Abstract
Background
Heart failure often leads to hospitalization and can directly impact other organs, such as the kidneys. Acute kidney injury (AKI) is a common complication in patients hospitalized for acute decompensated heart failure (ADHF) and is associated with worse outcomes. However, there are limited data on the magnitude of AKI among hospitalized ADHF patients in resource-limited settings such as Ethiopia. This study sought to determine the prevalence of AKI and the factors associated with AKI in ADHF patients in Northwest Ethiopia.
Method
A hospital-based cross-sectional study was conducted at the University of Gondar Hospital in Northwest Ethiopia from June 1 to September 30, 2022. A total of 239 participants were included using consecutive sampling. Demographic information was collected through patient interviews, and relevant clinical and laboratory data were obtained from the patients’ medical records. The data were analyzed using STATA version 15.0. Bivariate and multivariate logistic regression analyses were carried out to identify independently associated factors of AKI among patients with ADHF. A P value < 0.05 was considered to indicate statistical significance.
Results
The overall prevalence of AKI in ADHF patients was 25.1% (CI = 19.98–31.03). Older age ≥ 60 years(AOR = 2.95, 95%CI:1.34–6.21), diabetes mellitus (AOR = 9.55,95%CI:2.68–33.99),Hypertension (AOR = 2.34,95% CI:1.08–5.07), sepsis (AOR = 2.13,95%CI:1.09–4.8), use of loop diuretics (AOR = 4.03,95%CI:1.86–8.69) and previous history of AKI (AOR = 11.56,95%CI:4.02–33.26) were independently associated with the occurrence of AKI among ADHF patients.
Conclusion
A quarter of the patients admitted with ADHF developed AKI. Older age; comorbid diabetes mellitus, hypertension, or sepsis; a previous history of AKI; and the use of loop diuretics were associated with the occurrence of AKI. Such clinical characteristics available at hospital admission can be used to identify patients at increased risk for developing AKI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03914-2.
Keywords: Heart failure, acute kidney injury, prevalence, associated factor, Ethiopia
Background
Heart failure (HF) is a clinical syndrome characterized by typical symptoms such as breathlessness, ankle swelling and fatigue that may be accompanied by signs such as elevated jugular venous pressure, pulmonary crackles and peripheral edema. These symptoms are caused by a structural and/or functional cardiac abnormality, resulting in reduced cardiac output and/or elevated intracardiac pressure at rest or during stress [1]. Acute decompensated heart failure (ADHF) refers to previously diagnosed or new-onset HF presenting with rapid worsening of signs and symptoms of HF [2].
Heart failure is a global pandemic affecting 26 million people worldwide [3]. The prevalence of HF in developed countries is estimated to be 1–2% of the adult population [1]. In sub-Saharan Africa, there is a lack of population-based studies on the incidence and prevalence of HF. However, cardiovascular diseases account for 7–10% of all medical admissions, 3–7% of which are attributed to HF [3–5]. Most patients with HF in the sub-Saharan region are younger, productive part of the population, fail to receive proper treatment and tend to have New York Heart Association (NYHA) class IV HF [4]. The leading causes of HF in this region are hypertensive heart disease (HHD), dilated cardiomyopathy (DCMP) and rheumatic valvular heart disease (RVHD) [3–7].
In Ethiopia, hospital-based studies show that heart failure (HF) accounts for 16% of medical admissions and 23.9% of cardiovascular follow-up visits, primarily affecting middle-aged adults (48.3 to 53.6 years). A meta-analysis identified valvular heart disease (VHD) as the leading cause of HF (29.2–81%), followed by hypertensive heart disease and cardiomyopathy [8–10].
Heart failure has direct consequences on other organs, particularly the kidneys. The heart and kidneys interact in a complex, interdependent manner in both acute and chronic conditions, potentially leading to dual-organ dysfunction [11]. Acute kidney injury (AKI), which is defined as an abrupt decrease in the glomerular filtration rate (GFR) sufficient to eliminate nitrogenous waste products and other uremic toxins, is a common complication in patients admitted for ADHF [12].
The prevalence of AKI in admitted heart failure patients varies due to the different definitions used to define AKI. A systematic review and meta-analysis of studies primarily from developed countries (the U.S., Canada, Europe, and Israel) found that the prevalence of AKI in hospitalized patients with ADFF was 47.4% [13]. According to the Acute Decompensated Heart Failure National Registry of America, approximately 30% of patients admitted to the hospital for acute decompensated heart failure (ADHF) have acute or chronic renal insufficiency [14].
