Abstract
Background
Active cytomegalovirus (CMV) infection is associated with poor prognosis in septic patients with critical illness. Patients of septic category are highly likely to benefit from prophylactic antiviral therapy. Nevertheless, the clinical characteristics for CMV reactivation are lacking among septic patients requiring mechanical ventilation. The aim of this study was to investigate the incidence, risk factors, and clinical outcomes regarding active CMV infection in mechanically ventilated patients with sepsis.
Methods
A single-center, retrospective cohort study conducted from January 2021 to December 2023 that included septic patients on mechanical ventilation at the intensive care unit (ICU) of a national hospital. Study participants were divided into active and non-active CMV infection groups based on CMV DNAemia within a 28-day hospitalization period in ICU. Clinical features, laboratory findings, treatment measures, and clinical outcomes were compared between the two groups.
Results
Among 118 septic patients, 21 (17.8%) exhibited active CMV infection within 28-day ICU admission. Hemoglobin served as an independent risk factor and predictor for active CMV infection (P < 0.05). Moreover, the duration of mechanical ventilation and ICU stay in active CMV infection patients were significantly higher than in the comparison group (P < 0.05).
Conclusions
Active CMV infection is common and associated with adverse clinical outcomes in mechanically ventilated patients with sepsis. A low level of hemoglobin is an independent risk factor for active CMV infection. Further prospective studies are warranted to assess the efficacy of initiating prophylactic and preemptive antiviral therapies among patients with sepsis disorders.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10304-4.
Keywords: Active cytomegalovirus infection, Sepsis, Immunocompetent, Mechanical ventilation, Clinical characteristics
Background
Cytomegalovirus (CMV) is a ubiquitous latent viral infection prevalent throughout the general population [1]. CMV possesses the potential for reactivation under specific conditions, and the perilous impact is well-documented, particularly in immunocompetent patients with critical illness [2]. Among those patients, our previous study found the incidence of CMV reactivation could reach more than 30%, and were associated with prolonged duration of mechanical ventilation (MV), increased length of hospital stay, and elevated mortality [2–5].
Our recent research showed that when non-immunosuppressed critically ill patients admitted for sepsis (and/or septic shock), the incidence of CMV reactivation could be significantly increased, and similar results were found in other studies [4–7]. Moreover, sepsis is acknowledged as a significant risk factor for CMV reactivation, as the hyperinflammation can prompt the transition of CMV from latency to activation [2–7]. Patients of septic category are highly likely to benefit from prophylactic antiviral therapy [8]. However, research on active CMV infection in mechanically ventilated patients with sepsis remains scarce.
Therefore, this study aimed at investigating the incidence, risk factors, and clinical outcomes of active CMV infection among mechanically ventilated patients with sepsis. These results serve as an early warning for the identification of high-risk patients, thereby ameliorating the adverse clinical outcomes and providing guidance for antiviral therapy.
Methods
Study design and ethics
A single-center, retrospective cohort study included septic patients on mechanical ventilation at the respiratory intensive care unit (ICU) of a national hospital. The Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University approved the study protocol (No. ES-2024-K084-01). Informed consent was not required due to the retrospective nature of the study. The reporting of this study adheres to the STROBE Statement guidelines for observational research [9].
Participants and definitions
The research team conducted a comprehensive retrospective analysis of septic patients requiring MV admitted to the respiratory ICU at the First Affiliated Hospital of Guangzhou Medical University, China, from January 2021 to December 2023. Active CMV infection was ascertained through quantitative polymerase chain reaction (qPCR), utilizing blood plasma samples. Participants were classified into two groups based on CMV DNAemia levels during their 28-day ICU stay: active infection group (CMV DNAemia ≥ 500 copies/mL) and the non-active infection group (CMV DNAemia < 500 copies/mL). It should be noted that all participants in this study underwent CMV testing, and those with a CMV viral load of 0 copies/mL were not excluded. Active CMV infection was defined as viral load greater than or equal to 500 copies/mL in plasma [10–12]. The screening of the CMV viral load in the plasma was part of the routine clinical practice in the hospital (at least once per week).
The following inclusion and exclusion criteria were as follows: (1) inclusion criteria: ①Met the diagnostic criteria for sepsis (SEPSIS-3 criteria) [13] within 24 h ICU admission; ②Required MV support. (2) exclusion criteria: ①Aged < 18 years; ②Pregnant or lactation; ③ICU stay < 5 d or survival time < 72 h; ④Duration of MV < 5 d; ⑤Lack of CMV detection (CMV testing was not conducted in both plasma and endotracheal aspirates simultaneously); ⑥Readmitted to ICU or reintubation; ⑦Prior administration of antiviral therapy (ganciclovir, valganciclovir, aciclovir, valaciclovir, or foscarnet) before ICU entry; ⑧Immunocompromised: diagnosed with solid organ or bone marrow tumor, neutropenic, systemic glucocorticoids or cytotoxic drugs were used, transplantation, connective tissue disease, and HIV; ⑨Incomplete clinical data.
Data collection and quality control
Clinical data from the electronic medical records of those enrolled patients was extracted by two independent investigators who then adjudicated the accuracy of the data, including clinical features, laboratory findings, treatment measures, complications, and clinical outcomes. In cases of disagreement, a third reviewer (critical care specialist) resolved the differences. The collected data were then entered into an electronic database to facilitate subsequent statistical analysis.
