Abstract
Background
The American College of Cardiology, American Heart Association, and Centers for Medicare and Medicaid Services recommend shared decision-making (SDM) for patients with severe aortic stenosis choosing between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR). Although tools such as patient decision aids (DAs) and training in SDM have been shown to improve SDM, implementation of SDM and DAs is limited. The IMproving treatment decisions for Patients with AortiC stenosis Through Shared Decision Making (IMPACT SDM) study aims to (1) determine the effectiveness of the interventions (a DA and clinician SDM training) in achieving SDM (primary outcome) and improving the quality of decisions about aortic valve replacement, (2) determine the reach of the DAs and adoption of training, and (3) explore potential mechanisms of effectiveness and implementation at the patient-, clinician-, and clinic-level.
Methods
The study is a hybrid type II effectiveness-implementation study using a cluster randomized batched stepped wedge trial with 8 sites across the USA. Eligible patients will be surveyed before and after visits with the heart valve team; clinicians will be surveyed after visits. Reach of DAs and adoption of training will be tracked. Clinicians will be interviewed regarding barriers and facilitators to implementation.
Discussion
The IMPACT SDM Study seeks to provide evidence of the ability of the interventions to improve SDM and decision quality, and also to shed light on barriers and facilitators to SDM implementation to promote future implementation efforts.
Trial registration
ClinicalTrials.gov NCT06171737. Registered on December 15, 2023.
Keywords: Aortic valve stenosis, Decision-making, Shared, Decision aid, Continuing medical education, Cardiology, Transcatheter aortic valve replacement
Introduction
Background and rationale {6a}
The American College of Cardiology (ACC) and American Heart Association (AHA) recommend shared decision-making (SDM) for patients with severe aortic stenosis (AS) choosing between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR). SAVR was historically the only treatment for severe AS until 2011 when TAVR was approved for use in patients at prohibitive risk for surgery. TAVR indications have since expanded, including symptomatic patients at intermediate or high surgical risk (in 2016) and low surgical risk over the age of 65 (in 2019) [1]. These types of decisions, where a new, disruptive technology rapidly alters the decision landscape, add complexity for patients and clinicians. While the coverage decision does not mandate the use of a decision aid for SDM, the US Centers for Medicare and Medicaid Services (CMS) does require evaluation by a multidisciplinary heart valve team, including both a cardiac surgeon and interventional cardiologist, as a condition for reimbursement for TAVR [1, 2]. However, a recent systematic review of decision-making in severe AS found very few elements of SDM in routine care, highlighting barriers to SDM from the patients’ perspective including lack of information, not being included in decision-making, and dealing with multiple serious co-morbidities [3]. A broad Cochrane systematic review has identified common barriers to SDM implementation including time constraints, perceived lack of applicability, and lack of clinician support [4, 5]. Common organizational and system level barriers include lack of team-based culture, limited leadership support, and misaligned financial incentives [6–8]. Further, barriers specific to the context of severe AS include older patient age, limited patient activation, and severity of disease [3, 9]. Unfortunately, the guidelines recommending SDM have not provided any guidance for how to overcome these barriers to implementing SDM into routine care.
While the heart valve team approach mandated by CMS may address some of these known barriers to SDM in cardiology and cardiac surgery settings (e.g., financial incentives, leadership support, and clinician support), it is likely that other strategies will be needed to achieve fidelity to SDM in this setting. A recent survey of heart valve team physicians ranked clinician SDM skills training, leadership support, and patient decision aids as the top three priorities to effectively implement SDM [10–12]. A combination of clinician-directed strategies (e.g., training and educational materials) and patient-directed strategies (e.g., patient decision aids) makes more effective use of visit time and promotes shared decision-making [13].
There is a critical need for evidence to help clinics implement SDM into the pre-procedural assessment of patients with severe aortic stenosis. The goal of the proposal is to determine the effectiveness of the heart valve team alone compared to the heart valve team with formal patient-level (patient decision aids) and clinician-level (SDM skills training) SDM strategies. The protocol here is reported using the Trials structured Study Protocol template.
Objectives {7}
The goal for this study is to generate evidence of the effectiveness of a multi-faceted implementation strategy (a toolkit plus external facilitation) in promoting use of evidence-based SDM interventions (DAs and clinician training) to achieve SDM. The proposal will achieve the following aims:
- Determine the effectiveness of the interventions in achieving SDM and improving the quality of decisions about aortic valve replacement.
- Hypothesis 1: Patients in the intervention period will report higher SDM scores compared to those in comparator period.
- Research question 1a: Do SDM scores differ in vulnerable populations (e.g., low literacy, elderly, and racial/ethnic minorities)?
- Hypothesis 2: Patients in the intervention period will report higher knowledge and more informed, patient-centered decisions compared to those in comparator period.
- Research question 2a: Do knowledge or informed, patient-centered decisions outcomes differ in vulnerable populations (e.g., low literacy, elderly, and racial/ethnic minorities)?
- Determine the reach of the DAs and adoption of training.
- Hypothesis 3: Sites will reach at least 70% of eligible patients.
- Research question 3a: Does reach differ in vulnerable populations (e.g., low literacy, elderly, and racial/ethnic minorities)?
- Hypothesis 4: Sites will have at least 70% of clinicians adopt the training.
Explore potential mechanisms of effectiveness and implementation at the patient-, clinician-, and clinic-level and identify facilitators of and barriers to sustained use.
