Abstract
Purpose
Gastrointestinal (GI) dysfunction is common in critically ill patients and associated with poor outcomes. There is a lack of standardised methods for daily monitoring of GI function. COSMOGI aimed to develop a Core Outcome Set (COS) for daily monitoring of GI function to improve consistency and comparability in future studies in critically ill patients.
Methods
A modified Delphi consensus process engaging healthcare providers, clinical researchers, and patient representatives was performed. A systematic review identified existing parameters to monitor GI function, informing the development of potential outcomes. In Stage 1, participants rated outcomes (i.e., variables used for daily monitoring). In Stage 2, they refined and agreed on the definitions for the selected outcomes. The COS was ratified through consensus meetings.
Results
368 individuals registered for the Delphi process. 285 participants (77.4%) completed Stage 1, and 181 participants (63.5%) completed Stage 2. From 77 potential outcomes, 13 essential outcomes for daily monitoring of GI function in studies, each with an agreed-upon definition, were established: abdominal distension, bowel dilatation, intra-abdominal pressure, abdominal pain, stool passage, vomiting, GI bleeding (upper and lower), use of parenteral nutrition due to intolerance of enteral nutrition, prokinetics, postpyloric feeding due to gastroparesis, lower GI paralysis, gastroparesis, intolerance to enteral nutrition.
Conclusions
Using a modified Delphi consensus process, COSMOGI established a COS for monitoring GI function in critically ill patients in research. This COS and definitions provide a framework to guide future research, enabling comparability across studies and allowing for future definitions of GI dysfunction.
Trial registration: This project was registered at (www.comet-initiative.org) on 27.03.2023 (number 2609) and was an ESICM-endorsed research project.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05192-8.
Keywords: Gastrointestinal dysfunction, Enteral nutrition, Intensive care medicine, Delphi consensus process, Research standardisation
Introduction
Gastrointestinal (GI) dysfunction is common in critically ill patients and is associated with increased morbidity and mortality [1–4]. The range of definitions for GI function in the critically ill varies widely, and current approaches to monitoring GI dysfunction are limited [5]. This variability negatively impacts clinical research, complicates clinical assessment, and limits study comparability. In the absence of a gold standard to measure GI function, GI dysfunction is described by minor manifestations like dysmotility and severe complications such as abdominal compartment syndrome and gastrointestinal bleeding [6]. This variety of manifestations cannot be condensed into a single symptom, though complex clinical assessment of GI dysfunction is prone to subjectivity, and a standardised approach is lacking [7, 8]. Currently, different definitions of GI signs and symptoms are used in research, and standardising the reporting of these symptoms needs to be addressed [9].
No single sign or symptom of GI dysfunction significantly and independently correlates with mortality, which is commonly used as a surrogate marker in the absence of any gold standard to measure GI dysfunction. However, the associations with poor outcomes such as mortality, ICU length of stay, and organ dysfunction are strengthened when multiple parameters are considered together [1, 3]. Current approaches to monitoring GI function include the use of scoring systems [3, 10]. These scores assess symptoms of GI dysfunction, abdominal signs and interventions, including absent bowel sounds, abdominal distension, vomiting, gastric residual volume (GRV) measurement, intra-abdominal pressure (IAP) and application of prokinetics. While useful, these scores are not universally adopted, and there is no consensus on the key outcomes that should be monitored daily in clinical trials [9].
A recent systematic review structured the monitoring of GI dysfunction into six topics: abdominal signs and symptoms assessable at the bedside, estimates of gastric emptying, monitoring of intestinal motility, imaging techniques such as ultrasound or computerised tomography (CT), and measures of perfusion and biomarkers [9]. To enable comparison between studies, with the aim to meta-analyse available aggregated evidence, there is a need to reach a consensus on a minimum core outcome set (COS) for daily monitoring of GI function in the study setting. Therefore, we conducted a modified Delphi consensus process to establish a COS that should be reported in future clinical studies investigating GI dysfunction.
Methods
Research question
What is the COS for daily monitoring of GI function in studies assessing GI dysfunction or enteral nutrition in critically ill patients?
Objectives
Perform a modified Delphi consensus process to identify a COS for daily monitoring of GI function in adult critically ill patients. This COS should be reported in clinical trials assessing GI dysfunction or conducting nutritional research where GI dysfunction is an outcome.
Stakeholders and recruitment
Participants were representatives from three stakeholder groups: clinical researchers, ICU survivors and caregivers, and healthcare professionals. We invited international experts worldwide via the Feeding, Rehabilitation, Endocrinology & Metabolism (FREM) section of the European Society of Intensive Care Medicine (ESICM) to become steering committee members. This ensured an international and interprofessional steering committee and enabled us to reach the existing critical care networks to recruit participants.
Recruitment of healthcare professionals and clinical researchers with a combined interest and expertise in gastrointestinal function and intensive care medicine for the Delphi process was performed through contacts of the steering committee members, social media, and ESICM, which endorsed the project and sent out an invitation to all members of the Feeding, Rehabilitation, Endocrinology & Metabolism (FREM) section. Additionally, participants of previous projects were contacted [11]. Patient representatives were recruited through patient support groups and personal contacts.
We invited members of each stakeholder group via email to participate in the Delphi process. The email contained details about the project and outlined a timeline. The registration was conducted online, and each participant was assigned a unique identifier. Participants were not asked to declare any conflict of interest. Consent was implied at the beginning of the process through participation, and participants could withdraw at any point during the process. Data were used as stated in the invitation email, and consent, unless actively withdrawn, was inferred throughout the Delphi consensus process. Data could not be removed if active withdrawal occurred after data analysis. Responses were not used in any manner that allowed the identification of a participant.
Participants were asked to vote based on their personal/professional opinions rather than the organisation they represented. A recent systematic review published in 2020 provided the basis for the initial core domains and outcomes [9]. Based on the same protocol, an additional literature search was conducted to include any relevant literature published beyond November 2019. The search was completed (from December 2019 until January 2023) using the search terms published in the systematic scoping review on GI dysfunction in the critically ill [9]. Identified measures to monitor GI dysfunction were reduced to a standard taxonomy for COS development [12].
