Abstract
Background
The uptake of HIV partner status notification remains limited in low- and lower-middle-income countries. This mixed-methods systematic review aims to summarize the barriers and facilitators of HIV partner status notification in these settings.
Methods
We searched PubMed, Embase, CINAHL, PsychINFO, Scopus, and Web of Science from January 01, 2000, to August 31, 2023, for empirical qualitative and quantitative studies. Two independent reviewers completed the title, abstract, full-text screening, and data extraction. The risk of bias was assessed using a mixed-methods appraisal tool (MMAT), and the study findings were summarized narratively.
Results
Out of the 2094 studies identified, 59 relevant studies were included. Common barriers included fear of stigma and discrimination, violence, abandonment, breach of confidentiality and trust, low HIV-risk perception, and limited knowledge of HIV and HIV testing. Facilitators of HIV partner status notification were feelings of love and closeness in marital relationships, feelings of protecting self and partners, and HIV counseling services.
Conclusion
Efforts to improve HIV partner status notification in low- and lower-middle-income countries should consider barriers and facilitators across all its components, including notification, testing, and linkage to treatment. In addition, HIV partner services must be adapted to the unique needs of key populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10241-2.
Keywords: HIV and AIDS, Sexual partners, Notification, Low- and lower-middle-income countries
Introduction
To end the HIV pandemic, the Joint United Nations Programme on HIV (UNAIDS) has set ambitious 95-95-95 objectives to be achieved by 2030. These objectives include diagnosing 95% of people living with HIV, ensuring that 95% of diagnosed individuals are on antiretroviral therapy, and achieving viral suppression in 95% of those receiving antiretroviral therapy [1]. While significant progress has been made towards these targets, challenges persist. As of 2019, approximately 81% of people living with HIV globally were aware of their HIV status, with 82% of them on antiretroviral therapy and 88% of those on antiretroviral therapy achieving viral suppression, resulting in an overall viral suppression proportion of 59% [2]. The optimal implementation of HIV testing and treatment strategies has faced various obstacles, including structural, legal, and social barriers. These barriers contribute to inequities in access and uptake of HIV testing and treatment, limited retention in care, stigma and discrimination, suboptimal adherence to pre-exposure prophylaxis, limited access to key populations, and difficulties in meeting the UNAIDS targets for enrolling people living with HIV into treatment programs [3, 4].
One promising approach to achieving 95-95-95 objectives is supporting individuals to notify their HIV serostatus to others, particularly their partners [5]. HIV partner status notification plays a crucial role in identifying undiagnosed people living with HIV and those who have stopped attending clinics [6]. This process involves a voluntary two-step approach, where partners of people living with HIV are informed about their potential exposure probability and then supported in receiving testing services. The World Health Organization (WHO) has recognized the importance of incorporating partner status notification as an integral part of HIV testing services since 2012 [7]. HIV partner status notification has been shown to increase the rate of HIV testing and reduce transmission risk behaviors [8]. Also, HIV partner status notification in some situations can be different, for example, when the sexual partner has an undetectable viral load. If a partner with HIV is on treatment and has an undetectable viral load, they are unlikely to pass HIV on to others even if they do not use condoms. Based on previous studies, with appropriately scheduled viral monitoring, adherence counseling, and follow-up, these patients have a very low probability of viral rebound and HIV transmission to their sexual partners [9]. Also, it is not the individual’s responsibility to ensure their partner gets tested, but it would be ideal if they could suggest it without fear of recrimination [10]. Notably, partner notification is also a process whereby sexual partners of patients with sexually transmitted infections other than HIV are informed of their exposure to infection and the need to receive treatment. Partner notification for curable sexually transmitted infections may prevent re-infection of the patient and reduce the probability of complications and further spread [11]. However, while an undetectable viral load will most likely prevent transmission of HIV, it does not prevent other sexually transmitted infections or unintended pregnancy [10].
Several studies have explored the facilitators and barriers to HIV partner status notification in high-income countries, using both quantitative and qualitative approaches [7], and identified a range of facilitators (e.g., a supportive relationship) and barriers (e.g., stigma and discrimination) [12–15]. However, women living with HIV, may face greater barriers to HIV partner status notification than other groups. This is particularly significant given that women and girls constitute 53% of all people living with HIV globally [16]. Evidence shows that women living with HIV experience multiple, intersecting inequities related to gender, HIV status, violence, bodily autonomy, sexual and reproductive health rights, and economic dependence, among other factors [17].
Nonetheless, less is known about HIV partner status notification in the context of low- and lower-middle-income countries, which bear the highest burden of the HIV pandemic. In these regions, socio-cultural and economic factors may compromise the effectiveness of current control measures. Distinct epidemics of HIV have emerged in different geographical areas, characterized by variations in severity, affected population groups, associated risk behaviors, and viral strains. In addition to the significant human toll, the high burden of HIV has adverse social and economic impacts on many low- and lower-middle-income countries [18]. Compounded by these challenges, the uptake of HIV partner status notification remains limited in these settings. For example, rates of successful partner status notification and testing through passive HIV partner status notification services have been quite low in several low- and lower-middle-income countries [19]. Moreover, many benefits of HIV partner status notification depend on access to resources, services, and commodities that may not be readily available in these resource-constrained settings. Thus, program planners must carefully consider how to support individuals in disclosing their HIV status to others, aiming for positive outcomes while mitigating potential negative consequences [20]. Despite the existing body of literature on HIV partner status notification, there has been limited focus on ways to simplify this process and identify the associated obstacles, particularly in resource-limited settings. Therefore, this mixed-methods systematic review aims to explore the barriers and facilitators of HIV partner status notification, with a specific focus on low- and lower-middle-income countries.
Methods
Protocol and registration
We developed our review protocol and registered it in PROSPERO (CRD42022379427) [21]. We also followed the PRISMA reporting checklist to report our findings (S1 File) [22].
Eligibility criteria
Studies meeting the following eligibility criteria were included in this review: (i) Qualitative, quantitative, or mixed-methods empirical papers reporting barriers and/or facilitators for HIV partner status notification; (ii) Conducted in low- and lower-middle-income countries as defined by World Bank [23]; and (iii) Published in English between January 2000 and August 31, 2023. While the WHO formally recognized partner status notification as an integral part of HIV testing services in 2012, this practice had already been implemented and studied earlier. We included studies published since 2000 for four reasons: (i) The WHO’s report on the global HIV response from 2000 to 2015 indicated that partner status notification was feasible and effective in some settings before 2012 [24]; (ii) A 2016 WHO report identified 56 global studies on partner status notification services spanning the pre-2012 period, covering various populations and stakeholders [7]; (iii) A systematic review in 2002 reported strategies of HIV partner status notification [25]; and (iv) several studies showed existing partner status notification for preventing HIV before the year 2000 [26–28].
Eligible study populations included adults (≥ 18 years of age), people living with HIV, people most vulnerable to acquiring HIV (including gay men and other men who have sex with men, female sex workers, people who inject drugs [29], and healthcare providers. We also included studies conducted in multiple sites or countries if barriers/facilitators were separately analyzed and reported per site or country.
Information sources
In November 2022, we systematically searched six electronic databases: PubMed, Embase, CINAHL, Psych Info, Scopus, and Web of Science using predefined search terms (S2 File). We also reviewed the abstracts of the last two years of AIDS, the Center for Disease Control (CDC) STD Prevention Conference, the International Union against Sexually Transmitted Infections conferences, and the WHO website for relevant literature. The search was updated in August 2023 to identify additional literature published after November 2022. The key search terms included: (“HIV” OR “Acquired Immunodeficiency Syndrome” OR “AIDS”) AND (“contact tracing” OR “partner notification” OR “partner treatment” OR “partner testing” OR “partner referral” OR “provider referral” OR “passive referral” OR “contact referral” OR “patient referral”) AND (“Lower middle-income countries” OR “Low-income countries”). Additionally, grey literature search involved hand searches of unpublished research reports, policy literature, working papers, newsletters, government documents, speeches in Google and Google scholar (first 300 hits [30]). We used key phrases, such as “telling your partner,” “talking with a partner about HIV status”, or “sharing HIV status with partner”.
Study selection and data collection
We uploaded all identified citations into Endnote (v.20) reference management software and removed duplicates. Two independent reviewers (FT and MB) screened and assessed all titles and abstracts against our pre-defined eligibility criteria. Studies deemed non-relevant or reporting from high-income countries were excluded at this stage. If the exclusion decision was unclear, the study was included for full-text screening. Both reviewers independently assessed the full-texts of the remaining studies. Studies that did not meet all eligibility criteria were excluded, and the reasons for exclusion were recorded. Disagreements were resolved through discussion with a senior author (HSH). We developed and tested a data extraction table on three studies to ensure all relevant data items could be extracted. From each included study, we extracted the following data items: First author, year of publication, study location, study type, participants’ characteristics, sample size, study aims, and findings related to the review question (i.e. barriers and facilitators for HIV partner status notification). FT verified the extracted data for accuracy and made necessary additions or modifications. We compared the data we individually extracted and resolved any disagreement through discussion.
