Abstract
Mycobacterium avium is a common opportunistic pathogen in immunocompromised patients such as those infected with human immunodeficiency virus. Although M. avium is an intracellular organism replicating predominantly in macrophages, disseminated M. avium infection is seen in AIDS patients with CD4+ cell counts of <50 cells/μl, suggesting a possible involvement of a T cell-macrophage interaction for the elimination of M. avium. To determine whether CD40-CD40 ligand (CD40L) interactions play a role in M. avium infection, we studied the ability of CD40L to restrict M. avium replication in human monocyte-derived macrophages (MDM) in vitro. MDM were infected with M. avium and cocultured with CD40L-transfected 293 cells for 7 days. Intracellular growth of M. avium in these MDM was assessed by colony counting. CD40L-expressing cells inhibited growth of M. avium in MDM by 86.5% ± 4.2% compared to MDM cultured with control cells. These findings were verified by assays using purified, soluble recombinant human CD40L (CD40LT). CD40LT (5 μg/ml) inhibited intracellular growth of M. avium by 76.9% ± 18.0% compared to cells treated with medium alone. Inhibition by CD40LT was reduced by monoclonal antibodies (MAbs) against CD40 and CD40L. The inhibitory effect of CD40LT was not accompanied by enhancement of interleukin-12 (IL-12) production by M. avium-infected MDM, while CD40L-expressing cells stimulated IL-12 production by these cells. Treatment of M. avium-infected mice with MAb against murine CD40L resulted in recovery of larger numbers of organisms (0.8 to 1.0 log) from the spleens, livers, and lungs of these animals compared to infected mice which received normal immunoglobulin G. These results indicate that CD40-CD40L signaling may be an important step in host immune response against M. avium infection.
Mycobacterium avium infection is one of the most commonly encountered opportunistic infections in human immunodeficiency virus (HIV)-infected individuals (26). It remains difficult to treat and can be a significant cause of morbidity. M. avium predominantly infects and multiplies within macrophages (17). This organism is known to attach and enter macrophages with the help of specific receptors expressed on the surface of these cells (7, 37, 39). In vitro studies have shown that macrophages secrete several cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (19, 33) in response to infection with this organism. Some cytokines, such as TNF-α and GM-CSF, have also been shown to activate infected macrophages to kill this organism. Healthy individuals are able to control this infection easily. However, AIDS patients, particularly those who have CD4+ T-cell counts of less than 50 cells/μl, are at increased risk of developing disseminated infection due to M. avium (26). The fact that infection due to M. avium is seen predominantly in immunocompromised individuals with low CD4+ T cells suggests that T cells are critically important in controlling this infection and that a T-cell interaction with macrophages may play a role in preventing M. avium infection in healthy hosts.
T-cell products such as gamma interferon (IFN-γ) and IL-12 are known to be important for antimycobacterial activity of macrophages (20). In recent years it has been shown that T cells can stimulate macrophages by a non-cytokine-mediated, direct cell-cell contact-dependent pathway through CD40 ligand (CD40L). CD40L, also known as CD154, is expressed transiently on the surface of activated T cells and binds to surface CD40 molecules on antigen-presenting cells, including B cells, macrophages, and dendritic cells (5). CD40-CD40L signaling is essential for several immunoregulatory pathways, including cell-mediated host immune response against pathogens (24). Ligation of CD40L to CD40 on B cells has been shown to inhibit immunoglobulin (Ig) isotype switching (5) as well as primary and secondary humoral immune response to thymus-dependent (TD) antigens but not thymus-independent (TI) type II antigens (22). CD40-CD40L interactions are known to activate antigen-presenting cells, such as macrophages and dendritic cells (24). Ligation of CD40 with CD40L is also required for the microbicidal activity of macrophages. CD40-CD40L interactions have been reported to be important in resolution of infections by pathogens such as Leishmania major (10), Leishmania amazonensis (42), Cryptosporidium parvum (14), and Pneumocystis carinii (47). Patients suffering from hyper-IgM syndrome, who have a defect in their CD40L gene, are highly susceptible to intracellular pathogens such as Pneumocystis, Cryptococcus, and Histoplasma species (9, 35).
In this study, we examined the role of CD40L in M. avium infection, both in vitro and in vivo. We have determined whether CD40L plays a role in inhibiting intracellular growth of M. avium in human macrophages in vitro. Further, we evaluated the role of CD40-CD40L interactions in vivo, using monoclonal antibodies (MAbs) against CD40L to block this interaction in mice infected with M. avium.
MATERIALS AND METHODS
Reagents.
RPMI 1640 and Iscove’s modified Dulbecco’s medium were purchased from BioWhittaker (Walkersville, Md.). Fetal bovine serum (FBS) and normal human serum (NHS) were purchased from Irvine Scientific (Santa Ana, Calif.). Minimal essential medium with Eagle’s salts and FBS for culture of 293 cells were purchased from Quality Biological (Gaithersburg, Md.) and Summit Biotechnology (Fort Collins, Colo.), respectively. Murine MAbs against human CD40 (clone M3) (31) and recombinant human IFN-γ were purchased from Genzyme (Cambridge, Mass.). Control mouse IgG κ chain was purchased from PharMingen (San Diego, Calif.). Goat anti-mouse IgG-fluorescein isothiocyanate conjugate was obtained from Becton Dickinson (San Jose, Calif.). Purified recombinant soluble human trimeric CD40L (CD40LT) and murine MAb against human CD40L (clone M90) (4) were provided by Immunex Corporation (Seattle, Wash.). MR1 (hamster MAb specific for mouse CD40L) was purified from culture supernatants of hybridoma cells (HB-11048; American Type Culture Collection, Manassas, Va.) by Immunodynamics Inc. (San Diego, Calif.). Control hamster IgG was purchased from Pierce Chemical Co. (Rockford, Ill.). Alkaline phosphate-conjugated anti-mouse IgG was obtained from Sigma Chemical Co. (St. Louis, Mo.).
