Abstract
Background
The introduction of the Enterovirus A71 (EV-A71) vaccine in China in 2016 has led to a considerable decline in severe hand, foot, and mouth disease (HFMD) cases, with mild outpatient instances now representing the majority of HFMD cases in the country. Nevertheless, epidemiological investigations concerning mild outpatient cases remain scarce, resulting in inadequate descriptions of their clinical, etiological, and epidemiological characteristics. Our study aimed to analyze the clinical, etiological, and epidemiological characteristics of HFMD outpatients in Chengdu from 2019 to 2022 while identifying potential risk factors associated with the progression of outpatients requiring hospitalization.
Methods
A retrospective study was conducted to summarize the clinical, etiological, and epidemiological characteristics of outpatient HFMD cases in Chengdu from 2019 to 2022. Risk factors associated with progression to hospitalization of HFMD outpatients were evaluated using binomial logistic regression analysis.
Results
The study included 1,073 coxsackievirus A6 (CVA6), coxsackievirus A10 (CVA10), and coxsackievirus A16 (CVA16) HFMD nucleic acid test-positive outpatients. Among these, only 45 outpatients (4.19%) progressed to hospitalization. The median ages for CVA6, CVA10, and CVA16 infections were 25.23, 28.13, and 38.45 months, respectively (P < 0.001). CVA6 (76.51%, 821/1,073) has become the main serotype among outpatients in Chengdu, with the proportions from the second half of 2019 to 2022 being 45.59%, 95.17%, 77.67% and 80.71% respectively. EV-A71 cases even disappeared. Patients infected with CVA10 had a significantly higher likelihood of hospitalization (P < 0.05), while the presence of oral rash served as a protective factor (P < 0.05).
Conclusions
Our study highlights the critical need for enhanced surveillance of multiple HFMD pathogens, predominantly caused by the prevalent serotype CVA6. Clinically, enhanced surveillance of CVA10 is imperative to mitigate the hospitalization rate associated with HFMD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20909-8.
Keywords: Hand, foot, and mouth disease; Outpatients; Coxsackievirus; Epidemiology; Risk factors
Introduction
Hand, foot, and mouth disease (HFMD) is a globally recognized infectious disease primarily affecting children under the age of five, posing a significant threat to children's health [1–4]. Clinical images of typical HFMD patients are shown in Fig S1. Approximately twenty different enterovirus serotypes are responsible for HFMD, with enterovirus A71 (EV-A71), coxsackievirus A16 (CVA16), and coxsackievirus A6 (CVA6) being the most prevalent causative pathogens [5, 6]. The introduction of the EV-A71 vaccine in China in 2016 has led to substantial shifts in the pathogenic spectrum of HFMD [7]. Since the widespread vaccination of school-age with the EV-A71 vaccine in 2016, severe HFMD cases have significantly decreased in China [8]. However, the prevalence of HFMD caused by other serotypes remains high in China [9]. The total cumulative number of reported cases between 2019 and 2022 reached approximately 4.03 million, with an incidence rate of 96.0823/100,000 population in 2021 ( http://www.nhc.gov.cn/). Accessed 1 April 2023. Mild cases, predominantly caused by CVA6, CVA16, and coxsackievirus A10 (CVA10), have become the dominant form of HFMD in China [10, 11].
The latent infection rate of human enteroviruses (HEVs) in healthy people in China is high [12], most patients with enterovirus infection are mild or asymptomatic, and outpatient cases account for a relatively high proportion of HFMD, which is an important factor in the transmission of HFMD. Therefore, mild outpatient cases are at a critical stage in preventing and controlling the spread of the disease [12]. Nonetheless, a large number of previous studies of HFMD focus on hospitalized patients with severe and critical disease [13–15]. In addition, epidemiological studies investigating mild outpatient cases are relatively limited, and the epidemiology, clinical symptoms, and etiology are not well described in terms of milder HFMD [4].
Given that most current HFMD patients are outpatients. In this study, we examined the clinical, etiologic, and epidemiological characteristics of HFMD outpatients in Chengdu from 2019 to 2022, including, and identified potential risk factors associated with the progression of outpatient outcomes requiring hospitalization. This study contributes to the development of effective prevention and control measures for mild cases and provides evidence for further implementation of HFMD immunization programs.
Materials and methods
Definition
Laboratory-confirmed HFMD cases were diagnosed based on positive results for enterovirus detected by quantitative reverse transcription PCR (RT-qPCR). The admission criteria for hospitalization of HFMD outpatients aligned with the warning indicators for disease deterioration and critical condition outlined in the Chinese guidelines for HFMD diagnosis and treatment (2018 edition) [16].
