal, and caudodorsal parts of each lung. Results were expressed as the mean ± the standard deviation (SD) of the log10 CFU per gram of lung tissue. In each foal infected with strain 103−/415-VapA+, 10 randomly selected colonies were subcultured and analyzed for VapA expression by immunoblotting. Representative samples from the spleen and liver were also homogenized and cultured. Synovial fluid was collected from the left and right tibiotarsal and femoropatellar joints for bacterial cultures.
Immunohistochemistry for VapA expression.
Lung samples from foals infected with strains 103+, 103−, or 103−/415-VapA and euthanized 3 days postinfection were collected from the cranioventral lung lobes. Frozen 5-μm sections were placed on Superfrost Plus Slides (Fisher Scientific, Nepeon, Ontario, Canada), air dried, and fixed in 10% buffered formalin for 10 min. Endogenous peroxidases were quenched by incubation in 0.3% H2O2 in 100% methanol. The slides were rinsed three times in PBS, air dried, and incubated for 20 min with 10% normal rabbit serum as the blocking agent. The sections were incubated for 1 h with MAb103. After three rinses in PBS, the slides were incubated for 30 min with peroxidase-conjugated rabbit anti-mouse IgG (H+L; Jackson Immunoresearch Laboratories) diluted 1:500 in PBS. The slides were washed three times in PBS and stained by using the AEC Chromagen Kit (Sigma, St. Louis, Mo.) according to the manufacturer’s instructions. The lung sections were counterstained with 0.5% methyl green (in 0.1 M sodium acetate) for 3 min. Ten lung sections per foal were examined. The examiner was unaware of the experimental group.
Statistical analysis.
The foal data were analyzed by using the SAS general linear model procedure (22a). Least-square means were calculated by using the general linear model procedure, and comparison between bacterial groups at each time point was done by performing a t test on the least-square means.
RESULTS
Role of the 85-kb plasmid of R. equi in intracellular survival and replication in macrophages.
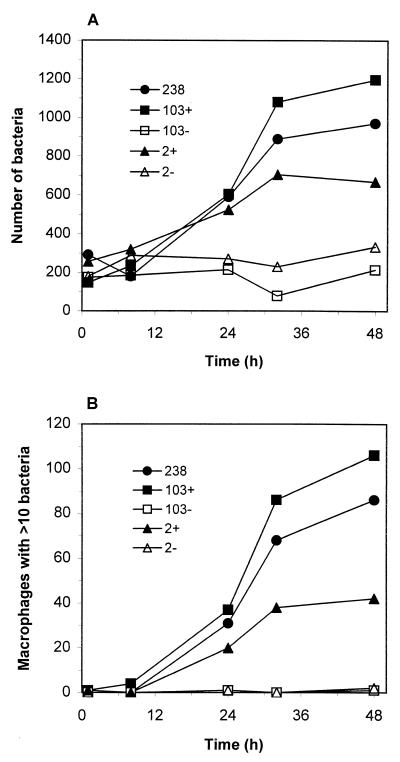
The virulent R. equi strains 2+ and 103+ containing an 85-kb plasmid and expressing VapA, together with their plasmid- cured isogenic derivatives 2− and 103−, were compared with respect to intracellular survival and replication within macrophages in vitro. A previously characterized (12) clinical isolate, strain 238, was used as a positive control in these experiments. Murine peritoneal macrophages were infected with R. equi for 30 min, and then unbound bacteria were removed by repeated washing. Gentamicin sulfate was then added to kill any remaining extracellular organisms. We had previously determined that the traditional CFU assay was not an accurate measure of intramacrophage rhodococcal growth due to bacterial chaining which occurred during replication (12). Thus, bacterial growth was monitored by using fluorescence microscopy and counting the number of bacteria associated with 200 macrophages and the number of macrophages containing 10 or more bacteria. The plasmid-containing isolates 238, 103+, and 2+ replicated efficiently within macrophages, increasing in number by three- to sixfold by 48 h postinfection (Fig. 1A). Likewise, the number of macrophages containing 10 or more bacteria increased with time postinfection (Fig. 1B). At the beginning of the experiment few, if any, of the infected macrophages contained as many as 10 bacteria of any strain. Forty-eight hours later, however, 56% of strain 103+-infected macrophages, 48% of strain 238-infected macrophages, and 21% of strain 2+-infected macrophages contained 10 or more R. equi. In contrast to the plasmid-positive strains, plasmid-cured derivatives failed to replicate in macrophages and bacterial numbers remained relatively constant throughout the infection (Fig. 1A). At 48 h few, if any, macrophages infected with the plasmid-cured strains contained as many as 10 organisms. In addition, bacterial growth and viability, assessed by evaluating the ability of the bacteria to incorporate tritiated uracil as described previously (12), yielded similar results (data not shown).
FIG. 1.
Infection of murine peritoneal macrophages with isogenic strains of R. equi. At various times postinfection, macrophage monolayers were fixed with methanol, and the bacteria were immunostained and visualized by fluorescence microscopy. (A) The numbers of bacteria (ordinate) associated with 200 macrophages were visually counted by fluorescence microscopy. (B) The numbers of macrophages with 10 or more bacteria were also recorded. Each strain was evaluated a minimum of three times with similar results.
Differential fixation of parallel macrophage monolayers with either paraformaldehyde or methanol as previously described (12) revealed that the lack of intracellular growth exhibited by the plasmid-cured strains was not the result of decreased phagocytosis on the part of the macrophage (data not shown). In addition, the failure of the virulence plasmid-cured strains to grow inside macrophages is not the result of some widespread replication defect or auxotrophy since both plasmid-positive and plasmid-negative strains grew well in both bacterial culture media or DMEM supplemented with 10% FCS, as well as on minimal agar plates containing only salts, glycerol as a carbon source, and MgSO4 (data not shown).