Culture of M. avium.
The previously studied M. avium strain 13 (32), isolated from an AIDS patient at the University of California, San Diego, was used in all experiments. It was cultured on Middlebrook 7H11 agar (Difco Laboratories, Detroit, Mich.) with oleic acid-albumin-dextrose complex (OADC) enrichment at 37°C in the presence of 5% CO2 for 2 weeks. Transparent colonies were selectively picked and further cultured on Middlebrook 7H11 plates for 2 more weeks. The resulting colonies, which were predominantly transparent (>90%), were then collected and washed two times with phosphate-buffered saline (PBS). The bacteria were finally resuspended in Middlebrook 7H11 broth (Difco Laboratories), and the optical density at 600 nm of the suspension was adjusted to 0.15 to 0.2. The suspension was aliquoted and stored at −70°C until use. The number of organisms per milliliter of this suspension was determined by the colony-forming unit (CFU) assay.
Isolation of human monocytes.
Monocytes were isolated from normal human buffy coats obtained from the San Diego Blood Bank by Ficoll-Hypaque and Percoll gradient centrifugation (25). Purity of the monocytes by this method was greater than 70%. The monocytes thus isolated were cultured for 5 to 7 days in Iscove’s modified Dulbecco’s medium supplemented with 10% NHS, 2 mM l-glutamine, and 50 U of penicillin-streptomycin per ml in Teflon beakers to yield monocyte-derived macrophage (MDM). MDM were further enriched by adherence to the wells of tissue culture plates before use in experiments. Purity of MDM after the second adherence as assessed by esterase staining was >95%. Viability determined by trypan blue exclusion was >97%.
Transfection of 293 cells with CD40L.
293 (human embryonic kidney) cells were transfected as described previously (31). Briefly, the cDNA for human CD40L was prepared by reverse transcription of mRNA isolated from peripheral blood mononuclear cells which were stimulated with an anti-CD-3 MAb and IL-2 and selected for surface CD40L expression by using an anti-CD40L MAb. This cDNA was amplified by PCR, and the PCR product was cloned into the expression vector pcDNA3.1(+) (Invitrogen, San Diego, Calif.). This plasmid, referred to as pcDNA3.1-CD40L, was transfected into 293 cells and selected in medium containing G418 (Genectin; GIBCO BRL, Grand Island, N.Y.). The CD40L-positive cells (CD40L-293 cells) were further selected by repeated cell sorting using a CD40L-specific antibody. The control 293 cells used in this study were prepared by transfection of 293 cells with the empty expression vector, pcDNA3.1(+) (pcDNA-293 cells). The ability of M. avium to infect 293 cells when added at a cell/bacterium ratio of 1:10 was tested; 0.37% ± 0.8% of the total number of M. avium added to the wells attached and/or invaded these cells. However, the organism did not replicate in 293 cells.
Infection of MDM with M. avium.
MDM were adhered to wells of a 48-well (2 × 105/well) or 96-well (6 × 104/well) tissue culture plate for 2 h, and nonadherent cells were removed by washing with serum-free RPMI 1640. After washing, adherent MDM were infected with M. avium at a multiplicity of infection of 10:1 for 2 h at 37°C. After 2 h, MDM were extensively washed with prewarmed RPMI 1640 to remove extracellular organisms. To determine whether priming of MDM with IFN-γ alters the effect of CD40L on intracellular growth of M. avium, in some experiments adherent MDM were incubated with IFN-γ (0.3 μg/ml; Genzyme) in RPMI 1640 containing 10% NHS for 3 days prior to infection with M. avium.
Treatment of M. avium-infected MDM with CD40L-293 cells or CD40LT.
CD40L-293 cells and pcDNA-293 cells were harvested and resuspended in RPMI 1640 containing 5% heat-inactivated FBS in the absence of antibiotics. Live CD40L-293 or pcDNA-293 cells were added to M. avium-infected MDM at a ratio of 0.5:1. M. avium-infected cells cultured with medium alone served as the untreated control. In experiments designed to study the effect of CD40LT, M. avium-infected MDM were incubated with CD40LT at 1 and 5 μg/ml. Infected cells cultured with medium alone served as controls.
On days 1, 3, and 7 postinfection, the supernatant was collected and set aside and the adherent cells were lysed. To account for all the mycobacteria in each well, corresponding lysates and supernatants were combined and used to determine the number of CFU (described below). Experiments were done in triplicate, and results were expressed as mean CFU per well ± standard deviation (SD).