Delphi consensus process
The modified Delphi consensus methodology is well-described and used extensively in COS-related projects [13]. It involves serial rounds of participants voting on recommendations related to a study question. Voting is based on the results of preceding rounds and is performed anonymously to prevent external influence [14]. All survey rounds were delivered electronically using DelphiManager software (COMET Initiative, University of Liverpool, UK). Consensus was reached via a two-stage process, with each stage containing two rounds and a final consensus meeting similar to previous projects [11].
In Stage 1, participants scored each suggested outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) scale. This scale ranges from 1 to 9 in terms of importance for inclusion. (1–3, not important for inclusion; 4–6, important but not critical; 7–9, critical to include). In Stage 2, participants scored each definition for each outcome according to the above GRADE scale.
Per protocol criteria for ‘essential’ inclusion was a ‘critical-to-include’ rating of 7–9 in ≥ 70% of all responses and ≤ 15% of all responses rating the definition as ‘not important’ (i.e., score ≤ 3) [15, 16]. Criteria for ‘recommended’ inclusion for outcomes and definitions was a ‘critical-to-include’ rating of 7–9 in ≥ 60% of all responses and ≤ 15% of all responses rating the outcome or definition as ‘not important’ (i.e., score ≤ 3). Outcomes and definitions received a’suggested’ status if a ‘critical-to-include’ rating of 7–9 in ≥ 50% of all responses and a ‘not important’ rating (i.e., score ≤ 3) in ≤ 15% of all responses. Criteria for exclusion were a ≥ 15% of all responses rating the outcome or definition as ‘not important’ (i.e., score ≤ 3).
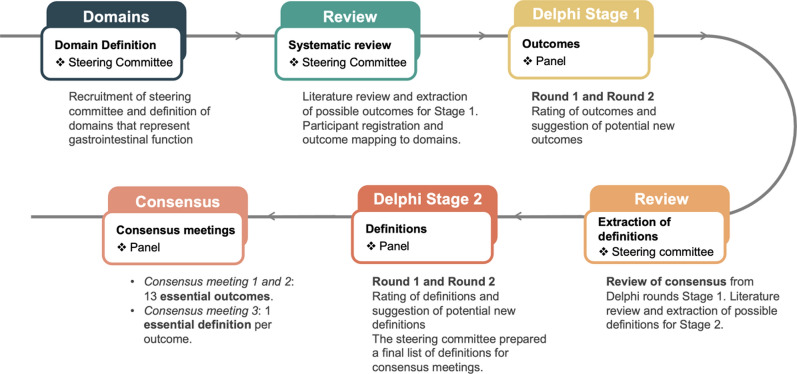
This project was registered with the COMET (Core Outcome Measures in Effectiveness Trials) initiative (https://www.comet-initiative.org/Studies/Details/2609), and the results from this study are reported following the COS-STAR Statement [12, 17]. A schematic representation of the stages is depicted in Fig. 1.
Fig. 1.
Schematic representation of the modified Delphi process applied in COSMOGI
Stage 1
Domains represented all GI functions [6] and were pre-specified through the steering committee at the FREM section meeting (22.03.2023, Brussels, Belgium).
Round 1: After confirmation and review by the steering committee, the outcomes extracted from the data sources were entered into the Delphi round. The outcome order was randomised. Each outcome was mapped to a domain, and some outcomes were mapped to multiple domains if appropriate. An explanatory document was provided for each outcome with a description of feasibility, impact on patient care, cost, accuracy, and any relevant literature [18]. Participants rated each outcome without considering the definition of the outcome. The opportunity to comment on existing or suggest new outcomes was provided during the first round. The steering committee reviewed all additional suggested outcomes after round 1 to ensure they provided a novel contribution to the next Delphi round.
Round 2: Scores for each outcome were distributed to the participants, and the average score, summarised by stakeholders, was presented. Participants were then asked to re-evaluate each outcome, including any new outcomes added to the round.
The study protocol contained the option for an additional round 3 to be held if ≥ 70% of responses from at least one stakeholder group rated ≥ 7 for a newly suggested outcome during round 2.
Stage 2
In Stage 2, definitions were sought for essential outcomes identified in Stage 1. Patient representatives were a priori excluded from Stage 2 due to the clinical nature of a monitoring set. After stage 1, the steering committee performed a literature search to gather all possible definitions of the outcome measures identified as essential to include during stage 1. Two investigators (KFB & ARB) then revised the definitions to ensure a consistent taxonomy and structure of presentation. The full list of all possible definitions was then presented to the steering committee, which voted to include or exclude the proposed definitions with a consensus cut-off of 70%. The final list of definitions was then used to populate round 1 of Stage 2. The Delphi process was performed as in Stage 1, with the same thresholds defining essential, recommended, and suggested definitions. Participants were again given an opportunity to suggest new definitions with an option for an additional round 3.
After the Delphi process of Stage 2, the steering committee prepared a final list of definitions for the consensus meeting. This list was based on the inputs from the consensus meeting of Stage 1, individual feedback from participants inputs and inputs from steering committee meetings with the goal of ensuring that the definitions are both meaningful and feasible.
Consensus meetings
Following the Delphi process, an online consensus meeting was held at the end of each stage to ratify the final COS contents and undertake any additional voting if required. All study participants who completed both rounds were invited to the consensus meeting of Stages 1 and 2, respectively. Additional voting was required if concerns regarding the list of outcomes identified during Delphi rounds were raised and if changes were suggested. Consensus on proposed changes, including changes to the results of the Delphi rounds, required more than 70% of participants at the consensus meeting to vote in favour of change.
Statistical analysis
Data are presented as median with interquartile ranges or numbers and percentages. Boxplots are used for visual representation of data. Linear regression models assessed differences between ratings of stakeholder groups for essential outcomes and definitions. A p-value of < 0.05 was considered statistically significant. All analyses were done using MatLab (r2024a, Natick, Massachusetts, USA).
Results
Stage 1
A total of 368 individuals registered on our website, https://cosmogi.site. 240 (65.2%) registered as healthcare providers, 115 (31.3%) registered as clinical researchers and 13 (3.5%) as patient representatives and/or caregivers. The panel contained 305 physicians (85.9%) and 50 dieticians/nutritionists (14.0%). The median years of experience for physicians was 15 [10 to 22] and 10 [6 to 13] years for non-physicians. 33 participants did not give any information about years of experience. 67.9% of participants were from Europe, 14.1% from Asia, 7.6% from North America, 4.6% from Oceania, 5.4% from South America and 1.1% from Africa. All registrations were migrated to the DelphiManager software. The completion rates for the first and second rounds were 285 (77.4%) and 252 (68.5%) participants, respectively (August 28th to November 12th 2023). Participants self-identified as 4% patient representatives or caregivers, 65% healthcare providers, and 31% clinical researchers. Participants who agreed to be acknowledged are listed (for more information, see Supplementary Content 1).