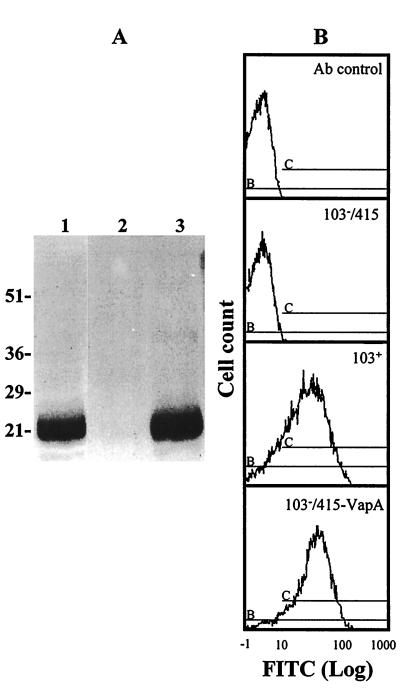
R. equi 103−/415-VapA expresses VapA as well as the wild-type strain 103+ and is stable in vitro.
A recombinant strain of R. equi was constructed to determine if expression of plasmid-encoded VapA is sufficient to promote replication within macrophages and virulence for both mice and foals. R. equi 103−, the plasmid-cured derivative of virulent R. equi 103+, was electroporated with pMH1, a shuttle vector in which vapA was subcloned, creating the strain 103−/415-VapA. Expression of the VapA protein by the recombinant strain 103−/415-VapA was confirmed by both immunoblotting (Fig. 2A) and quantitative flow cytometry (Fig. 2B). Immunoblotting also verified that the recombinant protein was of the correct molecular weight (Fig. 2A). The recombinant VapA protein was expressed on the bacterial surface at levels comparable to that of wild-type (Fig. 2B). The flow cytometric profile of the wild-type isolate 103+ showed that 81% of the bacterial population expressed VapA with a mean fluorescence intensity (MFI) of 7.4, while 89% of the recombinant strain 103−/415-VapA population expressed VapA with an MFI of 10.3. In addition, R. equi 103− electroporated with the pYUB415 vector only (strain 103−/415) exhibited a fluorescence profile indistinguishable from that of strain 103+ stained with an irrelevant antibody (Fig. 2B). The expression of VapA by the recombinant strain 103−/415-VapA, as assessed by flow cytometric analysis, remained constant over several subsequent passages in vitro, regardless of whether the recombinant strain was cultured in the presence or absence of hygromycin (Table 1). Thus, in the short term, antibiotic pressure was not necessary to maintain possession of the recombinant plasmid.
FIG. 2.
Expression of VapA by strains of R. equi. (A) Immunoblot of R. equi with a MAb against VapA. Lanes: 1, recombinant strain 103−/415-VapA; 2, strain 103−; 3, strain 103+. Sizes of protein molecular mass markers are indicated to the left in kilodaltons. (B) Flow cytometry profile showing the expression of VapA on the surface of R. equi. Bacteria were grown in liquid broth, washed, and stained with a MAb to VapA. The relative mean fluorescence values of wild-type isolate 103+, pYUB415 vector-electroporated plasmid-cured derivative (103−/415), and the recombinant pMH1-electroporated strain 103− (103−/415-VapA) were compared. In addition, all analyses included a comparison to bacteria stained with an irrelevant MAb (Ab control).
TABLE 1.
In vitro stability of VapA expression in recombinant R. equi
| Passage | % Positive (MFI)a for VapA in strain:
|
|||
|---|---|---|---|---|
| 103+ | 103−/415 | 103−/415-VapAb
|
||
| With hygromycin | Without hygromycin | |||
| 2 | 88.7 (8.64) | 3.4 (1.26) | 85.9 (6.55) | 83.3 (6.78) |
| 3 | 86.2 (8.39) | 3.7 (1.19) | 84.8 (5.95) | 82.6 (5.52) |
| 4 | 85.4 (8.06) | 1.6 (1.18) | 85.2 (5.46) | 86.8 (5.58) |
| 5 | 83.2 (8.53) | 1.6 (1.19) | 86.3 (6.11) | 85.7 (5.40) |
Percentage of bacterial cells staining positively with MAb103 minus cells stained with an irrelevant control antibody. MFI values are given as the log mean fluorescence intensity of positively gated cells.
Bacterial samples were taken from cultures grown with or without 150 μg of hygromycin per ml.
Infection of macrophages with the recombinant strain that expresses VapA.
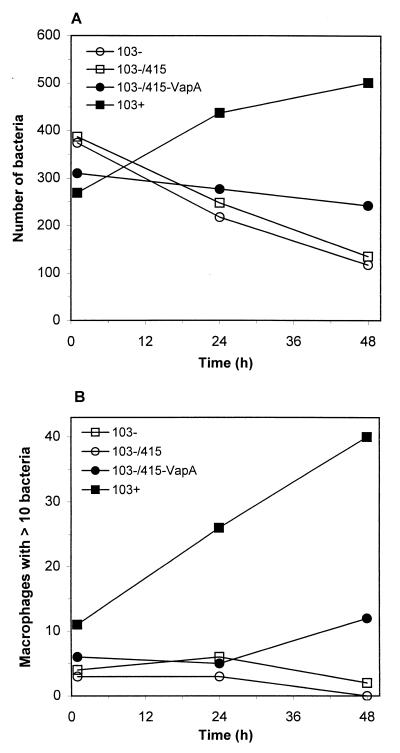
To assess whether the expression of the VapA protein is the sole determinant of R. equi intracellular survival and growth potential, parallel J774A.1 macrophage monolayers were infected with either wild-type strain 103+, its plasmid-cured derivative strain 103−, the virulence plasmid-cured, vector-electroporated strain 103−/415, or the virulence plasmid-negative, recombinant VapA-expressing strain 103−/415-VapA. Whereas the wild-type isolate 103+ exhibited the ability to replicate intracellularly, complementation of the virulence plasmid-cured strain 103− with vapA did not restore the capacity of this strain to grow inside macrophages (Fig. 3). The number of bacteria associated with macrophages, as well as the number of macrophages containing 10 or more bacteria, increased with time postinfection for monolayers infected with wild-type strain 103+ (Fig. 3). In contrast, the numbers of the recombinant strain 103−/415-VapA associated with macrophages remained static or decreased over time and were more similar to intracellular growth curves displayed by the virulence plasmid-cured strain 103−.