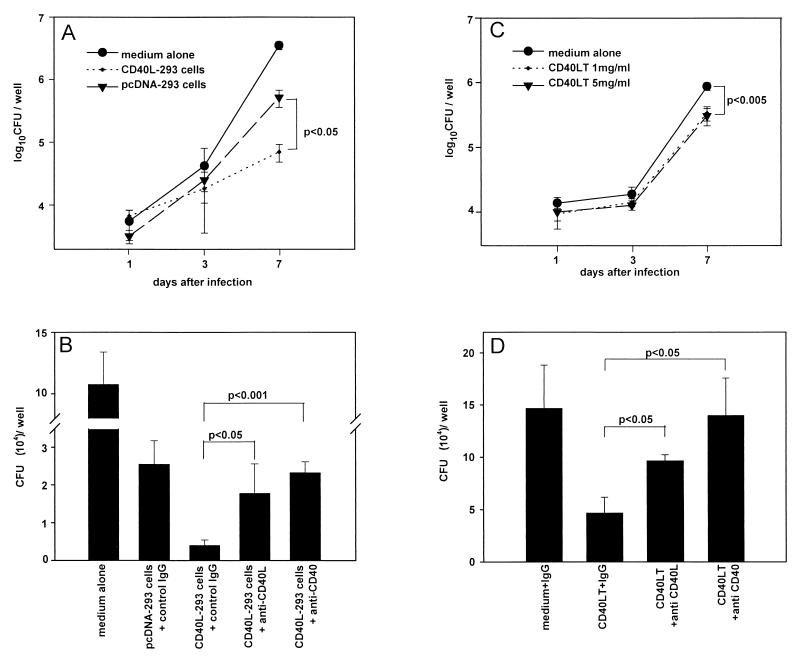
In some experiments, infected MDM were treated with CD40L-293 cells or CD40LT in the presence of MAb against CD40L (10 μg/ml) or CD40 (10 μg/ml) in order to prevent ligation of CD40L with CD40. In these experiments, total CFU in each well was determined only on day 7. Comparisons were made between M. avium-infected MDM treated with CD40L-293 cells and M. avium-infected MDM treated with pcDNA-293 cells or M. avium-infected MDM treated with CD40LT and medium alone.
CFU assay.
CFU were determined by colony counting as we described previously (36). Serial 10-fold dilutions of total cell lysates were performed in PBS; 10 μl of each dilution was plated on Middlebrook 7H11 plates supplemented with OADC enrichment, and plates were incubated up to 14 days at 37°C. The number of colonies were counted every alternate day from day 10 onward until no new colonies appear; this yields the number of CFU/10 μl. The number of CFU in each well was then calculated.
Measurement of IL-12 production by M. avium-infected MDM treated with CD40L-293 cells or CD40LT.
MDM infected with M. avium and uninfected MDM were cultured with CD40L-293 or pcDNA-293 cells in RPMI 1640 containing 5% heat-inactivated FBS in the absence of antibiotics. In some experiments, infected MDM were treated with 5 μg of CD40LT per ml. Cells treated with medium alone served as controls. On days 1, 3, and 7 postinfection, culture supernatant was collected and IL-12 (p70) was measured by enzyme-linked immunosorbent assay (ELISA) using human IL-12 ELISA kits (Endogen, Inc., Woburn, Mass.). Experiments were done in triplicate, and results are expressed as mean ± SD.
Infection of mice and administration of MAb against CD40L.
Seven- to eight-week-old, female C57BL/6 mice (Simonsen Laboratories, Gilroy, Calif.) were infected with 106 M. avium in 0.1 ml of PBS by intravenous tail vein injection and then divided into two groups of five each. Immediately after infection (day 0), as well as on days 2, 4, 6, 10, 13, 17, 20, 24, 27, 31, and 34, one group of mice received 250 μg of MAb against murine CD40L (MR1) intraperitoneally. The control group received 250 μg of normal hamster IgG at the same time points. On day 35 (5 weeks), all mice were euthanized and dissected. The liver, spleen, and lungs were collected and weighed. All procedures were performed under a biosafety cabinet in a biosafety level 2 facility. The organs were minced and homogenized for 30 s with 0.25% sodium dodecyl sulfate in PBS (1 ml/100 mg of tissue). The number of CFU in the tissue homogenates was determined by the CFU assay, and the results were expressed as CFU per organ.
As mentioned earlier, CD40-CD40L signaling is required for the generation of a humoral immune response against TD antigens. Anti-CD40L MAb treatment has been shown to inhibit the generation of an antibody response to these antigens (18). We used this observation as a tool to determine whether MR1 effectively blocks CD40L in our in vivo experiments. We therefore examined the humoral response against MR1, a hamster anti-mouse CD40L antibody which itself is a TD antigen (13). Serum IgG and IgM levels against MR1 in anti-CD40L-treated mice were compared with those of mice treated with control hamster IgG. Murine IgG specific for hamster IgG and IgM was assayed by ELISA. Blood samples were collected from each mouse on day 35 after infection with M. avium. ELISA plates (Nunc-Immuno Plate MaxiSorp plates; Nunc Inc., Naperville, Ill.) were coated overnight at 4°C with 10 μg of hamster IgG per ml. After blocking, 10-fold serial dilutions of the serum were performed and added into the wells. Mouse IgG or IgM specific for hamster IgG was detected by using alkaline phosphate-conjugated anti-mouse IgG or IgM, respectively.
Histologic examination.
Tissue sections of the spleen, liver, and lungs collected from each of the experimental and control mice were fixed overnight in 10% buffered formalin at room temperature and embedded in paraffin. The paraffin-embedded tissue was further sectioned (5-μm thickness), stained with Ziehl-Neelsen reagent, which specifically stains acid-fast bacilli, and also stained with hematoxylin-eosin. The sections were observed under an Olympus microscope. At least three specimens from each organ of each of the experimental and control mice were evaluated, and representative fields were photographed with Olympus microphotographic equipment (Olympus Optical Co., Lake Success, N.Y.) or a Zeiss Axiophot microscope (Carl Zeiss Inc., Thornwood, N.Y.) equipped with a Sony DKC5000 charge-coupled device camera. The images were captured and processed for presentation with Adobe Photoshop 4.0 and printed on a dye sublimation printer.