There is a paucity of data in Africa regarding the true magnitude of AKI and its associated factors among patients with HF. In the Sub-Saharan Africa Survey of Heart Failure (THESUS–HF), a multicenter observational study of patients with acute heart failure (AHF) across 9 sub-Saharan countries, including Ethiopia, the prevalence of renal dysfunction (eGFR < 30) was reported as 7.7%. However, the study was not specifically designed to investigate acute kidney injury (AKI), and only 10 patients from Ethiopia were included due to the late involvement of the country in the study [6]An institution-based cross-sectional study from Somalia found the prevalence of AKI in ADHF patients to be 8.3% [15]. Another hospital-based study performed at Tikur Anbesa Hospital in Ethiopia showed elevated serum Cr (Cr > 1.2 mg/dl) in 40% of patients admitted for ADHF [16].
Prior studies have identified various risk factors that could influence the occurrence of AKI in hospitalized ADHF patients. Comorbidities such as diabetes mellitus, hypertension, and dyslipidemia are among the most common risk factors for the development of AKI in patients with ADHF [17–20]. Additionally, increased baseline serum creatinine (SCr), a history of AKI, older age, hemodynamic abnormalities at admission, and sepsis are strong predictors of AKI [18, 21, 22].
Echocardiographic and electrocardiographic features, such as the presence of diastolic dysfunction and atrial fibrillation (AF), have also been reported to predict the occurrence of AKI [23, 24]. Acute kidney injury in patients with ADHF is linked to poorer short- and long-term outcomes, including higher mortality, hospitalization, and readmission rates and worsening of heart failure progression [11, 18, 25, 26]. A meta-analysis has shown that the presence of AKI in hospitalized ADHF patients is associated with a 5.14-fold increase in mortality [13]. Ethiopia is the second most populous country in Africa, with a population of more than 120 million people. The literature, mostly from developed nations, has indicated that AKI complicates a significant proportion of patients admitted for ADHF, with varying prevalence results [13, 22, 23, 26]. However, data on the magnitude of AKI in low-resource settings such as Ethiopia are lacking. The profile of AKI may differ from that of more developed countries. Studies on renal dysfunction in Western countries have involved mainly elderly patients with ischemic heart disease. However, the prevalence and predictors of AKI in younger, mainly hypertensive and rheumatic acute heart failure patients from Africa have not been well described. The aim of this study was to assess the magnitude and factors associated with AKI in patients with ADHF admitted to the University of Gondar Hospital (UoGH), Ethiopia.
Methods
Study area and setting
This study was conducted at the University of Gondar Hospital (UoGH) in Ethiopia. The hospital is located in Gondar town in the Amhara region, 748 km from the capital city, Addis Ababa. UoGH is a tertiary-level teaching and referral hospital that serves as the referral center for more than 12 district hospitals in the area, catering to a total catchment of 7 million people. The hospital has more than 800 beds and offers health services to patients with various diseases in the outpatient and inpatient departments. Basic diagnostic tests and treatment options for HF and AKI patients are available at the hospital. Imaging studies such as echocardiography, ECG, and CXR and serum biochemical tests such as kidney function tests and electrolytes are routinely performed. Hemodialysis services are available for patients with AKI and kidney failure. The hospital has no infrastructure for interventional cardiology or cardiac surgery.
Study design and population
This was a hospital-based cross-sectional study conducted from June 1st to September 30th, 2022. The source population included all patients with acute decompensated heart failure (ADHF) admitted to the UoGH medical ward and medical ICU. The study population included all ADHF patients admitted to the hospital during the specified period. All adult ADHF patients (aged > 18 years) admitted to UoGH were included, while patients with a previous history of known chronic kidney disease (CKD) were excluded.
Sample size and sampling procedures
The sample size was calculated using the single population proportion formula with the following assumptions: a confidence level of 95%, a 5% margin of error, and a prevalence of 47.4 from a previous meta-analysis report. Since the total population size was finite and less than 1000, a correction was applied.
With these assumptions and considering a 10% nonresponse rate, the sample size was calculated to be 257. All patients who fulfilled the prespecified criteria were recruited using consecutive sampling techniques at the time of discharge until the sample size was met.
Study variables and operational definitions
The dependent variable was the presence of AKI (yes/no). The independent variables included sociodemographic factors such as age and sex; clinical characteristics such as duration of HF, etiology of HF, previous history of AKI, and NYHA class; and medication such as diuretics, beta blockers, RAS inhibitors, and nephrotoxic medication. Comorbidities such as hypertension and diabetes; sepsis; dyslipidemia; duration of hospital stay; etiology of AKI; and laboratory results, including electrolytes and hemoglobin. Echocardiographic findings included ejection fraction, diastolic dysfunction, and pulmonary artery hypertension.