FIG. 3.
Infection of macrophages with the VapA-expressing recombinant strain. Bacteria were added to J774A.1 cells, a murine monocyte-macrophage-like cell line. At 1, 24, and 48 h postinfection, parallel monolayers were fixed, and bacteria were immunostained and visualized by fluorescence microscopy. Bacterial growth is expressed as both the total number of bacteria per 200 macrophages (A) and the number of macrophages (of 200 counted) containing 10 or more bacteria (B). Each data point represents the average of data from two coverslips. This figure is representative of four independent experiments.
In vivo infection of mice with R. equi 103+, 103−/415, or 103−/415-VapA.
We next determined whether expression of VapA would affect clearance of the bacteria in vivo. Immunocompetent BALB/c mice were intravenously infected with either R. equi 103+, 103−/415, or 103−/415-VapA, and the number of bacteria in the livers and spleens of these mice was quantified. In preliminary studies with a virulence plasmid-positive clinical isolate, it was determined that bacterial burdens in infected mice increased for the first 5 days postinfection and then decreased over the next week (data not shown). Thus, we limited our comparisons to the first 5 days postinfection. On day 5 postinfection, the mean bacterial counts in the livers and spleens of mice infected with the 85-kb plasmid- containing strain 103+ were 7.51 ± 0.24 and 7.62 ± 0.15 log10/g, respectively, whereas R. equi could not be recovered from 103−/415- and 103−/415-VapA-infected mice. Clearance of strain 103−/415-VapA was not the result of a loss of the recombinant plasmid in vivo, since all of the bacteria recovered on days 2 and 3 postinfection were hygromycin resistant (data not shown).
In vivo infection of foals.
Because virulence assessed by intravenous inoculation of an R. equi-resistant species such as mice may not necessarily reflect virulence in young horses which are naturally challenged by the respiratory route, studies were also performed in this native host species. To determine the role of the 85-kb plasmid and VapA in virulence of R. equi for foals, the virulent strain 103+ containing an 85-kb plasmid and expressing VapA, its plasmid-cured derivative strain 103−, and the plasmid-cured derivative containing a shuttle plasmid in which the vapA gene was subcloned (strain 103−/415-VapA) were used to intrabronchially infect pony foals. Noninfected foals kept under the same conditions were used as controls.
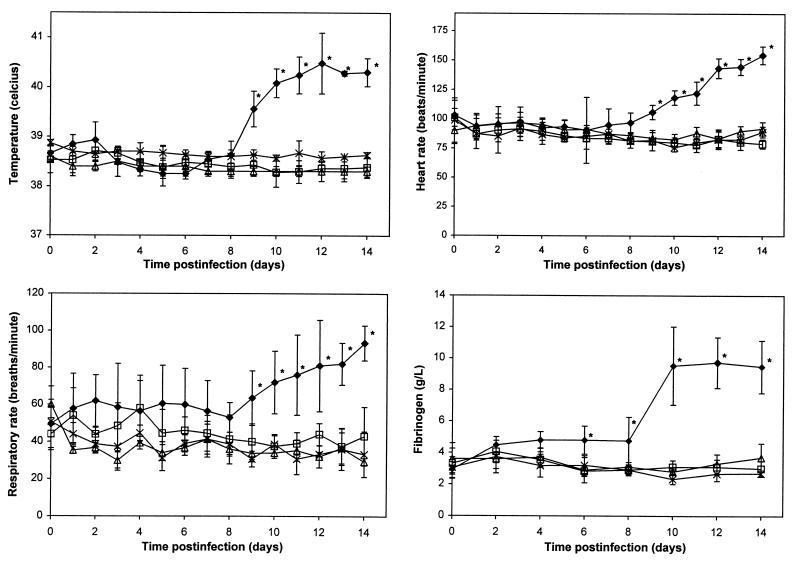
Beginning 9 days postinfection, foals infected with strain 103+ developed temperatures, heart rates, and respiratory rates significantly highter than their baseline values (day 0) and significantly higher than those of the animals of the other three experimental groups. In addition, starting at day 6, these same foals developed significantly higher fibrinogen concentrations (Fig. 4). The leukocyte counts of foals infected with strain 103+ were significantly higher than those of the other three groups only on days 6 and 8 postinfection. The heart rates, respiratory rates, temperatures, leukocyte counts, and fibrinogen concentrations of foals infected with strain 103− or 103−/415-VapA did not differ significantly from the baseline values or from the values of the control foals (Fig. 4). All foals infected with strain 103+ developed mild to moderate bilateral effusion of the hocks and stifles starting between day 6 and day 11 postinfection. Two foals infected with strain 103+ also developed bilateral effusion of the fetlocks and carpi. Despite effusion of multiple joints, the foals were not lame. Neither the foals infected with strain 103− or 103−/415-VapA nor the controls developed joint effusion.
FIG. 4.
Temperatures, heart rates, respiratory rates, and fibrinogen concentrations of control foals (▵) and foals infected with R. equi 103+ (⧫), 103− (□), or 103−/415-VapA (×). Values are shown as the means ± SD (error bars) within each group. An asterisk indicates that P is <0.05 compared to baseline values for the same group and the other three groups at the same time point.