Study outcomes
The primary outcomes included the risk factors (predictive value) and clinical outcomes for active CMV infection among those participants. Moreover, the secondary outcomes included clinical features, laboratory findings, treatment measures, and complications.
Data analysis
All statistical analyses and graphical representations for data interpretation and visualization were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) or GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). Continuous variables were described as the mean ± standard deviation (SD) or as median with interquartile ranges (IQRs), and were analyzed using the Wilcoxon rank-sum test for comparisons. Categorical variables were summarized as frequencies and percentages and were compared using the chi-square test or Fisher's exact test, where appropriate.
Univariate logistic regression analysis was used to identify risk factors associated with active CMV infection. Variables with a P-value of less than 0.05 were considered potential risk factors and were included in a multivariate logistic regression model to refine the analysis. The predictive model for active CMV infection was developed by calculating regression coefficients (β), odds ratios (ORs), and the corresponding 95% confidence intervals (CIs). The predictive accuracy of the model was assessed using the receiver operating characteristic (ROC) curve, with key metrics including the area under the ROC curve (AUC), 95% CI, P-value, optimal cut-off point, sensitivity, and specificity. Lengths of 30-day MV and IMV, 28-day ventilator-free days (VFD), and duration of 30-day ICU stay rates between two groups were compared using the Kaplan–Meier (KM) survival analysis, with statistical differences assessed using the log-rank test. Correlation coefficients between CMV load in plasma and endotracheal aspirates (ETA) were calculated using the Pearson’s correlation coefficient. Statistical significance was defined as a two-sided P-value less than 0.05.
Results
Incidence of active CMV infection
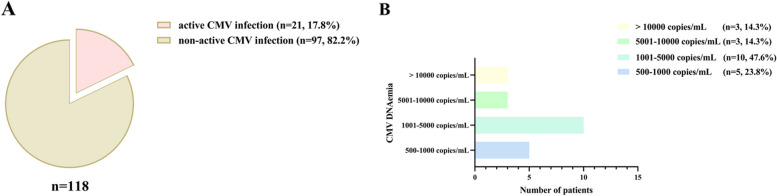
Within the 28-day ICU stay of the 118 septic patients receiving MV who were enrolled, active CMV infection was identified in 21 instances, representing 17.8% of the total (Fig. 1A, supplement files: Table E1). The percentages of the four levels of CMV DNAemia load were 23.8% (500–1000 copies/mL), 47.6% (1001–5000 copies/mL), 14.3% (5001–10000 copies/mL), and 14.3% (> 10,000 copies/mL), respectively (Fig. 1B). Out of those 21 individuals with CMV DNAemia, 19 (90.5%) tested positive upon their admission to the ICU early stage (day 0–7), and 2 cases (9.5%) showed positive results between days 15 and 21 (supplement files: Table E2). Additionally, the detection rate of CMV in lower respiratory tract specimens (ETA) was 55.9%, demonstrating a statistically significant positive correlation with CMV levels in plasma specimens (correlation coefficient, r = 0.916; P < 0.001) (supplement files: Table E2, Figure E2).
Fig. 1.
Incidence of Active CMV Infection and Level of CMV DNAemia Load. A Incidence of active cytomegalovirus (CMV) infection within 28-day intensive care unit (ICU) admission; B CMV DNAemia load within 28-day ICU admission
Patient clinical characteristics
Among the 118 enrolled septic patients, 94 were male (79.7%). The median age for all study participants was 67 years. The study participants median scores for APACHE II and SOFA were 23 and 9, respectively. The main cause of sepsis was severe community-acquired pneumonia (sCAP) (96.6%). The main comorbidities were hypertension (n = 58, 49.2%), diabetes (n = 51, 43.2%), and cardiovascular diseases (n = 33, 28.0%). Except for hypertension [28.6% vs. 53.6% (n); P = 0.037] and respiratory rates [22 vs. 20 (bpm, median); P = 0.047], differences in these clinical characteristics between the two groups were not significant (Table 1).
Table 1.