Trial design {8}
The study is a hybrid type II effectiveness-implementation study. The original design was a cluster randomized stepped wedge design with 8 steps at 8 sites across the USA [14]. The stepped wedge cluster randomized design was chosen for two main reasons. First, sites were hesitant to participate in the trial without a guarantee they would receive the intervention and this trial design allows all sites to participate in the intervention by the end of the trial. Second, the intervention necessitates a staggered rollout at each site given the time required to create site-specific workflows for DA delivery and train the necessary staff which is not feasible with a parallel trial design. In the study, each site will start in the heart valve team usual care comparator arm. All sites will start to enroll patients at the same time; a new site will switch over to the intervention period (where they will implement clinician training and DA delivery) every step (every 4 months).
Patients will complete surveys before the visit (T0) and shortly after the visits with both heart specialists (T1). We will randomly select one specialist to complete a short survey after the visit. The main hypotheses are that compared to patients in the comparator (usual care), patients in the intervention period will (hypothesis 1) report more SDM at T1 and (hypothesis 2) higher knowledge and preference-treatment concordant care at T1. Further, we will test whether the implementation (hypothesis 3) reaches > 70% and (hypothesis 4) > 70% of clinicians adopt the training. The third aim will use interviews with key participants at each site to explore experiences, contextual factors, resources, and barriers to help explain the quantitative findings.
Changes to the trial design: Shortly before enrollment started, two of the original sites dropped out and the PIs and statistician, after consultation with several experts, decided to adjust the design in order to be able to bring on two new sites. The design was altered to a batched stepped wedge design with two batches to accommodate different starting times for the sites [15]. The length of each step will be 5 months for the 6 sites in batch 1 and 9 months for the 2 sites in batch 2. The statistician re-randomized the sites in batch 1 according to the approach described previously. There were no changes to the hypotheses.
Involvement of patients and the community in the trial
Our lead patient partner will serve as co-investigator and will be heavily involved in proposal development; recruitment and training of other patient partners, design of study recruitment materials; training of research coordinators; development of toolkit videos; as well as interpretation and presentation of the results. Specifically, she will help to recruit a patient advisory panel of 6 diverse patient partners who will meet at least monthly in year 1 and then quarterly in subsequent years. Patient advisors will be selected to include representation from the key subgroups and we will ensure that we have at least two advisors who have had each treatment (SAVR or TAVR). Further, the lead patient partner will coordinate with the Patient and Family Advisory Councils at the participating sites to ensure that we have a broad range of local perspectives to support the patient voices in the study.
Methods: participants, interventions, and outcomes
Study setting {9}
This study is being undertaken at eight sites (6 academic hospitals and 2 private practice hospitals) across the USA. Participating sites include Massachusetts General Hospital (MGH) in Boston, MA, University of Colorado, Denver in Aurora, CO, Emory University in Atlanta, GA, Piedmont Heart Institute in Atlanta, GA, Providence Health in Portland, OR, University of North Carolina at Chapel Hill, University of California San Francisco, and University of Texas Southwestern in Dallas, TX.
Changes to the study setting: Two sites, Piedmont Heart Institute in Atlanta, GA and Providence Health in Portland, OR, withdrew from the study before enrolling any patients. Two new sites, University of Pennsylvania in Philadelphia, PA, and Washington University School of Medicine in St. Louis, MO, joined the study.
Eligibility criteria {10}
Clinicians: Heart valve team members including physicians and advanced practice providers will be eligible for the baseline assessment and debrief interviews. The interventional cardiologists and cardiac surgeons who consult with patients during the decision-making process will be eligible for the post-visit surveys.
Patients: In keeping with current indications for TAVR and SAVR, patient participants will be eligible if they are between 65 and 85 years old, have severe AS defined as an aortic valve area < 1 cm2, meet clinical indications for aortic valve replacement, and attend a scheduled visit with a participating interventional cardiologist and/or cardiac surgeon at one of the sites. Ineligibility criteria are as follows: prior valve replacement surgery, high surgical risk (e.g., Society of Thoracic Surgery (STS) score > 8%), prior coronary artery bypass surgery (CABG), end stage renal disease on dialysis, severe lung disease (COPD) requiring home oxygen, advanced cirrhosis, unable to read or write in English or Spanish (as the DAs are only available in English and Spanish), and unable to consent for self (proxy respondents are not allowed).
Who will take informed consent? {26a}
Patient will receive an invitation packet by mail and/or upon check in at the clinic which will contain an invitation letter signed by the site PI and/or heart valve clinic director, an information sheet describing the study and the risks and benefits, the pre-visit survey, and an incentive. Informed consent procedures for this minimal risk study vary from site to site. Initially, we had planned for Massachusetts General Hospital (MGH) to serve as the single IRB. However, MGH IRB determined that the study was exempt and as a result, it would not serve as the single IRB. Each site was then required to submit to their own IRB and that resulted in slight variations in recruitment and enrollment protocol at some sites. At most sites, completion of the pre-visit survey is taken to indicate consent; at two sites, a verbal or written consent process will be conducted by the research coordinator prior to collecting the pre-visit survey.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Not applicable. This trial does not involve collecting biological specimens for storage.
Interventions
Explanation for the choice of comparators {6b}
The study will compare a usual care comparator arm to an intervention (DAs and clinician SDM skills training) arm. In the “usual care” arm, all sites will take a multidisciplinary approach to treatment of severe AS and patients considering TAVR are seen by both an interventional cardiologist and a cardiac surgeon as required for payment by CMS. The intervention arm was chosen to have two components: (1) a DA and (2) clinician SDM skills training. Decision aids have been shown to help patients prepare for visits, increase patient involvement in decisions, as well as increase patients’ knowledge and clarify preferences [13, 16]. SDM training has been shown to increase confidence, improve attitudes toward SDM, and promote use of decision aids [13, 17, 18]. During the transition to the intervention arm, the coordinating center will provide sites with an implementation toolkit and external facilitation (1–1 support) as both are effective and scalable implementation strategies to promote integration of the DA into routine care [19, 20].