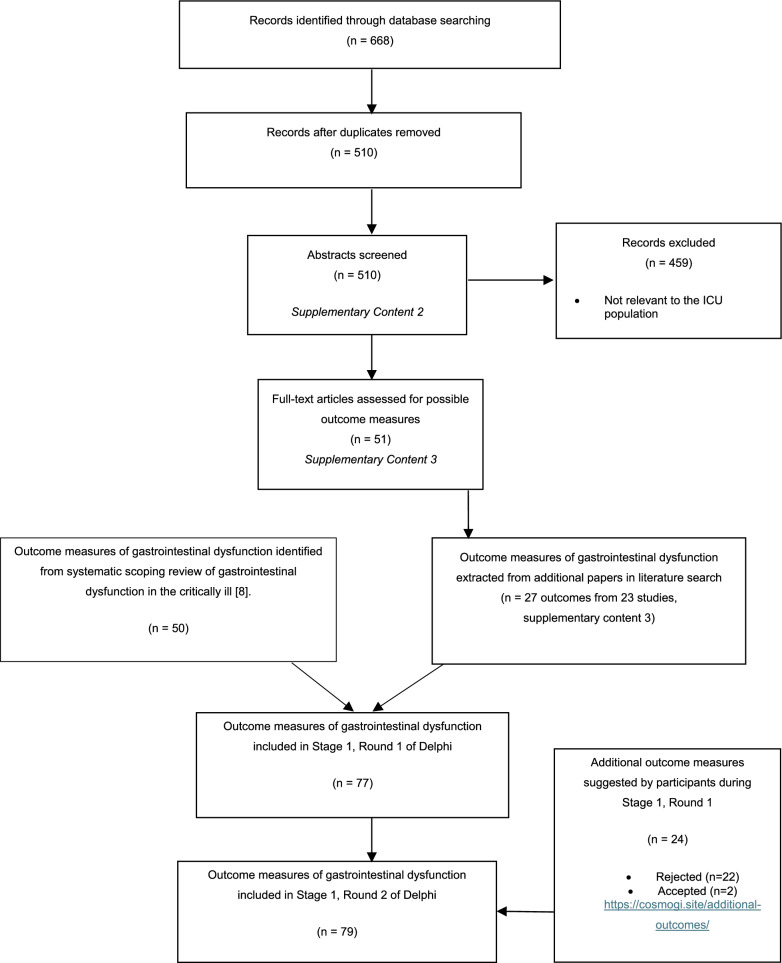
Fifty outcome measures were identified from the systematic scoping review on GI dysfunction in the critically ill [9]. Following the systematic review update, 27 potential outcomes were identified, providing 77 outcome measures to be included in Stage 1, Round 1 of the Delphi consensus process (Fig. 2, Table 1). These outcomes were grouped into the 11 domains of GI function [6]. Some outcomes were mapped to multiple domains of GI function, leading to 102 outcomes in the initial Delphi round (Supplementary Table 1). During Stage 1, Round 1, participants in the Delphi consensus suggested an additional 24 potential outcome measures. Two of those 24 were included in Stage 1, Round 2 of voting (Supplementary Table 2). The two additional outcomes did not reach a > 70% rating by any stakeholder group in round 2; therefore, a third round was unnecessary.
Fig. 2.
PRISMA flow diagram of included/excluded evidence and outcomes (Stage 1) [24]
Table 1.
Core outcome set of daily monitoring of GI function showing all 13 essential outcomes
| Outcome | Percentage of 7–9 (critical to include) rating in Stage 1 Delphi, Round 2 (%) | Consensus meeting vote to change to recommended outcome. (> 70% consensus needed) |
|---|---|---|
| Abdominal distension | 86.9 | 13% (87% to remain essential) |
| Bowel dilatation | 77.4 | 28% (73% to remain essential) |
| Intra-abdominal pressure | 74.2 | 38% (63% to remain essential) |
| Abdominal pain | 88.1 | 7% (93% to remain essential) |
| Stool passage | 80.6 | 7% (93% to remain essential) |
| Vomiting | 88.5 | 2% (98% to remain essential) |
| GI bleeding (upper and lower) | 82.1/77.4 | 3% (91% to remain essential) |
| Use of parenteral nutrition due to intolerance of enteral nutrition | 82.9 | 22% (78% to remain essential) |
| Prokinetics | 71.8 | 31% (69% to remain essential) |
| Postpyloric feeding due to gastroparesis | 79.4 | 22% (78% to remain essential) |
| Lower GI paralysis | 77.8 | 22% (78% to remain essential) |
| Gastroparesis | 83.3 | 11% (89% to remain essential) |
| Intolerance to enteral nutrition | 89.7 | 6% (94% to remain essential) |
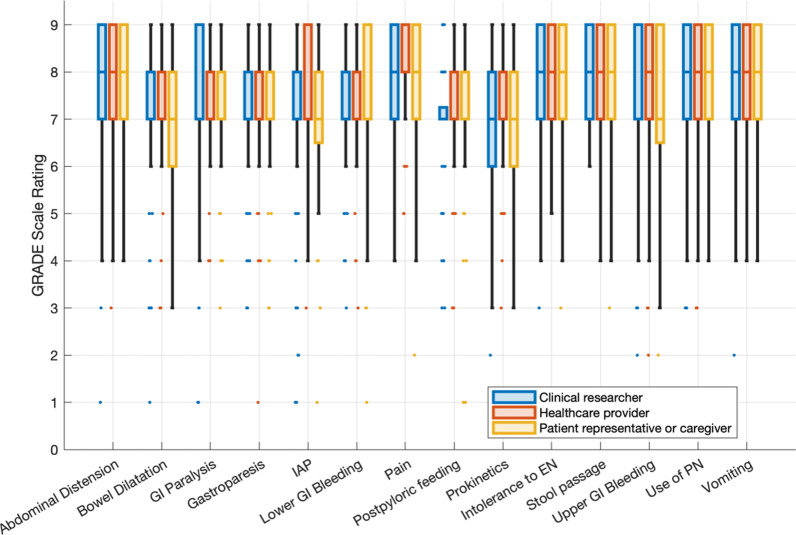
Following the second round of the Delphi process, 14 outcomes were identified as essential (Fig. 3). These outcomes include signs and symptoms (abdominal distension, bowel dilatation, IAP, abdominal pain, stool passage, vomiting & GI bleeding), treatment/interventional outcomes (use of parenteral nutrition, prokinetics and postpyloric feeding) as well as clinical entities (GI paralysis, gastroparesis and intolerance to enteral nutrition). Linear regression models did not identify any significant association between the stakeholder groups’ ratings (Supplementary Table 3). Additionally, the process identified 3 recommended and 6 suggested outcomes, while 46 outcomes were excluded (Table 2 and Supplementary Table 1 ). Ten outcomes did not reach any prespecified threshold (Supplementary Table 1). Based on a collective agreement, the steering committee refined the list of essential outcomes from 14 to 13, and adjusted the initial taxonomy: Upper and lower gastrointestinal bleeding (reaching a consensus of 82.1% and 77.4% respectively) were merged into one outcome (GI bleeding). The outcome «pain» was refined to denote «abdominal pain» specifically. Furthermore, GI paralysis was more precisely specified as «lower GI paralysis», including all intestinal sections below the pylorus within this outcome. Lastly, the outcome previously termed «response to enteral nutrition» was revised to «intolerance to enteral nutrition». This change was prompted by feedback during the Delphi rounds, which highlighted that "response" could imply metrics such as body composition, whereas «intolerance» more accurately addresses the capability of a patient to undergo enteral feeding, reflecting GI dysfunction.