Patients clinical features at ICU admission
|
Total n = 118 |
CMV DNAemia | P | ||
|---|---|---|---|---|
|
Yes (n = 21, 17.8%) |
No (n = 97, 82.2%) |
|||
| Age (yrs) | 67 (58–75) | 64 (58–74) | 68 (59–75) | 0.394 |
| Sex, n (%) | - | - | - | > 0.999 |
| Male | 94 (79.7) | 17 (81.0) | 77 (79.4) | - |
| Female | 24 (20.3) | 4 (19.0) | 20 (20.6) | - |
| Weight (kg) | 59.1 ± 14.0 | 56.2 ± 13.8 | 59.8 ± 14.0 | 0.295 |
| BMI (kg/m2) | 21.4 ± 4.5 | 20.8 ± 4.5 | 21.6 ± 4.4 | 0.482 |
| Score of disease severity | ||||
| APACHE II | 23 (18–29) | 23 (20–29) | 23 (18–28) | 0.921 |
| SOFA | 9 (7–12) | 8 (6–13) | 9 (7–12) | 0.924 |
| Causes of sepsis, n (%) | - | - | - | > 0.999 |
| sCAP | 114 (96.6) | 21 (100.0) | 93 (95.9) | - |
| Other | 4 (3.4) | 0 (0.0) | 4 (4.1) | - |
| Vital signs | ||||
| Average blood pressure (mmHg) | 85 ± 20 | 80 ± 18 | 86 ± 20 | 0.214 |
| Heart rate (bpm) | 100 (81–117) | 110 (85–120) | 98 (81–112) | 0.198 |
| Respiratory rate (bpm) | 20 (18–25) | 22 (20–26) | 20 (18–23) | 0.047# |
| Temperature (℃) | 36.8 (36.5–37.5) | 37.0 (36.5–37.8) | 36.8 (36.5–37.5) | 0.136 |
| Comorbidities, n (%) | ||||
| Hypertension | 58 (49.2) | 6 (28.6) | 52 (53.6) | 0.037# |
| Diabetes | 51 (43.2) | 10 (47.6) | 41 (42.3) | 0.654 |
| Cardiovascular diseases | 33 (28.0) | 7 (33.3) | 26 (26.8) | 0.546 |
| COPD | 33 (28.0) | 3 (14.3) | 30 (30.9) | 0.123 |
| CVA | 23 (19.5) | 5 (23.8) | 18 (18.6) | 0.805 |
| CKD | 17 (14.4) | 5 (23.8) | 12 (12.4) | 0.312 |
| ILD | 12 (10.2) | 3 (14.3) | 9 (9.3) | 0.772 |
ICU Intensive Care Unit, CMV Cytomegalovirus, DNAemia DNA detection by quantitative polymerase chain reaction (qPCR) on peripheral blood samples, BMI Body Mass Index, APACHE II Acute Physiology and Chronic Health Evaluation II, SOFA Sequential Organ Failure Assessment, sCAP Severe Community-acquired Pneumonia, COPD Chronic Obstructive Pulmonary Disease, CVA Cerebrovascular Accident, CKD Chronic Kidney Disease, ILD Interstitial Lung Disease
#P < 0.05; Categorical variables were expressed as n (%), Continuous variables were expressed as Mean ± SD or Median (IQRs); Bold font indicates the difference was statistically significant
Moreover, the active CMV infection group exhibited lower hemoglobin (Hb) [71 vs. 98 (g/L, median); P = 0.001], higher aspartate aminotransferase (AST) [49.2 vs. 35.9 (U/L, median); P = 0.046], and higher serum urea nitrogen (BUN) [13.2 vs. 10.0 (mmol/L, median); P = 0.047] levels. However, there were no significant differences for other laboratory findings between the two groups (Table 2).
Table 2.
Laboratory findings at ICU admission
|
Total n = 118 |
CMV DNAemia | P | ||
|---|---|---|---|---|
|
Yes (n = 21, 17.8%) |
No (n = 97, 82.2%) |
|||
| PaO2/FiO2 | 210 (144–286) | 119 (134–270) | 211 (146–287) | 0.439 |
| White blood cells (109/L) | 11.6 (8.1–15.8) | 9.9 (6.9–13.0) | 12.1 (8.2–16.2) | 0.098 |
| Neutrophils (109/L) | 9.9 (7.0–13.8) | 8.7 (5.4–12.1) | 10.3 (7.0–14.1) | 0.109 |
| Lymphocytes (109/L) | 0.5 (0.4–0.8) | 0.6 (0.3–0.8) | 0.5 (0.4–0.8) | 0.855 |
| Monocytes (109/L) | 0.5 (0.3–0.9) | 0.3 (0.2–0.8) | 0.5 (0.3–0.9) | 0.130 |
| Hemoglobin (g/L) | 92 (74–112) | 71 (69–91) | 98 (79–112) | 0.001# |
| Platelet (109/L) | 179 (126–265) | 159 (81–265) | 186 (127–262) | 0.536 |
| Procalcitonin (ng/mL) | 1.33 (0.25–8.04) | 1.59 (0.64–17.22) | 1.04 (0.25–6.00) | 0.209 |
| Blood lactate (mmol/L) | 1.6 (1.3–2.7) | 1.8 (1.3–2.7) | 1.6 (1.3–2.6) | 0.744 |
| PT (s) | 15.2 (14.3–16.4) | 15.9 (14.6–16.6) | 15.2 (14.2–16.3) | 0.373 |
| APTT (s) | 43.3 (37.6–51.7) | 44.3 (36.1–56.6) | 43.3 (37.8–51.4) | 0.933 |
| Cardiac troponins (ng/ml) | 41.6 (14.7–215.0) | 54.7 (26.4–218.4) | 39.3 (13.7–198.4) | 0.275 |
| NT-proBNP (pg/mL) | 1357 (445–5454) | 2518 (1256–11,358) | 1080 (412–4518) | 0.061 |
| AST (U/L) | 38.9 (21.9–77.7) | 49.2 (38.7–119.0) | 35.9 (20.6–74.0) | 0.046# |
| Albumin (g/L) | 31.5 ± 5.2 | 31.4 ± 5.5 | 31.5 ± 5.1 | 0.921 |
| T-BIL (μmol/L) | 13.7 (9.8–20.2) | 13.7 (10.5–20.2) | 13.5 (9.8–18.9) | 0.615 |
| Scr (μmol/L) | 90.5 (63.2–169.7) | 120.1 (71.0–213.7) | 89.2 (63.3–147.1) | 0.097 |
| BUN (mmol/L) | 10.3 (6.7–17.2) | 13.2 (10.2–19.7) | 10.0 (6.3–15.7) | 0.047# |
ICU Intensive Care Unit, CMV Cytomegalovirus, DNAemia DNA detection by quantitative polymerase chain reaction (qPCR) on peripheral blood samples, PT Prothrombin Time, APTT Activated Partial Thromboplastin Time, NT-proBNP N-terminus Precursor of B-Type Natriuretic Peptide, AST Aspartate Aminotransferase, T-BIL Total Bilirubin, Scr Serum Creatinine, BUN Serum Urea Nitrogen
#P < 0.05; Continuous variables were expressed as Mean ± SD or Median (IQRs); Bold font indicates the difference was statistically significant
Treatment measures and complications
Most of the patients with active CMV infection received antiviral therapy [85.7% vs. 28.9% (n); P < 0.001] after ICU admission. Furthermore, compared to the group without active CMV infection, blood transfusion [81.0% vs. 48.5% (n); P = 0.007] and gamma globulin infusions [66.7% vs. 29.9% (n); P = 0.002] were found to have been highly administered in the group with active CMV infection after ICU admission. Other therapeutic measures were not significantly different between the two groups (Table 3).