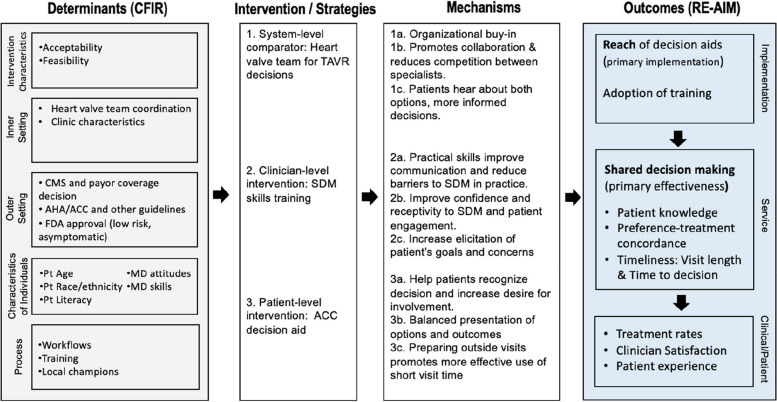
The overall project is informed by an integrated logic model adapted from methods by Smith et al. [21]. Fig. 1 presents our project logic model linking determinants (derived from the CFIR framework) and interventions with mechanisms and outcomes guided by RE-AIM.
Fig. 1.
Integrated CFIR and RE-AIM implementation research logic model for SDM in aortic stenosis
Intervention description {11a}
The patient DA: the ACC’s CardioSmart decision aid, “Treatment Options for Severe Aortic Stenosis for Patients Deciding Between TAVI and Surgery.” The DA was created using an iterative design process similar to that to create other specialty cardiology patient decision aids and is freely available online at. https://www.cardiosmart.org/topics/aortic-stenosis/assets/decision-aid/choosing-between-tavr-and-surgery [22, 23].
The clinician SDM skills training: a 60-min online training session that will provide practical tips, interactive case studies, and tools for conducting shared decision-making conversations covering core competencies [24]. The training will be available to all clinicians at participating sites involved in the study.
Criteria for discontinuing or modifying allocated interventions {11b}
The DA will be reviewed every 2 years, or as needed if new evidence is published, to ensure that it is up to date. Revisions will be recommended to the ACC/CardioSmart who is a stakeholder partner on this study. Similarly, the SDM training will be reviewed annually and updated as needed if the evidence changes substantially.
Strategies to improve adherence to interventions {11c}
Each site will identify a clinical and administrative champion to support the DA delivery. The primary study team will lead a 3-h design session with sites to develop the workflow for DA integration and to support training of clinic staff in the workflow and documentation of DA delivery. The primary study team will have short (20–30 min) check-in meetings with site champions every other week for the first 2 months, then monthly for the next 6 months to monitor reach, fidelity to the workflow, and troubleshoot any barriers. Of note, the DA delivery will be implemented by clinic staff as part of routine care; the research coordinators will not be distributing DAs to patients during the intervention period. This feature of the study is important to promote maintenance and sustainability after the study ends.
Study staff from sites will meet with the primary study team monthly to review adherence to study tracking activities which will include discussion of reach of DAs and clinician training. The online training course will be available to sites once they have switched to the intervention arm and can be used as new clinicians come on board over the course of the study.
Relevant concomitant care permitted or prohibited during the trial {11d}
Not applicable. This trial does not have any pre-specified standards for concomitant care permitted or prohibited during the trial.
Provisions for post-trial care {30}
Not applicable. There is no anticipated harm and compensation for trial participation.
Outcomes {12}
The outcomes were selected based on the importance to the heart valve team clinicians and leadership (primary decision-makers) and after incorporating patients’ perspectives based on interviews and literature review [25]. All stakeholders were interested in a meaningful shared decision-making conversation (primary outcome, patient-reported). Patients also felt it was important to be well-informed and to make sure that doctors listened to their goals and preferences. Clinicians were also interested in potential burden (e.g., increased visit length or time to decision) and impact on treatment rates. Additional implementation outcomes include reach of decision aids, adoption of the training, and maintenance of the decision aids once the trial is complete. Table 1 details the definition and sources for the outcomes in the logic model. Figure 2 details when key outcomes will be collected.
Table 1.
Primary and secondary outcomes
| Primary or secondary | Name of outcome | Specific measure to be used | Timepoints |
|---|---|---|---|
| Primary | Shared decision-making | Four-item Shared Decision-Making Process score [26] | Post-visit patient survey |
| Secondary | Reach | % of eligible patients who receive decision aid | Chart review |
| Secondary | Patient knowledge | Six-item multiple choice knowledge score [27] | Post-visit patient survey |
| Secondary | Preference-treatment concordance | % of patients who received preferred treatment within 6 months of visit | Preference from post-visit patient survey; treatment from chart review |
| Secondary | Patient experience | Five-item CAHPS MD-patient communication subscale and visit rating score [28] | Post-visit patient survey |
| Secondary | Clinician satisfaction | Single item, % of clinicians who mark “very” or “extremely” satisfied with visit | Post-visit clinician survey |
| Secondary | Adoption | % of eligible clinicians who complete the SDM skills training | 4 weeks from the start of intervention period |
| Secondary | Timeliness (time to decision) | Single item, % of patients who report stage of decision-making is “made a decision” | Post-visit patient survey |
| Secondary | Timeliness (burden) | Single item, % of visits where clinicians report visit length is “longer than normal” | Post-visit clinician survey |
Fig. 2.