Statistical analysis.
Results were expressed as mean ± SD. Statistical differences were determined by Student’s t test. A P value below 0.05 was considered to be statistically significant.
RESULTS
In vitro effect of CD40L against growth of M. avium in MDM.
To assess the effect of CD40-CD40L interaction on intracellular growth of M. avium in MDM, we treated M. avium-infected MDM with CD40L-293 cells and determined the number of CFU on days 1, 3, and 7 after infection (Fig. 1A). CD40L-293 cells were found to inhibit the intracellular growth of M. avium in MDM by 86.5% ± 4.2% compared to MDM treated with pcDNA-293 cells, which served as the control (P < 0.05). However, pcDNA-293 cells themselves were found to substantially inhibit intracellular growth of M. avium in MDM compared to infected MDM treated with medium alone. To confirm that the inhibitory effect observed with CD40L-293 cells was indeed due to CD40L and not merely the cells themselves, MAb specific for CD40L or CD40 was used to block ligation of CD40 with CD40L (Fig. 1B). The antimycobacterial effect of CD40L-293 cells was inhibited by 65% ± 23% (P < 0.05) in the presence of MAb against CD40L and by 90% ± 14% (P < 0.001) in the presence of MAb against CD40.
FIG. 1.
Effect of CD40L-293 cells and CD40LT on intracellular growth of M. avium in MDM. M. avium-infected MDM were cocultured with CD40L-293 cells or CD40LT in the presence and absence of MAbs against CD40L and CD40 for up to 7 days. Intracellular growth of M. avium was assessed by the CFU assay on days 1, 3, and 7 after infection. Each condition was tested in triplicate, and results are expressed as mean CFU per well ± SD. (A) M. avium-infected MDM cultured with CD40L-293 cells; (B) M. avium-infected MDM cultured with CD40L-293 cells or control pcDNA-293 cells in the presence and absence of MAb against CD40L (10 μg/ml), CD40 (10 μg/ml), or control mouse IgG κ chain (10 μg/ml); (C) M. avium-infected MDM cultured with recombinant CD40LT at 1 or 5 μg/ml or medium alone; (D) M. avium-infected MDM cultured with CD40LT in the presence and absence of MAb against CD40L (10 μg/ml), CD40 (10 μg/ml), or control mouse IgG κ chain (10 μg/ml). Results shown are representative of three experiments.
These data were further validated in assays using CD40LT. Intracellular growth of M. avium in MDM was assessed on days 1, 3, and 7 after infection in the presence and absence of CD40LT (Fig. 1C). On day 7, CD40LT at 1 and 5 μg/ml was found to inhibit intracellular growth of M. avium by 61.2% ± 9.6% (P < 0.001) and 65.0% ± 10.3% (P < 0.001), respectively, compared to growth of M. avium in MDM treated with medium alone. This inhibitory effect of CD40LT on the intracellular growth of M. avium was blocked by 49% ± 8% (P < 0.05) in the presence of MAb against CD40L and 82% ± 18% (P < 0.02) in the presence of MAb against CD40 (Fig. 1D). These findings clearly demonstrate that ligation of CD40L with CD40 inhibits intracellular growth of M. avium in infected MDM.
IFN-γ, an important T-cell product, is known to be a stimulant of antimycobacterial activity in macrophages (3). Further, IFN-γ is known to increase CD40 expression on MDM (1). We therefore determined whether priming of MDM with IFN-γ further enhances the inhibitory effect of CD40L. MDM were incubated with IFN-γ for 3 days prior to infection with M. avium and then exposed to CD40L-293 cells or CD40LT. Priming MDM with IFN-γ did not appear to modify the inhibitory effect of CD40L-293 cells or CD40LT on M. avium growth within these cells (data not shown).
CD40L-293 cells, but not CD40LT, enhance IL-12 production by M. avium-infected MDM.
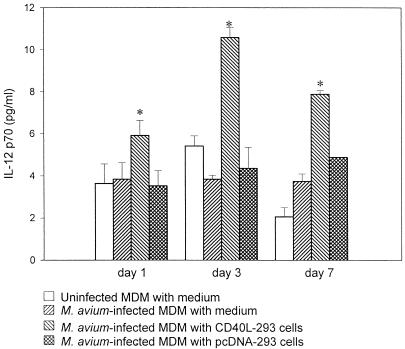
CD40-CD40L interaction is known to stimulate the production of IL-12 by macrophages (40). To determine whether IL-12 is responsible for the antimycobacterial effect of CD40L, culture supernatants of M. avium-infected MDM were assayed for IL-12 in the presence and absence of CD40L-293 cells or CD40LT. MDM were infected with M. avium and cultured with CD40L-293 cells or CD40LT. Cells incubated with pcDNA-293 cells and medium alone served as controls. On days 1, 3, and 7 after infection, IL-12 (p70) in the culture supernatant was measured by ELISA (Fig. 2).
FIG. 2.
IL-12 (p70) production by M. avium-infected MDM cocultured with CD40L-293 cells. M. avium-infected MDM and uninfected MDM were cultured with medium alone, pcDNA-293 cells, or CD40L-293 cells. On days 1, 3, and 7 after infection, culture supernatants were collected and assayed for IL-12 (p70) by ELISA. Experiments were done in triplicate, and results are expressed as mean ± SD. ∗, P < 0.03 compared to M. avium-infected MDM cultured with pcDNA-293 cells.