Study population
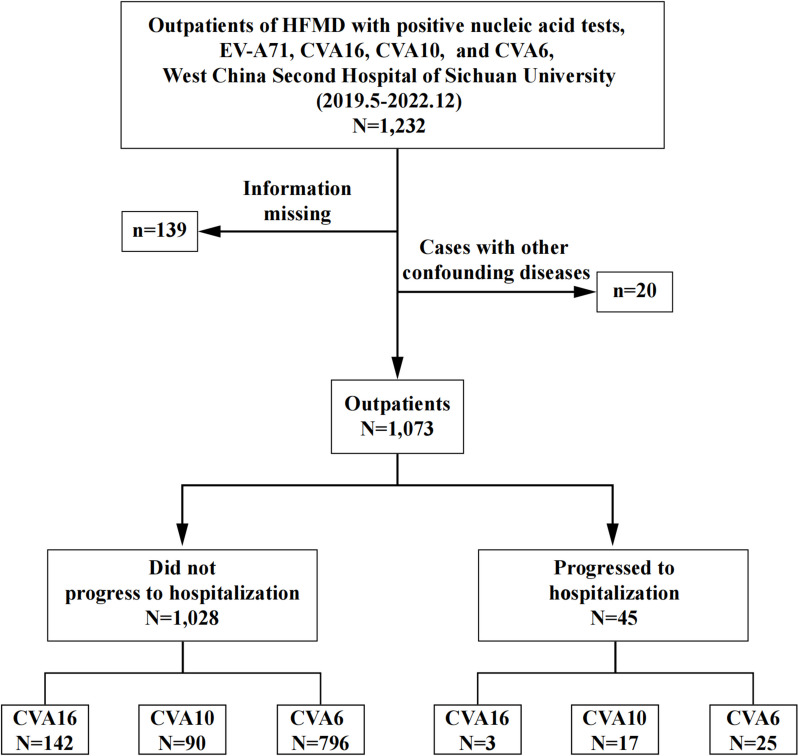
Throat swabs were collected from all outpatients on the day of the clinic, and RT-qPCR testing was performed within 24 h. All patients were tested for EV-A71, CVA16, CVA6, and CVA10 serotypes using a commercial RT-qPCR Kit for confirmation of diagnosis. A total of 1,232 HFMD patients who tested positive for EV-A71, CVA6, CVA10, or CVA16 nucleic acids in Chengdu were enrolled from May 1, 2019 to December 31, 2022. After excluding patients with other confounding diseases (such as other childhood exanthematous diseases, encephalitis or meningitis caused by other viruses, poliomyelitis, patients with underlying diseases, etc.) and patients with insufficient information, 1,073 outpatients included in the study (Fig. 1).
Fig. 1.
Flowchart illustrating the enrolled outpatients during the study period (from 1 May 2019 to 10 December 2022)
Data collection
We utilized a data abstraction form developed from a previous study [17] to extract essential clinical information from the clinical electronic medical record system of West China Second University Hospital, Sichuan University from May 1, 2019 to December 31, 2022. The extracted data encompassed key elements, including enterovirus serotypes, baseline characteristics, severity of illness during hospitalization, treatment regimens, and outcomes. Trained researchers performed the data extraction from the electronic medical records.
Statistical analysis
Quantitative variables were tested for normal (Gaussian) distribution using the Shapiro–Wilk normality test, and all variables displayed a nonnormal distribution in this study. Nonnormally distributed continuous variables were expressed as the median (25th percentile (P25), 75th percentile (P75)) and compared across groups by the Mann–Whitney U test or Kruskal–Wallis rank sum test. Nonnormal discrete variables were represented by means (minimum, maximum) and were subjected to Fisher’s exact probability test. Categorical variables were expressed as counts (n) and percentages (%) and compared across groups using the Chi-square test or Fisher’s exact probability test, as appropriate. Binomial logistic regression analysis was employed to analyze the risk factors influencing the clinical outcomes of outpatient HFMD cases. Established and evaluated a predictive model for the progression of outpatients with HFMD to inpatients using the Receiver Operating Characteristic (ROC) curve. All data were collected using Epidata (version 3.1, EpiData Association) and processed using Excel 2019 (version 2310, Microsoft). Statistical data analysis was conducted with SPSS (version 27, IBM). All statistical tests reported two-sided P values; P < 0.05 was considered significant.
Results
Patients and characteristics
During the study period from May 2019 to December 2022, a total of 1,073 HFMD outpatients were included in this study, all of whom were confirmed positive for CVA6, CVA10, or CVA16 through nucleic acid testing. It is worth noting that no case of EV-A71 was detected during the same period and no deaths. Among those, only 45 outpatients (4.19%) progressed to hospitalization, while 1,028 patients (95.81%) did not require hospitalization. For clinical symptoms, 846 cases (78.84%) reported fever symptoms, 433 cases (40.25%) experienced Hyperpyrexia, and 1,062 cases (98.97%) of outpatients had rash symptoms. Oral rash was the most common, accounting for 1,008 cases (93.94%) (Table 1).
Table 1.