SPIRIT figure
Clinician surveys and interviews: At the beginning of the study, the heart valve team clinicians will complete a baseline needs assessment survey that includes items regarding confidence in SDM skills, attitudes toward DAs and SDM, priority of SDM for key groups, and heart team integration [29]. For each patient enrolled, clinicians will be randomly selected to complete a brief survey after the visit and will include assessments of SDM process from the clinician’s perspective, perception of visit length, appropriateness of SDM, and satisfaction with the visit. Several months after implementation of the DAs, clinicians will be interviewed about their experiences.
Clinician post-visit survey: A brief survey will be administered after the visit and will include assessments of SDM process from the clinician’s perspective, perception of visit length, appropriateness of SDM, and satisfaction with the visit.
Patient pre- and post-visit surveys: A brief pre-visit patient survey will be administered before the visit, including items about patient treatment preference, stage of decision-making [30], literacy [31], and decision self-efficacy [32]. Patients will also be asked to complete a post-visit survey after visits with their heart valve team, including measures of SDM, knowledge, treatment preference, patient experience, and stage of decision-making. See Table 1 for more details.
Chart review: Study staff will conduct a chart review to collect clinical covariates, visit note, treatments (TAVR, SAVR, and/or other), and short-term outcomes. Baseline clinical covariates and 30-day clinical outcomes will be collected from CMS-mandated Transcatheter Valve Therapy (TVT) registry and the Society for Thoracic Surgery (STS) registry for TAVR and SAVR patients, respectively.
Participant timeline {13}
Clinicians: At the beginning of the trial, clinicians and leadership for the heart valve team at each site will complete the baseline needs assessment. Clinicians selected for a clinician post-visit survey will complete the survey after the visit with the enrolled patient. About 8 months after a site has transitioned to the intervention, we will invite clinicians, leadership, and staff from the heart valve team to participate in a post-implementation interview.
Patients: 2 weeks prior to a visit, research staff at each site will screen the clinic schedule, identify eligible patients, and send an invitation with an information sheet describing the study to patients in advance of the visit. Research staff will meet patients in clinic before their visit to review the information sheet, answer questions, obtain consent, and administer the short pre-visit survey. After patients complete the pre-visit survey, they will be given the post-visit survey to complete at home after they have seen both specialists. Staff will follow a modified Dillman approach with small incentives ($10 gift card) and planned phone and mailing reminders to ensure high response rates to the post-visit survey [33]. During the intervention period, the DA will be delivered to patients by clinic staff as part of routine care. Due to variation in routine care by site, DA delivery may vary but the DA will be delivered to the patient prior to completion of the post-visit survey.
Sample size {14}
For aim 1, using Hooper and Bourke approach to conduct the power analysis we find that with 8 steps and 16–17 patients per step per cluster, the design effect is 1.87 assuming an intra-class correlation of 0.4 [34]. With a total sample size of 1080, the effective sample size is 588. We will have 80% power to detect a small effect size (Cohen’s d = 0.23) for continuous variables (primary outcome: patient-reported SDM scores; secondary outcomes: patient knowledge/experience) with a two-sided significance level of 0.05.
Changes to sample size for aim 1: For batch 1, the design effect is 1.41 assuming a within-period ICC of 0.25 and a between-period ICC of 0.1 with 6 steps and 19–20 patients per step per cluster. For batch 2, the design effect is 6.71 assuming a within-period ICC of 0.2 and a between-period ICC of 0.08 with 2 steps and 37–38 patients per step per cluster. With a total sample size of 900, the effective sample size is 568. We will have 80% power to detect a small effect size (Cohen’s d = 0.26) for continuous variables (primary outcome: patient-reported SDM scores; secondary outcomes: patient knowledge/experience) with a two-sided significance level of 0.05.
For aim 2, the sample size for implementation reach outcome is 600 (limited to patients recruited during the exposed periods and assessed through chart review with no attrition). The width of the confidence interval of the estimate will be limited to 3.8% on each side using the exact (Clopper-Pearson) confidence limits. The sample size for adoption (% of eligible clinicians who complete the SDM skills training) is estimated to be 56 (assessed through administrative records with no attrition) and the width of the confidence interval of the estimate will be limited to 12% on each side.
Recruitment {15}
Clinicians: Sites will generate a list of eligible clinicians for the training. The Department Chief, site PI, and/or heart valve team director will send an email invitation and/or inform clinicians about the training at the team meeting. This invitation will be followed with individual email with link to the training sent by the primary study team. Each clinician will get three reminders to complete the training, including a second reminder from the chief or director if needed.
Clinicians will be given 1 month to complete the training. Depending on the site, they may also allocate specific time to complete the training, for example, having it be the focus for a regularly scheduled team meeting.
Patients: The goal is for each site to enroll 16–17 patients in each step (4-month period). We estimate that, in total, all sites will invite about 2000 patients, enroll 1400 (70% consent rate). As complete clinical information may not be available at the time of invitation, we expect that some patients will be deemed ineligible after consenting and will be withdrawn from the sample (estimate 14%) and some patients will not return the post-visit survey (estimate 10%). As a result, we expect to collect a post-visit survey (primary outcome) on 1080. Each site will enroll patients in the same way in the control and intervention periods.
There are three main vulnerable groups that we will work to enroll: Black patients, Hispanic patients, and older adults (≥ 75 years old; who may have visual, hearing, or mild cognitive impairments). To achieve high enrollment rates for these individuals, many steps will be taken. See Sect. 18b.