Table 3.
Treatment Measures, Complications, and Clinical Outcomes
|
Total n = 118 |
CMV DNAemia | P | ||
|---|---|---|---|---|
|
Yes (n = 21, 17.8%) |
No (n = 97, 82.2%) |
|||
| Treatment measures, n (%) | ||||
| Before ICU admission | ||||
| Glucocorticoids | 26 (22.0) | 4 (19.1) | 22 (22.7) | 0.941 |
| Blood transfusion | 12 (10.2) | 5 (23.8) | 7 (7.2) | 0.060 |
| Gamma globulin infusions | 12 (10.2) | 2 (9.5) | 10 (10.3) | > 0.999 |
| After ICU admission | ||||
| Glucocorticoids | 87 (73.7) | 14 (66.7) | 73 (75.3) | 0.417 |
| Blood transfusion | 64 (54.2) | 17 (81.0) | 47 (48.5) | 0.007# |
| Gamma globulin infusions | 43 (36.4) | 14 (66.7) | 29 (29.9) | 0.002# |
| Immunosuppressive drugsb | 10 (8.5) | 3 (14.3) | 7 (7.2) | 0.534 |
| Antiviral therapyc | 46 (39.0) | 18 (85.7) | 28 (28.9) | < 0.001# |
| IMV | 110 (93.2) | 19 (90.5) | 91 (93.8) | 0.942 |
| CRRT | 42 (35.6) | 11 (52.4) | 31 (32.0) | 0.076 |
| ECMO | 21 (17.8) | 5 (23.8) | 16 (16.5) | 0.631 |
| Complications, n (%) | ||||
| ARF | 104 (88.1) | 18 (85.7) | 86 (88.7) | 0.995 |
| MODS | 55 (46.6) | 12 (57.1) | 43 (44.3) | 0.286 |
| Septic shock | 42 (35.6) | 6 (28.6) | 36 (37.1) | 0.459 |
| AKI | 40 (33.9) | 8 (38.1) | 32 (33.0) | 0.654 |
| ARDS | 30 (25.4) | 6 (28.6) | 24 (24.7) | 0.715 |
| AECOPD | 30 (25.4) | 2 (9.5) | 28 (28.9) | 0.065 |
| AHF | 23 (19.5) | 4 (19.1) | 19 (19.6) | > 0.999 |
| DIC | 12 (10.2) | 1 (4.8) | 11 (11.3) | 0.613 |
| Clinical outcomes | ||||
| Length of MVa (d) | 20 (11–35) | 34 (17–39) | 20 (11–29) | 0.047# |
| Length of IMVa (d) | 18 (10–32) | 32 (12–39) | 17 (10–28) | 0.042# |
| 28-day VFDa (d) | 0 (0–3) | 0 (0–0) | 0 (0–4) | 0.087 |
| Duration of ICU stay (d) | 21 (14–41) | 35 (22–52) | 21 (13–36) | 0.013# |
| Duration of hospital stay (d) | 35 (20–48) | 39 (23–53) | 33 (19–46) | 0.125 |
| 28-day all-cause mortality, n (%) | 10 (8.5) | 1 (4.8) | 9 (9.3) | 0.809 |
| In-hospital all-cause mortality, n (%) | 17 (14.4) | 4 (19.1) | 13 (13.4) | 0.745 |
ICU Intensive Care Unit, CMV Cytomegalovirus, DNAemia DNA detection by quantitative polymerase chain reaction (qPCR) on peripheral blood samples, IMV Invasive Mechanical Ventilation, CRRT Continuous Renal Replacement Therapy, ECMO Extracorporeal Membrane Oxygenation, ARF Acute Respiratory Failure, MODS Multiple Organ Dysfunction Syndrome, AKI Acute Kidney Failure, ARDS Acute Respiratory Distress Syndrome, AECOPD Acute Exacerbation of Chronic Obstructive Pulmonary Disease, AHF Acute Heart Failure, DIC Disseminated Intravascular Coagulation, VFD Ventilator-Free Days
#P < 0.05; Categorical variables were expressed as n (%), Continuous variables were expressed as Mean ± SD or Median (IQRs); Bold font indicates the difference was statistically significant
aIn-hospital period
bIncluded Cyclophosphamide, Methotrexate, and Mycophenolate Mofetil
cIncluded Ganciclovir, Valganciclovir, or Sodium Phosphate
Acute respiratory failure (ARF) with a prevalence of 88.1% was found to be a major complication in septic patients undergoing MV. Comparison of complications between the two groups revealed no statistically significant differences (Table 3).