Fig. 3.
Boxplots with interquartile ranges, minimum/maximum and outliers showing stakeholder ratings of Delphi Stage 1, Round 2. IAP: Intra-abdominal pressure. GI: Gastrointestinal. EN: Enteral nutrition. PN: Parenteral nutrition
Table 2.
Recommended and suggested outcomes
| Outcome | Unable to rate n (%) | Percentage of 7 to 9 votes (critical to include) (%) | Percentage of 1 to 3 votes (not important) (%) | Status based on round 2 |
|---|---|---|---|---|
| Opioid use incl. Opioid antagonists | 4 (1.6%) | 63.9 | 3.6 | Recommended |
| L-Lactate | 17 (6.7%) | 60.7 | 7.9 | Recommended |
| Bacteremia with enteral microflora | 13 (5.2%) | 67.9 | 5.6 | Recommended |
| Acute intestinal Failure (ESPEN) | 13 (5.2%) | 57.9 | 4.4 | Suggested |
| Laxatives | 5 (2%) | 51.6 | 6.3 | Suggested |
| Treatment of hypermotility | 9 (3.6%) | 52 | 9.1 | Suggested |
| Clinical swallowing tests | 5 (2%) | 53.2 | 10.7 | Suggested |
| GRV (Gastric residual volume) | 25 (9.9%) | 55.2 | 7.1 | Suggested |
| Ascites | 5 (2%) | 59.1 | 4.8 | Suggested |
Results are from Round 2 of the Delphi Stage 1. These findings were not ratified at the final consensus meeting
The COS, including 13 essential outcomes, was presented at a consensus meeting attended by 55, aiming to ratify the COS with a consensus threshold of over 70% (Table 1). However, the consensus on the entire COS at once was not achieved, with only 66% agreement. The main concerns were the excessive burden for patients (e.g., radiation exposure) and healthcare professionals and the absence of clinical indication to daily measure all 13 outcomes in every patient enrolled in a clinical study. These concerns highlight the need for a more tailored and sustainable data collection approach to improve study feasibility. A subsequent consensus meeting with 50 participants involved voting on each outcome individually and considering whether to reclassify any outcome from essential to recommended (requiring over 70% agreement). All 13 essential outcomes received ratification at this second meeting (Table 1). It was recognised that daily measurement of some outcomes may not be necessary for all patients, and the specific definitions of these outcomes, which were developed in stage 2, should be formulated accordingly. The recommended and suggested outcomes, including the results from the Delphi voting, are presented in Table 2. The full outcome list, including excluded outcomes, can be found in Supplementary Table 1. The demographics of all consensus meetings can be found in the online supplement (Supplementary Content 1).
Stage 2
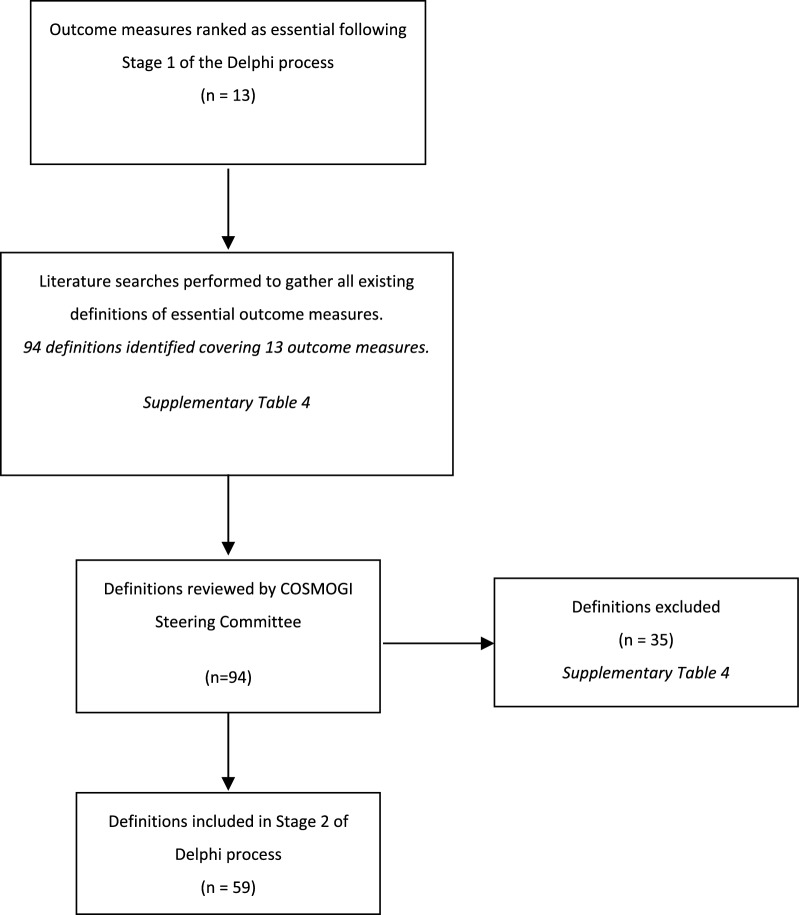
There were 242 participants for Stage 2, of whom 181 completed both rounds (January 21st to March 09th 2024). The literature review for Stage 2 produced 94 suggested definitions across the 13 outcome measures (Fig. 4). Consensus voting by the steering committee led to 59 definitions for inclusion (Supplementary Table 4). The participants were presented with these definitions in round 1 of Stage 2. Participants in this round suggested an additional 10 definitions, none of which were approved by the steering committee, rendering an additional round 3 unnecessary. At least one essential definition was identified per essential outcome measure, and in several cases, multiple definitions were considered essential for a single outcome (Supplementary Table 4). There were significant differences between the two stakeholder groups for 6/29 (20.7%) of the essential definitions (one for bowel dilatation, one for GI bleeding, one for parenteral nutrition, one for intolerance to enteral nutrition and two for vomiting, Supplementary Table 5). In all cases, healthcare providers rated the definitions higher than clinical researchers (Supplementary Table 5). Following a detailed review by the Steering Committee, informed by the consensus meetings and the Delphi results, a singular definition for each of the 13 essential outcomes was compiled. Based on the discussion in the consensus meeting of Stage 1, the definitions for IAP, abdominal pain, bowel dilatation, and GRV measurement in the context of gastroparesis were refined to apply only to specific study populations as outlined in the study protocols of future studies. The list of 13 definitions was then disseminated to all participants for final ratification during the consensus meeting, where all proposed definitions achieved consensus (Table 3). The definitions provide instructions on how the outcome should be assessed, what cut-offs should be considered and how the outcome should be documented (Table 3).