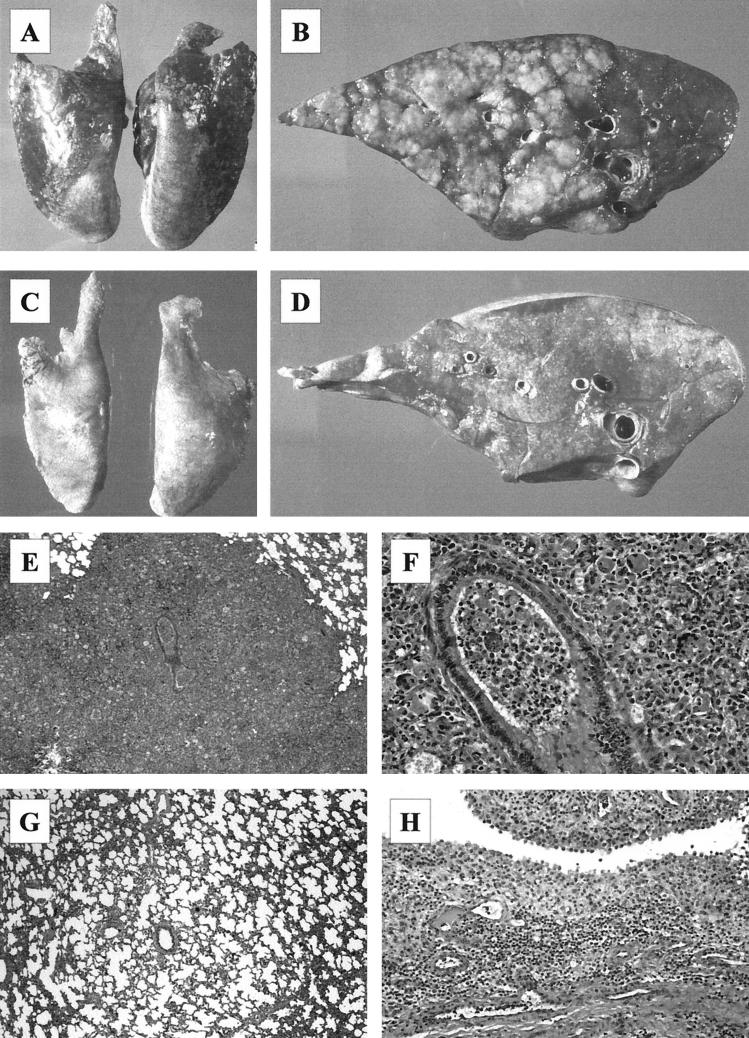
Immediately after euthanasia, the foals were subjected to a complete postmortem examination. Significant gross pathological findings were observed only in the thoracic cavity. All of the foals infected with strain 103+ had gross lung lesions ranging from mild to moderate consolidation of the cranioventral lobes on day 3 to severe consolidation involving 60 to 70% of the lung area on day 14 (Fig. 5A). On the cut section, the consolidated parenchyma comprised multiple, well-defined, tan nodular lesions up to 5 cm in diameter (Fig. 5B). Some of the nodules contained a central area of caseous necrosis. The bronchial lymph nodes of 103+-infected foals were slightly enlarged on day 3 and markedly enlarged on day 14. The lungs and bronchial lymph nodes of foals infected with strain 103− or 103−/415-VapA or of the PBS-treated controls were grossly normal (Fig. 5C and D). On day 3 postinfection the lung weight/body weight ratios of foals infected with R. equi 103+, 103−, or 103−/415-VapA were not significantly different from those of the control foals. On day 14, the lung weight/body weight ratios of foals infected with strain 103+ (4.58 ± 0.96%) were significantly greater than those of the other three groups (P < 0.0001). The ratios of foals infected with 103− (1.01 ± 0.13%) or 103−/415-VapA (1.09 ± 0.07%) were not significantly different from those of the control foals (1.13 ± 0.07%).
FIG. 5.
Pathological findings in foals infected intrabronchially with R. equi 103+ or 103−. (A) Severe bilateral consolidation of the lungs on day 14 postinfection in a foal infected with R. equi 103+. (B) Cross section of a cranioventral lung lobe in a foal infected with R. equi 103+ showing multiple well-defined nodular areas of pulmonary consolidation on day 14 postinfection. (C) Lungs of a foals infected with R. equi 103− showing no gross lesions. (D) Cross section of the lung shown in panel C. (E) Extensive bronchopneumonia in the lungs of a strain 103+-infected foal on day 14. Magnification, ×10. (F) Higher magnification of the lesion shown in panel A showing pyogranulomatous inflammation. Magnification, ×68. (G) Mild atelectasis and hypercellularity of the alveolar septi in a strain 103−-infected foal on day 14. Magnification, ×12. (H) Severe inflammation of the synovial membrane in the hock of a strain 103+-infected foal on day 14. Magnification, ×38.
All foals infected with R. equi 103+ developed histologic lesions of suppurative to pyogranulomatous bronchopneumonia. Lesions on day 3 postinoculation comprised areas of patchy to diffuse consolidation in which alveoli were distended with neutrophils and alveolar macrophages. Some bronchioles contained suppurative exudate. Lesions were more pronounced on day 14 postinoculation, with more intense bronchiolar inflammation and a more pronounced granulomatous component, including the presence of multinucleated giant cells (Fig. 5E and F). Bronchial associated lymphoid tissue (BALT), which was not apparent in the saline-treated controls, was moderately hyperplastic on day 3 and markedly hyperplastic in most of the foals euthanized on day 14. Foals infected with R. equi 103− developed minimal lesions: chiefly, mild atelectasis and a slight hypercellularity of the interalveolar septa (Fig. 5G). Isolated microscopic lesions were detected in three foals euthanized at day 14. In two of these the lesions were microscopic foci of resolving bronchopneumonia, and in one the lesion was a small granuloma. Mild to moderate hyperplasia of BALT was evident in the lungs of 103−-infected foals killed 14 days after infection. The lungs of four of the foals infected with R. equi 103−/415-VapA were indistinguishable microscopically from those of the PBS-treated controls. In two foals, one euthanized at day 3 and one at day 14, there were focal microscopic granulomatous lesions with rare giant cell formation. Mild BALT hyperplasia was noted in one foal. Apart from lung lesions, the most significant difference between foals infected with 103+ and the three remaining experimental groups was the development of significant suppurative synovitis in those foals euthanized 14 days postinfection (Fig. 5H).