Clinical outcomes
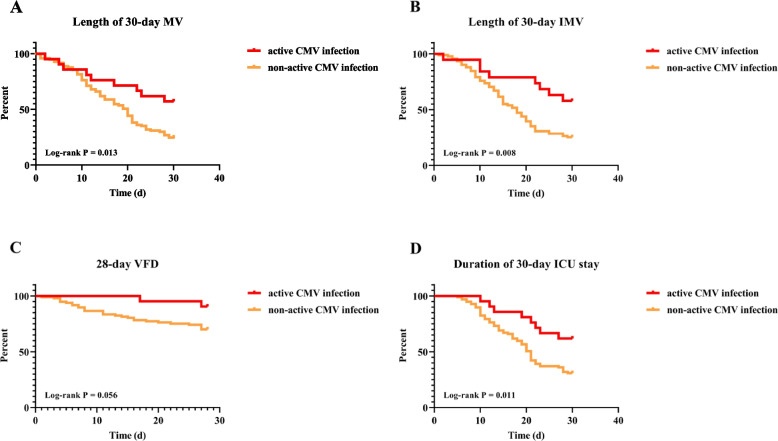
In-hospital duration of MV [34 vs. 20 (d, median); P = 0.047], IMV [32 vs. 17 (d, median); P = 0.042], and ICU stay [35 vs. 21 (d, median); P = 0.013] in the active CMV infection group were significantly higher than in the non-active CMV infection group (Table 3). Lengths of 30-day MV and IMV, and duration of 30-day ICU stay rates were high in the group with active CMV infection using the KM survival analysis (Log-rank P < 0.05) (Fig. 2). Other clinical outcomes between the two groups showed no statistically significant differences (Table 3).
Fig. 2.
Effect of Active CMV Infection on Duration of IMV and ICU Stay. A Effect of active CMV infection on length of 30-day mechanical ventilation (MV); B Effect of active CMV infection on length of 30-day invasive mechanical ventilation (IMV); C Effect of active CMV infection on duration of 28-day ventilator-free days (VFD); D Effect of active CMV infection on duration of 30-day ICU stay
Risk factors and predictive value
In the multivariate regression model, lower Hb level was associated with patients with active CMV infection (P < 0.05). Based on the regression coefficient (β), Hb [β: -0.03] was a protective factor (supplement files: Table E3). ROC curve was plotted for Hb level to assess the predictive value of active CMV infection, and found the AUC of Hb level was 0.73 (specificity: 66.7%, sensitivity: 82.5%; P = 0.001). Furthermore, we used the cut-off method to obtain 76 g/L as the threshold for predicting active CMV infection (Fig. 3, supplement files: Table E4).
Fig. 3.
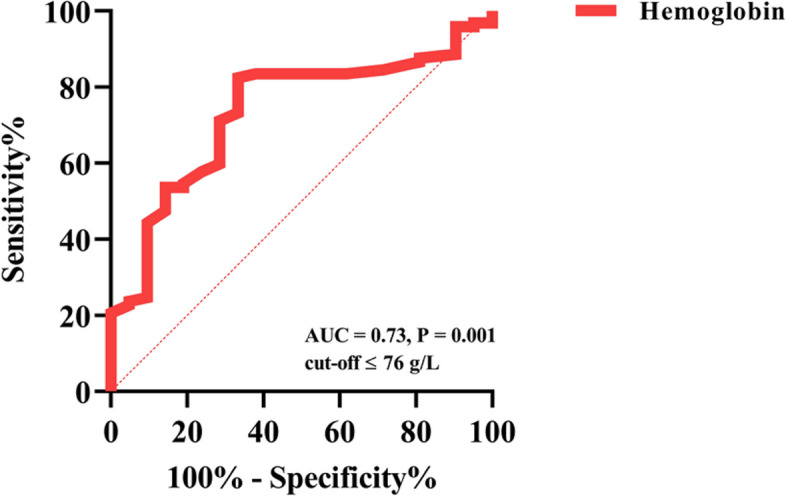
Predictive Value of Hemoglobin for Active CMV Infection
Discussion
Our study revealed incidence of active CMV infection in septic patients on mechanical ventilation, with 17.8% testing positive during the 28-day ICU stay. Hb level emerged as a significant predictor, with lower levels indicating a higher risk of active CMV infection. Patients with active CMV infection also exhibited longer durations of MV, IMV, and ICU stay. These findings highlight CMV as a crucial factor in septic patients, emphasizing the necessity for early intervention strategies.
Active CMV infection is a relatively frequent occurrence among non-immunosuppressed patients with critical illness. Our research team's systematic review and meta-analysis not only showed that the incidence of CMV reactivation in immunocompetent individuals could reach as high as 31%, but also revealed sepsis as an independent risk factor for CMV reactivation [3], consistent with other research [2]. Furthermore, our two clinical studies demonstrated that among critically ill patients complicating sepsis or septic shock, the incidence of active CMV infection significantly improved [4, 5]. This phenomenon was further confirmed in a review from the authoritative team [6]. The increased incidence of CMV reactivation in sepsis patients may be attributed to the hyperinflammatory state of sepsis, which triggers the replication of latent CMV, leading to reactivation. This hypothesis has been validated in animal experiments showing that sepsis-associated mediators (lipopolysaccharide, IL-6, IL-1β, TNF-α) promote the occurrence and progression of CMV reactivation [14, 15].