Characteristics of 1,073 outpatients with HFMD in Chengdu, China, 2019–2022
| Total (n = 1,073) | Did not progress to hospitalization (n = 1,028) | Progressed to hospitalization (n = 45) | P-Value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 636(59.27%) | 606(95.28%) | 30 (4.72%) | 0.012a |
| Female | 437(40.73%) | 422(96.57%) | 15(3.43%) | |
| Age (months) | 22(15 ~ 36) | 22(15 ~ 36) | 19(14 ~ 34) | 0.28b |
| Serum type | ||||
| CVA6 | 821(76.51%) | 796(96.95%) | 25(3.05%) | < 0.001a |
| CVA10 | 107(9.97%) | 90(84.11%) | 17(15.89%) | |
| CVA16 | 145(13.52%) | 142(97.93%) | 3(2.07%) | |
| TIOC (days) | 1.48(0 ~ 22) | 1.47(0 ~ 22) | 1.77(0 ~ 11) | < 0.01c |
| TICA (days) | NA | NA | 1.31(0 ~ 5) | NA |
| TIOA (days) | NA | NA | 7.28(0 ~ 42) | NA |
| Clinical manifestations | ||||
| Fever | 846(78.84%) | 804(95.04%) | 42(4.96%) | 0.022a |
| Hyperpyrexia (> 39℃) | 433(40.25%) | 407(94.00%) | 26(6.00%) | 0.007a |
| Rash | 1,062(98.97%) | 972 (91.53%) | 40 (3.77%) | 0.005c |
| Site of rash | ||||
| Oral | 1,008(93.94%) | 972 (96.43%) | 36(3.57%) | 0.012a |
| Hand | 878 (81.83%) | 852 (97.04%) | 26(2.96%) | < 0.001a |
| Foot | 704 (65.61%) | 677 (96.16%) | 27(3.84%) | 0.909a |
| Buttocks | 463 (43.15%) | 443 (95.68%) | 20(4.3%) | 0.706a |
| Trunk | 217 (20.22%) | 204 (94.01%) | 13(5.99%) | 0.126a |
| Lower limbs | 270 (25.16%) | 248 (91.85%) | 22(8.15%) | < 0.001a |
| Upper limbs | 208 (19.38%) | 196 (94.23%) | 12(5.77%) | 0.194a |
| Face | 231 (21.53%) | 218 (94.37%) | 13(5.63%) | 0.199a |
| Blood results | ||||
| White blood cell count(109/L) | 10.30(8.10 ~ 13.10) | 10.30(8.20 ~ 13.10) | 9.40(7.05 ~ 13.10) | 0.204b |
| Neutrophil (%) | 55.30(41.35 ~ 65.10) | 55.40(42.40 ~ 65.10) | 50.40(33.05 ~ 71.20) | 0.437b |
| Lymphocyte (%) | 32.00(23.20 ~ 45.30) | 32.00(23.35 ~ 44.80) | 36.70(17.10 ~ 50.00) | 0.646b |
| Neutrophil count (109/L) | 5.68(3.58 ~ 8.29) | 5.78(3.69 ~ 8.24) | 4.98(2.45 ~ 8.86) | 0.276b |
| Monocyte count (109/L) | 1.03(0.76 ~ 1.35) | 1.04(0.77 ~ 1.36) | 0.88(0.67 ~ 1.26) | 0.018b |
| Lymphocyte count (109/L) | 3.23(2.37 ~ 4.29) | 3.26(2.40 ~ 4.31) | 2.80(2.00 ~ 3.94) | 0.125b |
| Eosinophil count (109/L) | 0.09(0.03 ~ 0.21) | 0.09(0.03 ~ 0.20) | 0.11(0.02 ~ 0.26) | 0.943b |
| C-reactive protein (mg/L) | 12.50(4.80 ~ 24.60) | 12.70(5.10 ~ 24.98) | 4.50(0.80 ~ 21.35) | 0.007b |
All the data indicated in bold are values of P < 0.05 that are considered statistically significant.
CV Coxsackievirus, TIOC time interval from onset to clinic, TICA time interval from clinic visit to admission, TIOA time interval from onset to admission, NA not applicable.
aChi-square test, b Mann–Whitney U test, c Fisher’s exact probability test.
Temporal patterns of enterovirus serotypes
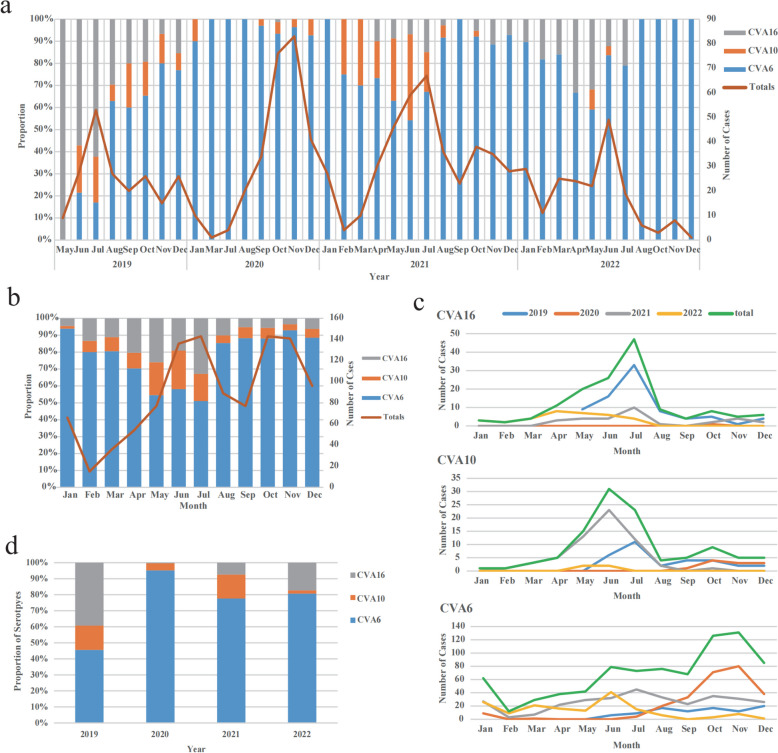
A total of 1,073 outpatients with HFMD were confirmed by nucleic acid testing. The annual incidence of HFMD remained relatively stable over the study period. The main peak of HFMD outpatients occurred between March and August, persisting until September in 2019, 2021, and 2022 (Fig. 2a). A similar peak was also observed from September to November (Fig. 2b). However, in 2020, there was only one peak observed (Fig. 2a). What’s more, a decline in the number of outpatient cases of HFMD was observed from 2021 to 2022 (Fig. 2a).