ADHF was defined as a patient with previously diagnosed or new-onset heart failure who showed rapid worsening of signs and symptoms of heart failure [1] and whose primary admission diagnosis was ADHF. The definition and staging of AKI were performed in accordance with the Kidney Disease Initiative Global Outcome (KDIGO) guidelines [27]. AKI was defined as an increase in serum creatinine (SCr) of ≥ 0.3 mg/dl within 48 h after admission or an increase in SCr to ≥ 1.5-fold from baseline, which is known or presumed to have occurred within the prior 7 days. AKI was graded as stage 1 (SCr 1.5–1.9 times the baseline or ≥ 0.3 mg/dl increase), stage 2 (SCr 2.0–2.9 times the baseline) or stage 3 (SCr 3.0 times the baseline or SCr ≥ 4.0 mg/dl or need for dialysis). The likely cause of AKI was determined based on the clinical judgment of the treating physician. Heart failure was further classified as heart failure with reduced ejection fraction (HFrEF) (if ejection fraction < 40%), heart failure with mildly reduced ejection fraction (if ejection fraction 40 − 50%), or heart failure with preserved ejection fraction (HFpEF) (if ejection fraction > 50%) [28].
Pulmonary artery hypertension was considered present if the pulmonary artery pressure measured by echocardiography was > 30 mmHg.
Data collection, tools and analysis
The data were collected using an investigator-administered questionnaire. The questionnaire was tested for accuracy in 12 heart failure patients. The data collectors were three trained general practitioners. Before the actual data collection, data collectors received one day training on the content of data extraction, from and how to collect the data. The principal investigator supervised every activity of the data collection process. Completeness of the data was checked on each day of data collection by the principal investigator. Patients were interviewed to gather demographic information. Clinical parameters such as diagnosis, comorbidities, treatments, laboratory results, and echocardiographic reports were collected from individual patient records. The data were entered into EPI data version 4.6 and then transferred to STATA version 15.0 for analysis. Data cleaning was conducted before performing the descriptive analysis. The baseline characteristics are presented as numbers and percentages. The findings are summarized in tables and figures. Binary regression was employed to assess the difference between independent variables among patients with or without AKI. Both bivariate and multivariate logistic regression analyses were used to identify independently associated factors of AKI in ADHF patients. Variables with a P value < 0.2 in the bivariate analysis were included in the multivariate analysis to control for the potential effect of confounders. The adjusted odds ratio (AOR) with 95% CI was used to describe associations, with P < .05 considered to indicate statistical significance.
Results
Sociodemographic characteristics
A total of 239 patients participated in the study out of 262 patients approached, resulting in a 91.2% response rate. The mean age was 53.1 ± 18.2 years, with the majority being females, accounting for 161 (59%) of the respondents. More than two-thirds, 165 (69.04%), lived in rural areas, and 179 (74.9%) were unable to read and write. (See Table 1).
Table 1.
Sociodemographic characteristics of patients with ADHF admitted to UOG Hospital, Gondar, Ethiopia; N = 239
| Socio -demographic characteristics | Category | Frequency | |
|---|---|---|---|
| n | % | ||
| Sex | Male | 98 | 41 |
| Female | 141 | 59 | |
| Age (years) | < 60 | 133 | 55.65 |
| ≥ 60 | 106 | 44. 35 | |
| Marital status | Single | 23 | 9.62 |
| Married | 179 | 74.9 | |
| Divorced | 10 | 4.18 | |
| Widowed | 27 | 11.3 | |
| Educational status | Unable to read and write | 175 | 73.22 |
| Able to read and write | 16 | 6.69 | |
| Primary school | 23 | 9.62 | |
| Secondary school | 14 | 5.86 | |
| College and above | 11 | 4.6 | |
| Occupation | Farmer | 72 | 30.13 |
| Housewife | 113 | 47.28 | |
| Merchant | 9 | 3.77 | |
| Student | 15 | 6.28 | |
| Government employee | 12 | 5.02 | |
| Others | 18 | 7.53 | |
| Residence | Urban | 74 | 30.96 |
| Rural | 165 | 69.04 | |
ADHF: acute decompensated heart failure; UoG: University of Gondar
Prevalence of AKI and clinical characteristics of participants with and without AKI
Among the study participants, 60 (25.1%) (CI: 19.98–31.03) patients developed AKI; 47 were diagnosed at the time of admission, and 13 developed AKI after admission. Of those who developed AKI, 66.6% were older than 60 years. The mean age of patients who developed AKI was greater than that of patients who did not (61.4 ± 16.4 vs. 50.3 ± 18.0 years). Among 27 patients with a previous history of AKI, 70.37% developed AKI during current admission. The majority of the study participants were newly diagnosed HF patients, 79.9% of whom were NYHA class IV at presentation. The major etiology of HF was IHD, followed by RVHD and corpulmonale. Patients with AKI had longer hospital stays than did those without AKI (10.6 ± 8.6 vs. 7.9 ± 6.1 days) (Table 2).
Table 2.