Data transformation
We extracted data on barriers and facilitators to HIV partner status notification from the included studies. Qualitative findings, including the qualitative component of mixed methods studies, were extracted as presented in the original research papers, capturing themes and paragraphs of textual description. Quantitative findings, including the quantitative component of mixed methods studies, were transformed into textual descriptions [31]. Finally, we merged qualitative findings and transformed study findings into a single dataset.
Risk of bias in individual studies
To assess the methodological quality of the included studies, we used the Mixed Methods Appraisal Tool (MMAT) [32]. This tool, widely used in systematic reviews, offers the advantage of assessing interdependent qualitative and quantitative elements of mixed-methods research. We independently identified the categories of study design using the MMAT tool and then appraised each study against the corresponding methodological quality criteria [32]. We discussed potential exclusion for studies failing to meet more than one quality criterion. However, we were inclined towards inclusion to avoid omitting potentially crucial insights to comprehensively understand the phenomenon under study [33, 34].
Mixed methods synthesis
We applied framework synthesis, a highly transparent and deductive approach recommended for the synthesis of evidence on complex interventions [35]. This approach combines critical, realistic, and subjective idealistic epistemology elements. We analyzed our data set using Excel software [36]. The analysis involved iterative coding and sub-coding of the extracted results, with individual definitions, cross-checking, discussion, and refinement of the code system. We resolved disagreements through discussion. We applied a modified version of Song et al.’s approach [36] to analyze and present barriers and facilitators of HIV partner status notification. The primary aim of this study was to evaluate barriers and facilitators of HIV partner status notification in low- and lower-middle-income countries, along with extracting these findings for testing and linkage to treatment if available. First, we assigned each extracted result (i.e. barrier or facilitator) to one of the three components based on their definition level: Notification of sexual partners by people living with HIV, testing of sexual partners after they have been notified of possible HIV exposure, and linking partners living with HIV to treatment services. Second, we thematically analyzed each barrier or facilitator, considering its contextual description. Third, to summarize the comprehensive barrier or facilitator descriptions, we applied meta-summary—a quantitatively oriented aggregation of qualitative findings first proposed by Sandalowsky, Barroso, and Voils (2007) [34]. After familiarizing ourselves with the extracted dataset, reading and re-reading the identified barriers and facilitators, and exploring underlying patterns, we identified the barriers and facilitators of HIV partner status notification.
Results
Study selection
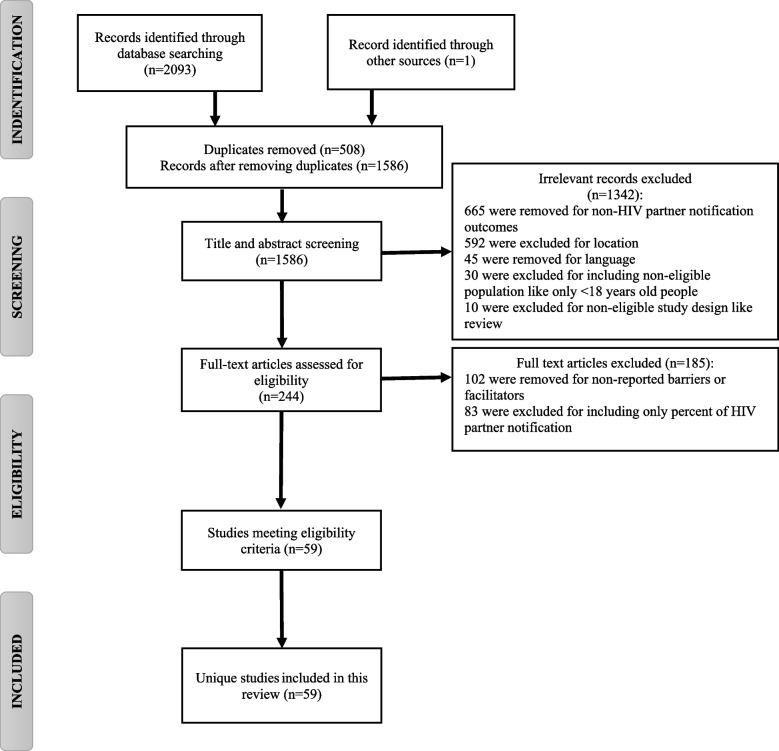
Of the 2094 included studies in the primary search, 59 studies were included in the final step. The PRISMA Flow Diagram presents the number of papers included throughout the selection process, along with the reasons for exclusion (Fig. 1).
Fig. 1.
PRISMA flow diagram for selection of studies on HIV partner status notification
Description of studies included in the review
Table 1 provides an overview of the characteristics of the included studies. The studies were conducted in various countries, including Kenya (n = 14) [37–50], Uganda (n = 10) [51–60], Malawi (n = 8) [61–68], Tanzania (n = 6) [69–74], Ethiopia (n = 4) [75–78], Cameroon (n = 3) [79–81], Mozambique (n = 2) [82, 83], Zambia (n = 2) [84, 85], South Africa (n = 2) [86, 87], Nigeria (n = 1) [88], Malawi and Tanzania (n = 1) [89], Guinea-Bissau (n = 1) [90], India (n = 1) [91], Rwanda (n = 1) [92], Burkina Faso ( n = 1) [93], Lesotho (n = 1) [94], and Iran (n = 1) [95]. Among the 59 included studies, 23 were qualitative, 12 were cross-sectional, 11 were clinical trials, seven were mixed methods, and six were cohort studies. Also, the age range of participants in the included studies was 19–78, and the range of the sample sizes was 14 in qualitative studies to 9,022 in quantitative studies.
Table 1.
Summary of included studies reporting on barriers and facilitators to HIV partner status notification by study type in low and lower-middle-income countries, 2000–2023
| Study type | First author (Year of publication) | Location | Year of data collection | Study population | Sample size | Age (Year) | Quality score (out of 5) | |
|---|---|---|---|---|---|---|---|---|
| Clinical trial | Joseph Davey et al., 2022 [87] | South Africa | 2021 | Women living with HIV | 176 | Mean: 35 | 2 | |
| Dovel et al., 2022 [62] | Malawi | 2018–2020 | People living with HIV on antiretroviral therapy | 365 |
Mean: 37 SDa: 11.6 |
4 | ||
| Mutale et al., 2021 [84] | Zambia | 2019–2020 | Pregnant women | 316 | NRb | 4 | ||
| Jeremiah et al., 2021 [89] | Malawi and Tanzania | 2010 | Pregnant women | 535 | Mean: 27 | 4 | ||
| Choko et al., 2019 [61] | Malawi | 2016–2017 | Pregnant women and their male partners | 2349 | Mean (SD)a Women: 24.8 (5.4), male partners: 29.6 (7.5) | 5 | ||
| Korte el al., 2019 [57] | Uganda | 2018 | Pregnant women and their primary male partners | Pregnant women: 824 and male partners: 896 | NRb | 3 | ||
| Krakowiak et al., 2016 [48] | Kenya | 2013–2014 | Pregnant women and their primary male partners | 601 | Mean (SD)a: 24.7, 4.9 | 4 | ||
| Masters et al., 2016 [50] | Kenya | 2015–2016 | Pregnant women (Antenatal and postpartum) | 600 | Mean: 24 | 5 | ||
| Osoti et al., 2014 [39] | Kenya | 2014 | Pregnant women | 300 | Mean: 22 | 4 | ||
| Brown et al., 2012 [68] | Malawi | 2012 | People newly diagnosed with HIV | 170 | NRb | 2 | ||
| Brown et al., 2011 [67] | Malawi | 2008–2009 | People newly diagnosed with HIV | 267 | Median: 28, IQRc: 24–33 | 4 | ||
| Cross- sectional study | Remera et al., 2022 [92] | Rwanda | 2018–2019 | People newly diagnosed with HIV or people living with HIV on antiretroviral therapy (index client) | 6336 | NRb | 5 | |
| Pashaei et al., 2022 [95] | Iran | NRb | Women living with HIV | 192 | Mean (SD)a: 41.59, 8.7 | 4 | ||
| Markos Kachero., et al., 2021 [78] | Ethiopia | 2020 | All male partners of pregnant women | 798 | Mean (SD)a: 37.5 (7.1) | 5 | ||
| Tih et al., 2019 [80] | Cameroon | 2007–2015 | People living with HIV (index client) and their sexual partners’/contact persons | 9022 | Median:36, IQRc: 30–43 | 5 | ||
| Buhikire et al., 2018 [51] | Uganda | 2016 | People newly diagnosed with HIV | 464 | Mean: 32 | 4 | ||
| Kahabuka et al., 2017 [71] | Tanzania | 2015 | People newly diagnosed with HIV | 384 | Mean: 33.2 | 5 | ||
| Omunakwe et al., 2015 [88] | Nigeria | 2015 | People living with HIV on antiretroviral therapy | 250 | Mean: 37.1, SDa: 8.8 | 3 | ||
| Myers et al., 2016 [83] | Mozambique | 2014 | People newly diagnosed with HIV | 206 | Median: 29, IQRc: 24–34 | 4 | ||
| Alemayehu et al., 2017 [75] | Ethiopia | 2014 | Male partners | 422 | Range: 30–39 | 3 | ||
| Henley et al., 2013 [79] | Cameroon | 2009–2010 | People living with HIV (index clients) diagnosed in antenatal care, voluntary counseling and testing, and inpatient facilities and partners | 1463 People living with HIV, 1607 sexual partners | Median: 31, range: 15–70 | 5 | ||
| Makoleka et al., 2012 [85] | Zambia | NRb | Pregnant women | 120 | Range: 18–29 | 4 | ||
| Otieno et al., 2010 [40] | Kenya | 1999–2005 | pregnant women living with HIV | 116 | Median: 30, IQRc: 23–38 | 3 | ||