CD40L-293 cells were found to significantly enhance production of IL-12 by M. avium-infected MDM on days 1, 3, and 7 postinfection (P < 0.03) compared to MDM treated with medium only or pcDNA-293 cells. However, CD40L-293 cells did not affect IL-12 production by uninfected MDM (data not shown). These results indicate that CD40-CD40L ligation enhances IL-12 production by M. avium-infected MDM but not by uninfected MDM. In contrast, CD40LT did not enhance the production of IL-12 by infected or uninfected MDM. Pretreatment of MDM with IFN-γ did not alter the IL-12 response of MDM (data not shown).
In vivo effect of CD40L against M. avium infection in mice. (i) Assessment of bacterial load.
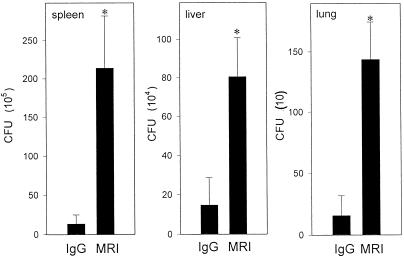
To study the role of CD40-CD40L interaction in vivo, growth of M. avium in the livers, spleens, and lungs of mice that received MAbs against CD40L was analyzed and compared with that of mice which received control hamster IgG. Mice were infected with M. avium and treated with 250 μg of MR1 or control hamster IgG intraperitoneally for 35 days as described previously by Wiley and Harmsen for mice infected with P. carinii (47). Based on their studies, administration of MAbs at this concentration was found to be sufficient to inhibit resolution of P. carinii infection in mice by blocking CD40-CD40L ligation.
The spleens, livers, and lungs were collected from M. avium-infected mice at 5 weeks, homogenized, and used to determine the number of CFU as described in Materials and Methods. The number of viable bacteria recovered from the livers, lungs, and spleens of mice treated with MR1 was 0.8 to 1.0 log higher than that recovered from mice treated with the control antibody (P < 0.01) (Fig. 3). Measurement of the immune response (serum IgG and IgM levels) of these mice to hamster IgG indicated that mice receiving MR1 had significantly lower serum IgG and IgM levels compared to mice that received control hamster IgG (data not shown). These results indicate that the MAb MR1 administered to the mice in our study was sufficient to block CD40L function in vivo and that the inhibition of M. avium growth observed in the spleens, livers, and lungs of mice was indeed due to blockade of CD40L by this antibody.
FIG. 3.
M. avium growth in mice treated with anti-CD40L MAb. Mice were infected with 106 M. avium and treated with MR1 (anti-CD40L MAb) or control IgG. M. avium growth in the spleen, liver, and lungs was determined by the CFU assay on day 35. Results shown are mean of CFU per organ ± SD. ∗, P < 0.01 compared to CFU in the organs of M. avium-infected mice treated with control hamster IgG.
(ii) Histological examination.
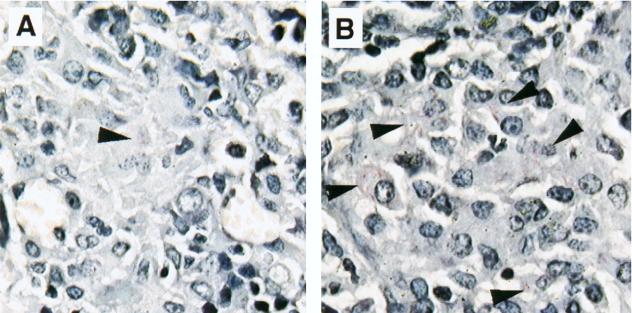
Tissue sections from the spleens of M. avium-infected mice treated with MR1 and control IgG were fixed and stained with Ziehl-Neelsen reagent (Fig. 4). The number of acid-fast bacilli observed in sections of the spleen from MR1-treated mice was higher than that observed in the sections from mice treated with control IgG (Fig. 4). These findings correlate with the CFU data described earlier.
FIG. 4.
Mycobacterial burden in tissue from M. avium-infected mice treated with anti-CD40L MAb. Mice were infected with M. avium and treated with anti-CD40L MAb or control IgG. Mice from both groups were sacrificed on day 35. The spleen was collected from each mouse, sectioned (5 μm), and stained with Ziehl-Neelsen acid-fast stain to visualize mycobacteria. The sections were viewed at a magnification of ×630. Shown are spleen sections from M. avium-infected mice treated with control IgG (A) and anti-CD40L MAb (B). Arrowheads indicate acid-fast bacilli.
Tissue sections of the spleen and liver from M. avium-infected mice treated with MR1 as well as normal IgG were stained with hematoxylin-eosin and compared with tissue sections prepared from uninfected mice of comparable age (Fig. 5). The white pulp of the spleen appeared to be disrupted in M. avium-infected mice (Fig. 5B and C) but not in the uninfected mice (Fig. 5A). However, there were no significant pathological differences between MR1-treated mice (Fig. 5C) and control IgG-treated M. avium-infected mice (Fig. 5B). The livers from uninfected mice did not show any granulomas (Fig. 5D), in contrast to livers from M. avium-infected mice (Fig. 5E and F). The sizes and numbers of granulomas in the liver from MR1-treated and control IgG-treated mice were similar (Fig. 5E and F). Although there do not appear to be significant pathological differences between the two groups of mice, blockade of CD40-CD40L interaction in M. avium-infected mice with MAb abrogates the control and restriction of this organism in vivo. The size and number of granulomas in the liver do not appear to reflect bacterial load in this organ.