Clinical studies focusing on immunocompetent sepsis patients as a specific research subject have a history of nearly 35 years since 1990, during which approximately eight studies have clarified the role of CMV reactivation in non-immunosuppressed patients with sepsis [7, 16–22]. The incidence of active CMV infection in this study was 17.8%, which is lower compared to 24%—41% incidence of active CMV infection observed in other studies involving patients with sepsis not caused by immunosuppression [6, 7, 16–22]. The incidence of active CMV infection in our study may differ from previous findings due to disease background of study subjects, varying criteria and detection methods. Particularly, the timing and duration of CMV testing in other research could influence the reported rates. As a retrospective study, ours might have underestimated the incidence by not capturing every time point. Concurrently, the discovery that 90.5% of study participants experienced CMV reactivation within the early stage of their ICU admission indicated that these individuals might have already had CMV reactivation prior to their ICU admission. Therefore, CMV detection should be conducted throughout the entire hospitalization cycle, not just upon admission to the ICU. Early recognition of CMV reactivation, coupled with timely interventional therapy, will improve poor prognosis in mechanically ventilated patients with sepsis. In addition, among acute respiratory distress syndrome (ARDS) patients with sepsis, those patients who received MV for more than 4 days had a 27% cumulative risk of CMV reactivation [21]. CMV reactivation in mechanically ventilated patients is a matter of concern, especially since the causal relationship between the two needs to be further verified by basic research.
Consistent with our findings, prior research has established a link between active CMV infection and certain clinical parameters, including low Hb levels, elevated AST and BUN. This is further supported by evidence that CMV infection can impair both cardiac and renal functions, leading to an increase in these biochemical indicators [23, 24]. The physiological disruption caused by CMV extends beyond these parameters, potentially contributing to a broader spectrum of clinical manifestations and complications in affected patients.
Our research, through a multivariate logistic regression analysis, identified hemoglobin levels as an independent risk factor for active CMV infection, offering a moderate predictive value. This correlation seems unprecedented, as existing literature lacks similar findings for sepsis patients undergoing MV. The decline in hemoglobin may signify the severity of illness, given that critically ill patients are often in a state of myelosuppression, leading to a loss of hematopoietic and immune capabilities, which in turn are associated with CMV reactivation [25]. Additionally, CMV infection, particularly in sepsis or septic shock patients, is a significant cause of myelosuppression [26–28]. This situation can lead to a reduction in hemoglobin levels, increasing the risk of blood transfusions, which have been shown to be closely related to CMV reactivation through direct transmission of the virus from donors and indirect factors such as allogeneic stimulation [29]. Our study also observed a higher transfusion rate in the group with active CMV infection, which to some extent corroborates the possibility that transfusions may facilitate CMV reactivation. However, it should be noted that due to the limited sample size in this study, there exists the potential for a type II error.
Most of the findings suggested that active CMV infection was related to the clinical prognoses of non-immunosuppressed septic patients, which is consistent with our findings, including longer durations of MV, IMV, and ICU stay. The factors contributing to these unfavorable outcomes are diverse, encompassing both direct injury (such as CMV pneumonia) and indirect injury (such as immune disorder) [25]. Therefore, the treatment of active CMV infection is considered to be of significant importance. Certainly, the timing of when clinical physicians initiate antiviral therapy and the choice of medications used may impact the final outcomes. Clinical trials about prophylactic and pre-emptive antiviral treatment strategies did not evidently prove the benefit in immunocompetent critically ill patients with active CMV infection [8, 30]. Nevertheless, patients in the sepsis category may derive significant advantages from prophylactic antiviral therapy, although the therapeutic benefits must be carefully weighed against their potential side effects of the antiviral medications [8, 31].
In addition, there are several issues that warrant attention. Firstly, the assessment of CMV should be expanded to multiple specimens (lower respiratory tract specimens), and our study found that the detection rate of CMV in the lower respiratory tract is higher, and its viral load correlates with that in the blood. A study suggest that CMV positivity in the airway is closely related to adverse outcomes [32]. Secondly, the role of plasma CMV IgG positivity in septic patients could be further evaluated in the future, as recent studies have shown that CMV IgG positivity is associated with both short-term and long-term adverse clinical outcomes [33, 34]. Lastly, sepsis itself is a significant cause of induced immunosuppression, and the pathogenicity of CMV may vary at different stages of sepsis [28, 35, 36]. In summary, this study elucidates the incidence, risk factors (predictive value), and clinical outcomes of active CMV infection among mechanically ventilated patients with sepsis. These results serve as an early warning for the identification of high-risk patients, thereby ameliorating adverse clinical outcomes and providing guidance for antiviral therapy.
This study has several limitations. First, as it was retrospective in nature, it encountered limitations in pinpointing the exact time points for accurate viral detection. Meanwhile, constraints imposed by a limited sample size and the presence of unaccounted confounders contributed to the research's shortcomings. Second, the detection of CMV DNAemia indicated active infecton in most cases, so the serostatus of CMV should have been further evaluated. Third, there was an absence of robust statistical analyses to address the deficiency in CMV sampling among patients who subsequently passed away or were discharged from the facility. Fourth, as a retrospective study, it lacks assessment of CMV viral load prior to ICU admission, co-infections, and CMV-related organ failures during hospitalization. Therefore, prospective, multicenter, propensity score-matched studies are to be carried out. More importantly, subsequent studies should evaluate the efficacy and therapeutic timing of prophylactic and preemptive anti-CMV therapies in immunocompetent mechanically ventilated patients with sepsis.