| Mixed- methods study | Klabbers et al., 2021 [56] | Uganda | 2019 | People living with HIV (index client) and sexual partners, health care workers | Index clients: 882, sexual partners: 1126, healthcare providers: 32 | Mean (SD)a: Index clients: 35 (9.46), Sexual partners: 34 (9.04), health worker care workers: 32 (7.52) | 4 | |
| Offorjebe et al., 2020 [66] | Malawi | 2017–2018 | People living with HIV | 404 | Mean: 37.6, IQRc: 30–44 | 2 | ||
| Welty et al., 2020 [46] | Kenya | 2018 | People newly diagnosed with HIV And health care workers |
people living with HIV: 532, Health care workers: 17 |
NRb | 4 | ||
| Klabbers et al., 2020 [55] | Uganda | 2018 | People living with HIV (index client) and sexual partners | 33 qualititative, 882 quantitiative | Mean (SD)a: 35 (9.5) | 4 | ||
| Plotkin et al., 2018 [72] | Tanzania | 2015 | People newly diagnosed with HIV | 391 index clients and 249 sexual partners of index clients | Range: 23–34 | 4 | ||
| Selvaraj et al., 2017 [91] | India | 2011–2015 | Married people living with HIV | 3884 | Mean: 38 | 5 | ||
| Falnes et al., 2011 [70] | Tanzania | 2007–2008 | Health personnel, women and their partners | 426 | Mean: 27 | 4 | ||
| Qualitative study | van der Elst et al., 2022 [45] | Kenya | 2018–2020 | People newly diagnosed with HIV | 24 | Median: 26, IQRc: 19–39 | 2 | |
| Liu et al., 2022 [38] | Kenya | 2020 | HIV testing service providers | 14 | Mean: 35 | 3 | ||
| Lofgren et al., 2021 [58] | Uganda | 2017 |
People living with HIV and Health care workers |
people living with HIV:58, Health care workers: 20 |
Median: 32 IQR3: 20–55 (people living with HIV) |
5 | ||
| Sanga et al., 2021 [73] | Tanzania | 2019 | People living with HIV | 7 FGDsd and 30 in-depth interviews | Range: 31–45 | 2 | ||
| Sircar et al., 2020 [43] | Kenya | NRb | Health care workers | 52 | NRb | 2 | ||
| Madiba et al., 2020 [94] | Lesotho | 2019 | Pregnant women | 15 | Mean: 20 | 2 | ||
| Monroe-Wise et al., 2019 [47] | Kenya | 2015 | People living with HIV | 47 | NRb | 2 | ||
| Van Der Elst et al. 2019 [44] | Kenya | 2013–2016 | People living with HIV (subtype-1)and sex workers | 23 |
Women: 28 Men: 26 |
4 | ||
| Conserve et al., 2019 [69] | Tanzania | 2015 | Key implementers of HIV and AIDS interventions, healthcare providers, secondary school boys and members of the community | Men: 23 | Mean: 27.3, SDa: 6.5 | 2 | ||
| Daniels et al. 2019 [86] | South Africa | 2017 | pregnant women living with HIV | 28 | Mean: 28.7 | 3 | ||
| Odoyo et al., 2019 [49] | Kenya | 2017–2018 | Health care workers | 71 | Mean: 32 | 4 | ||
| Sanga et al., 2019 [74] | Tanzania | 2014–2015 | People newly diagnosed with HIV and health care workers | 38 | NRb | 5 | ||
| Quinn et al., 2018 [60] | Uganda | 2018 | Fishermen and sex workers in fishing communities, male and female mainland community members, and healthcare providers | 64 Interview and 6 FGDsd |
Median: Fishing communities: 28, Mainland communities: 26, Healthcare providers: 32 |
3 | ||
| Hino et al., 2018 [63] | Malawi | 2018 | People living with HIV | 40 | Mean: 28, range (18–51) | 4 | ||
| Matoga et al., 2018 [65] | Malawi | 2016 | Health care workers and clinic observations | 15 | Median: 32, IQRc: 28–38 | 2 | ||
| Carrasco et al., 2017 [82] | Mozambique | 2009–2012 |
People living with HIV; peer educators working for community-based organisations, providing services to people living with HIV, community members, people who had family members living with HIV and AIDS, Couples. |
227 | NRb | 4 | ||
| Goyette et al., 2016 [37] | Kenya | 2015 | Clients, health advisors, HIV testing and counseling counselors | 20 | Median: 40 | 2 | ||
| Kamanga et al., 2015 [64] | Malawi | 2008–2009 | People newly diagnosed with HIV, healthcare providers | 28 | NRb | 5 | ||
| Nakigozi et al., 2013 [59] | Uganda | 2010–2011 | People living with HIV and health care workers | Patients: 48, health care worker:12 | IQRc: 15–49 | 3 | ||
| Netsanet et al., 2013[77] | Ethiopia | 2012 | People newly diagnosed with HIV | 15 | Mean: 32 | 5 | ||
| Njozing et al., 2011 [81] | Cameroon | 2009 | HIV andTB patients | 32 | Mean: 36 | 3 | ||
| Issiaka et al., 2010 [93] | Burkina Faso; West Africa | 2010 | Women living with HIV | 79 | Mean: 24.5 | 5 | ||
| King et al., 2009 [54] | Uganda | 2004 | People living with HIV | 48 qualitative semi-structured interviews and 1092 structured interviews | Median: 37 for women and 40 for men | 5 | ||
| Cohort study | Madsen et al., 2020 [90] | Guinea-Bissau | 2018 | People newly diagnosed with HIV | 495 | Median: 37 (women), IQRc: 32–45 | 4 | |
| Pintye et al., 2019 [41] | Kenya | 2017–2018 | Male partners of women | 3620 | Median: 24, IQRc: 21–28 | 5 | ||
| Kiene et al., 2017 [52] | Uganda | 2017 | Sexual partners | 304 (152 females, 152 males) |
Mean (SD)a: Male: 35.20 (18.8), Female: 32.54 (8.9) |
5 | ||
| Kim et al., 2014 [53] | Uganda | 2012 | Pregnant women | 188 | Median: 24, IQRc: 20–29 | 4 | ||
| Sharma et al., 2021 [42] | Kenya | 2018–2019 | Women living with HIV | 1050 | Mean: 29, range (15–74) | 5 | ||
| Tegegne et al., 2022 [76] | Ethiopia | 2015–2020 | People living with HIV on antiretroviral therapy | 792 | Mean: 64.3, range: 48–78 | 5 | ||
aStandard deviation
bNot reported
cInterquartile range
dFocus group discussion
Risk of bias and quality appraisal
Most of the included studies were of high quality (n = 39; 66.1%), while some were moderate (n = 9; 15.3%) or weak quality (n = 11; 18.6%). The weakest element in the qualitative studies was the lack of detail necessary to evaluate whether the data substantiated the interpretation of results. As most of the quantitative studies were conducted among the key populations whom outside researchers often find hard to reach, information bias due to non-response to some sensitive questions was the main issue in these studies. The weakest element in the mixed-methods studies was a lack of consideration of divergence between qualitative and quantitative results.
Synthesis of results
The following narrative synthesis of results summarizes identified barriers and facilitators overall and by key population. Socio-demographic characteristics and behaviors associated with HIV partner status notification are presented separately, given that they represent individual-level drivers of notification uptake rather than external barriers or facilitators. The integrated quantitative and qualitative data were converged in this study. Figure 2 presents an overview of the barriers and facilitators to HIV partner status notification.
Fig. 2.
Overview of barriers and facilitators to HIV partner status notification
Barriers and facilitators to HIV partner status notification
Notification of sexual partners by people living with HIV
Barriers
In the context of notifying sexual partners by people living with HIV, several barriers to HIV partner status notification emerged across multiple settings: Fear of stigma and discrimination [37, 38, 47, 56, 73, 74, 76, 77, 88, 90, 95], fear of separation and abandonment of a sexual partner [37, 56, 58, 59, 63, 64, 72, 73, 76, 77, 80, 86, 88, 90, 93, 95], fear of violence (e.g., physical, emotional, sexual, otherwise) [41–43, 47, 55, 56, 58, 73, 76, 79, 80, 86], fear of partner reactions, including blame [37, 41, 63, 64, 76, 77, 93], fear of rejection and abuse [59, 73], fear of breach of confidentiality and trust [37, 38, 56, 95], having multiple partners [38, 41, 51, 63, 72], insufficient HIV knowledge [47, 71, 74], fear of loss of financial support [37, 44, 47, 80], lack of support from partner [47, 59], feelings of shame [88, 95] and denial [74, 90], social-ecological factors (e.g., culture, traditional gender roles and fear of criminalization) [44, 47, 56, 71, 77], lack of access to partner contact information and uncertainty of how to notify them [74], geographical barriers [76], and fear of isolation and job loss [37].