Fig. 2.
Distribution of enterovirus genotypes over the study period. a Serotype distribution of Enteroviruses positive HFMD outpatients in Chengdu, China, 2019–2022. b Monthly distribution of enterovirus serotypes of HFMD outpatients in Chengdu, China, 2019–2022. c Monthly distribution of HFMD related to CVA6, CVA10, and CVA16 in Chengdu, China, 2019–2022. d Annual serotype distribution of HFMD outpatients in Chengdu, China, 2019–2022
From 2019 to 2022, CVA6 had the highest prevalence in 2021, with noticeable monthly fluctuations. In contrast, CVA16 had its peak prevalence in 2019, while CVA10 had its highest prevalence in 2021, but with fewer monthly fluctuations (Fig. 2c). CVA6 predominantly occurred from September to December, while the CVA10 and CVA16 were more prevalent from May to August (Fig. 2c). In fact, CVA6 also had a higher number of cases from May to August than CVA10 and CVA16, it was the sudden large number of CVA6-related cases arising from September to December 2020 that caused the peak incidence months shift.
CVA6 was found to be the prevailing serotype in outpatients, with the proportions from the second half of 2019 to 2022 being 45.59%, 95.17%, 77.67%, and 80.71% respectively. In 2019, CVA16 had the highest proportion at 39.21%. However, its proportion dropped significantly in the following years (2020–2022), registering percentages of 0.38%, 7.44%, and 17.26%, respectively (Table S1). CVA10 accounted for a high proportion in 2019 and 2021, 15.20% and 14.89%, respectively, compared to a small proportion of 4.45% and 2.03% in 2020 and 2022 (Fig. 2d). Pathogen distribution of HFMD outpatients from May 2019 to December 2022 is shown in supplementary Table S1.
Age distribution of outpatients with HFMD
The analysis of age distribution revealed that children under 5 years old accounted for over 93.20% of HFMD outpatients. Specifically, among the outpatients, the largest age group was represented by 1- to 2-year-olds, accounting for 41.93%, followed by 2- to 3-year-olds (20.41%) and 3- to 5-year-olds (19.01%). Notably, children under 1 year old accounted for 11.83% of outpatients, while those over 5 years old constituted only 6.80% of outpatients (Table 2). The age distribution of 45 inpatients progressing from outpatients with HFMD is shown in supplementary Fig S2.
Table 2.
Serotype distribution among single-enterovirus-infected HFMD patients in different sex and age groups in Chengdu, China, 2019–2022
| Sex | Age (Year) | Age (Month) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotype | Total (n = 1,073) | Male (n = 636) | Female (n = 437) | MR | < 1 (n = 127) | 1 ~ 2 (n = 450) | 2 ~ 3 (n = 219) | 3 ~ 5 (n = 204) | 5 ~ 7 (n = 45) | ≥ 7 (n = 28) | M (P25, P75) |
| CVA6 | 821 | 491 (77.20%) | 330 (75.51%) | 1.49 | 100 (78.74%) | 372 (82.67%) | 168 (76.71%) | 146 (71.57%) | 22 (48.89%) | 13 (46.43%) | 25.23 (15.00, 33.00) |
| CVA10 | 107 | 54 (8.49%) | 53 (12.13%) | 1.02 | 17 (13.39%) | 38 (8.44%) | 21 (9.59%) | 23 (11.27%) | 6 (13.33%) | 2 (7.14%) | 28.13 (17.00, 36.00) |
| CVA16 | 145 | 91 (14.31%) | 54 (12.36%) | 1.69 | 10 (7.87%) | 40 (8.89%) | 30 (13.70%) | 35 (17.16%) | 17 (37.78%) | 13 (46.43%) | 38.45 (20.25, 48.00) |
| P-value | 0.119a | < 0.001a | < 0.001b | ||||||||
All the data indicated in bold are values of P < 0.05 that are considered statistically significant.
a Chi-square test; b Kruskal–Wallis rank sum test; MFR, male to female ratio; M, median; CV, Coxsackievirus.
Distribution among outpatients with HFMD of different sex and age groups
From 2017 to 2022, among the 1,073 outpatient cases, 821 (76.51%) patients were infected with CVA6, 145 (13.52%) were positive for CVA16, and only 107 (9.97%) were positive for CVA10 (Table 1). There were differences in sex distribution among the three serotypes, with ratios of 1.49 for CVA6, 1.02 for CVA10, and 1.69 for CVA16. Of all male patients (n = 636), 77.20% (491 out of 636), 8.49% (54 out of 636), and 14.31% (91 out of 636) were caused by CVA6, CVA10, and CVA16, respectively. Interestingly, there was no significant difference in sex distribution among patients with a single HFMD infection (P > 0.05). Age distribution analysis revealed statistically significant differences among patients with different serotypes (P < 0.001). The median age of all patients was 22.00 months (Table 1). The median ages for CVA6, CVA10, and CVA16 infections were 25.23, 28.13, and 38.45 months, respectively. It is worth noting that patients with HFMD caused by CVA6 were younger than those caused by the other two serotypes, with a median age of 25.23 months (15.00, 33.00) (Table 2).