Changes to original recruitment: The goal is for each site in batch 1 to enroll 19–20 eligible patients in each step (5-month period) for a total of about 750 eligible enrolled. For batch 2 sites, their goal is to enroll 41–42 patients per step (9-month period) for a total of about 250 eligible enrolled. As shown in Fig. 3, we estimate that, in total, all sites will invite about 1850 patients, enroll 1300 (70% consent rate). As complete clinical information may not be available at the time of invitation, we expect about 23% will be deemed ineligible after the visit such that only 1000 of the 1300 enrolled will be asked to complete the post-visit survey. Of the 1000, we expect to collect a post-visit survey (primary outcome) on 900. The attrition rate including dropouts and post-enrollment ineligibility is 30.7% (400/1300).
Fig. 3.

Flow of activities and data collection estimates for the trial
Assignment of interventions: allocation
Sequence generation {16a}
Sites will be randomized in pairs, with sites with similar volume of racial and ethnic minority patients in opposite orders to maintain balance in the key subgroup across periods. For the clinician survey, we will use computer-generated random assignment to select one specialist to receive the post-visit survey for each patient participant.
Changes to the original sequence generation: For the 6 sites included in batch 1, we will randomly select one sequence that provides similar volumes in the total number, age ≥ 75, and minority subjects between the two arms from all possible permutations (6! = 720). We will use a simple random number generator to determine the order for the 2 sites included in batch 2.
Concealment mechanism {16b}
Allocation of sites to the timing of the transition to the intervention phase will be temporarily concealed. Sites will be notified about 6 months before their transition to the intervention arm in order to facilitate planning for rollout and site visits.
Implementation {16c}
Sites will be randomly assigned to the step at which they begin the intervention period by the statistician.
Assignment of interventions: blinding
Who will be blinded {17a}
There is limited blinding in the trial due to practical limitations. Patients will not be blinded to the materials they receive. Sites will be notified about 6 months before their transition to the intervention arm in order to facilitate planning for rollout and site visits. Clinicians, staff, and research staff will not be blinded to the study arm. The statistician conducting analyses will be blinded to group assignment at each step.
Procedure for unblinding if needed {17b}
Not applicable. The design is open label with participants and clinicians not blinded so unblinding will not occur.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Baseline needs assessment surveys and clinician will be completed online or on paper. Patient pre-visit and post-visit surveys can be completed either online or on paper. Paper surveys will be entered into REDCap database by study staff upon receipt. Post-implementation interviews will be audio-recorded and professionally transcribed for analysis. Study staff will conduct chart reviews and enter data into centralized REDCap databases. The TVT registry and the STS registry data will be collected for patients undergoing TAVR or SAVR, respectively.
Plans to promote participant retention and complete follow-up {18b}
We will follow the guidelines for research studies targeting underserved populations (such as the Evaluate, Engage, Reflect and Carefully Match or EERC) [35]. We have also identified several resources such as the health literacy guidance from CMS, training videos from the palliative care network for research coordinators to support effective communication with older populations, and expertise from our own co-investigators. We have also designed a study protocol that anticipates and overcomes known barriers (e.g., reducing fear or mistrust, reducing logistical barriers, avoiding placebo), uses known facilitators (e.g., personal contact and remuneration), and then employs systems to ensure accountability by monitoring results and making adjustments in timely fashion to ensure strong recruitment and retention.
Data management {19}
All data will be centrally housed with the primary study team. The primary study team will create centralized REDCap surveys for all sites for all surveys. Data entered from paper surveys will be double entered for 10% of the data to ensure quality; paper surveys will be scanned, stored, and disposed of following each site’s internal guidelines.
Confidentiality {27}
Surveys and any notes from the interviews will not contain any identifying information and will be coded by unique study ID number only. Any study data that includes identifiers will be kept on password-protected servers or in locked file cabinets.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable. See above 26b there will be no biological specimens collected.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Aim 1
With the stepped wedge design, the outcomes during the intervention periods will be compared to outcomes during the control periods using an intention to treat approach [36]. Our primary analyses will be conducted excluding missing data. For patient-reported outcomes, we will use generalized linear mixed models with a normal link for continuous outcomes (e.g., shared decision-making) and a logit link for the binary outcomes (e.g., preference-treatment concordance). The models will include a random effect to account for clustering within each site and a fixed effect for calendar time.
Changes to original protocol: The fixed period effect (7 in batch 1 and 3 in batch 2) will be estimated separately. The treatment effect will be an weighted sum of estimates obtained for each batch separately, weighted by its variance.
Aim 2
We will test hypotheses that at least 70% of patients will receive the decision aid, with a similar rate in vulnerable populations and that at least 70% of clinicians will adopt the SDM training. We will calculate site and clinician adoption rates and maintenance (number of sites that continued to use the decision aids in year 5).
Aim 3
We will explore potential mechanisms of effectiveness and implementation outcomes using mixed methods at the clinic-, clinician-, and patient-level and identify barriers and facilitators for maintenance of the strategies. The baseline needs assessment survey, field notes from meetings with the sites, and post-implementation interviews will support these analyses. These analyses will be guided by constructs from CFIR and will be hypothesis generating with a particular focus on identifying key predictors of successful implementation.
Interim analyses {21b}
Not applicable. There are no anticipated problems that are detrimental to the participants so there will be no stopping guidelines or interim analyses.
Methods for additional analyses (e.g., subgroup analyses) {20b}
Aim 1
We will explore heterogeneity of the treatment effect (HTE). The key pre-specified subgroups include site, patient age, patient sex as a biological variable, and vulnerable populations. We will also explore determinants such as heart valve team integration (measured with teamwork, organizational learning, and communication subscales collected at the baseline needs assessment) and clinician attitudes toward SDM (from baseline needs assessment). We will test the interaction between treatment status and subgroup in the models. For clinician reported outcomes, we will use the same modeling approach with an additional random effect to take into account the repeated measures from the same clinician.