Facilitators
Several factors facilitated HIV partner status notification: Using assisted partner notification [43, 60, 77, 80, 83, 86, 92, 95], feelings of love and closeness in relationships, and maintaining trust [54, 63, 64, 68, 71, 79, 85], moral duty and sense of responsibility [54, 86], feeling of self-care [54], incentives and services like counseling and support [86, 94], positive cultural practices, such as education and change of the social norms, such as supporting communities to support each other, people living with HIV, and people from key populations, adopting a pro-testing and pro-treatment strategy and improving communication and relationship skills [68, 85], and partner support [94].
Testing of sexual partners after they have been notified of possible HIV exposure
Barriers
Common barriers related to the testing of sexual partners were: Fear of the consequences of a positive diagnosis [56], fear of stigma and discrimination from a partner, family, health care workers, and society [43, 82, 90, 91], fear of intimate partner violence [46, 56, 66], or abandonment [67, 82, 90], afraid of telling an HIV status with sexual partners or other people, and loss of social status [82]. Also, people who belonged to key populations (e.g., gay men and other men who have sex with men, female sex workers, people who inject drugs) [43, 91] and people with extramarital or multiple sexual partners [56, 69] were more reluctant to HIV testing of their partners. Some barriers were related to the refusing partner to HIV testing [51, 71, 90], lack of trust in health care workers or counselors [37, 43, 66], fear of being tested [56, 75, 78], ignorance of the testing [78], ignorance of the importance of HIV testing due to incorrect information [56, 75], or having limited time and financial resources [59]. Some others were related to not being able to contact partner [90] due to their partner living far from of the HIV testing site [51, 90], the partner went to another clinic for testing, the partner died or was too sick, relationships dissolved [90], geographically distant [56] partner not retained in care [51]. Health system barriers included inconvenient clinic hours of operation, lack of incentives, lack of space and limited trained staff and poor staff attitude [65], inadequate resources, including room setup, lack privacy, lack of a mechanism to trace a partner, problems related to working hours, absence of an independent gender-sensitive unit and support groups [75], poorly organized clinic procedures and visit schedules, overcrowding, long waiting times, and distance and transport costs to HIV care centers [74]. Some barriers for healthcare providers to refuse HIV status notification were difficulty obtaining partners’ accurate contact information, low salaries, lack of equipment, and high workload [38]. In addition, there were also barriers, such as harmful gender norms and inequities (e.g., women’s fear to request their spouse for HIV testing, refusal by partners and gender-based violence) [66, 70, 91].
Facilitators
In the component of HIV testing of partners, common facilitators were: Increasing access to HIV testing [91], such as direct access to HIV counseling and testing, availability of free services, counseling services offered by multiple stakeholders [46, 91], assisted partner notification [83], HIV self-testing [41, 50, 62, 66, 84, 87] with financial incentives [61], safe home visits and HIV testing of pregnant women and their couples [39, 48, 53, 57], encouraged to test and support by their partners [52, 69, 85, 89]. Also, some factors like uncertainty about acquiring HIV [83], disease symptoms [72], risky sexual behavior [69], and afraid of the transmission of HIV [76] were facilitators of HIV testing. Health system factors in this component were: Collaboration of expert clients and local leadership [56], availability of facilities [65], training, spreading awareness of HIV testing, communicating with patience and nonjudgmental attitude and assuring confidentiality [37, 38, 81] counseling strategies including emphasizing personal benefits [56] health education materials (such as pamphlets, posters, video and audio productions), HIV knowledge [78] and allowing time to process HIV test results [56].
Linking partners living with HIV to treatment services
Barriers
Barriers related to linking partners living with HIV to treatment services were HIV stigma, partner negligence or violence [40, 82], and loss of retention in care [51]. This component was also noted from the side of health care workers; inconsistent and under-resourced training, individual attitudes of providers [43], and inappropriate services, such as lack of ability of staff to link partners living with HIV to treatment services [51] were also in this component.
Facilitators
Support and encouragement from partners [40, 45, 94], family, relatives, and social system [45, 74] support and good patient-staff relationship, conducting multiple counseling sessions [45, 74], the importance of peer-supported linkages to HIV care and the need for respectful, high-quality care [65] referral procedures and well-organized clinic procedures [45, 74], initiating antiretroviral therapy on the same day as testing [62], also having symptoms of the disease [74] can be a reason for linking people living with HIV to treatment.
Results by location, study type and key population
While our review included a limited number of studies from Asia, potentially limiting comparability with studies conducted in Africa, stigma emerged as a pervasive barrier across countries. Notably, the barriers identified in Asian settings appeared to be more pronounced in the context of HIV notification and testing components of partner status notification services. Regarding the study types, no significant differences were observed in the reported facilitators and barriers, with the exception of clinical trials. These intervention studies frequently evaluated strategies, such as increasing access to HIV testing, including HIV self-testing, as a facilitator for partner status notification.
A large number of studies focused on people living with HIV (n = 20). Other populations included healthcare providers, HIV testing and counseling counselors (n = 15), sexual partners of people living with HIV or pregnant women (n = 14), people newly diagnosed with HIV (n = 13), pregnant women (n = 12), community members (n = 2), key populations like gay men and other men who have sex with men, transgender people, female sex workers, incarcerated people (n = 3) and women living with HIV (n = 4). Results are presented in Table 2, where the findings are categorized by key population, emphasizing the distinct needs and contexts of each group in the context of low- and lower-middle-income countries.
Table 2.
Barriers and facilitators of HIV partner status notification by key population in low and lower-middle-income countries, 2000–2023
| Population type | Notification of sexual partners by people living with HIV | Testing of sexual partners after they have been notified of possible HIV exposure | Linking partners living with HIV to treatment services | |||
|---|---|---|---|---|---|---|
| Barriers | Facilitators | Barriers | Facilitators | Barriers | Facilitators | |
|
People living with HIV |
1) Fear of Stigma and Discrimination 2) Fear of Violence 3) Fear of Relationship Loss 4) Fear of Breach of Confidentiality 5) Fear of Rejection and Abuse 6) Fear of Negative Repercussions 7) Fear of Loss of Financial Support 8) Lack of HIV Knowledge and Low Risk Perception 9) Social-Ecological Factors 10) Challenges in Communication 11) Time Constraints 12) Lack of Partner Contact Information 13) Lack of Support from Partner 14) Shame and Denial |
1) Emotional Factors 2) Moral Duty and Responsibility 3) Assisted Partner Notification |
1) Fear of Consequences of a Positive Diagnosis 2) Fear of Stigma and Discrimination 3) Fear of Intimate Partner Violence 4) Fear of Testing Itself 5) Relationship Dissolution and Distance 6) Lack of Trust in Healthcare providers 7) Gender Norms and Inequality 8) Reluctance of Key Populations |
1) Increased Access to HIV Testing 2) Assisted Partner Notification 3) Community-Based HIV Testing 4) Health System Factors |
1) HIV Stigma 2) Spouse Negligence or Violence |
1) Initiating antiretroviral therapy on the Same-day as Testing |
|
People newly diagnosed with HIV |
1) Fear of Stigma and Discrimination 2) Fear of Relationship Loss 3) Fear of Negative Repercussions 4) Challenges in Communication 5) Lack of HIV Knowledge and Low Risk Perception 6) Lack of Partner Contact Information 7) Social-Ecological Factors 8) Shame and Denial |
1) Emotional Factors 2) Positive Cultural Practices 3) Assisted Partner Notification |
1) Fear of Stigma and Discrimination 2) Fear of Intimate Partner Violence 3) Fear of Consequences of a Positive Diagnosis 4) Refusal by Partners 5) Health System Barriers 6) Relationship Dissolution and Distance |
1) Increased Access to HIV Testing 2) Community-Based HIV Testing 3) Assisted Partner Notification |
1) Loss of retention in care or Migration 2) Inappropriate Services |
1) Support from Family, Relatives, and the Social System 2) Positive Patient-Staff Relationship 3) Well-Organized Clinic Procedures 4) Presence of Disease Symptoms |
|
Women living with HIV |
1) Fear of Stigma and Discrimination 2) Fear of Relationship Loss 3) Fear of Negative Repercussions 4) Fear of Rejection and Abuse 5) Fear of Breach of Confidentiality 6) Shame and Denial 7) Lack of HIV Knowledge and Low Risk Perception 8) Time Constraints |
1) Emotional Factors 2) Moral Duty and Responsibility |
NRa | 1) HIV Self-Testing | NRa | NRa |
|
Pregnant women |
1) Fear of Relationship Loss 2) Fear of Violence |
1) Emotional Factors 2) Positive Cultural Practices 3) Partner Support 4) Moral Duty and Responsibility 5) HIV Counseling Services 6) Incentives and Facilities |
NRa |
1) HIV Self-Testing 2) Community-Based HIV Testing 3) Partner Support |
1) HIV Stigma 2) Spouse Negligence or Violence, |
1) Partner Support |
|
Key populations including gay men and other men who have sex with men, transgender people, sex workers, people in prisons |
1) Fear of Stigma and Discrimination 2) Fear of Loss of Financial Support 3) Fear of Rejection and Abuse 4) Fear of Violence 5) Fear of Negative Repercussions 6) Fear of Breach of Confidentiality 7) Fear of Relationship Loss 8) Social-Ecological Factors 9) Challenges in Communication 10) Lack of Support from Partner |
1) Assisted Partner Notification 2) Emotional Factors 3) Moral Duty and Responsibility |
NRa |
1) Assisted Partner Notification 2) Increased Access to HIV Testing 3) Community-Based HIV Testing 4) Health System Factors |
NRa | NRa |
|
Sexual partners of people living with HIV or pregnant women |
1) Fear of Stigma and Discrimination 2) Fear of Violence 3) Fear of Negative Repercussions 4) Fear of Relationship Loss 5) Fear of Rejection and Abuse 6) Fear of Breach of Confidentiality 7) Fear of Loss of Financial Support 8) Challenges in Communication 9) Social-Ecological Factors |