Fig. 4.
PRISMA flow diagram Stage 2 of Delphi consensus process [24]
Table 3.
COSMOGI Definitions and documentation practice with information as to how the definition was created
| Essential outcome | Definition | Documented daily as | How the definition was created | Consensus at final meeting (> 70% needed) (%) | Subjectivity rating |
|---|---|---|---|---|---|
| Abdominal distension | Abdominal distension is defined as a visibly enlarged abdomen that appears expanded at palpation and/or percussion | Yes/No | Based on the essential definitions found in the Delphi rounds | 94.7 | High (Estimation) |
| Abdominal pain | Abdominal pain should be assessed by numeric rating scale (NRS) or visual analogue scale (VAS) scale for patients who are able to communicate. For patients who are unable to communicate, behavioural pain scale (BPS) should be used during clinical examination of the abdomen to provoke an abdominal response. Pain should be documented as no pain, mild pain, moderate pain or severe pain. Assessment is only necessary in patients with suspected abdominal pain as defined in the study protocol | Yes (with a pain intensity)/No/Not applicable. Independent of the scale (NRS/VAS/BPS), pain intensity should be documented as "mild", "moderate" or "severe" pain |
Based on the essential and recommended definitions found in the Delphi rounds. To ensure that patients who are unable to communicate can also be assessed, the BPS was included Based on the consensus meeting, assessment was limited to the relevant population as defined per study protocol |
100 | Moderate (Subjective measure, but clear scaling available) |
| Bowel dilatation | Bowel dilatation is defined as small bowel diameter > 3 cm or large bowel diameter > 6 cm (caecum > 9 cm) measured by any imaging modality; and associated with symptoms referring to bowel distension (pain; nausea; vomiting; IAH) and/or GI paralysis. Bowel dilatation is only assessed in patients at risk as defined in the study protocol of each study | Yes/No/Not applicable | Based on the essential definitions (combining both large and small bowels) and consensus meeting, where assessment was only deemed necessary as per study protocol and not in every patient | 92.5 | Low (Imaging measures) |
| Gastroparesis | Gastroparesis is defined by high gastric residual volumes (GRV) (i.e. > 500mL; measured or estimated by ultrasound) or vomiting due to delayed gastric emptying in the absence of mechanical obstruction. Indication for GRV measurement,GRV cut-off and measurement frequency need to be defined in the study protocol and reported in the manuscript | Yes/No | Based on the essential definitions. Based on the consensus meeting, GRV measurement only needs to be assessed as per study protocol | 92.7 | High (GRV is quantifiable, but cut-offs may vary and interpretation of signs and symptoms may be subjective) |
| Gastrointestinal bleeding | Gastrointestinal bleeding is defined as the loss of blood from any location within the gastrointestinal tract, evidenced by the macroscopic presence of blood in vomited fluids, gastric aspirate, or stools, and/or by evidence of active bleeding on CT scan, angiography, or endoscopy. Clinically important GI bleeding is defined as overt bleeding associated with significant hemodynamic changes, the need for blood transfusions (two or more units of blood over a 24h period), significant changes in hemoglobin level (at least 2g/dl), or the necessity for surgical, endoscopic or endovascular intervention to control bleeding | Yes (specify if any bleeding or clinically important GI bleeding)/No | Based on the essential definitions from the Delphi rounds | 92.7 | Low (Evidence of bleeding) |
| Intraabdominal pressure | Intra-abdominal hypertension (IAH) is defined as a sustained or repeated pathologic elevation of intra-abdominal pressure (IAP) ≥ 12 mmHg. The severity of IAH is graded into four categories based on maximum daily IAP values: Grade I (12–15 mmHg), Grade II (16–20 mmHg), Grade III (21–25 mmHg), and Grade IV (> 25 mmHg). IAP only needs to be measured in patients at risk as defined in the study protocol of each study | Yes (specify grade)/No/Not applicable | Based on the essential definitions and consensus meeting, where assessment was only deemed necessary as per study protocol and not in every patient | 100 | Low in paralysed patients (quantifiable). Moderate in spontaneously breathing patients (interpretation may depend on experience) |
| Stool passage | Documentation of daily frequency and stool consistency using Bristol stool form scale. Absence of stool passage used as a part of definition of lower GI paralysis is the absence of stool for 3 days or longer despite current administration of laxatives and/or prokinetics | Yes (specify times per day with Bristol scale)/No | Essential and recommended definitions were combined | 88.6 | Low (Quantifiable, clear scaling) |
| Lower GI paralysis | Paralysis of lower GI tract (paralytic ileus) is defined as the inability of the bowel to pass stool due to impaired peristalsis. Clinical signs include the absence of stool for three or more consecutive days without mechanical obstruction and despite current administration of laxatives and/or prokinetics. Bowel sounds may or may not be present | Yes/No/Not applicable (cannot be measured in the first two days) | Based on the definition of absence of stool, medication was added to the definition | 100 | Moderate (Stool passage is quantifiable, but associated symptom interpretation may vary) |
| Prokinetics | Prokinetic therapy is defined as use of any prokinetics alone or in combination in case of feeding intolerance | Yes/No | Based on essential definitions. A full list of medication is provided in supplement (Supplementary Content 4) | 100 | Moderate (Indication depends on clinical judgement) |
| Intolerance to enteral nutrition | Intolerance is defined as the occurrence of GI symptoms (e.g. abdominal distension, vomiting/regurgitation, pain, diarrhoea or elevated GRV or IAP), leading to discontinuation or reduction of enteral feeding to the extent that nutritional targets cannot be met via the enteral route | Yes/No | Based on the essential definitions. Additionally, discontinuation or reduction of EN due to clinical signs & symptoms clinically defines feeding intolerance, | 100 | High (Decision to interrupt EN depends on clinical judgement) |