Factors associated with progression to hospitalization
Forty-five (4.19%) outpatients progressed to inpatients. Outpatient cases with different follow-up outcomes exhibited marked differences in demographic characteristics, clinical features, and laboratory test results. Notably, outpatients who did not progress to hospitalization were more likely to display oral and hand rashes as well as elevated monocyte counts and C-reactive protein levels (P < 0.05). Correspondingly, outpatients who progressed to hospitalization had a longer time interval from onset to clinic and were more likely to develop fever, hyperthermia, and lower limbs rashes (P < 0.05) (Table 1). The distribution of serotypes among outpatients with different outcomes is shown in supplementary Fig S3.
To better understand the risk factors influencing the progression to hospitalization of HFMD outpatients, we conducted a binomial logistic regression analysis using the variables outlined in Table 3. According to the analysis, patients with the CVA10 viral genotype were significantly associated with an increased risk of progression (OR: 6.273, 95% CI: 1.663–23.655, P = 0.007), while the presence of an oral rash was associated with protection from progression (OR: 0.231, 95% CI: 0.101–0.532, P = 0.001). Other variables, including the CVA6 genotype, sex, fever, hyperpyrexia, and rash on the lower limbs, did not show significant associations with outpatient outcomes risk (Table 3). We plotted the receiver operating characteristic curve (ROC) and analyzed the joint indicators of specificity and sensitivity (Fig S4). The results showed that the area under the curve (AUC) for these joint indicators was 0.762, with a 95% confidence interval of 0.692–0.832, indicating statistical significance. It is worth noting that at the maximum Youden index, the sensitivity was 0.778 and the specificity was 0.611.
Table 3.
Binomial logistic regression analysis of factors associated with the progression of hospitalized patients with HFMD in Chengdu, China, 2019–2022
| Total (n) | B | S.E | Wald | df | P-value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Virus genotype | |||||||||
| CVA6 | 821 | 0.049 | 0.648 | 0.006 | 1 | 0.940 | 1.050 | 0.295 | 3.738 |
| CVA10 | 107 | 1.836 | 0.677 | 7.351 | 1 | 0.007 | 6.273 | 1.663 | 23.655 |
| Demographic characteristics | |||||||||
| Sex | 636 | 0.384 | 0.337 | 1.296 | 1 | 0.255 | 1.468 | 0.758 | 2.841 |
| Clinical manifestations | |||||||||
| Fever | 846 | 0.841 | 0.656 | 1.646 | 1 | 0.199 | 2.320 | 0.642 | 8.387 |
| Hyperpyrexia | 433 | 0.482 | 0.342 | 1.982 | 1 | 0.159 | 1.619 | 0.828 | 3.168 |
| Site of rash | |||||||||
| Oral | 1008 | -1.464 | 0.425 | 11.868 | 1 | 0.001 | 0.231 | 0.101 | 0.532 |
| Lower limbs | 270 | 0.314 | 0.359 | 0.764 | 1 | 0.382 | 1.368 | 0.677 | 2.764 |
| Constant | 1073 | -3.529 | 0.842 | 17.558 | 1 | 0.000 | 0.029 | - | - |
All the data indicated in bold are values of P < 0.05 that are considered statistically significant. B, Regression coefficient; S.E, Standard error; Wald, Wald statistic; df, Degrees of freedom; OR, odds ratio; CI, confidence interval.
Discussion
This study presents a comprehensive investigation into the epidemiology, etiology, and clinical features of HFMD outpatient cases caused by CVA6, CVA10, and CVA16 serotypes in the Chengdu, 2019–2022, thereby addressing a gap in epidemiological research on mild outpatient cases. We observed that CVA6 (76.51%, 821/1,073) has become the main serotype in recent years. Moreover, some outpatient cases were at high risk of progressing to severe hospitalization, with CVA10-induced cases posing the highest risk. These findings contribute valuable information to HFMD clinical practice, facilitating improved prevention and treatment outcomes.
Our study discovered that the monthly peak incidence of HFMD in 2019, 2021, and 2022 primarily occurred from March to August, aligning with previous studies conducted in other Chinese provinces [18–22]. However, the monthly peak incidence in 2020 spanned September to November because of the absence of HFMD case admissions from April to June as well as February of that year. This observation might be attributed to the strict prevention and control measures implemented in Chengdu, China, during the COVID-19 pandemic, which reduced social gatherings and consequently the transmission of HFMD.
Moreover, the monthly distribution of CVA10 and CVA16 serotypes in HFMD outpatient cases predominantly occurred from May to August. Conversely, the monthly distribution of the CVA6 serotype demonstrated more significant fluctuations, which were dispersed throughout all months. It is noteworthy that during 2020, patients induced by CVA6 (95.17%, 256/269) were primarily concentrated from September to December, analogous to another study focusing on hospitalized cases in Chengdu, China [23]. Substantial differences in the median age of patients infected with CVA6, CVA10, and CVA16 were observed, with CVA6-associated HFMD patients being younger than those affected by other serotypes and exhibiting a median (P25, P75) age of 25.23 months (15.00, 33.00). The findings of our study underscore the significance of prioritizing the prevention and control of HFMD caused by distinct pathogens based on monthly and seasonal patterns, while concurrently formulating personalized vaccination strategies according to age and gender disparities.
