Abstract
The kinetics of ethanol oxidation by NAD+, and acetaldehyde and butyraldehyde reduction by NADH, catalysed by yeast alcohol dehydrogenase, were studied in the pH range 4.9--9.9 at 25 degrees C and in the temperature range 14.8--43.5 degrees C at pH 7.05. The kinetics of reduction of acetaldehyde by [4A-2H]NADH at pH 7.05 and pH 8.9 at 25 degrees C were also studied. The results of the kinetic experiments indicate that the mechanism of catalysis, previously proposed on the basis of studies at pH 7.05 and 25 degrees C (Dickinson & Monger, 1973), applies over the wide range of conditions now tested. Values of some of the initial-rate parameters obtained were used to deduce information about the pH- and temperature-dependence of the specific rates of combination of enzyme and coenzymes and of the dissociation of the enzyme--coenzyme compounds. Primary and secondary plots of initial-rate data are deposited as Supplementary Publication SUP 50043 (20 pages) with the British Library (Lending Division), Boston Spa, Wetherby, Yorks. LS23 7BQ, U.K., from whom copies may be obtained under the terms indicated in Biochem. J. (1975) 145, 5.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley P., Dickinson F. M. A study of the kinetics and mechanism of rabbit muscle L-glycerol 3-phosphate dehydrogenase. Biochem J. 1974 Oct;143(1):19–27. doi: 10.1042/bj1430019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The preparation and properties of crystalline alcohol dehydrogenase from liver. Biochem J. 1961 Aug;80:440–445. doi: 10.1042/bj0800440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem J. 1966 Jul;100(1):34–46. doi: 10.1042/bj1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
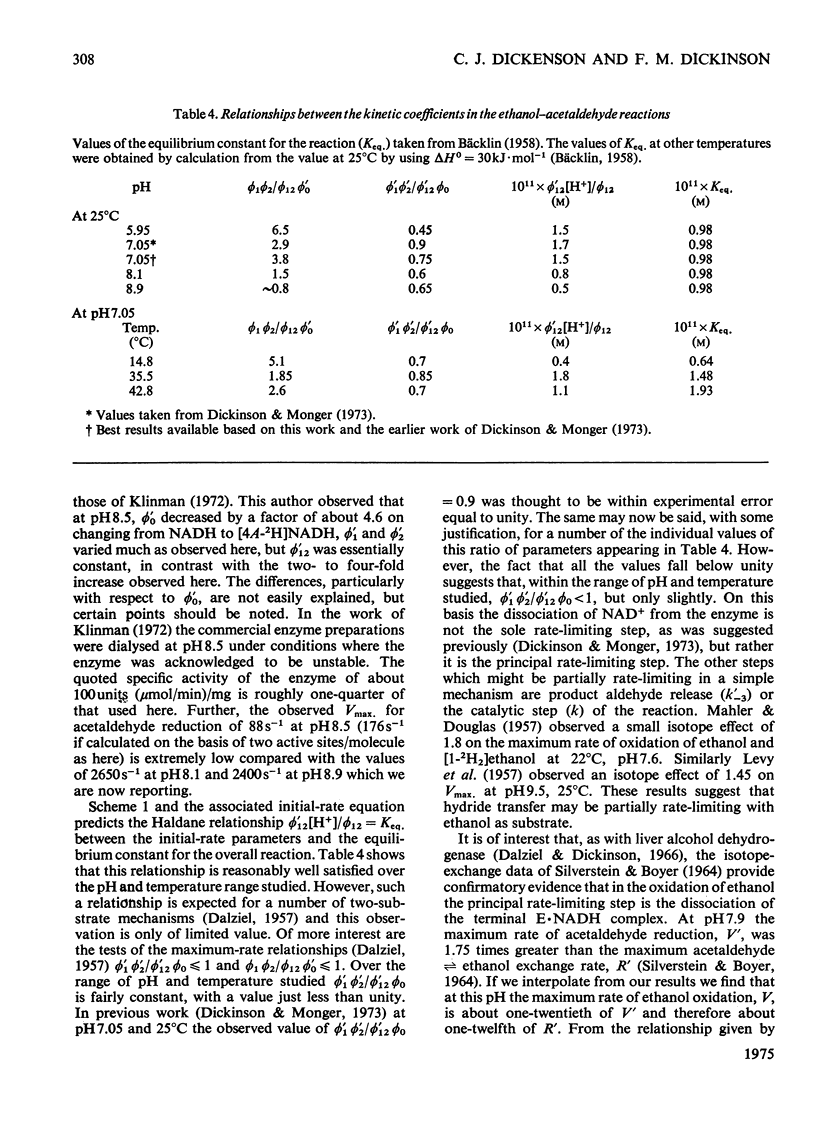
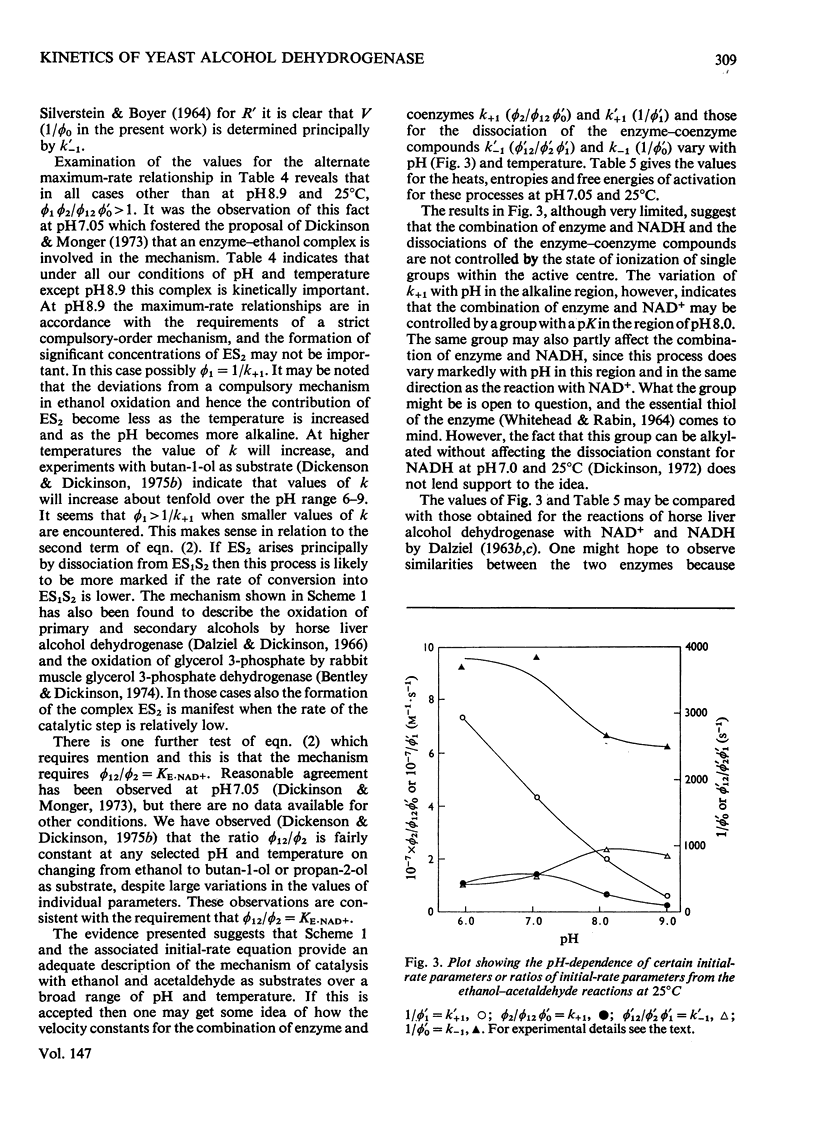