| Use of parenteral nutrition | Parenteral nutrition is defined as artificial nutrition administered through a venous access due to absolute contraindications for receiving enteral nutrition or due to enteral nutrition intolerance. It can be total or supplementary | Yes (specify 1: reason (contraindication or feeding intolerance) and 2: full or supplemental)/No | All essential definitions were combined. Specific list of contraindications can be found in the supplement (Supplementary Content 4) | 97.5 | Moderate (Definition for EFI as indication for parenteral nutrition may vary) |
| Vomiting | Vomiting or emesis is defined as the occurrence of any visible regurgitation of gastric content irrespective of the amount | Yes (independent if either vomiting or regurgitation)/No | All essential definitions were combined | 97.3 | Low (Observable) |
| Postpyloric feeding | Postpyloric feeding is defined as enteral feeding delivered distal to the stomach due to gastroparesis, obstruction or oesophageal or gastric surgery | Yes/No | Based on the essential definition | 92.1 | Moderate (Indication depends on clinical judgement) |
The authors rated the subjectivity of each outcome/definition: The subjectivity scale assesses the potential bias from assessor dependency, categorized as low (objective and reproducible), moderate (some interpretation or variability), or high (heavily reliant on subjective judgment). Outcomes with moderate or high subjectivity may require further exploration and refinement through additional consensus processes to improve standardization and reliability
Discussion
This modified Delphi consensus process in a large cohort of healthcare providers, clinical researchers, and patient representatives successfully produced the first COS of daily monitoring of GI function in studies in critically ill patients. The unified outcomes (i.e. monitored variables) will enable the comparability between future studies and help identify which variables contribute to GI dysfunction. Ultimately, research groups may better define GI dysfunction and plan new clinical trials more adequately based on the identified unified core of outcome measures. This core outcome set targets future trials or observational studies in critically ill patients where GI dysfunction is a primary or secondary outcome, including trials on vasopressors, enteral nutrition or pharmacological interventions. While enteral nutrition was part of the initial research question without specification, it was agreed that research dedicated exclusively to enteral nutrition (e.g., metabolomics studies), without explicitly examining GI dysfunction as an outcome, falls outside the scope of the COSMOGI COS. We provide the set of outcomes (i.e., variables) to be monitored with a consensus definition for each outcome. This includes directions on the methodology of outcome assessment, how assessment may be limited by study protocols of future studies (Table 3), and the recommended documentation practices. Essential outcomes that may involve additional measurements or radiation exposure, such as bowel distension (requiring imaging) or intra-abdominal pressure (requiring a bladder catheter), will be evaluated by the investigators of each individual study (Table 3). Based on the consensus definitions, their application should be determined in the specific study protocol, which will be reviewed and approved by the respective ethical committees. The COS was developed with the perspective of future researchers developing a case report form for their study. While we provide distinct recommendations on the documentation process, the exact summary and reporting of these variables in future manuscripts is at the researchers’ discretion; we suggest that summaries of daily data on GI dysfunction are made available through online supplements or data repositories.
Most of the available parameters for monitoring GI dysfunction remain observer-dependent, necessitating consensus definitions. As there is no gold standard to measure GI function up to this point in time, prediction of mortality has been used as a measurable surrogate for GI dysfunction, based on the rationale that each organ dysfunction is a part of multiple organ dysfunction in the critically ill and therefore increases mortality. GI dysfunction in critically ill patients has been associated with mortality [1, 19]. The Acute Gastrointestinal Injury grading has been proposed based on consensus, descriptively comprising multiple variables to reflect GI dysfunction [8]. Later, the development of the Gastrointestinal Dysfunction Score (GIDS) showed that higher scores on a scale from 0 to 4 were significantly associated with mortality when added to the SOFA score [3]. For 28-day mortality, the hazard ratio (HR) was 1.39 (95% CI 1.05 to 1.84), and for 90-day mortality, the HR was 1.42 (95% CI 1.11 to 1.82) when GIDS was included alongside the total SOFA score. The GIDS consists of multiple variables, as no single variable predicted mortality sufficiently [3]. Many of the score’s variables have also found their way into this COS, such as vomiting, abdominal distension, prokinetic use, intra-abdominal pressure, GI bleeding, and GI paralysis [3]. Notably, the presence of one single GI symptom in patients receiving oral nutrition was not associated with increased mortality compared to the absence of all studied GI symptoms [3]. However, clear and unified definitions for these parameters were unavailable, limiting the GIDS’s value.
COSMOGI consists of 13 variables, all identified by the panel members of the Delphi process. While it was discussed during the consensus meeting that the number of variables might be too high, it was agreed that a number of variables are needed to assess GI function; in line with previous results [3], a single variable may not represent GI function adequately, and a “comprehensive” clinical picture is necessary to provide sufficient data for future definitions and research. Accordingly, it was decided against changing the initial criteria for consensus defining “essential” to force a reduced number of outcomes. At the consensus meeting, the steering committee and the panel raised concerns that specific variables (i.e., GRV in the context of gastroparesis, abdominal pain, IAP, and bowel dilatation) should not be recorded in all patients daily because of a low pretest probability and absent clinical indication with a potentially excessive burden. In Stage 2, we adapted the definitions of these outcomes to allow future researchers to define the study population (i.e., the patients at risk and/or those with a high pretest probability) in whom these measurements should be done daily. Although these outcomes are still considered “essential”, the specific definitions, which all reached consensus at the final meeting, ensure their feasibility (Table 3).