Conclusions
Active CMV infection is frequently observed to be correlated with several adverse clinical outcomes in mechanically ventilated patients with sepsis. A low hemoglobin level may serve as a risk factor and valuable predictor for CMV reactivation. To improve the poor prognosis in those patients, further prospective studies are necessary to establish the efficacy of initiating preventive and preemptive antiviral therapies.
Supplementary Information
Acknowledgements
Not applicable.
Accordance statement
All methods were performed in accordance with the relevant guidelines and regulations.
Abbreviations
- ICU
Respiratory Intensive Care Unit
- CMV
Cytomegalovirus
- DNAemia
DNA detection by quantitative polymerase chain reaction (qPCR) on peripheral blood samples
- qPCR
Quantitative Polymerase Chain Recation
- BMI
Body Mass Index
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- SOFA
Sequential Organ Failure Assessment
- sCAP
Severe Community-acquired Pneumonia
- COPD
Chronic Obstructive Pulmonary Disease
- CVA
Cerebrovascular Accident
- CKD
Chronic Kidney Disease
- ILD
Interstitial Lung Disease
- PT
Prothrombin Time
- APTT
Activated Partial Thromboplastin Time
- NT-proBNP
N-terminus Precursor of B-Type Natriuretic Peptide
- AST
Aspartate Aminotransferase
- T-BIL
Total Bilirubin
- Scr
Serum Creatinine
- BUN
Serum Urea Nitrogen
- IMV
Invasive Mechanical Ventilation
- CRRT
Continuous Renal Replacement Therapy
- ECMO
Extracorporeal Membrane Oxygenation
- ARF
Acute Respiratory Failure
- MODS
Multiple Organ Dysfunction Syndrome
- AKI
Acute Kidney Failure
- ARDS
Acute Respiratory Distress Syndrome
- AECOPD
Acute Exacerbation of Chronic Obstructive Pulmonary Disease
- AHF
Acute Heart Failure
- DIC
Disseminated Intravascular Coagulation
- VFD
Ventilator-Free Days
- ETA
Endotracheal Aspirates
- BALF
Bronchoalveolar Lavage Fluid
- β
Regression Coefficient
- OR
Odds Ratio
- 95% CI
95% Confidence Interval
- AUC
Area Under Curve
Authors’ contributions
ZHZ, XQL, and YML conceived and designed the study; ZHZ, JRZ, SD, XYF, YHL, JLS, LSC, TTS, SZL, JJZ, XSL, RZ, DDL, and YHX collected and aggregated data; ZHZ, JRZ, SD, XYF, and YHL analyzed the data and wrote the manuscript; XQL and YML reviewed and revised the manuscript. XQL and YML contributed equally to this work. All authors read and approved the final manuscript.
Funding
The study was funded by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (Nos. SQ2023AAA031364, 2023ZD0517300), National Natural Science Foundation of China (No. 82070084), Science and Technology Program of Guangzhou (Nos. SL2023A04J00179, 2024A04J3312), and Guangzhou Clinical Specialty Program (No. 2023C-TS01).
Data availability
The datasets generated during and analyzed during the current study are not publicly available due to patients privacy but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committees of the First Affiliated Hospital of Guangzhou Medical University approved the protocol (No. ES-2024-K084-01). Due to the retrospective nature of this study, the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University also waived the requirement for informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Zhihui Zhang, Jierong Zhang, Shuang Dai, Xueying Fan and Yuhua Liu are co-first authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoqing Liu and Yimin Li contributed equally to this work.
Contributor Information
Yimin Li, Email: dryiminli@vip.163.com.
Xiaoqing Liu, Email: lxq1118@126.com.
References
- 1.Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis[J]. Rev Med Virol. 2019;29(3):e2034. 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 2.Al-Omari A, Aljamaan F, Alhazzani W, et al. Cytomegalovirus infection in immunocompetent critically ill adults: literature review[J]. Ann Intensive Care. 2016;6(1):110. 10.1186/s13613-016-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Huang Y, Xu Z, et al. Cytomegalovirus infection and outcome in immunocompetent patients in the intensive care unit: a systematic review and meta-analysis[J]. BMC Infect Dis. 2018;18(1):289. 10.1186/s12879-018-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Li R, Chen Y, et al. Association between active cytomegalovirus infection and lung fibroproliferation in adult patients with acute respiratory distress syndrome: a retrospective study[J]. BMC Infect Dis. 2022;22(1):788. 10.1186/s12879-022-07747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Liu X, Sang L, et al. Cytomegalovirus reactivation in immunocompetent mechanical ventilation patients: a prospective observational study[J]. BMC Infect Dis. 2021;21(1):1026. 10.1186/s12879-021-06698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marandu T, Dombek M, Cook CH. Impact of cytomegalovirus load on host response to sepsis[J]. Med Microbiol Immunol. 2019;208(3–4):295–303. 10.1007/s00430-019-00603-y. [DOI] [PubMed] [Google Scholar]
- 7.Imlay H, Dasgupta S, Boeckh M, et al. Risk factors for cytomegalovirus reactivation and association with outcomes in critically ill adults with sepsis: a pooled analysis of prospective studies[J]. J Infect Dis. 2021;223(12):2108–12. 10.1093/infdis/jiaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limaye AP, Stapleton RD, Peng L, et al. Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial[J]. JAMA. 2017;318(8):731–40. 10.1001/jama.2017.10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies[J]. Lancet. 2007;370(9596):1453–7. 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Fryer JF, Heath AB, Anderson R, et al. Collaborative study to evaluate the proposed 1st WHO International Standard for Human Cytomegalovirus (HCMV) for nucleic acid amplification (NAT)-based assays[S]. WHO/BS/10/2138. World Health Organization, Geneva, Switzerland.