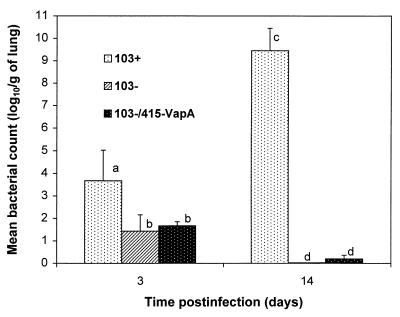
On day 3 postinfection, the mean number of R. equi in the lungs of foals infected with strain 103+ was significantly higher than in those infected with strain 103− or strain 103−/415-VapA (Fig. 6). On day 14, the strain 103+ numbers had increased significantly whereas, by contrast, strain 103− could no longer be cultured. Small numbers of strain 103−/415-VapA were cultured from the lungs of only one foal infected with that strain. Both on day 3 and on day 14, the mean bacterial numbers in the lungs of foals infected with strain 103− were not significantly different from those of foals infected with 103−/415-VapA (Fig. 6). R. equi was not cultured from the control foals. R. equi was cultured from the spleens of three of four foals infected with strain 103+ and euthanized 14 days postinfection. R. equi was also cultured from the liver of one of those foals and from at least one joint in all four foals. R. equi was not cultured from the spleens, livers, or synovial fluids of foals infected with strain 103− or strain 103−/415-VapA, and it was not cultured on day 3 from foals infected with strain 103+.
FIG. 6.
Bacterial numbers in the lungs of foals infected with R. equi 103+, 103−, or 103−/415-VapA. Numbers of R. equi in four dispersed and preselected areas of both lungs were determined by culturing serial dilutions of lung homogenates and counting the CFU. Letters differing between bacterial groups or time points indicate a statistically significant difference in bacterial numbers (P < 0.01).
Strain 103−/415-VapA is stable and expresses VapA in vivo.
To confirm that the lack of virulence of strain 103−/415-VapA was not caused by a loss of the shuttle plasmid in vivo, the lung homogenates of foals infected with that strain were cultured on TSA plates with or without hygromycin. On day 3, the mean bacterial count on hygromycin-containing plates (1.67 ± 0.18 log10 CFU/g of lung) was not significantly different from that obtained on plates lacking hygromycin (1.70 ± 0.17). On day 14, the mean bacterial count on hygromycin-containing plates was 0.11 ± 0.16, whereas R. equi was not cultured from plates lacking hygromycin. Ten R. equi colonies from each foal infected with strain 103−/415-VapA and euthanized on day 3 (five from plates with hygromycin and five from plates without hygromycin), and all of the colonies obtained from foals euthanized on day 14 were subcultured and analyzed for VapA expression by immunoblotting. They all produced strong VapA expression.
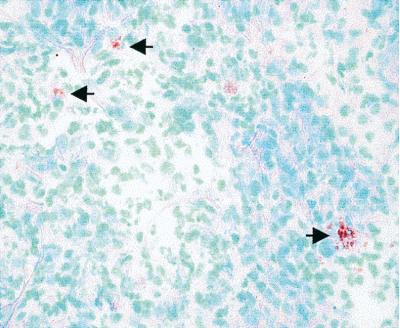
To confirm that strain 103−/415-VapA also expressed VapA in vivo, frozen lung sections from foals euthanized on day 3 were analyzed by immunohistochemistry by using a primary MAb to VapA. 103+-infected foals had large numbers of VapA-expressing R. equi in their lungs. The lungs of foals infected with 103−/415-VapA were also positive for VapA expression (Fig. 7). VapA expression was not detected in the lungs of foals infected with strain 103−.
FIG. 7.
Immunohistochemical staining of the lungs of a foal infected with strain 103−/415-VapA showing VapA expression in vivo (arrows). Magnification, ×400.
DISCUSSION
Several facultative intracellular pathogens, including Shigella spp., Salmonella spp., and Yersinia spp., possess large plasmids encoding a variety of virulence loci (10, 22, 24). Among other important contributions to virulence, these large plasmids play a role in invasion, in intracellular survival, and/or in the ability to cause systemic infections (10, 22, 24). The 85-kb plasmid of R. equi is of particular interest because of its thermoregulation of a virulence-associated protein and its likely role in response to other environmental influences, including pH (26, 27). The present study shows that the 85-kb plasmid of R. equi is essential for intracellular survival and replication in macrophages (Fig. 1). We demonstrated that loss of the plasmid affected bacterial replication in macrophages not only for strain 103 but also for strain 2, suggesting that this is a general effect of plasmid loss. In a recent study, strain 103+ was somewhat resistant to phagocytosis but could survive in macrophages (8). In contrast, strain 103− was phagocytized extensively and rapidly cleared by macrophages (8). In mice, functional T lymphocytes are absolutely required for in vivo clearance of plasmid-containing virulent strains of R. equi, and T lymphocyte-deficient athymic nude mice develop a severe granulomatous pneumonia (15). In contrast, T lymphocyte-deficient athymic nude mice can progressively clear infection with plasmid-cured derivatives (15). These in vivo results in mice support the in vitro findings reported here that plasmid-cured derivatives fail to grow in macrophages and are cleared by innate defense mechanisms such as growth inhibition or killing by macrophages.