Importantly, it must be clearly acknowledged that many of these variables included in the final COS are observer- and practice-dependent. Despite the best effort to produce meaningful and feasible definitions, it is impossible to account for all components possibly influencing the measurement of these variables in research or clinical practice. However, the current consensus is needed to obtain comparable information from future studies, whereas a search for more robust tools, e.g., monitors for GI motility, biomarkers for absorption, etc., needs to continue. COSMOGI may be updated based on future research findings, and additional consensus processes may be needed to further refine some of these outcomes. Including consistent outcomes in all studies will ensure that both significant and nonsignificant findings are captured. Prediction tools for specific entities of gastrointestinal dysfunction such as enteral feeding intolerance may be helpful to identify study populations, where the benefit or potential harm of specific management strategies (e.g. enteral nutrition strategies such as early low dose enteral nutrition, delayed EN or prokinetics) on patient-centered outcome may be studied. So far, efforts to develop such prediction tools are limited by unclear nomenclature of GI dysfunction [20]. Comprehensive data obtained through dynamic monitoring of all aspects of GI dysfunction are needed for multiple reasons: (1) To describe different patterns of GI dysfunction, (2) To describe response of GI function to EN challenge, contributing to more precise definition of enteral feeding intolerance, (3) To identify patient groups for interventional studies targeting GI dysfunction and (4) To monitor treatment effect in interventional studies. Eventually, this should allow for data-based identification and management of GI dysfunction.
Many biomarkers have been proposed as GI dysfunction markers and added to our initial Delphi round [21]. While some are promising [22, 23], they are not widely available and have not been validated sufficiently [21]. Two of these biomarkers assessed in the iSOFA study (citrulline and intestinal fatty acid-binding protein) did not prove their potential and were not included in the final score (GIDS) [3]. Future biomarker validation should also be supported by the availability of consensus on clinical variables reflecting GI dysfunction. The panel’s informed rating has agreed with these limitations and has not identified any of the proposed biomarkers as essential.
Some items in the final COS are GI symptoms or abdominal signs, assessable as a single variable, whereas some are clinical entities combining several items, and some are interventions applied to manage GI dysfunction, including management of enteral feeding intolerance. Such an approach may appear complex; however, considering the inability of each variable alone to determine GI dysfunction, combining variables may be helpful to formulate a clinically applicable definition, such as including severity grades of GI dysfunction as a part of multiple organ dysfunction. Therefore, it was decided not to merge outcomes further, maintaining the level of granularity deemed essential by the panel throughout the process. While most of the identified outcomes are patient-centered, some, such as use of prokinetics, postpyloric feeding and parenteral nutrition reflect treatment decisions taken by health care providers. However, these treatment decisions are expected to reflect a reaction to anticipated or detected intolerance to oral and gastric feeding. Accordingly, considering the paucity of robust and observer-independent variables reflecting GI dysfunction, these may remain important components of the overall assessment of GI dysfunction. In addition to the outcomes, future reporting of inter-observer variability within subjective outcomes may help to identify the need for potential updates in the COS.
Generating a larger data pool is needed to evaluate the importance of each component of this core outcome set as a part of GI dysfunction leading to increased mortality or other unfavorable patient-centered outcomes. Unfortunately, direct validation of these items and definitions against GI function cannot be immediately planned due to the absence of a gold standard. However, in future studies, several of these items can be tested against specific measurements (e.g. instrumental monitoring of motility, biomarkers). At the same time, studies developing or validating clinical tools to measure GI dysfunction are provided with consensus definitions for symptoms and signs potentially used in these tools.This project has several limitations: Participants were not asked to express their conflicts of interest in the study, but the large number of participants should reduce any bias this may have introduced. Our drop-out rate of around 30% per Stage may have introduced further bias but was not outside the scope of other Delphi consensus processes [11]. As for any research in this area, this study is limited by the lack of a gold standard for measuring GI function and by the fact that most of the variables considered to reflect GI dysfunction are observer-dependent.
Conclusions
In this modified Delphi consensus process in a large cohort of international healthcare providers, we identified 13 essential outcomes reflecting GI dysfunction in the critically ill that are proposed to be monitored and reported in future studies. Consensus was also reached on specific definitions for each outcome, providing clear instructions on how to measure each variable and defining the appropriate study population for reporting. COSMOGI facilitates an improved and unified reporting of currently available variables describing different aspects of GI dysfunction, thereby serving as a basis for the future development of an evidence-based definition of GI dysfunction.
Supplementary Information
Author contributions
KFB and ARB were this study’s primary and senior investigators. KFB, ARB, JG, ZAP, TD drafted the study protocol. All authors have read and agreed to the study protocol before commencing the study. KFB, ARB, and BJ extracted and produced the outcomes for Stage 1. KFB, JS, AV, JCLD, MK, ARB, ZC, RM, ADM, AC, DB, BJ, CL, SB, GB, AVZ, BJ, RM, JCLD, MK, KG, SK, SK, KG, SP prepared the definitions for Stage 2. All authors reviewed and revised the definitions for Stage 2. All authors participated in the Delphi consensus process. KFB, BJ, ARB drafted the first version of the manuscript. All authors revised the manuscript and have read and approved the final version of the manuscript.
Funding
Partially funded by the University of Tartu, from the Estonian Research Council Grant PRG1255.
Availability of data and materials
Data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable, no ethical approval was needed for this study. Participants were invited and informed via participant information sheets available at https://cosmogi.site/documents.
Consent for publication
All participants were informed about the aims of this study and the intent to publish. Participants who chose to be listed personally can be found in the online supplement.