Clinical characteristics of patients with ADHF admitted to UOG Hospital, Gondar, Ethiopia
| Variable | Category | Acute kidney injury | |
|---|---|---|---|
| Yes | No | ||
| Frequency, n (%) | Frequency, n (%) | ||
| Age, Mean(± SD) in years | 61.4 ± 16.4 | 50.3 ± 18.0 | |
| Sex | Male | 24, (24.49) | 74, (75.51) |
| Female | 26, (25.53) | 105,(74.47) | |
| History of AKI | Yes | 19, (70.37) | 8, (29.63) |
| No | 41 ,(19.34) | 171, (80.66) | |
| Duration of HF (months) | New | 32, (23.88) | 102, (76.12) |
| < 12 | 9, (29.03) | 22, (70.97) | |
| > 12 | 19, (25.68) | 55,(74.32) | |
| Etiology of HF | IHD | 22, (30.14) | 51,(69.86) |
| DCMP | 5, (18.52) | 22, (81.48) | |
| RVHD | 5, (11.90) | 37,(88.10) | |
| HHD | 12, (52.17) | 11, (47.83) | |
| DVHD | 7, (30.43) | 16, (69.57) | |
| Corpulmonale | 7, (17.07) | 34, (82.93) | |
| Others | 2, (20.00) | 8, (80.00) | |
| NYHA class | III | 9, (18.00) | 41,(82.00) |
| IV | 51, (26.98) | 138,(73.02) | |
| Place of admission | Ward | 57, (24.26) | 178, (75.74) |
| MICU | 3, (75.00) | 1, (25.00) | |
| Length of hospital stay(days) | < 7 | 23, (20.00) | 92,(80.00) |
| ≥ 7 | 179, (70.16) | 60,(29.84) | |
AKI: acute kidney injury; NYHA: New York Heart Association; HF: heart failure; IHD: ischemic heart disease; DCMP: dilated cardiomyopathy; RVHD: rheumatic valvular heart disease; HHD: hypertensive heart disease; DVHD: degenerative heart disease; MICU: medical intensive care unit; UoG: University of Gondar
Comorbidities among participants with ADHF
Nearly half of the participants in the study (n = 119, 49.79%) had preexisting chronic comorbidities, with hypertension, diabetes mellitus, and atrial fibrillation being the most commonly reported comorbidities. A significant number of patients also experienced sepsis during their current admission (Table 3).
Table 3.
Proportion of comorbidities among patients with and without AKI. N = 119
| Comorbidities | AKI | |
|---|---|---|
| Yes | No | |
| Frequency, n (%) | Frequency, n (%) | |
| Hypertension | 28(40.58) | 41(59.42) |
| Diabetes Mellitus | 13(72.22) | 5(27.78) |
| Dyslipidemia | 4(100) | 0(0) |
| Atrial Fibrillations | 10(19.23) | 42(80.77) |
| Sepsis | 37(31.9) | 79(68.1) |
| Others | 17(16.67) | 45(72.58) |
AKI: acute kidney injury
Stages and etiologies of AKI
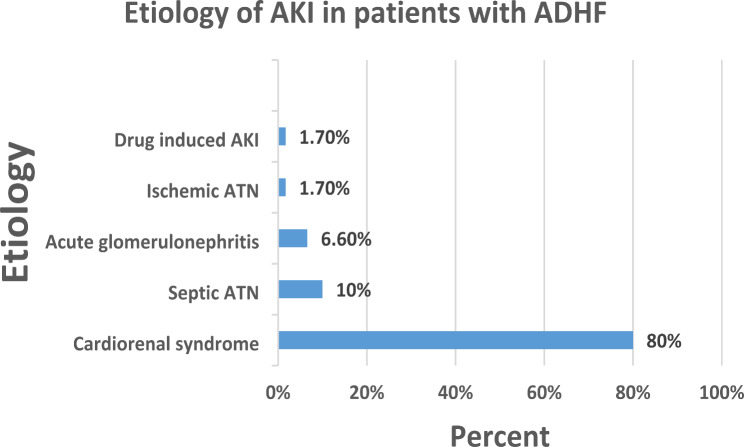
Based on the KDIGO 2012 criteria, 72% of patients developed stage I AKI (Fig. 1), and the etiology of AKI was found to be cardiorenal syndrome in 80% of patients (Fig. 2).
Fig. 1.
The stages of AKI among patients with ADHF who developed AKI. N = 60
AKI: acute kidney injury; ADHF: acute decompensated heart failure
Fig. 2.
Etiologies of AKI in ADHF patients admitted to UOG hospital. N = 60. ATN: acute tubular necrosis, AKI: acute kidney injury, ADHF: acute decompensated heart failure
Laboratory and echocardiography characteristics of ADHF patients with and without AKI
Serum electrolytes, including sodium and potassium, were measured in 118 patients in the present study. Hyponatraemia was found in 20 patients (16.9%), and hypokalemia was found in 25 patients (21.2%). Anemia was present in 70 patients (29.35%). The ejection fraction decreased in 88 patients (36.8%), and 124 patients (51.9%) had pulmonary arterial hypertension.(See Table 4).
Table 4.