1) Emotional Factors 2) Assisted Partner Notification 3) Partner Support |
1) Fear of Stigma and Discrimination 2) Fear of Consequences of a Positive Diagnosis 3) Fear of Intimate Partner Violence 4) Fear of Testing Itself 5) Refusal by Partners 6) Relationship Dissolution and Distance 7) Gender Norms and Inequality 8) Health System Barriers 9) Reluctance of Key Populations |
1) HIV Self-Testing 2) Increased Access to HIV Testing 3) Partner Support 4) Assisted Partner Notification 5) Health System Factors |
1) HIV Stigma 2) Spouse Negligence or Violence, |
NRa |
| Health care workers, health advisors, HIV testing and counseling counselors [37, 38, 43, 46, 49, 56, 58–60, 64, 65, 69, 70, 74, 82] |
1) Fear of Stigma and Discrimination 2) Fear of Relationship Loss 3) Fear of Violence 4) Fear of Rejection and Abuse 5) Fear of Negative Repercussions 6) Fear of Breach of Confidentiality 7) Fear of Loss of Financial Support 8) Fear of Isolation and Job Loss 9) Challenges in Communication 10) Social-Ecological Factors 11) Lack of Support from Partner 12) Lack of HIV Knowledge and Low Risk Perception 13) Shame and Denial 14) Lack of Partner Contact Information |
1) Emotional Factors 2) Assisted Partner Notification |
1) Fear of Consequences of a Positive Diagnosis 2) Fear of Stigma and Discrimination 3) Fear of Intimate Partner Violence 4) Fear of Testing Itself 5) Relationship Dissolution and Distance 6) Health System Barriers 7) Gender Norms and Inequality 8) Lack of Trust in Healthcare providers 9) Reluctance of Key Populations |
1) Increased Access to HIV Testing 2) Assisted Partner Notification 3) HIV Self-Testing 4) Community-Based HIV Testing 5) Health System Factors 6) Partner Support |
1) HIV Stigma 2) Spouse Negligence or Violence 3) Inconsistent and Under-Resourced Training |
1) Importance of Peer-Supported Linkages to HIV Care 2) Support from Family, Relatives, and the Social System 3) Conducting Multiple Counseling Sessions 4) Well-Organized Clinic Procedures 5) Presence of Disease Symptoms |
aNot reported
Discussion
In this mixed-methods systematic review, we analyzed data from 59 studies conducted in low- and lower-middle-income countries to comprehensively document and understand recent and emerging barriers and facilitators to HIV partner status notification. Our findings revealed common barriers across key populations, such as pervasive fear of stigma and discrimination and their negative consequences, including violence, abandonment, and breach of confidentiality and trust. This review also identified several facilitators, including emotional factors, such as feelings of love and closeness in relationships, the dynamics of marital relationships, and a sense of self and partner protection. Additionally, we identified innovative testing modalities that can enhance the effectiveness of HIV partner status notification, including increased access to HIV testing through methods through HIV self-testing.
Our findings underscore how stigma and discrimination function as primary barriers to HIV status disclosure across key populations. The complex decision to discuss HIV sero-status prevents many individuals from seeking HIV testing and counseling services or disclosing their status upon diagnosis [82]. Women face particular challenges, including fears of family stigmatization, loss of child custody, property rights, and spousal support [96, 97]. These barriers are compounded by concerns about violence, abandonment, breaches of confidentiality, and limited HIV. Legal frameworks, particularly HIV-specific criminal laws [98, 99], create additional obstacles, especially for marginalized populations including lesbian, gay, bisexual, transgender, queer/ questioning, intersex, and asexual (LGBTQIA+) people, people who use drugs, and people involved in sex work, undermining the principle that sexual health is a shared responsibility between sexual partners [100, 101]. Women living with HIV face further challenges through forced sterilization and criminalization of transmission [99, 102].
The context-specific nature of these barriers varies between resource settings, with low- and lower-middle-income countries struggling with limited resources, weak healthcare infrastructure, entrenched cultural norms, and low-risk perception [4, 12], while high-income countries grapple with legal and ethical issues affecting marginalized populations, such as undocumented refugees, immigrants, Indigenous people, and people of color. Despite these differences, successful interventions across contexts share common elements, such as community-based approaches, support services, and capacity-building efforts [97, 98, 100, 103]. Addressing context-specific barriers while harnessing facilitators tailored to local realities is crucial for effective HIV partner status notification strategies globally [5, 97, 104]. Until healthcare professionals and communities effectively address HIV stigma and its consequences, these barriers will continue to fuel the spread of HIV. Therefore, developing supportive strategies that assist people living with HIV in disclosing their HIV status and implementing community initiatives to transform attitudes towards HIV stigma and discrimination offer the most promising path forward.
Our review also identified different aspects of partner support that contribute to the process of HIV partner status notification, including emotional connections, such as love and closeness in marital relationships and motivations to protect oneself and one’s partner. Previous studies have highlighted the potential positive outcomes associated with HIV partner status notification, including a desire for a closer relationship [103, 105]. Partner notification could also lead to greater relationship closeness and stability [106]. Effective communication between partners, during which people living with HIV encourage their partners to consider screening or treatment, has been identified as a critical component of successful partner notification strategies [11]. Service providers should therefore consider these factors (e.g., stability, closeness, intimacy) in the HIV counseling process. While these interventions primarily aim to modify individual behavior, their system-level impacts could be viewed as secondary effects resulting from these behavioral changes [107]. This multifaceted approach ensures that interventions address both individual and systemic aspects of behavior change, maximizing their potential impact.
Our review identified several innovative testing approaches that could enhance HIV partner status notification and early diagnosis, including HIV self-testing, community-based HIV testing, mobile testing, and home-based testing. The WHO guidelines on HIV self-testing and partner notification highlight these novel approaches as promising methods for reaching people with undiagnosed HIV and strengthening partner engagement and relationship bonds [7]. However, it is important to acknowledge that implementing HIV self-testing in low and lower-middle-income countries faces challenges, such as cost barriers and user error rates [108]. Notably, despite the concept of undetectable = untransmittable (U = U) being established since at least 2018 [109], none of the studies in our analysis discussed it, suggesting healthcare workers’ reluctance to discuss this critical information with patients. Although a 2019 study advocated for all healthcare workers to discuss U = U with all people living with HIV [110], our findings suggest this recommendation has not been widely implemented. This oversight is particularly concerning as it potentially infringes upon patients’ rights to access the most current and relevant health information [111]. The lack of U = U discussions represents a critical gap in HIV care and education that warrants immediate attention from healthcare providers and policymakers. Future research should prioritize this aspect, specifically examining the implementation of U = U discussions in clinical settings and evaluating healthcare providers’ adherence to their duty of sharing and discussing this crucial information with patients. Addressing these issues by tailoring innovative approaches to the specific contexts of low and lower-middle-income countries and providing education and training programs is crucial to effectively identify and control HIV in key populations, and preventing its transmission to others. Studies have shown that community-based and non-governmental organizations (NGOs) play a crucial role in supporting HIV services engagement, complementing traditional healthcare settings. These organizations implement various effective programs, including HIV testing promotion among key populations, reduction of HIV-related and marginalized-group stigma, and addressing testing-related fears. They also work to promote pro-testing peer and social norms while ensuring non-judgmental, culturally competent HIV counseling and testing services [112]. Additionally, programs that combine critical reflection on gender norms with information sharing (e.g., antiretroviral therapy benefits) and skill-building (e.g., communication) have proven effective in supporting people living with HIV and key populations in their engagement with HIV services [113].
Strengths and limitations
A major strength of this review is its integration of quantitative and qualitative data to address a complex research question. This approach allows for a comprehensive synthesis of diverse perspectives, providing decision-makers with results directly relevant to their work. Additionally, the broad scope of this review enables the identification of emerging and lesser-known barriers and facilitators, offering a comprehensive view across diverse populations.
Nevertheless, this review has several limitations. First, despite our comprehensive search strategy, it is possible that some relevant studies were not identified in our search, potentially leading to the omission of certain barriers or facilitators that may be absent from this synthesis. Second, the nature of the data precludes the establishment of causality for the identified barriers and facilitators. Third, while results are presented across various populations, some key populations and territories are underrepresented, potentially limiting the generalizability of findings. Fourth, this study could not identify any differences in barriers and facilitators of HIV partner status notification between older and more recent studies, despite the possibility that challenges in HIV care may have evolved over time. This aspect should be considered in future research, especially considering the game-changing context of U = U. Lastly, in this study, we included only people aged 18 or older and our findings are not generalizable to minors living with HIV.