Because virulence in an R. equi-resistant species such as the mouse might not necessarily reflect virulence in the natural host, we elected also to assess virulence in foals. In the present study, the plasmid-cured derivative of a virulent R. equi strain failed to induce bronchopneumonia in foals and was rapidly cleared from the lungs within 2 weeks of infection. In contrast, the parent plasmid-containing strain induced a severe granulomatous pneumonia and replicated considerably in the lungs of infected foals (Fig. 5 and 6). These results show that the 85-kb plasmid of R. equi is absolutely required for its virulence in foals, its only naturally occurring immunocompetent host. However, because a concomitant chromosomal mutation in strain 103− cannot be totally excluded, irrefutable proof awaits reintroduction of the plasmid into the plasmid-cured strain with full restoration of virulence. The lack of phenotypic markers other than VapA, however, makes selection of transformants extremely difficult (4). Wada et al. (35) recently reported the lack of clinical signs and pathology at 28 and 42 days postinfection in two foals infected with the plasmid-cured derivative of a different strain of R. equi, supporting the conclusion that the lack of virulence of strain 103− is due to plasmid loss rather than to chromosomal mutation. However, the present study is more definitive and extends these findings by the use of a different strain and considerably larger numbers of foals which were not colostrum deprived and which were all infected at the same age rather than between 27 and 83 days of age. In addition, this study demonstrated the rapid clearance of the plasmid-cured strain, a finding which contrasted with the progressive increase in numbers of the plasmid-positive parent strain.
Previous models of experimental infection of foals with R. equi have yielded equivocal results. In some studies, aerosol infection of foals with R. equi resulted in severe lesions (16), whereas in other studies administration of 1010 CFU of a virulent strain on four consecutive days failed to induce significant lesions (20). Intrabronchial administration of R. equi in saline is a more reproducible way of inducing the disease (13). The experimental infection model described here was highly reproducible, causing a disease resembling the subacute form of naturally occurring R. equi pneumonia, and will likely prove to be useful in future studies in the immunization of foals against R. equi infections. Although occasionally seen in naturally infected foals, the septic arthritis and polysynovitis observed in 103+-infected foals (Fig. 5H) had, to our knowledge, never been reproduced experimentally. The results of the present virulence study in foals concur well with in vitro intracellular survival and replication in macrophages and with organ clearance in mice, supporting the value of these two models in assessing the virulence of R. equi.
The histological lesions of foals infected with the plasmid-cured derivative consisted mainly of mild atelectasis and hypercellularity of the alveolar septi (Fig. 5G). However, rare mild granulomas were observed in a few 103−- and 103−/415-VapA-infected foals. Inoculation of mice with killed R. equi ATCC 33701 or its killed plasmid-cured derivative both resulted in the formation of granulomas (30). In another study, inoculation of mice with purified mycolic acid-containing glycolipids extracted from the R. equi cell wall resulted in granuloma formation with the glycolipids containing the longer carbon chain mycolic acid resulting in the more severe lesions (9). Combined with the findings reported here, these results suggest that although the 85-kb plasmid is absolutely necessary for the pathogenicity of R. equi, the nature of the glycolipids in the R. equi cell wall appear to be essential for the development of the typical granulomas in vivo. Nevertheless, our study has shown that a plasmid-cured strain of R. equi was rapidly cleared by foals, thus showing that mycolic acids and other putative virulence factors such as the polysaccharide capsule, the cholesterol oxidase, and other phospholipases (18) are insignificant in comparison to plasmid-mediated functions.
As opposed to foals where the 85-kb plasmid is absolutely required for virulence, opportunistic infections in immunocompromised human patients do not always result from R. equi strains containing the large plasmid and expressing VapA. In a recent study, the majority of R. equi isolates from patients with AIDS tended either to be virulent for mice, to possess 85- or 90-kb plasmids and to express VapA (24% of isolates), or to have intermediate virulence for mice and to contain one of four distinct large plasmids encoding a 20-kDa antigen related to but distinct from VapA (48% of isolates); 28% were avirulent. In contrast, most (80%) of the non-AIDS isolates were avirulent for mice, lacked plasmids, and did not express these antigens (28, 31). Since the plasmid-cured strain was rapidly cleared from the foals described here, our findings suggest that the foal is not a suitable model for R. equi pneumonic disease in immunocompromised human patients because the factors predisposing these patients to infection appear to be absent in the foal. It would be of interest, however, to examine the virulence of intermediately virulent R. equi in foals by using the reproducible model of infection described here.
This study is the first assessment of the individual role of VapA in intracellular survival and virulence. Because of the similarities between R. equi and Mycobacterium spp., we correctly hypothesized that vectors used for Mycobacterium spp. would also be stable in R. equi. The Mycobacterium-E. coli shuttle plasmid pYUB415 in which vapA was subcloned (strain 103−/415-VapA) was stable in R. equi in vitro and expressed VapA to an extent similar to that of the wild-type strain 103+ (Fig. 2 and Table 1). This shuttle plasmid was also stable in R. equi in vivo, despite the lack of hygromycin selection. Strain 103−/415-VapA expressed VapA in vivo as assessed by immunohistochemistry on lung tissue (Fig. 7), although the level of expression was not as strong as that observed in foals infected with strain 103+. This reduced expression was likely the result of the considerably lower (100-fold) bacterial numbers in their lungs compared to those of foals infected with strain 103+. Expression of VapA without any other plasmid-encoded products did not restore the ability to survive or replicate in macrophages, and it did not increase the virulence of the plasmid-cured strain for either mice or foals. Thus, expression of VapA alone is not sufficient for virulence (Fig. 3 and 6). However, these results do not totally rule out a role for VapA in virulence. Creation of a vapA-deleted strain and characterization of that mutant will determine whether VapA is a true virulence factor. Recent sequencing adjacent to vapA in the 85-kb plasmid of R. equi has revealed three open reading frames with 30 to 40% overall amino acid identity to vapA (2). All three genes were transcribed when R. equi was cultured in vitro, and at least one of these gene products was recognized by serum from a naturally infected foal (2). Simultaneous expression of all the vap-like genes may be necessary for virulence.
Although VapA alone is not sufficient for reverting a plasmid-cured strain to virulence, several lines of evidence suggest that VapA and possibly other Vap proteins are protective antigens. First, a MAb to VapA and serum from horses immunized with partially purified VapA had opsonizing activity (20, 34). Second, purified immunoglobulins obtained from horses vaccinated with partially purified VapA protected mice against intraperitoneal challenge with R. equi when compared with mice given either a placebo or immunoglobulins from nonimmunized horses (6). Third, intravenous administration to foals of plasma obtained from horses immunized with partially purified VapA resulted in significantly lower bacterial counts in their lungs compared to foals administered plasma with no detectable antibody to VapA (20). Finally, evidence from mouse studies also supports the ability of VapA-enriched antigens to produce a Th1 immune response as assessed by liver clearance, delayed-type hypersensitivity, and immunoglobulin isotype response (21). Because it is avirulent for foals and expresses VapA in vivo, the recombinant strain 103−/415-VapA may prove useful as a live attenuated vaccine.