Laboratory and echocardiography findings of patients with ADHF admitted to UOG Hospital, Gondar, Ethiopia
| Variables | Category | AKI | |
|---|---|---|---|
| Yes, n (%) | No, n (%) | ||
| Sodium | < 135 | 7(35) | 13(65) |
| > 135 | 29(29.59) | 69(70.41) | |
| Potassium | Hypokalemia | 10(40) | 15(60) |
| Normal | 25(27.78) | 65(72.22) | |
| Hyperkalemia | 2(66.67) | 1(33.67) | |
| Hemoglobin | Anemia | 21(30) | 49(70) |
| Normal | 39(23.08) | 130(76.92) | |
| Ejection fraction | Reduced | 21(23.86) | 67(76.14) |
| Mid-range | 3(15.79) | 16(84.21) | |
| Preserved | 36(27.27) | 96(72.73) | |
| Pulmonary arterial hypertension | Yes | 24(19.39) | 100(80.65) |
| No | 36(31.3) | 79(68.7) | |
AKI: acute kidney injury; ADHF: acute decompensated heart failure; UoG: University of Gondar
Factors associated with the occurrence of acute kidney injury
According to our bivariate analysis, the development of AKI was associated with variables such as hypertension, diabetes mellitus, previous history of AKI, older age (> 60 years), sepsis at admission, NYHA class IV disease, and receiving loop diuretics immediately before admission. Multivariate analysis revealed that participants with diabetes and hypertension were at increased risk of AKI, with adjusted odds ratios (AORs) of 9.55 (95% CI = 2.68–33.99) and 2.34 (95% CI = 1.08–5.07), respectively. Older patients aged ≥ 60 years were also at a significantly greater risk of developing AKI than were those younger than 60 years, with an AOR of 2.95 (95% CI: 1.34–6.21). Those with a previous history of AKI also had an increased risk of developing AKI, with an AOR of 11.56 (95% CI: 4.02–33.26). Participants who had been taking loop diuretics before admission were also found to be at greater risk of AKI than were those who were not taking loop diuretics, with an AOR of 4.03 (95% CI: 1.86–8.69) (Table 5).
Table 5.
Bivariate and multivariate logistic regression analysis of patients with ADHF admitted to UOG Hospital, Gondar, Ethiopia
| Variables | Category | AKI | COR | AOR | |
|---|---|---|---|---|---|
| Yes, N (%) | No, N (%) | ||||
| Age | < 60 | 20(15.04) | 113(84.96) | 1 | 1 |
| >=60 | 40(37.74) | 66(62.26) | 3.42,(1.85–6.34)*** | 2.95(1.34–6.21)*** | |
| History of AKI | No | 41(19.34) | 171(80.66) | 1 | 1 |
| Yes | 19{70.37) | 8(29.6) | 9.9 (4.05–24.21)*** | 11.56(4.02–33.26)*** | |
| NYHA class | Class III | 9(18) | 41(82%) | 1 | 1 |
| Class IV | 51(26.98) | 138(73.02) | 1.68 (0.76–3.71)* | 1.32 (0.49–3.5) | |
| Loop diuretics | No | 28(18.06) | 127(81.94) | 1 | 1 |
| Yes | 32(38.10) | 52(61.90) | 2.79 (1.53–5.09)*** | 4.03(1.86–8.69)*** | |
| Hypertension | No | 32(18.82) | 138(81.18) | 1 | 1 |
| Yes | 28(40.58) | 41(59.42) | 2.94 (1.59–5.45)*** | 2.34(1.08–5.07)** | |
| Diabetes mellitus | No | 47(21.27) | 174(78.73) | 1 | 1 |
| Yes | 13(72.22) | 5(27.78) | 9.62 (3.27–28.36)*** | 9.55(2.68–33.99)*** | |
| Sepsis | No | 23(18.70) | 100(81.30) | 1 | 1 |
| Yes | 37(31.9) | 79(68.10) | 2.04(1.12–3.70)** | 2.13(1.09–4.8)** | |
*** p < .01, ** p < .05, * p < .1
P = .254, for Hosmer–Lemeshow goodness of fit test
AKI: acute kidney injury; NYHA: New York Heart Association; COR: crude odds ratio; AOR: adjusted odds ratio; UoG: University of Gondar
Discussion
This study in Ethiopia is one of the first to assess the prevalence of AKI in ADHF patients. A total of 239 patients were recruited, 60 of whom developed AKI, resulting in a prevalence of 25.1%. This finding is comparable to the reported prevalence from a meta-analysis of 28 studies that reported a 23% prevalence [18] and a study from Winthrop University Hospital, New York (21%) [24]. The prevalence in our study was lower than that in reports from eight European countries (25%) [23] and a study from the Netherlands (30%) [22]. Another meta-analysis of 6 studies reported a prevalence of 47.4% [13]. The prevalence of AKI in the present study was greater than that in a study from the USA, in which the prevalence of AKI was 17.8% [29]. The sub-Saharan Africa Survey of Heart Failure (THESUS-HF), which included 1006 patients, reported that 30.6% had an estimated glomerular filtration rate < 60 ml/min/1.73 m2 [30]. To the best of our knowledge, no studies have specifically examined the prevalence and associated factors of AKI in ADHF patients in Ethiopia. However, a study conducted to assess the outcomes of HF patients admitted to Tikur Anbessa Specialized Hospital revealed that 40% of patients had increased Cr (> 1.2 mg/dl) [16]. The variation in prevalence is likely due to the use of different criteria and cutoff points for defining AKI. Different studies have used different serum creatinine increases ranging from 0.2 to 0.5 mg/dl, as well as the AKIN, RIFLE, and KDIGO AKI definitions. Additionally, differences in the demographics of the studied population, healthcare facility settings, and underlying causes of heart failure across centers may contribute to the varying rates of AKI.