Conclusion
HIV partner status notification is essential to effective HIV prevention and treatment. Our findings highlight several important factors that can be leveraged to increase HIV partner status notification in resource-limited settings and provide valuable evidence for shaping practice, policy, and future research aiming to advance global HIV targets. A notable gap in the reviewed studies was the discussion of U=U. Healthcare providers have a responsibility to discuss U=U with all people living with HIV, and this critical issue warrants further research attention. Future studies would also benefit from actively incorporating the perspectives of people living with HIV, as their insights are essential for informing policies and programs. People living with HIV have long advocated for the removal of punitive policies and laws that create barriers to status disclosure, even in the context of U=U. The intersection of criminalization, discrimination, and various vulnerabilities further complicates this situation. Therefore, policymakers should carefully consider the legal and ethical implications of partner notification requirements at the global level.
Supplementary Information
Acknowledgements
We appreciate all researchers who provided complementary data needed from their studies. We also would like to thank the Student Research Committee, Kerman University of Medical Sciences.
Abbreviations
- MMAT
Mixed-methods appraisal tool
- WHO
World Health Organization
- CDC
Center for Disease Control
- NGO
non-governmental organization
- IQR
Interquartile range
- SD
Standard deviation
- NR
Not reported
- FGD
Focus group discussion
Authors’ contributions
FT, MK and HSH designed the study; AB conducted a literature review/search; FT and MB screened the title/abstract and full-texts, and extracted data; FT and MB conducted data synthesis. The initial draft of the manuscript was prepared by FT and revised by MK. All authors reviewed and approved the final version of the manuscript.
Funding
The study was supported by Kerman University of Medical Sciences (Grant number: 401000288).
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.HIV/AIDS JUNPo. Fast-track: ending the AIDS epidemic by 2030. Geneva: UNAIDS; 2014. [Google Scholar]
- 2.UNAIDS. Prevailing against pandemics by putting people at the centre—World AIDS Day report 2020. https://www.unaids.org/en/resources/documents/2020/prevailing-against-pandemics. Accessed 23 Oct 2024.
- 3.HIV/AIDS JUNPo, Ending AIDS. Progress towards the 90-90-90 targets. Global AIDS Update. 2017. https://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. Accessed 23 Oct 2024.
- 4.Joulaei H, Shooshtarian S, Dianatinasab M. Is UNAIDS 90–90-90 target a dream or a reality for Middle East and North Africa Region on ending the AIDS Epidemic? A review study. AIDS Rev. 2018;20(2):83. [DOI] [PubMed] [Google Scholar]
- 5.Smith R, Rossetto K, Peterson BL. A meta-analysis of disclosure of one’s HIV-positive status, stigma and social support. AIDS Care. 2008;20(10):1266–75. [DOI] [PubMed] [Google Scholar]
- 6.Muessig KE, Tucker JD, Wang B-X, Chen X-S. HIV and syphilis among men who have sex with men in China: the time to act is now. Sex Transm Dis. 2010;37(4):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. World Health Organization; 2016. [PubMed] [Google Scholar]
- 8.Simoni JM, Pantalone DW. Secrets and safety in the age of AIDS: does HIV disclosure lead to safer sex? Top HIV Med. 2004;12:109–18. [PubMed] [Google Scholar]
- 9.Min S, Gillani FS, Aung S, Garland JM, Beckwith CG, editors. Evaluating HIV viral rebound among persons on suppressive antiretroviral treatment in the era of undetectable equals untransmittable (U = U). Open Forum Infect Dis. 2020;7(12):ofaa529. [DOI] [PMC free article] [PubMed]
- 10.Prevention Access Campaign. 2024 [ https://www.preventionaccess.org/faq/].
- 11.Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev. 2013;10:CD002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Zhao J, Li X, Chen X, Wang H, Williams AB, et al. Perceived facilitators and barriers regarding partner notification in people living with HIV in Hunan, China: a qualitative study from the patient perspective. J Assoc Nurses AIDS Care. 2019;30(6):658–67. [DOI] [PubMed] [Google Scholar]
- 14.Wang AL, Peng R-R, Tucker JD, Cohen MS, Chen X-S. Partner notification uptake for sexually transmitted infections in China: a systematic literature review. Sex Transm Infect. 2012;88(5):386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semple SJ, Pines HA, Strathdee SA, Vera AH, Rangel G, Magis-Rodriguez C, et al. Uptake of a partner notification model for HIV among men who have sex with men and transgender women in Tijuana, Mexico. AIDS Behav. 2018;22:2042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global HIV and AIDS statistics—Fact sheet. 2021. https://www.unaids.org/en/resources/fact-sheet. Accessed 23 Oct 2024.
- 17.Dunaway K, Brion S, Hale F, Alesi J, Assan H, Chung C, et al. What will it take to achieve the sexual and reproductive health and rights of women living with HIV? Women’s Health. 2022;18:17455057221080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, Williamson C. The HIV-1 epidemic: low-to middle-income countries. Cold Spring Harbor Perspect Med. 2012;2(3):a007187. [DOI] [PMC free article] [PubMed]
- 19.Dalal S, Johnson C, Fonner V, Kennedy CE, Siegfried N, Figueroa C, et al. Improving HIV test uptake and case finding with assisted partner notification services. AIDS. 2017;31(13):1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy CE, Fonner VA, Armstrong KA, O’reilly KR, Sweat MD. Increasing HIV serostatus disclosure in low-and middle-income countries: a systematic review of intervention evaluations. AIDS. 2015;29(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PROSPERO. International prospective register of systematic reviews 2022 [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022379427]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 23.The World Bank. 2023 [ https://www.blogs.worldbank.org/en/opendata/new-world-bank-group-country-classifications-income-level]
- 24.World Health Organization. Global health sector response to HIV, 2000–2015: focus on innovations in Africa: progress report. 2015. https://www.afro.who.int/publications/global-health-sector-response-hiv-2000-2015-focus-innovations-africa. Accessed 23 Oct 2024.
- 25.Mathews C, Coetzee N, Zwarenstein M, Lombard C, Guttmacher S, Oxman A, et al. A systematic review of strategies for partner notification for sexually transmitted diseases, including HIV/AIDS. Int J STD AIDS. 2002;13(5):285–300. [DOI] [PubMed] [Google Scholar]
- 26.Golden MR. HIV partner notification: a neglected prevention intervention. Sex Transm Dis. 2002;29(8):472–5. [DOI] [PubMed] [Google Scholar]
- 27.Golden MR. Assisted partner services for HIV: ready to go global. AIDS. 2017;31(13):1891–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toomey KE, Cates W Jr. Partner notification for the prevention of HIV infection. AIDS. 1989;3(1):S57–62. [DOI] [PubMed] [Google Scholar]
- 29.UNAIDS. [ https://www.unaids.org/en/topic/key-populations]
- 30.Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS ONE. 2015;10(9):e0138237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmond JA, Kirkpatrick P, Loveday H. Mixed methods systematic reviews. Int J Evid Based Healthc. 2015;13(3):121–31. [DOI] [PubMed]
- 32.Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed methods appraisal tool (MMAT), version 2018. Registration Copyr. 2018;1148552(10):285–91.
- 33.Hannes K, Noyes J, Booth A, Harden A, Harris J, Lewin S, et al. Critical appraisal of qualitative research. 2011;19(4):364–7.