Our study did not identify any HFMD cases caused by EV-A71, and the proportion of outpatient cases progressing to hospitalization was relatively small (4.19%, 45/1,073). As the patients in this study were all from the period following the large-scale promotion of the inactivated EV-A71 vaccine in China starting in 2016 [8], we speculate that widespread vaccination with the inactivated EV-A71 vaccine may have played a role in reducing both proportions of EV-A71 associated cases and severe HFMD cases. However, further research is necessary to establish a more definitive and direct link between the EV-A71 vaccine and its impact in reducing severe cases. In line with previous research in other Chinese provinces [20, 24, 25], our study demonstrated that CVA6 has emerged as the prevailing serotype in Chengdu's HFMD outpatient cases, with CVA16 and CVA10 potentially being moderately prevalent serotypes. This finding highlights the urgent need for the development of multivalent vaccines to prevent and control emerging enterovirus infections.
Our research concluded that patients with CVA10 genotype infection were more likely to progress to hospitalization, consistent with the findings of Liu et al. of outpatients [26]. Nevertheless, a study in Zunyi, China, focusing on hospitalized cases, reported that the incidence of high fever and severe infection was higher with CVA2, CVA5, and CVA6 [27], suggesting that further evidence regarding the relationship between viral genotypes and HFMD severity is needed. It is necessary to explore the underlying biological mechanisms.
In this study, we observed that outpatient cases that did not progress to hospitalization were more likely to exhibit oral and hand rashes (P < 0.05), aligning with recent research on severe HFMD in Guangxi, China, and a previous study of inpatients and outpatients in southern Vietnam [4, 28]. We also found that the presence of oral rashes was associated with a reduced risk of hospitalization, similar to a study in Chongqing, China, which focused on hospitalized cases, implying that the presence of vesicles on the mouth or cheeks might act as a protective factor [29]. The presence of oral rashes may indicate an active local immune response [30]. However, the extent to which this response influences viral replication and disease progression in HFMD is not well-established and warrants further investigation. Besides, the presence of a rash in the mouth or other areas of the body may also motivate parents to bring their children to the doctor for active medical treatment, and doctors may be able to diagnose it more easily, thus reducing the risk of severe HFMD and reducing the risk of hospitalization. Prior studies have also identified breastfeeding, early diagnosis, and heightened vigilance as protective factors against severe HFMD mortality [14, 31]. These findings can help clinicians assess case risks during treatment, leading to more effective treatment strategies. Further research should focus on investigating these factors in larger samples and broader contexts to better guide prevention and intervention strategies.
This study had some limitations. First, only typing of four main HFMD viruses (EV-A71, CVA6, CVA10, and CVA16) in the hospital may limit the generalizability of the study results for other enterovirus serotypes. However, these serotypes are the most frequently identified causes of HFMD worldwide and are accountable for the majority of severe cases and fatalities, thereby making them of particular clinical importance. Future research may benefit from a more expansive focus that includes a broader range of enteroviruses. Second, the strict prevention and control measures implemented in China during the COVID-19 pandemic may have motivated an underestimation of the incidence of HFMD outpatient cases. Third, there was a potential for bias because of asymptomatic patients may not seek hospital-based care, inherently constraining the completeness of our datasets. Lastly, because the data originated from a single medical institution, the study results may not be generalizable to the entire region. Further multi-center studies would help validate and generalize our findings.
Conclusions
This study offers crucial insights into the epidemiological, etiological, and clinical features of HFMD outpatient cases induced by multiple viral serotypes, providing valuable reference information for public health departments in the prevention and control of HFMD, and laying the groundwork for the development of future multivalent vaccines. Vigilant monitoring of mild HFMD cases in outpatients, predominantly caused by the prevalent serotype CVA6, is crucial for outbreak prevention. Clinically, enhanced surveillance of CVA10 is imperative to mitigate the hospitalization rates associated with HFMD.
Supplementary Information
Supplementary Material 1: Table S1. Pathogen distribution of HFMD outpatients in Chengdu, China, 2019-2022. Fig S1. Clinical images of typical HFMD patients. Rashes and ulcerations on the a) hand and b-c) foot of typical HFMD patients. Fig S2. Age distribution of 45 inpatients progressing from outpatients with HFMD. Fig S3. Distribution of serotypes among outpatients with different outcomes. Fig S4. ROC Curve for Predicting Hospitalization in HFMD Patients.
Acknowledgements
Our sincere gratitude extends to the West China Second University Hospital, Sichuan University, for their generous support in providing essential clinical data that greatly facilitated this study.
Abbreviations
- CV
Coxsackievirus
- EV
Enterovirus
- HFMD
Hand, foot and mouth disease
- HEVs
Human enteroviruses
- MFR
Male to female ratio
- NA
Not applicable
- rRT-PCR
Real-time reverse-transcription polymerase chain reaction
- TICA
Time interval from clinic visit to admission
- TIOC
Time interval from onset to clinic
- TIOA
Time interval from onset to admission
Authors’ contributions
LL made significant contributions to the study's conceptualization, design, literature searches, knowledge content definition, manuscript editing, and review. MYX, YZ, JL, and SRZ conceptualized and designed the study, conducted literature searches, collected and analyzed data, performed statistical analysis, and prepared the manuscript. DHY and PG conducted literature searches and collected data. SHZ conducted preliminary and statistical analyses. GYJ assisted with literature searches and data collection. YC was responsible for result collation and preliminary analysis. JTM, ZHC, YL, XJL, YLZ, and CYZ contributed to manuscript editing and review. All authors reviewed the manuscript.