The majority (72%) of patients had stage 1 AKI, with cardiorenal syndrome (CRS) being the most common cause, followed by septic ATN. These findings are in line with those of previous studies [13]. Bidirectional acute and chronic disorders of heart and kidney are today classified as cardiorenal syndromes (CRS) types 1–5. Type 1 CRS is characterized by a rapid worsening of cardiac function leading to AKI and most frequently appears in the setting of ADHF [31]. The development and progression of CRS are driven by complex hemodynamic, neurohumoral, inflammatory, and oxidative mechanisms. Reduced cardiac output and arterial filling pressure, along with elevated central venous pressure from systemic venous congestion and decreased renal perfusion, activate maladaptive neurohumoral mechanisms such as the renin-angiotensin-aldosterone system, the sympathetic nervous system, and antidiuretic hormone secretion [32].
Age was found to be independently associated with the occurrence of AKI. Participants aged > 60 years were found to have a 3-fold greater risk of AKI than were those aged < 60 years.
Older age is a known risk factor for the occurrence of AKI, and these findings are supported by similar previous studies [18, 22].
Other factors associated with the occurrence of AKI in this study included underlying comorbidities such as hypertension and diabetes mellitus. Among participants who developed AKI, 27.6% were diabetic and 46.6% were hypertensive. In this study, diabetes and hypertension increased the odds of AKI by 9 and 2X, respectively. This finding is consistent with the findings of previous studies from the US [24, 33], and a meta-analysis by Damman et al. revealed the same finding [18]. Taken together, these findings suggest that noncommunicable diseases (NCDs), such as diabetes and hypertension, are becoming common risk factors for developing AKI in low-income countries, heralding the epidemiologic shift toward NCDs. Sepsis was also found to be a significant risk factor for the development of AKI, and this finding was supported by similar previous studies [21].
Participants who were receiving diuretic treatment before admission were found to have a 4-fold increase in the prevalence of AKI. This was supported by previous meta-analysis reports [18]. Our study also revealed that participants with a previous history of AKI were 11 times more likely to develop AKI during their current admission than were their counterparts. There is evidence of an association between AKI and adverse long-term outcomes, such as recurrent AKI episodes [34].
Previous studies have indicated that systolic dysfunction, anemia and hyponatraemia can increase the occurrence of AKI [24, 35]. Nevertheless, in our current study, we did not observe a connection between these factors and AKI. A considerable number of patients had incomplete echocardiography and electrolyte measurement data, which could have impacted the findings.
Due to the high prevalence of AKI in patients with ADHF, interventions to prevent the development of acute decompensated heart failure should focus on addressing precipitating factors, ensuring medication adherence, and treating underlying heart disease. Policymakers should allocate resources to support the diagnosis and treatment of AKI, including providing hemodialysis in hospitals treating patients with ADHF.
Limitations
Incomplete echocardiography and laboratory reports hinder the assessment of certain variables, such as diastolic dysfunction, pulmonary artery hypertension, and hyponatremia. Ischemic heart disease followed by idiopathic dilated cardiomyopathy were found to be leading causes of HF. This could be due to epidemiologic transition of diseases or misclassification. Diagnostic tests such as angiography and stress ECG tests are not available in the hospital and use of transthoracic echocardiography alone may result in misclassification of some patients either way. The etiology of AKI was determined by the treating physician based on clinical parameters, which may be inaccurate for some patients. Certain etiologies of AKI need kidney biopsy for confirmation and lack of availability of routine kidney biopsy in the study setting may lead to misclassification of causes of AKI. Information on history of previous AKI was obtained from patients’ medical records, but this may be underestimated due to incomplete documentation and the inclusion of patients referred from other hospitals. The impact of AKI on patient outcomes, such as hospital stay, readmission, and mortality, has not been evaluated.