In conclusion, this study has shown that the 85-kb plasmid is essential for virulence of R. equi for foals, its natural host, and that bacterial clearance in mice and intracellular survival or replication in mouse macrophages are adequate models for assessing the virulence of R. equi. The Mycobacterium-E. coli shuttle plasmid pYUB415 is stable in R. equi both in vitro and in vivo and is a useful tool for investigating the potential virulence genes of R. equi. Expression of VapA without any other plasmid-encoded products is not sufficient for virulence. Further studies are required to identify the virulence genes of the 85-kb plasmid of R. equi and their functions, as well as the role of VapA in this infection.
ACKNOWLEDGMENTS
S. Giguère and M. K. Hondalus contributed equally to the content of this study.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (J.F.P.), by the Ontario Ministry of Agriculture, Food and Rural Affairs (J.F.P.), and by the Grayson Jockey Club Research Foundation (D.M.M.). S. Giguère is the recipient of a fellowship from the Medical Research Council of Canada.
We thank Vivian Nicholson, Robert Rinfret, Meegan Larsen, and Duane Robinson for technical assistance and Kathleen Hooper-McGrevy for assistance with immunohistochemical staining.
REFERENCES
- 1.Arlotti M, Zoboli G, Moscatelli G L, Magnati G, Maserati R, Borghi V, Andreoni M, Libanore M, Bonazzi L, Piscina A, Ciammarughi R. Rhodococcus equi infection in HIV-positive subjects: a retrospective analysis of 24 cases. Scand J Infect Dis. 1996;28:463–467. doi: 10.3109/00365549609037941. [DOI] [PubMed] [Google Scholar]
- 2.Byrne B A, Prescott J F, Palmer G H, Hines S A. Conference handbook of the 8th International Conference on Equine Infectious Diseases, Dubai, United Arab Emirates. 1998. Characterization of a virulence-associated gene family in Rhodococcus equi, abstr. P-63; p. 236. [Google Scholar]
- 3.De la Pena-Moctezuma A, Prescott J F. Association with HeLa cells by Rhodococcus equi with and without the virulence plasmid. Vet Microbiol. 1995;46:383–392. doi: 10.1016/0378-1135(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 4.De la Pena-Moctezuma A, Prescott J F. Attempts to find phenotypic markers of the virulence plasmid of Rhodococcus equi. Can J Vet Res. 1996;60:29–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Donisi A, Suardi M G, Casari S, Longo M, Cadeo G P, Carosi G. Rhodococcus equi infection in HIV-infected patients. AIDS. 1996;10:359–362. doi: 10.1097/00002030-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez A S, Prescott J F, Nicholson V M. Protective effect against Rhodococcus equi infection in mice of IgG purified from horses vaccinated with virulence associated protein (VapA)-enriched antigens. Vet Microbiol. 1997;56:187–192. doi: 10.1016/s0378-1135(97)00087-4. [DOI] [PubMed] [Google Scholar]
- 7.Giguère S, Prescott J F. Clinical manifestations, diagnosis, treatment, and prevention of Rhodococcus equi infections in foals. Vet Microbiol. 1997;56:313–334. doi: 10.1016/s0378-1135(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 8.Giguère S, Prescott J F. Cytokine induction in murine macrophages infected with virulent and avirulent Rhodococcus equi. Infect Immun. 1998;66:1848–1854. doi: 10.1128/iai.66.5.1848-1854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoh K, Mitsuyama M, Imaizumi S, Kawamura I, Yano I. Mycolic acid-containing glycolipid as a possible virulence factor of Rhodococcus equi for mice. Microbiol Immunol. 1991;35:175–185. doi: 10.1111/j.1348-0421.1991.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 10.Guiney D G, Libby S, Fang F C, Krause M, Fierer J. Growth-phase regulation of plasmid virulence genes in Salmonella. Trends Microbiol. 1995;3:275–279. doi: 10.1016/s0966-842x(00)88944-1. [DOI] [PubMed] [Google Scholar]
- 11.Harvey R L, Sunstrum J C. Rhodococcus equi infections in patients with and without human immunodeficiency virus infection. Rev Infect Dis. 1991;13:139–145. doi: 10.1093/clinids/13.1.139. [DOI] [PubMed] [Google Scholar]
- 12.Hondalus M K, Mosser D M. Survival and replication of Rhodococcus equi in macrophages. Infect Immun. 1994;62:4167–4175. doi: 10.1128/iai.62.10.4167-4175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J A, Prescott J F, Markham R J F. The pathology of experimental Corynebacterium equi infection in foals following intrabronchial challenge. Vet Pathol. 1983;20:440–449. doi: 10.1177/030098588302000407. [DOI] [PubMed] [Google Scholar]
- 14.Labidi A, Dauget C, Goh K S, David H L. Plasmid profiles of Mycobacterium fortuitum complex isolates. Curr Microbiol. 1984;11:235–240. [Google Scholar]
- 15.Madarame H, Takai S, Matsumoto C, Minamiyama K, Sasaki Y, Tsubaki S, Hasegawa Y, Nakane A. Virulent and avirulent Rhodococcus equi infection in T-cell deficient athymic nude mice: pathologic, bacteriologic and immunologic responses. FEMS Immunol Med Microbiol. 1997;17:251–262. doi: 10.1111/j.1574-695X.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 16.Martens R J, Fiske R A, Renshaw H W. Experimental subacute foal pneumonia induced by aerosol administration of Corynebacterium equi. Equine Vet J. 1982;14:111–116. doi: 10.1111/j.2042-3306.1982.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 17.Mosser D M, Hondalus M K. Rhodococcus equi: an emerging opportunistic pathogen. Trends Microbiol. 1996;4:29–33. doi: 10.1016/0966-842x(96)81502-2. [DOI] [PubMed] [Google Scholar]