Competing interests
Varsha Asrani: No conflict of interest. Kaspar Felix Bachmann: No conflict of interest. Danielle E. Bear: Consulting fees from Baxter Healthcare, Fresenius Kabi, and Nestle Health Science. Giuliano Bolondi: No conflict of interest. Sabrina Boraso: No conflict of interest. Michael Casaer: Supported by the Research Foundation—Flanders, Belgium through a senior clinical investigator fellowship (1832822N). Zhigang Chang: No conflict of interest. Craig M. Coopersmith: Supported by the National Institutes of Health (R35GM148217). Antonella Cotoia: No conflict of interest. Thomas Davies: No conflict of interest. Angelique De Man: No conflict of interest. Gunnar Elke: No conflict of interest. Kursat Gundogan: No conflict of interest. Jan Gunst: Supported by the Research Foundation—Flanders, Belgium through a senior clinical investigator fellowship (1842724N). Bethan Jenkins: No conflict of interest. Slavica Kvolik: No conflict of interest. Marcus Laube: No conflict of interest. Matthias Lindner: No conflict of interest. Juan Carlos Lopez-Delgado: No conflict of interest. Cecilia Loudet: No conflict of interest. Ram Matsa: No conflict of interest. Emmanuel Pardo: EP received a research grant from Nestle Health Science. Simone Piva: No conflict of interest. Zudin Puthucheary: ZP has received honoraria for consultancy from Fresenius Kabi and Nutricia. Annika Reintam Blaser: ARB received speaker fee from Nutricia, consultancy fees from Fresenius Kabi and Nestle Health Science. Todd Rice: TWR has received consultancy fees from Nestle, Baxter, Abbott, and Fresenius Kabi. Sergio Ruiz-Santana: No conflict of interest. Stefan J. Schaller: SJS received grants and non-financial support from Baxter, Fresenius, and Nutricia. Joel Starkopf: No conflict of interest. Christian Stoppe: No conflict of interest. Arthur Van Zanten: ARHvZ reported receiving honoraria for advisory board membership from Baxter, Nutricia, and Fresenius Kabi, and research funding from Baxter.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reintam Blaser A, Poeze M, Malbrain ML, Bjorck M, Oudemans-van Straaten HM, Starkopf J, et al. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med. 2013;39(5):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H, Lu J, Li H, Duan A, Wang Y, Zhang D. Association between nutrition support and acute gastrointestinal injury in critically ill patients during the first 72 hours. Clin Nutr. 2021;40(1):217–21. [DOI] [PubMed] [Google Scholar]
- 3.Reintam Blaser A, Padar M, Mändul M, Elke G, Engel C, Fischer K, et al. Development of the gastrointestinal dysfunction score (GIDS) for critically ill patients—a prospective multicenter observational study (iSOFA study). Clin Nutr. 2021;40(8):4932–40. [DOI] [PubMed] [Google Scholar]
- 4.Zhang D, Li Y, Ding L, Fu Y, Dong X, Li H. Prevalence and outcome of acute gastrointestinal injury in critically ill patients: A systematic review and meta-analysis. Medicine. 2018;97(43): e12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moonen PJ, Reintam Blaser A, Starkopf J, Oudemans-van Straaten HM, Van der Mullen J, Vermeulen G, et al. The black box revelation: monitoring gastrointestinal function. Anaesthesiol Intensive Ther. 2018;50(1):72–81. [DOI] [PubMed] [Google Scholar]
- 6.Reintam Blaser A, Bachmann KF, Deane AM. Gastrointestinal function in critically ill patients. Curr Opin Clin Nutr Metab Care. 2023;26(5):463–9. [DOI] [PubMed] [Google Scholar]
- 7.Asrani VM, Brown A, Huang W, Bissett I, Windsor JA. Gastrointestinal dysfunction in critical illness: a review of scoring tools. J Parenter Enter Nutr. 2020;44(2):182–96. [DOI] [PubMed] [Google Scholar]
- 8.Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM working group on abdominal problems. Intensive Care Med. 2012;38(3):384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reintam Blaser A, Preiser JC, Fruhwald S, Wilmer A, Wernerman J, Benstoem C, et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and nutrition of the European society of intensive care medicine. Crit Care. 2020;24(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asrani VM, Brown A, Huang W, Bissett I, Windsor JA. Gastrointestinal dysfunction in critical illness: a review of scoring tools. JPEN J Parenter Enteral Nutr. 2020;44(2):182–96. [DOI] [PubMed] [Google Scholar]
- 11.Davies TW, van Gassel RJJ, van de Poll M, Gunst J, Casaer MP, Christopher KB, et al. Core outcome measures for clinical effectiveness trials of nutritional and metabolic interventions in critical illness: an international modified Delphi consensus study evaluation (CONCISE). Crit Care. 2022;26(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorst SL, Gargon E, Clarke M, Blazeby JM, Altman DG, Williamson PR. Choosing important health outcomes for comparative effectiveness research: an updated review and user survey. PLoS ONE. 2016;11(1): e0146444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8(1): e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, Tunis S, et al. Core outcome set-standards for development: the COS-STAD recommendations. PLoS Med. 2017;14(11): e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET handbook: version 1.0. Trials. 2017;18(Suppl 3):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkham JJ, Gorst S, Altman DG, Blazeby JM, Clarke M, Devane D, et al. Core outcome set-standards for reporting: the COS-STAR statement. PLoS Med. 2016;13(10): e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachmann KF. COSMOGI Information Sheets 2024 [Available from: https://cosmogi.site/information-sheets/
- 19.Zhang D, Fu R, Li Y, Li H, Li Y, Li H. Comparison of the clinical characteristics and prognosis of primary versus secondary acute gastrointestinal injury in critically ill patients. J Intensive Care. 2017;5(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Li Y, Wang H, Li H, Li Y, Zhang L, et al. Development and validation of a nomogram for predicting enteral feeding intolerance in critically ill patients (NOFI): mixed retrospective and prospective cohort study. Clin Nutr. 2023;42(12):2293–301. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins B, Calder PC, Marino LV. A scoping review considering potential biomarkers or functional measures of gastrointestinal dysfunction and enteral feeding intolerance in critically ill adults. Clin Nutr ESPEN. 2022;52:331–9. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Chen Y, Huo F, Wang Y, Zhang D. Association between acute gastrointestinal injury and biomarkers of intestinal barrier function in critically ill patients. BMC Gastroenterol. 2017;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reintam Blaser A, Padar M, Tang J, Dutton J, Forbes A. Citrulline and intestinal fatty acid-binding protein as biomarkers for gastrointestinal dysfunction in the critically ill. Anaesthesiol Intensive Ther. 2019;51(3):230–9. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.