Conclusion
AKI is a common complication among study participants admitted with ADHF and is reported in a quarter of the patients. Older age; comorbidities such as diabetes mellitus and hypertension; sepsis; a previous history of AKI; and the use of loop diuretics were independently associated with AKI occurrence. These clinical characteristics, available at hospital admission, can help identify patients at increased risk for developing AKI. Routine and frequent screening for AKI in ADHF patients, particularly those who are older, have comorbidities, sepsis, or are on loop diuretics, may lead to improved patient outcomes. However, further studies are needed to assess the outcomes of patients with AKI who are admitted for ADHF.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the College of Medicine and Health Sciences, University of Gondar, for providing financial assistance for the study. We also acknowledge the study participants, the data collectors and the health personnel involved in patient care.
Abbreviations
- ADHF
Acute decompensated heart failure
- AF
Atrial fibrillation
- AKIN
Acute kidney injury network
- AKI
Acute kidney injury
- AOR
Adjusted odds ratio
- BUN
Blood urea nitrogen
- HF
Congestive heart failure
- CKD
Chronic kidney disease
- Cr
Creatinine
- DCMP
Dilated cardiomyopathy
- DVHD
Degenerative valvular heart disease
- ECG
Electrocardiography
- EF
Ejection fraction
- eGFR
Estimated glomerular filtration rate
- HFrEF
Heart failure with reduced ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- IHD
Ischemic heart disease
- KDIGO
Kidney disease improving global outcomes
- MICU
Medical intensive care unit
- NYHA
New York Heart Association
- COR
Crude odds ratio
- AOR
Adjusted odds ratio
- RHD
Rheumatic heart disease
- RIFLE
Risk, Injury, Failure, loss, Endstage
- UoGH
University of Gondar Hospital
Author contributions
YB contributed to the conception, design, data collection, analysis, initial manuscript writing, and review of the manuscript. WH contributed to the conception, design, analysis, writing and review of the manuscript. SA, MA, and HS contributed to the conception, design, analysis and review of the manuscript. All the authors read and approved the final manuscript and approved its submission for publication.
Funding
Funding for this research was obtained from the ‘Research and Publication Office’ of the College of Medicine and Health Sciences, University of Gondar. The funding body had no role in the design of the study, data collection, or analysis and interpretation of the data.
Data availability
All the data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request (email: workhailu@yahoo.com).
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the University of Gondar, School of Medicine Ethics Committee (Ref No.71207/2021 Date: 04/07/2021). Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–m2200. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, et al. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur J Heart Fail. 2008;10(10):933–89. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. [DOI] [PubMed]
- 3.Savarese G, Lund LH. Global Public Health Burd Heart Fail Eoidemiology. 2017;7–11. [DOI] [PMC free article] [PubMed]
- 4.Ntusi NB, Mayosi BM. Epidemiology of Heart Failure in sub-Saharan Africa. Expert Rev Cardiovasc Ther. 32457;7(2):169–80. [DOI] [PubMed]
- 5.Damasceno A, Cotter G, Dzudie A, Sliwa K, Mayosi BM. Heart Failure in Sub-Saharan Africa: Time for Action. J Am Coll Cardiol. 2007;50(17):1688–93. [DOI] [PubMed] [Google Scholar]
- 6.Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries: Results of the sub-Saharan Africa survey of heart failure. Arch Intern Med. 2012;172(18):1386–94. [DOI] [PubMed] [Google Scholar]
- 7.Dokainish H, Teo K, Zhu J, Roy A, Alhabib KF, Elsayed A et al. Heart Failure in Africa, Asia, the Middle East and South America: The INTER-CHF study. Int J Cardiol [Internet]. 2016;204:133–41. 10.1016/j.ijcard.2015.11.183 [DOI] [PubMed]
- 8.Tsega TA, Demissei BG. A systematic review of epidemiology, treatment and prognosis of heart failure in adults in Ethiopia. J Cardiovasc Med. 2018;19(3):91–7. [DOI] [PubMed] [Google Scholar]
- 9.Bane A, Bayisa T, Adamu F, Abdissa SG. Medical Admissions and Outcomes at Saint Paul’s Hospital, Addis Ababa, Ethiopia: A retrospective study. Ethiop J Heal Dev. 2016;30(1):50–6. [Google Scholar]
- 10.Tefera Y, Abebe T, Abegaz T, Ab M. The Changing Trend of Cardiovascular Disease And Its Clinical Characteristics In Ethiopia:Hospital-Based Observational Study. Vasc Health Risk Manag. 2017;20(9):A602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, Von Haehling S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat Rev Nephrol [Internet]. 2016;12(10):610–23. 10.1038/nrneph.2016.113 [DOI] [PubMed]
- 12.Jurgen Floege RJ, Johnson JF. Comprehensive Clinical Nephrology. Fourth edi. Saunders Elsevier; 2010. p. 797.