Aim 2
We will explore HTE among subgroups as in aim 1 described above.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
For patient-reported outcomes (shared decision-making, knowledge, decision aid usage, etc.), missing data items will be handled according to established protocols for the validated surveys. For item-specific analysis, our primary analyses will be conducted excluding patients with missing data. We will conduct a sensitivity analysis using the multiple imputation approach. For outcomes assessed via chart review, such as visits and treatment received, we do not expect any missing data. If there is no documented treatment or visit note, then we will assume it did not happen (i.e., neither TAVR nor SAVR received). For demographics and other sample characteristics, we will include an unknown category for missing race/ethnicity, education, literacy, or other features.
Plans to give access to the full protocol, participant-level data, statistical code, and materials {31c}
The full protocol, de-identified data (where possible), and statistical code will be included in a public registry within 12 months of completion of the project.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The coordinating center study team is made up of both PIs as well as the co-investigators, project manager, data manager, and statistician from MGH. The team is responsible for the overall organization and oversight of the trial. Each of the 8 site study teams is made up of at least one site PI along with their clinical and administrative staff, who are responsible for the day-to-day operations for the trial. One patient partner is a co-investigator on the study. Four patient partners and one caregiver partner are advisors and provide feedback and lived expertise to support study design, protocols, outcomes, and interventions. The study team includes experts in shared decision-making, aortic stenosis, decision science, mixed-methods, and implementation science. Additional collaborators and advisors include the ACC/AHA, Heart Valve Voice US, Heart Valve Collaboratory, and the Food and Drug Administration.
Composition of the data monitoring committee, its role and reporting structure {21a}
For this minimal risk study, the PIs and statistician will meet every 6 months and to monitor data on this project.
Regulatory reporting
Adverse event reporting and harms {22}
No serious adverse events are expected based on the minimal risk in the trial. If a serious adverse event occurs, the PI will report the event to the Institutional Review Board (IRB) within 24 h and file appropriate paperwork. If a mild or moderate adverse event occurs, the PI will summarize the event in the annual continuing review progress report.
Frequency and plans for auditing trial conduct {23}
The coordinating center will be reviewing site-level data monthly in order to provide monthly CONSORT reports to the funder once enrollment begins.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
All sites will be alerted to protocol amendments within 1 week of amendments being introduced to the primary study site IRB. The coordinating center will provide rationale and all documents for study amendments to sites.
Dissemination plans {31a}
We will use a multi-pronged approach to disseminate the findings to academic, clinical, and consumer audiences. Findings will be disseminated through manuscript and presented at local, regional, and national meetings of key groups and societies. To reach practicing clinicians, we will work closely with our stakeholder partners to disseminate findings through webinars, conferences, and newsletters. These materials will also be posted on the MGH Health Decision Sciences Center website. Further, we will work with our patient partners and Heart Valve Voice US to prepare lay summaries of the results to share with the patient participants in the study, as well as communications (radio shows, blogs, newsletters, local presentations at community centers) to reach the wider patient community. We will also generate a press packet to be used by MGH and other sites’ public relations/media services teams to generate extensive coverage in the popular press and on social media.
Discussion
Shared decision-making (SDM) is a recommended approach for patients with severe aortic stenosis considering valve replacement. Although tools such as patient decision aids (DAs) and training in SDM have been shown to improve SDM, implementation of SDM and DAs is limited. The objectives of this hybrid effectiveness-implementation study are (1) to determine the effectiveness of interventions (a DA and clinician SDM training) in achieving SDM and improving the quality of decisions about aortic valve replacement; (2) to determine the reach of the DAs and adoption of training; and (3) to explore potential mechanisms of effectiveness and implementation at the patient-, clinician-, and clinic-level.
The FDA’s public health component to their mission, with core value of promoting patient engagement and improved health equity, is very interested in how these findings may impact regulatory decisions, particularly for new devices where there is limited long-term evidence but promising short-term results. Further, the study may shed light on the effectiveness of a heart valve team, requiring consultation with multiple specialists, in other settings where the introduction of a new, disruptive technology pits one specialist against another. Thus, this study will add to our understanding of how team-based care, alongside SDM interventions, may impact patient decisions and care.
We note that the unique and unprecedented impact of the COVID pandemic has highlighted the potential weaknesses of stepped wedge designs due to secular trends associated with a public health emergency and a related universal disruption in health care delivery. However, stepped wedge designs prior to the COVID pandemic were successfully used to support rigorous research findings, especially in settings for which standard randomized designs were not optimal, nor clearly feasible. We will work closely with sites to develop contingency plans to mitigate any disruptions that may arise from secular trends or disruptions.
Trial status
Not yet enrolling. Protocol version 3 03/19/2024. Target enrollment to run from April 1, 2024 through March 28, 2027.