- 11.Ljungman P, Chemaly RF, Khawaya F, et al. Consensus definitions of cytomegalovirus (cmv) infection and disease in transplant patients including resistant and refractory cmv for use in clinical trials: 2024 update from the transplant associated virus infections forum[J]. Clin Infect Dis. 2024;ciae321. 10.1093/cid/ciae321. [DOI] [PMC free article] [PubMed]
- 12.Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation[J]. Transplantation. 2018;102(6):900–31. 10.1097/TP.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3)[J]. JAMA. 2016;315(8):801–10. 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook CH, Trgovcich J, Zimmerman PD, et al. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice[J]. J Virol. 2006;80(18):9151–8. 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook CH, Zhang Y, McGuinness BJ, et al. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice[J]. J Infect Dis. 2002;185(10):1395–400. 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 16.Domart Y, Trouillet JL, Fagon JY, et al. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery[J]. Chest. 1990;97(1):18–22. 10.1378/chest.97.1.18. [DOI] [PubMed] [Google Scholar]
- 17.Kutza AS, Muhl E, Hackstein H, et al. High incidence of active cytomegalovirus infection among septic patients[J]. Clin Infect Dis. 1998;26(5):1076–82. 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 18.von Müller L, Klemm A, Weiss M, et al. Active cytomegalovirus infection in patients with septic shock[J]. Emerg Infect Dis. 2006;12(10):1517–22. 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heininger A, Haeberle H, Fischer I, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis[J]. Crit Care. 2011;15(2):R77. 10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walton AH, Muenzer JT, Rasche D, et al. Reactivation of multiple viruses in patients with sepsis[J]. PLoS ONE. 2014;9(2):e98819. 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong DSY, Spitoni C, Klein Klouwenberg PMC, et al. Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome[J]. Intensive Care Med. 2016;42(3):333–41. 10.1007/s00134-015-4071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh N, Inoue M, Osawa R, et al. Inflammasome expression and cytomegalovirus viremia in critically ill patients with sepsis[J]. J Clin Virol. 2017;93:8–14. 10.1016/j.jcv.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Peng G, Bai J, He B, Huang K, Hu X, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta-analysis of prospective studies up to 2016[J]. J Am Heart Assoc. 2017;6(7):e005025. 10.1161/JAHA.116.005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raval AD, Kistler KD, Tang Y, Murata Y, Snydman DR. Epidemiology, risk factors and outcomes associated with cytomegalovirus in adult kidney transplant recipients: a systematic literature review of real-world evidence[J]. Transpl Infect Dis. 2020;e13483. 10.1111/tid.13483. [DOI] [PubMed]
- 25.Papazian L, Hraiech S, Lehingue S, et al. Cytomegalovirus reactivation in ICU patients[J]. Intensive Care Med. 2016;42(1):28–37. 10.1007/s00134-015-4066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Rodriguez S, Wang L, et al. Sepsis induces hematopoietic stem cell exhaustion and myelosuppression through distinct contributions of TRIF and MYD88[J]. Stem Cell Reports. 2016;6(6):940–56. 10.1016/j.stemcr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skirecki T, Drechsler S, Jeznach A, et al. An early myelosuppression in the acute mouse sepsis is partly outcome-dependent[J]. Front Immunol. 2021;12:708670. 10.3389/fimmu.2021.708670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Moldawer LL, Opal SM, et al. Sepsis and septic shock[J]. Nat Rev Dis Primers. 2016;2:16045. 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziemann M, Thiele T. Transfusion-transmitted CMV infection - current knowledge and future perspectives[J]. Transfus Med. 2017;27(4):238–48. 10.1111/tme.12437. [DOI] [PubMed] [Google Scholar]
- 30.Papazian L, Jaber S, Hraiech S, et al. Preemptive ganciclovir for mechanically ventilated patients with cytomegalovirus reactivation[J]. Ann Intensive Care. 2021;11(1):33. 10.1186/s13613-020-00793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowley NJ, Owen A, Shiels SC, et al. Safety and efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically ill patients: a randomized clinical trial[J]. JAMA Intern Med. 2017;177(6):774–83. 10.1001/jamainternmed.2017.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang L, Zhang X, Pang L, et al. Viral reactivation in the lungs of patients with severe pneumonia is associated with increased mortality, a multicenter, retrospective study[J]. J Med Virol. 2023;95(1):e28337. 10.1002/jmv.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unterberg M, Ehrentraut SF, Bracht T, et al. Human cytomegalovirus seropositivity is associated with reduced patient survival during sepsis[J]. Crit Care. 2023;27(1):417. 10.1186/s13054-023-04713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X, Meng Y, Hu X, et al. Association between cytomegalovirus seropositivity and all-cause mortality: an original cohort study[J]. J Med Virol. 2024;96(2):e29444. 10.1002/jmv.29444. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy[J]. Nat Rev Immunol. 2013;13(12):862–74. 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong DSY, Chong GM, Chemaly RF, et al. Comparative clinical manifestations and immune effects of cytomegalovirus infections following distinct types of immunosuppression[J]. Clin Microbiol Infect. 2022;28(10):1335–44. 10.1016/j.cmi.2022.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available due to patients privacy but are available from the corresponding author on reasonable request.