- 13.Vandenberghe W, Gevaert S, Kellum JA, Bagshaw SM, Peperstraete H, Herck I, et al. Acute kidney injury in cardiorenal syndrome type 1 patients: A systematic review and meta-analysis. CardioRenal Med. 2015;6(2):116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams KF, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–16. [DOI] [PubMed] [Google Scholar]
- 15.Jeele MOO, Hussein AA, Mohamud MA, Adani AA, Mohamud MFY. Spectrum and prevalence of renal dysfunction among heart failure patients attending tertiary care hospital: first report from Somalia. Egypt J Intern Med [Internet]. 2023;35(1). 10.1186/s43162-023-00253-w
- 16.Tirfe M, Nedi T, Mekonnen D, Berha AB. Treatment outcome and its predictors among patients of acute heart failure at a tertiary care hospital in Ethiopia: A prospective observational study. BMC Cardiovasc Disord. 2020;20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS et al. Incidence, Predictors at Admission, and Impact of Worsening Renal Function among Patients Hospitalized with Heart Failure. J Am Coll Cardiol [Internet]. 2004;43(1):61–7. 10.1016/j.jacc.2003.07.031 [DOI] [PubMed]
- 18.Damman K, Valente MAE, Voors AA, O’Connor CM, Van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur Heart J. 2014;35(7):455–69. [DOI] [PubMed] [Google Scholar]
- 19.Gudsoorkar PS, Thakar CV. Acute Kidney Injury, Heart Failure, and Health Outcomes. Cardiol Clin [Internet]. 2019;37(3):297–305. 10.1016/j.ccl.2019.04.005 [DOI] [PubMed]
- 20.Ronco C, Bellasi A, Di Lullo L. Implication of Acute Kidney Injury in Heart Failure. Heart Fail Clin [Internet]. 2019;15(4):463–76. 10.1016/j.hfc.2019.05.002 [DOI] [PubMed]
- 21.Correa A, Patel A, Chauhan K, Shah H, Saha A, Dave M et al. National Trends and Outcomes in Dialysis-Requiring Acute Kidney Injury in Heart Failure: 2002–2013. J Card Fail [Internet]. 2018;24(7):442–50. 10.1016/j.cardfail.2018.05.001 [DOI] [PubMed]
- 22.Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G et al. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre-RELAX-AHF. Eur J Hear Fail. 2011;961–7. [DOI] [PubMed]
- 23.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: Results of the prospective outcomes study in heart failure (POSH). Eur Heart J. 2006;27(10):1216–22. [DOI] [PubMed] [Google Scholar]
- 24.Chittineni H, Miyawaki N, Gulipelli S, Fishbane S. Risk for acute renal failure in patients hospitalized for decompensated congestive heart failure. Am J Nephrol. 2007;27(1):55–62. [DOI] [PubMed] [Google Scholar]
- 25.Berra G, Garin N, Stirnemann J, Jannot AS, Martin PY, Perrier A, et al. Outcome in acute heart failure: Prognostic value of acute kidney injury and worsening renal function. J Card Fail. 2015;21(5):382–90. [DOI] [PubMed] [Google Scholar]
- 26.Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, et al. Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J. 2013;77(3):687–96. [DOI] [PubMed] [Google Scholar]
- 27.ISN. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(1):3. [Google Scholar]
- 28.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [DOI] [PubMed] [Google Scholar]
- 29.Patel UD, Greiner MA, Fonarow GC, Phatak H, Hernandez AF, Curtis LH. Associations between worsening renal function and 30-day outcomes among Medicare beneficiaries hospitalized with heart failure. Am Heart J [Internet]. 2010;160(1):132–138.e1. 10.1016/j.ahj.2010.03.033 [DOI] [PMC free article] [PubMed]
- 30.Sani MU, Davison BA, Cotter G, Sliwa K, Edwards C, Liu L, et al. Renal dysfunction in African patients with acute heart failure. Eur J Heart Fail. 2014;16(7):718–28. [DOI] [PubMed] [Google Scholar]
- 31.Haase M, Müller C, Damman K, Murray PT, Kellum JA, Ronco C, et al. Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: Workgroup statements from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol. 2013;182i:99–116. [DOI] [PubMed] [Google Scholar]
- 32.Chahal RS, Chukwu CA, Kalra PR, Kalra PA. Heart failure and acute renal dysfunction in the cardiorenal syndrome. Clin Med J R Coll Physicians Lond. 2020;20(2):146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147(2):331–8. [DOI] [PubMed] [Google Scholar]
- 34.Gameiro J, Marques F, Lopes JA. Long-term consequences of acute kidney injury: A narrative review. Clin Kidney J. 2021;14(3):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eren Z, Ozveren O, Buvukoner E, Kaspar E, Degertekin M, Kantarci G. A Single-Centre Study of Acute Cardiorenal Syndrome: Incidence, Risk Factors and Consequences. Cardiorenal Med. 2012;2(3):168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request (email: workhailu@yahoo.com).