Abbreviations
- IMPACT SDM
IMproving treatment decisions for Patients with AortiC stenosis Through Shared Decision Making
- SDM
Shared decision making
- TAVR
Transcatheter aortic valve replacement
- SAVR
Surgical aortic valve replacement
- DA
Decision aid
- AS
Aortic stenosis
- ACC
American College of Cardiology
- AHA
American Heart Association
- CMS
Centers for Medicare and Medicaid Services
- STS
Society of Thoracic Surgery
- CABG
Coronary artery bypass grafting
- MGH
Massachusetts General Hospital
- CFIR
Consolidated framework for implementation research
- RE-AIM
Reach, Effectiveness, Adoption, Implementation, and Maintenance
- TVT
Transcatheter Valve Therapy
- EERC
Evaluate, Engage, Reflect and Carefully match
- HTE
Heterogeneity of treatment effect
Authors’ contributions {31b}
All authors read and approved the final manuscript. KS: conceptualization, methodology, investigation, resources, writing—original draft, writing—reviewing and editing, visualization, supervision, project administration, funding acquisition. SE: conceptualization, methodology, investigation, resources, writing—original draft, writing—reviewing and editing, funding acquisition. KV: investigation, writing—original draft, writing—reviewing and editing, project administration, funding acquisition. YC: conceptualization, methodology, investigation, resources, writing—original draft, writing—reviewing and editing, visualization, supervision, project administration, funding acquisition. MC: investigation, resources, writing—reviewing and editing, supervision, project administration. CD: investigation, resources, writing—reviewing and editing, supervision, project administration. ND: investigation, resources, writing—reviewing and editing, supervision, project administration. CK: investigation, resources, writing—reviewing and editing, supervision, project administration, funding acquisition. EK: investigation, writing—reviewing and editing. KG: investigation, resources, writing—reviewing and editing, supervision, project administration. JM: investigation, resources, writing—reviewing and editing, supervision, project administration. DK: investigation, resources, writing—reviewing and editing, supervision, project administration. DM: investigation, resources, writing—reviewing and editing, supervision, project administration, funding acquisition. VT: investigation, writing—reviewing and editing. SS: conceptualization, methodology, investigation, resources, writing—original draft, writing—reviewing and editing, supervision, funding acquisition. AN: investigation, resources, supervision, project administration. NQ: investigation, resources, supervision, project administration. AB: investigation, resources, supervision, project administration.
Funding {4}
Funded by the Patient-Centered Outcomes Research Institute (PCORI) CDR-2022C1-26333. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing.
Data availability {29}
The full protocol, de-identified data (where possible), and statistical code will be included in a public registry within 12 months of completion of the project.
Declarations
Ethics approval and consent to participate {24}
Ethical approval for the study will be provided by each site individually. The majority of sites approved waiver of written consent to participate in this minimal risk trial, and consent is implied by completion of the survey.
Consent for publication {32}
At 6 sites, participants will receive an information sheet reviewing options, benefits, and risks of the trial and consent will be implied by return of the pre-visit survey. At one site, research staff will obtain written consent and at one site research staff will obtain and document verbal consent.
Competing interests {28}
NL reports being a consultant for Edwards Lifesciences. CK reports a K23 from NHLBI (K23HL153892). CD reports paid consulting fees from Medtronic and Record Medical. EK reports receiving honoraria from Abbott, Boston Scientific, Edwards, and Medtronic. ND receives research support from the American Heart Association, NIH, AHRQ, and Merck and research funding and consulting for Abiomed. KS reports receiving funding for shared decision-making research from PCORI, AHRQ, and NIH, outside submitted work. KS developed the Shared Decision-Making Process scale (copyright Massachusetts General Hospital) that is being used as an outcome measure in the study. KV reports receiving support for shared decision-making research from PCORI and AHRQ outside submitted work and receiving research funding from Google LLC outside the submitted work. SE reports receiving research funding and consulting fees from Edwards Lifesciences and Medtronic. VT reports advising and research with Artivion, AtriCure, Boston Scientific, Abbott Vascular, CroiValve, Edwards Lifesciences, Medtronic, JenaValve, HighLife, Innovalve, and DASI Simulations. JM reports institutional grant support from the University of Colorado School of Medicine: Philips Medical Systems, Medtronic Corporation. SS reports travel sponsored by the ACC, Global Heart Hub, TCT, and Heart Valve Collaboratory and significant financial interests in Heart Valve Voice US. AN has received institutional research funding and speaker fees from Edwards Lifesciences, which is involved in products for the treatment of aortic stenosis. AB reports receiving honoraria for teaching a course at Edwards Lifesciences. DM, MC, YC, KG, NQ, and DK report no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jacques L, Jensen T, Schafer J, Fulton S, Schott L, Baldwin J. Decision memo for transcatheter aortic valve replacement (TAVR). US Centers for Medicare and Medicaid Services (CMSgov). Published online 2012:1–68. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=257.
- 2.Bavaria J, Tommaso C, Brindis R, et al. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;73(3):340–74. [DOI] [PubMed] [Google Scholar]
- 3.van Beek-Peeters J, van Noort E, Faes M, et al. Shared decision making in older patients with symptomatic severe aortic stenosis: a systematic review. Heart. 2020;106:647–55. [DOI] [PubMed] [Google Scholar]
- 4.Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–35. 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(S2):S14. 10.1186/1472-6947-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13(1):1–22. 10.1186/s13012-018-0731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munro S, Manski R, Donnelly KZ, et al. Investigation of factors influencing the implementation of two shared decision-making interventions in contraceptive care: a qualitative interview study among clinical and administrative staff. Implement Sci. 2019;14(1):1–16. 10.1186/s13012-019-0941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd A, Joseph-Williams N, Edwards A, Rix A, Elwyn G. Patchy, “coherence”: using normalization process theory to evaluate a multi-faceted shared decision making implementation program (MAGIC). Implement Sci. 2013;8(1):1–9. 10.1186/1748-5908-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhouli M, Holmes DR, Carroll JD, et al. Racial disparities in the utilization and outcomes of TAVR: TVT registry report. JACC Cardiovasc Interv. 2019;12(10):936–48. 10.1016/j.jcin.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Lindeboom JJ, Coylewright M, Etnel JRG, Nieboer AP, Hartman JM, Takkenberg JJM. Shared decision making in the heart team: current team attitudes and review. Structural Heart. 2021;5(2):163–7. 10.1080/24748706.2020.1859660. [Google Scholar]