- 34.Sandelowski M, Barroso J, Voils CI. Using qualitative metasummary to synthesize qualitative and quantitative descriptive findings. Res Nurs Health. 2007;30(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petticrew M, Rehfuess E, Noyes J, Higgins JP, Mayhew A, Pantoja T, et al. Synthesizing evidence on complex interventions: how meta-analytical, qualitative, and mixed-method approaches can contribute. J Clin Epidemiol. 2013;66(11):1230–43. [DOI] [PubMed] [Google Scholar]
- 36.Song W, Mulatu MS, Rao S, Mendoza MC, Kudon HZ, Rorie M. Factors associated with partner notification, testing, and positivity in HIV partner services programs in the United States, 2013 to 2017. Sex Transm Dis. 2022;49(3):197–203. [DOI] [PubMed] [Google Scholar]
- 37.Goyette M, Wamuti BM, Owuor M, Bukusi D, Maingi PM, Otieno FA, et al. Understanding barriers to scaling up HIV assisted partner services in Kenya. AIDS Patient Care STDs. 2016;30(11):506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Wamuti BM, Owuor M, Lagat H, Kariithi E, Obong’o C, et al. It is a process–a qualitative evaluation of provider acceptability of HIV assisted partner services in western Kenya: experiences, challenges, and facilitators. BMC Health Serv Res. 2022;22(1):616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osoti AO, John-Stewart G, Kiarie J, Richardson B, Kinuthia J, Krakowiak D, et al. Home visits during pregnancy enhance male partner HIV counseling and testing in Kenya: a randomized clinical trial. AIDS. 2014;28(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otieno PA, Kohler PK, Bosire RK, Brown ER, Macharia SW, John-Stewart GC. Determinants of failure to access care in mothers referred to HIV treatment programs in Nairobi, Kenya. AIDS Care. 2010;22(6):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pintye J, Drake AL, Begnel E, Kinuthia J, Abuna F, Lagat H, et al. Acceptability and outcomes of distributing HIV self-tests for male partner testing in Kenyan maternal child health and family planning clinics. AIDS. 2019;33(8):1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma M, Kariithi E, Kemunto E, Otieno G, Lagat H, Wamuti B, et al. High acceptability of assisted partner notification services among HIV-positive females in Kenya: results from an ongoing implementation study. J Acquir Immune Defic Syndr. 2021;86(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sircar NR, Maleche AA. Assessing a human rights-based approach to HIV testing and partner notification in Kenya: a qualitative study to examine how Kenya’s policies and practices implement a rights-based approach to health. Health Hum Rights. 2020;22(2):167. [PMC free article] [PubMed] [Google Scholar]
- 44.Van Der Elst E, Kombo B, Mugo P, Thiong’o A, Kanungi J, Wahome E, et al. Adjustment to acute or early HIV-1 infection diagnosis to prompt linkage to care and ART initiation: qualitative insights from coastal Kenya. Psychol Health Med. 2019;24(5):631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Elst EM, Abuna M, Agutu C, Ogada F, Galole A, Shikuku J, et al. Facilitating HIV status adjustment: qualitative insights from the Tambua Mapema proof-of-concept study in Kenya. PLoS ONE. 2022;17(1):e0261255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welty S, Motoku J, Muriithi C, Rice B, de Wit M, Ashanda B, et al. Brief report: recent HIV infection surveillance in routine HIV testing in Nairobi, Kenya: a feasibility study. JAIDS J Acquir Immune Defic Syndr. 2020;84(1):5–9. [DOI] [PubMed] [Google Scholar]
- 47.Monroe-Wise A, Maingi Mutiti P, Kimani H, Moraa H, Bukusi DE, Farquhar C. Assisted partner notification services for patients receiving HIV care and treatment in an HIV clinic in Nairobi, Kenya: a qualitative assessment of barriers and opportunities for scale‐up. J Int AIDS Soc. 2019;22:e25315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krakowiak D, Kinuthia J, Osoti AO, Asila V, Gone MA, Mark J, et al. Home-based HIV testing among pregnant couples increases partner testing and identification of serodiscordant partnerships. J Acqu Immune Defic Syndr (1999). 2016;72(Suppl 2):S167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odoyo JB, Morton JF, Ngure K, O’Malley G, Mugwanya KK, Irungu E, et al. Integrating pr EP into HIV care clinics could improve partner testing services and reinforce mutual support among couples: provider views from a pr EP implementation project in Kenya. J Int AIDS Soc. 2019;22:e25303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masters SH, Agot K, Obonyo B, Napierala Mavedzenge S, Maman S, Thirumurthy H. Promoting partner testing and couples testing through secondary distribution of HIV self-tests: a randomized clinical trial. PLoS Med. 2016;13(11):e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buhikire K, Voss J, Kigozi J, Nyakato P, Ankunda N, Kalebbo B, et al. Reaching the first 90 in Uganda: predictors of success in contacting and testing the named sexual partners of HIV + index clients in Kiboga District. AIDS Behav. 2018;22:2458–67. [DOI] [PubMed] [Google Scholar]
- 52.Kiene SM, Gbenro O, Sileo KM, Lule H, Wanyenze RK. How do we get partners to test for HIV? Predictors of uptake of partner HIV testing following individual outpatient provider initiated HIV testing in rural Uganda. AIDS Behav. 2017;21:2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim LH, Arinaitwe E, Nzarubara B, Kamya MR, Clark TD, Okong P, et al. Acceptability and feasibility of serial HIV antibody testing during pregnancy/postpartum and male partner testing in Tororo, Uganda. AIDS Care. 2014;26(3):360–6. [DOI] [PubMed] [Google Scholar]
- 54.King R, Lifshay J, Nakayiwa S, Katuntu D, Lindkvist P, Bunnell R. The virus stops with me: HIV-infected ugandans’ motivations in preventing HIV transmission. Soc Sci Med. 2009;68(4):749–57. [DOI] [PubMed] [Google Scholar]
- 55.Klabbers RE, Muwonge TR, Ayikobua E, Izizinga D, Bassett IV, Kambugu A, et al. Understanding the role of interpersonal violence in assisted partner notification for HIV: a mixed-methods study in refugee settlements in West Nile Uganda. J Global Health. 2020;10(2):020440. [DOI] [PMC free article] [PubMed]
- 56.Klabbers RE, Muwonge TR, Ayikobua E, Izizinga D, Bassett IV, Kambugu A, et al. Health worker perspectives on barriers and facilitators of assisted partner notification for HIV for refugees and Ugandan nationals: a mixed methods study in West Nile Uganda. AIDS Behav. 2021;25:3206–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korte JE, Strauss M, Ba A, Buregyeya E, Matovu JK, Kisa R, et al. HIV testing preferences among pregnant women attending antenatal care and their male partners: a discrete choice experiment in Uganda. Afr J AIDS Res. 2019;18(4):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lofgren SM, Tsui S, Atuyambe L, Ankunda L, Komuhendo R, Wamala N, et al. Barriers to HIV care in Uganda and implications for universal test-and-treat: a qualitative study. AIDS Care. 2022;34(5):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakigozi G, Atuyambe L, Kamya M, Makumbi FE, Chang LW, Nakyanjo N, et al. A qualitative study of barriers to enrollment into free HIV care: perspectives of never-in-care HIV-positive patients and providers in Rakai Uganda. BioMed Res Int. 2013;2013:470245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quinn C, Nakyanjo N, Ddaaki W, Burke VM, Hutchinson N, Kagaayi J, et al. HIV partner notification values and preferences among sex workers, fishermen, and mainland community members in Rakai, Uganda: a qualitative study. AIDS Behav. 2018;22:3407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choko AT, Corbett EL, Stallard N, Maheswaran H, Lepine A, Johnson CC, et al. HIV self-testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: an adaptive multi-arm, multi-stage cluster randomised trial. PLoS Med. 2019;16(1):e1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dovel KL, Balakasi K, Phiri K, Shaba F, Offorjebe OA, Gupta SK, et al. A randomized trial of index HIV self-testing for sexual partners of ART clients in Malawi. medRxiv. 2022:2022–09. [DOI] [PMC free article] [PubMed]
- 63.Hino S, Grodensky C, Rutstein SE, Golin C, Smith MK, Christmas L, et al. HIV status disclosure during acute HIV infection in Malawi. PLoS ONE. 2018;13(7):e0201265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamanga G, Brown L, Jawati P, Chiwanda D, Nyirenda N. Maximizing HIV partner notification opportunities for index patients and their sexual partners in Malawi. Malawi Med J. 2015;27(4):140–4. [PMC free article] [PubMed] [Google Scholar]
- 65.Matoga M, Mmodzi P, Massa C, Bula A, Hosseinipour M, Chasela C. Health system factors influencing partner notification for STIs and HIV in Lilongwe Malawi. A pre-intervention phase assessment for a quality improvement project. J Infect Dis Med. 2018;3(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Offorjebe OA, Hoffman RM, Shaba F, Balakasi K, Davey DJ, Nyirenda M, et al. Acceptability of index partner HIV self-testing among HIV-positive clients in Malawi: a mixed methods analysis. PLoS ONE. 2020;15(7):e0235008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56(5):437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown LB, Miller WC, Kamanga G, Kaufman JS, Pettifor A, Dominik RC, et al. Predicting partner HIV testing and counseling following a partner notification intervention. AIDS Behav. 2012;16:1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conserve DF, Issango J, Kilale AM, Njau B, Nhigula P, Memiah P, et al. Developing national strategies for reaching men with HIV testing services in Tanzania: results from the male catch-up plan. BMC Health Serv Res. 2019;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Falnes EF, Moland KM, Tylleskär T, de Paoli MM, Msuya SE, Engebretsen IM. It is her responsibility: partner involvement in prevention of mother to child transmission of HIV programmes, northern Tanzania. J Int AIDS Soc. 2011;14(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahabuka C, Plotkin M, Christensen A, Brown C, Njozi M, Kisendi R, et al. Addressing the first 90: a highly effective partner notification approach reaches previously undiagnosed sexual partners in Tanzania. AIDS Behav. 2017;21:2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plotkin M, Kahabuka C, Christensen A, Ochola D, Betron M, Njozi M, et al. Outcomes and experiences of men and women with partner notification for HIV testing in Tanzania: results from a mixed method study. AIDS Behav. 2018;22:102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanga E, Nampewo Z, PrayGod G, Wringe A. HIV positive status disclosure to sexual partners: a qualitative study to explore experiences and challenges among clients attending HIV care services in North-Western Tanzania. AIDS Care. 2023;35(7):953–60. [DOI] [PubMed] [Google Scholar]