- 18.Prescott J F. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4:20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott J F, Fernandez A S, Nicholson V M, Patterson M A, Yager J A, Viel L, Perkins G. Use of a virulence-associated protein based enzyme-linked immunosorbent assay for Rhodococcus equi serology in horses. Equine Vet J. 1996;28:344–349. doi: 10.1111/j.2042-3306.1996.tb03103.x. [DOI] [PubMed] [Google Scholar]
- 20.Prescott J F, Nicholson V M, Patterson M C, Zandona Meleiro M C, Caterino de Araujo A, Yager J A, Holmes M A. Use of Rhodococcus equi virulence-associated protein for immunization of foals against R. equi pneumonia. Am J Vet Res. 1997;58:356–359. [PubMed] [Google Scholar]
- 21.Prescott J F, Patterson M C, Nicholson V M, Morein B, Yager J A. Assessment of the immunogenic potential of Rhodococcus equi virulence associated protein (VapA) in mice. Vet Microbiol. 1997;56:213–225. doi: 10.1016/s0378-1135(97)00090-4. [DOI] [PubMed] [Google Scholar]
- 22.Sasakawa C, Buysse J M, Watanabe H. The large virulence plasmid of Shigella. Curr Top Microbiol Immunol. 1992;180:21–44. doi: 10.1007/978-3-642-77238-2_2. [DOI] [PubMed] [Google Scholar]
- 22a.SAS Institute, Inc. SAS/STAT user’s guide, version 6. Cary, N.C: SAS; 1990. [Google Scholar]
- 23.Sekizaki T, Takai S, Egawa Y, Ikeda T, Ito H, Tsubaki S. Sequence of the Rhodococcus equi gene encoding the virulence-associated 15–17-kDa antigens. Gene. 1995;155:135–136. doi: 10.1016/0378-1119(95)00009-u. [DOI] [PubMed] [Google Scholar]
- 24.Tabrizi S N, Robins-Browne R M. Influence of a 70 kilobase virulence plasmid on the ability of Yersinia enterocolitica to survive phagocytosis in vitro. Microb Pathog. 1992;13:171–179. doi: 10.1016/0882-4010(92)90018-j. [DOI] [PubMed] [Google Scholar]
- 25.Takai S, Anzai T, Sasaki Y, Tsubaki S, Kamada M. Virulence of Rhodococcus equi isolated from lesions of infected foals. Bull Equine Res Inst. 1993;30:9–14. [Google Scholar]
- 26.Takai S, Fukunaga N, Kamisawa K, Imai Y, Sasaki Y, Tsubaki S. Expression of virulence-associated antigens of Rhodococcus equi is regulated by temperature and pH. Microbiol Immunol. 1996;40:591–594. doi: 10.1111/j.1348-0421.1996.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 27.Takai S, Iie M, Watanabe Y, Tsubaki S, Sekizaki T. Virulence-associated 15- to 17-kilodalton antigens in Rhodococcus equi: temperature-dependent expression and location of the antigens. Infect Immun. 1992;60:2995–2997. doi: 10.1128/iai.60.7.2995-2997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takai S, Imai Y, Fukunaga N, Uchida Y, Kamisawa K, Sasaki Y, Tsubaki S, Sekizaki T. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. J Infect Dis. 1995;172:1306–1311. doi: 10.1093/infdis/172.5.1306. [DOI] [PubMed] [Google Scholar]
- 29.Takai S, Koike K, Ohbushi S, Izumi C, Tsubaki S. Identification of 15- to 17-kilodalton antigens associated with virulence of Rhodococcus equi. J Clin Microbiol. 1991;29:439–443. doi: 10.1128/jcm.29.3.439-443.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takai S, Madarame H, Matsumoto C, Inoue M, Sasaki Y, Hasegawa Y, Tsubaki S, Nakane A. Pathogenesis of Rhodococcus equi infection in mice: roles of virulence plasmids and granulomagenic activity of bacteria. FEMS Immunol Med Microbiol. 1995;11:181–190. doi: 10.1111/j.1574-695X.1995.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 31.Takai S, Sasaki Y, Ikeda T, Uchida Y, Tsubaki S, Sekizaki T. Virulence of Rhodococcus equi isolates from patients with and without AIDS. J Clin Microbiol. 1994;32:457–460. doi: 10.1128/jcm.32.2.457-460.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takai S, Sekizaki T, Ozawa T, Sugawara T, Watanabe Y, Tsubaki S. Association between large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immun. 1991;59:4056–4060. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takai S, Watanabe Y, Ikeda T, Ozawa T, Matsukura S, Tamada Y, Tsubaki S, Sekizaki T. Virulence-associated plasmids in Rhodococcus equi. J Clin Microbiol. 1993;31:1726–1729. doi: 10.1128/jcm.31.7.1726-1729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan C, Prescott J F, Patterson M C, Nicholson V M. Molecular characterization of a lipid-modified virulence-associated protein of Rhodococcus equi and its potential in protective immunity. Can J Vet Res. 1995;59:51–59. [PMC free article] [PubMed] [Google Scholar]
- 35.Wada R, Kamada M, Anzai T, Nakanishi A, Kanemaru T, Takai S, Tsubaki S. Pathogenicity and virulence of Rhodococcus equi in foals following intratracheal challenge. Vet Microbiol. 1997;56:301–312. doi: 10.1016/s0378-1135(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 36.Yager J A, Prescott C A, Kramar D P, Honnah H, Balson G A, Croy B A. The effect of experimental infection with Rhodococcus equi on immunodeficient mice. Vet Microbiol. 1991;28:363–376. doi: 10.1016/0378-1135(91)90071-m. [DOI] [PubMed] [Google Scholar]