- 11.Matlock DD, McIlvennan CK, Thompson JS, et al. Decision aid implementation among left ventricular assist device programs participating in the DECIDE-LVAD stepped-wedge trial. Med Decis Making. 2020;40(3):289–301. 10.1177/0272989X20915227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoepke CE, Wallace BC, Allen LA, et al. Experiences implementing a suite of decision aids for implantable cardioverter defibrillators: qualitative insights from the DECIDE-ICD trial. Circ Cardiovasc Qual Outcomes. 2022;15(11):e009352. 10.1161/CIRCOUTCOMES.122.009352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7:CD006732. 10.1002/14651858.CD006732.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens J, Mills SD, Millett TJ, Lin FC, Leeman J. Design of a dual randomized trial in a type 2 hybrid effectiveness—implementation study. Implementation Sci. 2023;18(1):64. 10.1186/s13012-023-01317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasza J, Bowden R, Hooper R, Forbes AB. The batched stepped wedge design: a design robust to delays in cluster recruitment. Stat Med. 2022;41(18):3627–41. 10.1002/sim.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Consumers and Communication Group, ed. Cochrane database of systematic reviews. Published online April 12, 2017. 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed]
- 17.Sepucha KR, Simmons LH, Barry MJ, Edgman-Levitan S, Licurse AM, Chaguturu SK. Ten years, forty decision aids, and thousands of patient uses: shared decision making at Massachusetts General Hospital. Health Aff. 2016;35(4):630–6. 10.1377/hlthaff.2015.1376. [DOI] [PubMed] [Google Scholar]
- 18.Simmons L, Leavitt L, Ray A, Fosburgh B, Sepucha K. Shared decision making in common chronic conditions: impact of a resident training workshop. Teach Learn Med. 2016;28(2):202–9. 10.1080/10401334.2016.1146600. [DOI] [PubMed] [Google Scholar]
- 19.Yamada J, Shorkey A, Barwick M, Widger K, Stevens BJ. The effectiveness of toolkits as knowledge translation strategies for integrating evidence into clinical care: a systematic review. BMJ Open. 2015;5(4):e006808. 10.1136/bmjopen-2014-006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell BJ, Beidas RS, Lewis CC, et al. Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res. 2017;44(2):177–94. 10.1007/s11414-015-9475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JD, Li DH, Rafferty MR. The implementation research logic model: a method for planning, executing, reporting, and synthesizing implementation projects. Implementation Sci. 2020;15(1):84. 10.1186/s13012-020-01041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a decision aid for patients with advanced heart failure considering a destination therapy left ventricular assist device. JACC Heart Fail. 2015;3(12):965–76. 10.1016/j.jchf.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace BC, Jones J, Masoudi FA, et al. Development and piloting of four decision aids for implantable cardioverter-defibrillators in different media formats. Pacing Clinical Electrophis. 2021;44(11):1842–52. 10.1111/pace.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sepucha K, Simmons LH. Shared decision making: skills for clinical practice. Published online 2021. https://cmecatalog.hms.harvard.edu/shared-decision-making-skills-clinical-practice. Accessed 20 Jan 2022.
- 25.van Beek-Peeters JJAM, van Noort EHM, Faes MC, et al. Shared decision making in older patients with symptomatic severe aortic stenosis: a systematic review. Heart. 2020;106(9):647–55. 10.1136/heartjnl-2019-316055. [DOI] [PubMed] [Google Scholar]
- 26.Valentine KD, Vo H, Fowler FJ, Brodney S, Barry MJ, Sepucha KR. Development and evaluation of the shared decision making process scale: a short patient-reported measure. Med Decis Making. 2021;41(2):108–19. 10.1177/0272989X20977878. [DOI] [PubMed] [Google Scholar]
- 27.Valentine K, Marques F, Selberg A, et al. Abstract 397: shared decision making in cardiology: measures of shared decision making in patients with severe aortic stenosis considering valve replacement. Circ Cardiovasc Qual Outcomes. 2020;13:A397-. [Google Scholar]
- 28.Dyer N, Sorra JS, Smith SA, Cleary PD, Hays RD. Psychometric properties of the Consumer Assessment of Healthcare Providers and Systems (CAHPS®) clinician and group adult visit survey. Med Care. 2012;50:S28–34. 10.1097/MLR.0b013e31826cbc0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorra JS, Dyer N. Multilevel psychometric properties of the AHRQ hospital survey on patient safety culture. BMC Health Serv Res. 2010;10(1):199. 10.1186/1472-6963-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor A. User manual stage of decision making. User manual - stage of decision making. 2000, modified 2003. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Stage_Decision_Making.pdf. Accessed 2 June 2021.
- 31.Brice JH, Foster MB, Principe S, et al. Single-item or two-item literacy screener to predict the S-TOFHLA among adult hemodialysis patients. Patient Educ Couns. 2014;94(1):71–5. 10.1016/j.pec.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor A. User manual - decision self-efficacy scale [document on the Internet]. Published online 1995modified 2002. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decision_SelfEfficacy.pdf. Accessed 13 Dec 2021.
- 33.Dillman D, Smith J, Christian LM. Internet, phone, mail and mixed-mode surveys: the tailored design method. 4th ed. Ltd: John Wiley & Sons; 2014. [Google Scholar]
- 34.Hooper R, Bourke L. Cluster randomised trials with repeated cross sections: alternatives to parallel group designs. BMJ. 2015;350(jun08 8):h2925–h2925. 10.1136/bmj.h2925. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda Y, Brooks JL, Beeber LS. Guidelines for research recruitment of underserved populations (EERC). Appl Nurs Res. 2016;32:164–70. 10.1016/j.apnr.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes J, Granston T, Heagerty PJ. Current issues in the design and analysis of stepped wedge trials. Contemp Clin Trials. 2015;45(Pt A):55–60. 10.1038/s41395-018-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full protocol, de-identified data (where possible), and statistical code will be included in a public registry within 12 months of completion of the project.