Funding
This work received support from the National Natural Science Foundation of China (Grant 81903375), the China Postdoctoral Science Foundation (Grant 2018M643509), the Chengdu Science and Technology Bureau (Grant 2019-YF05-01080-SN), and the National College Students Innovation and Entrepreneurship Training Program (Grant 202310610212).
Data availability
The datasets used and analyzed in the current study are not publicly available due to restrictions applied to the availability of these data but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the West China Second University Hospital, Sichuan University. Due to the fact that this study was a retrospective study with no direct contact with human beings or human tissue samples and the data used in the study were medical record data, the need for informed consent from individual patients was waived by the Ethics Committee of the West China Second University Hospital, Sichuan University. No confidential information was involved in this research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maoyao Xia, Yu Zhu, Juan Liao and Shirong Zhang contributed equally and shared the first authorship.
References
- 1.Song C, Li Y, Zhou Y, Liang L, Turtle L, Wang F, et al. Enterovirus genomic load and disease severity among children hospitalised with hand, foot and mouth disease. EBioMedicine. 2020;62:103078. 10.1016/j.ebiom.2020.103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao K, Duan L, Peng Y, Wu M, Mai G, Yan Z, et al. Epidemiologic features of enterovirus associated with hand, foot and mouth disease in 2013 and 2014 in Shenzhen, China. Sci Rep. 2019;9(1):3856. 10.1038/s41598-019-40402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Chen J, Lu Z, Huang S, Zhang S, Cai J, et al. Enterovirus A71 utilizes host cell lipid beta-oxidation to promote its replication. Front Microbiol. 2022;13:961942. 10.3389/fmicb.2022.961942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoang MTV, Nguyen TA, Tran TT, Vu TTH, Le NTN, Nguyen THN, et al. Clinical and aetiological study of hand, foot and mouth disease in southern Vietnam, 2013–2015: Inpatients and outpatients. Int J Infect Dis. 2019;80:1–9. 10.1016/j.ijid.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi K, Koike S. Cellular receptors for enterovirus A71. J Biomed Sci. 2020;27(1):23. 10.1186/s12929-020-0615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thammasonthijarern N, Kosoltanapiwat N, Nuprasert W, Sittikul P, Sriburin P, Pan-Ngum W et al. Molecular Epidemiological Study of Hand, Foot, and Mouth Disease in a Kindergarten-Based Setting in Bangkok, Thailand. Pathogens. 2021;10(5). 10.3390/pathogens10050576. [DOI] [PMC free article] [PubMed]
- 7.Zhu P, Ji W, Li D, Li Z, Chen Y, Dai B, et al. Current status of hand-foot-and-mouth disease. J Biomed Sci. 2023;30(1):15. 10.1186/s12929-023-00908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Jiang L, Zhang C, He W, Tan Y, Ning C. The changes in the epidemiology of hand, foot, and mouth disease after the introduction of the EV-A71 vaccine. Vaccine. 2021;39(25):3319–23. 10.1016/j.vaccine.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wang Q, Chen Z, Duan X, Han Y, Luan R, et al. Viral shedding in patients with hand, foot and mouth disease induced by EV71, CA16, or CA6: A protocol for systematic review and meta analysis. Medicine (Baltimore). 2020;99(29):e21258. 10.1097/md.0000000000021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Zhou J, Xie G, Zheng S, Lou B, Chen Y, et al. The Epidemiological and Clinical Characteristics of Hand, Foot, and Mouth Disease in Hangzhou, China, 2016 to 2018. Clin Pediatr (Phila). 2020;59(7):656–62. 10.1177/0009922820910822. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Dai B, Han S, Duan G, Yang H, Chen S et al. Arising Concerns of Atypical Manifestations in Patients with Hand, Foot, and Mouth Disease. Vaccines (Basel) 2023, 11(2). 10.3390/vaccines11020405. [DOI] [PMC free article] [PubMed]
- 12.Liu B, Luo L, Yan S, Wen T, Bai W, Li H, et al. Clinical Features for Mild Hand, Foot and Mouth Disease in China. PLoS ONE. 2015;10(8):e0135503. 10.1371/journal.pone.0135503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Ding S, Xie W, Wang T, Qin Y, Zheng J, et al. Epidemiological and etiological characteristics of mild hand, foot and mouth disease in children under 7 years old, Nanjing, China, 2010–2019. Arch Public Health. 2022;80(1):220. 10.1186/s13690-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long L, Chen Z. Risk factors for death in children with severe hand, foot, and mouth disease in rural area in Sichuan. China International Journal of Infectious Diseases. 2018;73:213–213. 10.1016/j.ijid.2018.04.3898. [Google Scholar]
- 15.Li Y, Zhou Y, Cheng Y, Wu P, Zhou C, Cui P, et al. Effectiveness of EV-A71 vaccination in prevention of paediatric hand, foot, and mouth disease associated with EV-A71 virus infection requiring hospitalisation in Henan, China, 2017–18: a test-negative case-control study. Lancet Child Adolesc Health. 2019;3(10):697–704. 10.1016/S2352-4642(19)30185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XW, Ni X, Qian SY, Wang Q, Jiang RM, Xu WB et al. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J Pediatr. 2018;14(5):437–447. 10.1007/s12519-018-0189-8. [DOI] [PubMed]