FIG. 5.
Histopathology of tissue sections from liver and spleen of M. avium-infected mice. Mice were infected with M. avium and treated with anti-CD40L MAb or control IgG. Mice from both groups were sacrificed on day 35. The spleen and liver was collected from each mouse, sectioned (5 μm), and stained with hematoxylin-eosin. The sections were viewed at a magnification of ×100. (A) Spleen from uninfected mouse showing intact white pulp; (B) spleen from M. avium-infected mouse treated with control IgG showing the disrupted white pulp; (C) spleen from M. avium-infected mouse treated with anti-CD40L MAb also showing disrupted white pulp; (D) liver from uninfected mouse; (E) liver from control IgG-treated M. avium-infected mouse showing granulomas; (F) liver from anti-CD40L treated M. avium-infected mouse showing granulomas.
DISCUSSION
CD40-CD40L interactions are known to mediate host immune responses and T-cell-mediated effector functions (24). Binding of CD40L to CD40 on MDM is critical in the interaction of antigen presenting cells with T cells and during infections due to intracellular pathogens such as Leishmania and P. carinii (24). In this study, we have shown that CD40-CD40L interactions also play an important role in host immune response against M. avium infection. Ligation by CD40L of CD40 on the surface of M. avium-infected macrophages reduced intracellular growth of this organism in vitro. These studies used two different forms of human CD40L, CD40L-expressing cells and recombinant soluble human CD40L. Both forms of CD40L were found to inhibit M. avium growth in MDM. To confirm that the inhibitory effect of CD40L-293 cells was indeed due to CD40L and not merely the transfected cells themselves, we used MAb specific for CD40L or CD40 to block ligation of CD40 with CD40L. The antimycobacterial effects of CD40L-expressing cells was significantly reduced, suggesting that this effect was partially due to CD40L on the surface of the cells. It is likely that the inhibition observed in the case of the control cells transfected with the empty vector is due to the rapid growth of these cells in culture or due to the secretion of other cytokines by the cells. To further substantiate this finding, we performed studies using CD40LT, the soluble form of CD40L. CD40LT was found to significantly inhibit intracellular growth of M. avium compared to control cells treated with medium alone.
The in vitro experiments with CD40L (either on the membrane of 293 cells or as a soluble trimeric protein) suggest that this molecule acts directly on cultured human macrophages by inducing them to restrict the growth of M. avium in vitro. The mechanism of this effect is not yet clear and will require further study. For example, nitric oxide can be induced in murine macrophages by CD40L (27). Ligation of CD40L on T cells to CD40 on macrophages has been shown to induce nitric oxide production (45), and nitric oxide generation by macrophages is known to be a major pathway by which CD40L mediates immunity against L. amazonensis (42). Yet, it has been reported that nitric oxide has no antimycobacterial effects against M. avium either in murine macrophages in vitro or in mice in vivo (16).
Ligation of CD40L with CD40 has been reported to induce IL-12 production by IFN-γ-primed murine peritoneal macrophages (28, 40). IL-12 is a multifunctional cytokine which is produced mainly by macrophages and monocytes, which in turn stimulate production of IFN-γ, GM-CSF, and TNF-α by T cells. All of these cytokines are known to stimulate macrophages to restrict intracellular growth of M. avium (3, 15). IFN-γ has been shown to be critical for the control of mycobacterial infections. Mice with deletions of the IFN-γ gene or mice treated with anti-IFN-γ antibody are highly susceptible to infection by M. avium (2, 16, 21), and humans with genetic defects of the IFN-γ receptor are vulnerable to infection by M. avium (34). Consequently, it is understandable that mice with deletions of the IL-12 gene or treated with anti-IL-12 antibody are also susceptible to infection by M. avium (12). IL-12 alone, however, is not sufficient to induce cultured human macrophages to control M. avium (8). TNF-α is another cytokine which is important in controlling M. avium replication in vitro in macrophages (15) and in vivo in mice (16). CD40L also induces TNF production by both mouse (43) and human (1, 29) monocytes and macrophages. Further studies are needed to elucidate the possible involvement of TNF-α in the antimycobacterial activity induced in macrophages by CD40L.
IL-12 production is known to be essential for effective clearance of M. avium (12, 30). We analyzed IL-12 production by M. avium-infected MDM in the presence of CD40L-expressing cells and CD40LT. Interestingly, the antimycobacterial effects of CD40L-expressing cells were accompanied with enhancement of IL-12 production by M. avium-infected MDM. On the contrary, CD40LT, which is the soluble form of CD40L, failed to enhance IL-12 production by M. avium-infected MDM in conjunction with inhibition of intracellular M. avium growth. Previous studies by Fanslow et al. (18) have shown that the biological function of membrane-associated CD40L is different from that of soluble CD40L. This may account for the lack of stimulation of IL-12 production by M. avium-infected MDM treated with soluble CD40L in our study. Although we do not have direct evidence, it appears that IL-12 may be partly involved in the antimycobacterial activity of CD40L in vivo. In the case of L. major (10), cell-mediated immunity has been shown to be due to production of IL-12 by infected macrophages. In this study, which used CD40L-knockout (KO) mice, it was also shown that T cells from these mice are unable to induce IL-12 production by macrophages.