- Dickinson F. M., Monger G. P. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261–270. doi: 10.1042/bj1310261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. Role of the essential thiol groups of yeast alcohol dehydrogenase. Biochem J. 1972 Jan;126(1):133–138. doi: 10.1042/bj1260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. The binding of dihydronicotinamide--adenine dinucleotide and pyridine-3-aldehyde--adenine dinucleotide by yeast alcohol dehydrogenase. Biochem J. 1970 Dec;120(4):821–830. doi: 10.1042/bj1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson M. Measurements of the concentration of active sites in preparations of yeast alcohol dehydrogenase. Eur J Biochem. 1974 Jan 3;41(1):31–36. doi: 10.1111/j.1432-1033.1974.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. The use of ternary complexes to study ionizations and isomerizations during catalysis by lactate dehydrogenase. Biochem J. 1973 Apr;131(4):739–748. doi: 10.1042/bj1310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Partial similarities between yeast and liver alcohol dehydrogenases. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2295–2298. doi: 10.1073/pnas.70.8.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman J. P. The mechanism of enzyme-catalyzed reduced nicotinamide adenine dinucleotide-dependent reductions. Substituent and isotope effects in the yeast alcohol dehydrogenase reaction. J Biol Chem. 1972 Dec 25;247(24):7977–7987. [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF LIVER AND YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1964 Nov;239:3908–3914. [PubMed] [Google Scholar]
- Shore J. D., Gutfreund H., Brooks R. L., Santiago D., Santiago P. Proton equilibria and kinetics in the liver alcohol dehydrogenase reaction mechanism. Biochemistry. 1974 Sep 24;13(20):4185–4191. doi: 10.1021/bi00717a019. [DOI] [PubMed] [Google Scholar]
- Whitehead E. P., Rabin B. R. The thiol groups of yeast alcohol dehydrogenase. Biochem J. 1964 Mar;90(3):532–539. doi: 10.1042/bj0900532. [DOI] [PMC free article] [PubMed] [Google Scholar]