- 74.Sanga ES, Mukumbang FC, Mushi AK, Lerebo W, Zarowsky C. Understanding factors influencing linkage to HIV care in a rural setting, Mbeya, Tanzania: qualitative findings of a mixed methods study. BMC Public Health. 2019;19(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alemayehu M, Haidar J. Male involvement in prevention of mother-to-child transmission of HIV in the context of partner testing in Goba town, Ethiopia: a facility-based cross-sectional study. South Afr Med J. 2017;107(10):864–70. [DOI] [PubMed] [Google Scholar]
- 76.Tegegne AS. Prevalence for the disclosure of HIV status to sexual partners and its determinants among adults under cART in Amhara Region, Northwest Ethiopia. J Trop Med. 2022;2022:9941380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Netsanet F, Dessie A. Acceptance of referral for partners by clients testing positive for human immunodeficiency virus. HIV/AIDS-Res Palliat Care. 2013;5:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kachero Markos M, Kinfe Arba A. Utilization of HIV Test Service among pregnant women’s partners and its Associated factors in selected sub-cities of Addis Ababa, 2019: a community-based cross-sectional study. HIV/AIDS-Res Palliat Care. 2021;135:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis. 2013;40(12):909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tih PM, Temgbait Chimoun F, Mboh Khan E, Nshom E, Nambu W, Shields R, et al. Assisted HIV partner notification services in resource-limited settings: experiences and achievements from Cameroon. J Int AIDS Soc. 2019;22:e25310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Njozing BN, Edin KE, Sebastián MS, Hurtig A-K. If the patients decide not to tell what can we do?-TB/HIV counsellors’ dilemma on partner notification for HIV. BMC Int Health Hum Rights. 2011;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carrasco MA, Arias R, Figueroa ME. The multidimensional nature of HIV stigma: evidence from Mozambique. Afr J AIDS Res. 2017;16(1):11–8. [DOI] [PubMed] [Google Scholar]
- 83.Myers RS, Feldacker C, Cesár F, Paredes Z, Augusto G, Muluana C, et al. Acceptability and effectiveness of assisted human immunodeficiency virus partner services in Mozambique. Sex Transm Dis. 2016;43(11):690–5. [DOI] [PubMed] [Google Scholar]
- 84.Mutale W, Freeborn K, Graybill LA, Lusaka MM, Mollan KR, Mweemba O, et al. Addition of HIV self-test kits to partner notification services to increase HIV testing of male partners of pregnant women in Zambia: two parallel randomised trials. Lancet Global Health. 2021;9(12):e1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Makoleka MM. Factors affecting partner notification of HIV status. Afr J Midwifery Women’s Health. 2012;6(3):145–8. [Google Scholar]
- 86.Daniels J, De Vos L, Mogos W, Olivier D, Shamu S, Mudau M, et al. Factors influencing sexually transmissible infection disclosure to male partners by HIV-positive pregnant women in Pretoria townships, South Africa: a qualitative study. Sex Health. 2019;16(3):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joseph Davey DL, Wall KM, Naidoo N, Naidoo D, Xaba G, Serao C, et al. HIV testing and linkage to ART following secondary distribution of HIV self-test kits to male partners of women living with HIV: a pilot randomized control trial in Mpumalanga, South Africa. J Int AIDS Soc. 2022;25(6):e25937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Omunakwe HE, Okoye H, Efobi C, Onodingene M, Chinenye S, Nwauche CA. Disclosure amongst adult HIV patients on antiretroviral therapy in Port Harcourt, Nigeria. Int J STD AIDS. 2015;26(10):729–32. [DOI] [PubMed] [Google Scholar]
- 89.Jeremiah RD, Patel DR, Chirwa E, Kapito E, Mei X, McCreary LL, et al. A randomized group antenatal care pilot showed increased partner communication and partner HIV testing during pregnancy in Malawi and Tanzania. BMC Pregnancy Childbirth. 2021;21(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madsen T, Jespersen S, Medina C, Té DD, Wejse C, Laursen AL, et al. Acceptance and feasibility of partner notification to HIV infected individuals in Guinea-Bissau. AIDS Behav. 2020;24:1476–85. [DOI] [PubMed] [Google Scholar]
- 91.Selvaraj K, Kumar A, Chawla S, Shringarpure K, Thekkur P, Palanivel C, et al. Are partners of HIV-infected people being tested for HIV? A mixed-methods research from Gujarat, India. Public Health Action. 2017;7(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Remera E, Nsanzimana S, Chammartin F, Semakula M, Rwibasira GN, Malamba SS, et al. Active HIV case finding in the City of Kigali, Rwanda: assessment of voluntary assisted partner notification modalities to detect undiagnosed HIV infections. J Acqu Immune Defic Syndr (1999). 2022;89(4):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Issiaka S, Cartoux M, Ky-Zerbo O, Tiendrebeogo S, Meda N, Dabis F, et al. Living with HIV: women’s experience in Burkina Faso, West Africa. AIDS Care. 2001;13(1):123–8. [DOI] [PubMed] [Google Scholar]
- 94.Madiba S, Putsoane M. Testing positive and disclosing in pregnancy: a phenomenological study of the experiences of adolescents and young women in Maseru Lesotho. AIDS Res Treat. 2020;2020:6126210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pashaei Z, Oskouie F, Moradi-Lakeh M, Jahanfar S, Haghani S. HIV serostatus disclosure to sexual partner: a survey among women in Tehran, Iran. Eur J Med Res. 2022;27(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.French H, Greeff M, Watson MJ, Doak CM. HIV stigma and disclosure experiences of people living with HIV in an urban and a rural setting. AIDS Care. 2015;27(8):1042–6. [DOI] [PubMed] [Google Scholar]
- 97.Sowell RL, Phillips KD. Understanding and responding to HIV/AIDS stigma and disclosure: an international challenge for mental health nurses. Issues Ment Health Nurs. 2010;31(6):394–402. [DOI] [PubMed] [Google Scholar]
- 98.Lehman JS, Carr MH, Nichol AJ, Ruisanchez A, Knight DW, Langford AE, et al. Prevalence and public health implications of state laws that criminalize potential HIV exposure in the United States. AIDS Behav. 2014;18:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jürgens R, Cohen J, Cameron E, Burris S, Clayton M, Elliott R, et al. Ten reasons to oppose the criminalization of HIV exposure or transmission. Reprod Health Matters. 2009;17(34):163–72. [DOI] [PubMed] [Google Scholar]
- 100.DeBeck K, Cheng T, Montaner JS, Beyrer C, Elliott R, Sherman S, et al. HIV and the criminalisation of drug use among people who inject drugs: a systematic review. Lancet HIV. 2017;4(8):e357–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Platt L, Grenfell P, Meiksin R, Elmes J, Sherman SG, Sanders T, et al. Associations between sex work laws and sex workers’ health: a systematic review and meta-analysis of quantitative and qualitative studies. PLoS Med. 2018;15(12):e1002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.UNAIDS. 2024 [ https://www.unaids.org/en/resources/presscentre/featurestories/2024/july/20240724_confronting-coercion]
- 103.Chaudoir SR, Fisher JD. The disclosure processes model: understanding disclosure decision making and postdisclosure outcomes among people living with a concealable stigmatized identity. Psychol Bull. 2010;136(2):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomnay JE, Hulme-Chambers A, Bilardi J, Fairley CK, Huffam S, Chen MY. A qualitative study of means to improve partner notification after an HIV diagnosis among men who have sex with men in Australia. AIDS Patient Care STDs. 2017;31(6):269–74. [DOI] [PubMed] [Google Scholar]
- 105.Greene K, Derlega VJ, Mathews A. Self-disclosure in personal relationships. Camb Handb Person Relat. 2006;409:427. [Google Scholar]
- 106.Kissinger PJ, Niccolai LM, Magnus M, Farley TA, Maher JE, Richardson-Alston G, et al. Partner notification for HIV and Syphilis: effects on sexual behaviors and relationship stability. Sex Transm Dis. 2003;30:75–82. [DOI] [PubMed] [Google Scholar]
- 107.Chater N, Loewenstein G. The i-frame and the s-frame: how focusing on individual-level solutions has led behavioral public policy astray. Behav Brain Sci. 2023;46:e147. [DOI] [PubMed] [Google Scholar]
- 108.Rivera AS, Hernandez R, Mag-Usara R, Sy KN, Ulitin AR, O’Dwyer LC, et al. Implementation outcomes of HIV self-testing in low-and middle-income countries: a scoping review. PLoS ONE. 2021;16(5):e0250434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.UNAIDS. undetectable-. untransmittable 2018 [https://www.unaids.org/en/resources/presscentre/featurestories/2018/july/undetectable-untransmittable]
- 110.Calabrese SK, Mayer KH. Providers should discuss U = U with all patients living with HIV. Lancet HIV. 2019;6(4):e211–3. [DOI] [PubMed] [Google Scholar]
- 111.Ferguson L, Jardell W, Gruskin S. Leaving no one behind: human rights and gender as critical frameworks for U = U. Health Hum Rights. 2022;24(2):1. [PMC free article] [PubMed] [Google Scholar]
- 112.Woodford MR, Chakrapani V, Newman PA, Shunmugam M. Barriers and facilitators to voluntary HIV testing uptake among communities at high risk of HIV exposure in Chennai, India. Glob Public Health. 2016;11(3):363–79. [DOI] [PubMed] [Google Scholar]
- 113.Leddy AM, Gottert A, Haberland N, Hove J, West RL, Pettifor A, et al. Shifting gender norms to improve HIV service uptake: qualitative findings from a large-scale community mobilization intervention in rural South Africa. PLoS ONE. 2021;16(12):e0260425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.