Our studies using direct contact between M. avium-infected human macrophages and CD40L-expressing 293 cells model the cell-cell contact pathway of macrophage activation by activated T cells. Direct contact with activated T cells has been shown to be important for the control of mycobacterial infection in macrophages from both mice (44) and humans (41). CD40L on the membranes of activated T cells is the major factor in the cell-cell contact pathway of macrophage activation in a variety of systems (43). Consequently, it is plausible that some of the reversal of antimycobacterial immunity that we found after administering anti-CD40L antibody to M. avium-infected mice results from blocking CD40L-macrophage interactions.
In vivo, however, the administration of anti-CD40L antibody could affect other parts of the immune system. Blockade of CD40-CD40L interaction by anti-CD40L MAbs is known to suppress humoral immunity against TD antigen (46) and in turn against pathogens such as P. carinii, where host response is predominantly mediated by humoral immune responses (47). Humoral immunity against mycobacteria was reevaluated by Glatman-Freedman and coworkers, who demonstrated that antibody responses do play a role in modulating the course of mycobacterial infection (23). Low levels of antibody production against M. avium in M. avium-infected mice injected with MAbs against CD40L may have some role to play in elevated bacterial burden in these mice.
In in vivo experiments described in the present study, M. avium-infected mice treated with anti-murine CD40L were found to have higher number of organisms in the spleen, liver, and lungs compared to control M. avium-infected mice which received a control antibody. Our results suggest that development of granulomas during mycobacterial infection, which is the result of a series of complex inflammatory events, is independent of mycobacterial burden. Although the bacterial burden in organs harvested from M. avium-infected mice which received anti-CD40L MAbs was significantly higher than that in organs from mice treated with control IgG, there were no significant differences in the histopathological response between the two groups. There were no significant differences in the weights of the livers and spleens collected from the two groups of mice. Further, the size and number of granulomas in the liver were similar in the two groups.
While the CD40-CD40L pathway has been reported to be critical in elimination of infection due to pathogens such as Leishmania and P. carinii (10, 42, 47), Campos-Neto et al. (11) reported that resistance to Mycobacterium tuberculosis infection is achieved independently of CD40L. Their studies were carried out with CD40L-KO mice. In addition, the number of organisms injected were lower than that used in our study. However, they report that when mice were infected with 106 organisms as in the case of our study, and followed for longer periods of time, there was a significant difference between CD40L-KO and control mice with respect to the bacterial burden in the spleen and liver. This disparity between their findings and ours may be due to differences in the strain of mice used (C57BL/6 versus CD40L-KO) and size of the inoculum. CD40L-KO mice are known to have slight increases in the percentage of CD4+ T cells and slight decreases in the percentage of double-positive thymocytes compared to the wild-type mice. Although mice used in our study as well as the CD40L-KO mice have similar humoral and cellular immune responses against TD and TI antigens (38, 48), it is possible that host immune responses to M. tuberculosis and M. avium are quite different. For example, in humans, M. avium infection is commonly seen in HIV-infected individuals with mean CD4+ T-cell counts of less than 50 cells/μl (26), while M. tuberculosis infection occurs in all stages of HIV infection (6).
In summary, we have shown that ligation of CD40-CD40L plays a role in intracellular growth of M. avium in vitro, and treatment of mice with anti-CD40L MAb increased M. avium growth in the lungs, livers, and spleens of mice. We propose that interaction of CD40 with CD40L is an important pathway involved in the restriction of M. avium growth in mice. These results are highly consistent with the observation that interaction of T cells with M. avium-infected macrophages is required for resistance to M. avium infection in HIV-infected individuals. These findings suggest that administration of CD40L may serve as an alternate therapeutic means to inhibit M. avium infection in individuals who have low T-cell counts. Further studies to establish the mechanism of CD40L-mediated signaling leading to the restriction of M. avium infection are required.
ACKNOWLEDGMENTS
This research was supported by funds from the Universitywide AIDS Research Program, University of California, grant R97-SD-057 to A. Catanzaro, and NIH grants AI35258 and HL57911 to Richard S. Kornbluth.
We thank Immunex Corp for providing recombinant soluble human trimeric CD40L and E. K. Thomas and W. C. Fanslow (Immunex Corp.) for valuable suggestions during the course of the study and manuscript preparation. We also thank J. Yi and N. Varki for assistance with the histology studies.