- 17.Spratling R, Powers E. Development of a Data Abstraction Form: Getting What You Need From the Electronic Health Record. J Pediatr Health Care. 2017;31(1):126–30. 10.1016/j.pedhc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Shan J, Ji H, Zhang J, Yang H, Shen Q, et al. Study of the epidemiology and etiological characteristics of hand, foot, and mouth disease in Suzhou City, East China, 2011–2014. Arch Virol. 2016;161(7):1933–43. 10.1007/s00705-016-2878-8. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Zhang X, Chen X, Cheng YP, Wu YD, Shu Q, et al. Epidemiology of childhood enterovirus infections in Hangzhou. China Virol J. 2015;12:58. 10.1186/s12985-015-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu HY, Cai MY, Ge W, Li XL, Qiao R. Epidemiological characteristics and etiological monitoring results of hand, foot, and mouth disease in a hospital in Shanghai from 2016 to 2018. Laboratory Medicine and Clinic. 2023;20(1):4–8. 10.3969/j.issn.1672-9455.2023.01.002.[inChinese]. [Google Scholar]
- 21.Cai J, Lv H, Lin J, Chen Z, Fang C, Han J. Enterovirus infection in children attending two outpatient clinics in Zhejiang Province. China J Med Virol. 2014;86(9):1602–8. 10.1002/jmv.23884. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Huang C, Luo D, Yang J, Shi Y, Li D, et al. Clinical Characteristics and Treatment Overview in Hand-Foot-and-Mouth Disease Using Real-World Evidence Based on Hospital Information System. Evid Based Complement Alternat Med. 2022;2022:9156186. 10.1155/2022/9156186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan X, Zhang C, Wang X, Ren X, Peng H, Tang X, et al. Molecular epidemiology and clinical features of hand, foot and mouth disease requiring hospitalization after the use of enterovirus A71 inactivated vaccine in chengdu, China, 2017–2022: a descriptive study. Emerg Microbes Infect. 2022;11(1):2510–9. 10.1080/22221751.2022.2125346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui P, Li Y, Zhou CC, Zhou YH, Song CL, Qiu Q, et al. Clinical analysis of 555 outpatient cases of hand, foot, and mouth disease caused by different enteroviruses. Chinese Journal of Pediatrics. 2019;57(6):445–51. 10.3760/cma.j.issn.0578-1310.2019.06.009.[inChinese]. [DOI] [PubMed] [Google Scholar]
- 25.Hu HY, Cai MY, Ge W. Epidemiological characteristics analysis of hand, foot, and mouth disease pathogens from 2016 to 2019. Chin J Exp Clin Virol. 2021;35(3):291–5. 10.3760/cma.j.cn112866-20200720-00210.[inChinese]. [Google Scholar]
- 26.Liu Y, Ren XM, Tian ML. Clinical characteristics analysis of hand, foot, and mouth disease caused by three different Coxsackieviruses. Journal of Shandong First Medical University (Shandong Academy of Medical Sciences. 2022;43(04):277–281. 10.3969/j.issn.2097-0005.2022.04.007.
- 27.Ai Y, Zhang W, Wu J, Zhang J, Shen M, Yao S, et al. Molecular Epidemiology and Clinical Features of Enteroviruses-Associated Hand, Foot, and Mouth Disease and Herpangina Outbreak in Zunyi, China, 2019. Front Med (Lausanne). 2021;8:656699. 10.3389/fmed.2021.656699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Y, He W, Zheng Z, Pan P, Ju Y, Lu Z, et al. Factors related to the mortality risk of severe hand, foot, and mouth diseases (HFMD): a 5-year hospital-based survey in Guangxi, Southern China. BMC Infect Dis. 2023;23(1):144. 10.1186/s12879-023-08109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Zhao H, Ou R, Zhu H, Gan L, Zeng Z, et al. Epidemiological and clinical characteristics of severe hand-foot-and-mouth disease (HFMD) among children: a 6-year population-based study. BMC Public Health. 2020;20(1):801. 10.1186/s12889-020-08961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hickman HD, Moutsopoulos NM. Viral infection and antiviral immunity in the oral cavity. Nat Rev Immunol. 2024. 10.1038/s41577-024-01100-x. [DOI] [PubMed] [Google Scholar]
- 31.Long L, Gao L-D, Hu S-X, Luo K-W, Chen Z-H, Ronsmans C, et al. Risk factors for death in children with severe hand, foot, and mouth disease in Hunan. China Infectious Diseases. 2016;48(10):744–8. 10.1080/23744235.2016.1185801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1. Pathogen distribution of HFMD outpatients in Chengdu, China, 2019-2022. Fig S1. Clinical images of typical HFMD patients. Rashes and ulcerations on the a) hand and b-c) foot of typical HFMD patients. Fig S2. Age distribution of 45 inpatients progressing from outpatients with HFMD. Fig S3. Distribution of serotypes among outpatients with different outcomes. Fig S4. ROC Curve for Predicting Hospitalization in HFMD Patients.
Data Availability Statement
The datasets used and analyzed in the current study are not publicly available due to restrictions applied to the availability of these data but are available from the corresponding author on reasonable request.