REFERENCES
- 1.Alderson M R, Armitage R J, Tough T W, Strockbine L, Fanslow W C, Spriggs M K. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology. 1994;191:520–525. doi: 10.1016/S0171-2985(11)80458-4. [DOI] [PubMed] [Google Scholar]
- 3.Appelberg R, Castro A G, Pedrosa J, Silva R A, Orme I M, Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armant M, Armitage R, Boiani N, Delespesse G, Sarfati M. Functional CD40 ligand expression on T lymphocytes in the absence of T cell receptor engagement: involvement in interleukin-2-induced interleukin-12 and interfering production. Eur J Immunol. 1996;26:1430–1434. doi: 10.1002/eji.1830260705. [DOI] [PubMed] [Google Scholar]
- 5.Armitage R J, Maliszewski C R, Alderson M R, Grabstein K H, Spriggs M K, Fanslow W C. CD40L: a multi-functional ligand. Semin Immunol. 1993;5:401–412. doi: 10.1006/smim.1993.1046. [DOI] [PubMed] [Google Scholar]
- 6.Barnes P T, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 7.Bermudez L E, Young L S, Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991;59:1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermudez L E, Wu M, Young L S. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–4104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callard R E, Armitage R J, Fanslow W C, Spriggs M K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Maliszewski C R. CD40 Ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 11.Campos-Neto A, Ovendale P, Bement T, Koppi T A, Fanslow W C, Rossi M A, Alderson M R. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–2041. [PubMed] [Google Scholar]
- 12.Castro A G, Silva R A, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–2019. [PubMed] [Google Scholar]
- 13.Cohn M, Blomburg B. The self-nonself discrimination: a one- or two signal mechanism? Scand J Immunol. 1975;4:1–24. doi: 10.1111/j.1365-3083.1975.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 14.Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward A R. Requirement for CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect Immun. 1998;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on generation of reactive nitrogen intermediates. J Leukoc Biol. 1991;49:380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- 16.Doherty T M, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 17.Edwards D, Kirkpatrick C H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986;134:1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- 18.Fanslow W C, Srinivasan S, Paxton R, Gibson M G, Spriggs M K, Armitage R J. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994;6:267–278. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- 19.Fattorini L, Xiao Y, Li B, Santoro C, Ippoliti F, Orefici G. Induction of IL-1β, IL-6, TNF-α, GM-CSF, and G-CSF in human macrophages by smooth transparent and smooth opaque colonial variants of Mycobacterium avium. J Med Microbiol. 1994;40:129–133. doi: 10.1099/00222615-40-2-129. [DOI] [PubMed] [Google Scholar]
- 20.Flesch I E, Hess J H, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann S H. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon γ and tumor necrosis factor α. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florido M, Appelberg R, Orme I M, Cooper A M. Evidence for a reduced chemokine response in the lungs of beige mice infected with Mycobacterium avium. Immunology. 1997;90:600–606. doi: 10.1046/j.1365-2567.1997.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foy T M, Shepherd D M, Durie F H, Aruffo A, Ledbetter J A, Noelle R J. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grewal I S, Flavell R A. The CD40 ligand. At the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Catanzaro A, Rao S P. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect Immun. 1997;65:5262–5271. doi: 10.1128/iai.65.12.5262-5271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horsburgh C R., Jr Mycobacterium avium complex in the acquired immunodeficiency syndrome (AIDS) N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 27.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy M K, Picha K S, Fanslow W C, Grabstein K H, Alderson M R, Clifford K N, Chin W A, Mohler K M. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur J Immunol. 1996;26:370–378. doi: 10.1002/eji.1830260216. [DOI] [PubMed] [Google Scholar]
- 29.Kiener P A, Moran-Davis P, Rankin B M, Wahl A F, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 30.Kobayashi K, Kasama T, Yamazaki J, Hosaka M, Katsura T, Mochizuki T, Soejima K, Nakamura R M. Protection of mice from Mycobacterium avium infection by recombinant interleukin-12. Antimicrob Agents Chemother. 1995;39:1369–1371. doi: 10.1128/aac.39.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornbluth R S, Kee K, Richman D D. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive β-chemokines. Proc Natl Acad Sci USA. 1998;95:5205–5210. doi: 10.1073/pnas.95.9.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meylan P R, Richman D D, Kornbluth R S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect Immun. 1990;58:2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman G W, Gan H X, McCarthy P L, Jr, Remold H G. Survival of human macrophages infected with Mycobacterium avium intracellulare correlates with increased production of tumor necrosis factor-α and IL-6. J Immunol. 1991;147:3942–3948. [PubMed] [Google Scholar]
- 34.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 35.Notarangelo L D, Duse M, Ugazio A G. Immunodeficiency with hyper-IgM (HIM) Immunodefic Rev. 1992;3:101–122. [PubMed] [Google Scholar]
- 36.Ogata K, Linzer B A, Zuberi R I, Ganz T, Lehrer R I, Catanzaro A. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao S P, Ogata K, Catanzaro A. Mycobacterium avium-M. intracellulare binds to the integrin receptor αVβ3 on human monocytes and monocyte-derived macrophages. Infect Immun. 1993;61:663–670. doi: 10.1128/iai.61.2.663-670.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renshaw B R, Fanslow III W C, Armitage R J, Campbell K A, Liggitt D, Wright B, Davison B L, Maliszewski C R. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roecklein J A, Swartz R P, Yeager H., Jr Nonopsonic uptake of Mycobacterium avium complex by human monocytes and alveolar macrophages. J Lab Clin Med. 1992;119:772–781. [PubMed] [Google Scholar]
- 40.Shu U, Kiniwa M, Wu C Y, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 41.Silver R F, Li Q, Boom W H, Ellner J J. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–2417. [PubMed] [Google Scholar]
- 42.Soong L, Xu J, Grewal I S, Kima P, Sun J, Longley B J, Jr, Ruddle N H, McMahon-Pratt D, Fravell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 43.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 44.Sypek J P, Jacobson S, Vorys A, Wyler D J. Comparison of gamma interferon, tumor necrosis factor, and direct cell contact in activation of antimycobacterial defense in murine macrophages. Infect Immun. 1993;61:3901–3906. doi: 10.1128/iai.61.9.3901-3906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian L, Noelle R J, Lawrence D A. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995;25:306–309. doi: 10.1002/eji.1830250152. [DOI] [PubMed] [Google Scholar]
- 46.Van den Eertwegh A J M, Noelle R J, Roy M, Shepherd D M, Aruffo A, Ledbetter J A, Boersma W J A, Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interaction. J Exp Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiley J A, Harmsen A G. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J Immunol. 1995;155:3525–3529. [PubMed] [Google Scholar]
- 48.Xu J, Foy T M, Laman J D, Elliott E A, Dunn J J, Waldschmidt T J, Elsemore J, Noelle R J, Flavell R A. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]