Dear Editor,
We would like to thank Wei et al. [1] for their interest in our recently published correspondence in Critical Care [2]. The authors share our enthusiasm for the comparison of baricitinib and tocilizumab therapies in patients with coronavirus disease 2019 (COVID-19) receiving mechanical ventilation (MV) and agree that the study findings are important. However, they raised several issues with respect to the methodology regarding confounding by indication.
The authors commented that the tocilizumab group had a higher severity of illness, which might have led to a bias in the outcome assessment of the baricitinib and tocilizumab groups, even after propensity score (PS) matching. Indeed, the tocilizumab group was more likely to exhibit higher Charlson Comorbidity Index and renal dysfunction, along with a greater frequency of renal replacement therapy than those of the baricitinib group (Table S1 in the paper) [2]. However, contrary to the authors’ concerns, the patients in the baricitinib group were more likely to receive neuromuscular blocking agents and extracorporeal membrane oxygenation. Hence, we respectfully disagree, at least in part, with their claim that tocilizumab was preferentially administered to patients with rapidly progressing or refractory conditions. In the Korean National Health Insurance Service database [3], it is not feasible to temporarily associate MV with drug administration (baricitinib or tocilizumab) during hospitalization due to the lack of timestamps. Hence, it was not possible to assess whether the duration of MV prior to drug administration was associated with the outcomes in our study.
We agree with the authors’ opinion that the study design was vulnerable to unmeasured confounders, although the groups were balanced with regard to the measured confounders using a robust model such as PS analysis. However, the current guidelines are based on the results of analyses that do not include direct comparison between the two drugs [4, 5]. Thus, observational studies are useful for providing data regarding the effectiveness of baricitinib and tocilizumab in patients with critical COVID-19. We also agree that the differences in treatment duration and pharmacodynamics may have resulted in a more favorable response to baricitinib. In fact, multiple oral administrations of baricitinib may potentially exhibit consistent drug concentrations, even in cases of gastrointestinal dysfunction commonly observed in critically ill patients [6].
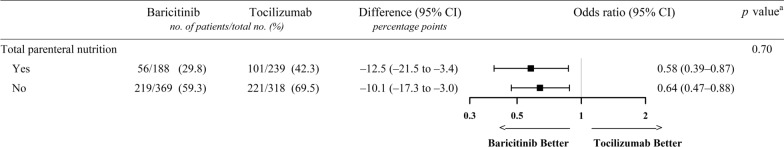
Since the intolerance to enteral nutrition might reflect a more critical condition, we conducted a subgroup analysis of 30-day mortality according to total parenteral nutrition (TPN) therapy (yes or no) in response to the authors’ suggestion regarding the exclusion of patients who received TPN. TPN use was identified using the relevant procedure codes (aseptic preparation fee of parenteral nutrition [J0042] and/or nutrition support team consultation fee [AI600 and AI700]) [7]. A higher percentage of patients in the tocilizumab group received TPN than that in the baricitinib group (239/557 [42.9%] vs 188/557 [33.8%], respectively; standardized mean difference = 1.01). However, regardless of TPN use, patients who received baricitinib exhibited significantly lower mortality rates than of those who received tocilizumab (Fig. 1). Notably, patients who received TPN experienced lower mortality rates, thereby indicating that intravenous therapies may not be ideal surrogates of disease severity. This finding may also be attributed to the difficulties in providing active nutritional support during the COVID-19 pandemic [8].
Fig. 1.
Association of baricitinib therapy on 30-day mortality according to total parenteral nutrition. Odds ratios (represented by squares) and 95% CIs (corresponding lines through them) were calculated for the propensity score-matched baricitinib (n = 557) and tocilizumab (n = 557) groups. a p values are for the interaction term. CI confidence interval
In conclusion, our study demonstrates that baricitinib may be a promising therapy for the treatment of patients with COVID-19 on MV. However, we agree with the authors’ observation that future studies would require more granular data, such as vital signs and laboratory values, to evaluate the association with baseline severity between the baricitinib and tocilizumab groups. Additionally, data on the timing of MV initiation and drug administration would be helpful in assessing the effects of early or late administration of baricitinib in patients requiring oxygen or MV. Finally, baricitinib or tocilizumab concentrations and inflammatory cytokine levels should be measured to enhance our understanding of the relationship between drugs and clinical response.
Acknowledgements
This study used the database of the Korea Disease Control and Prevention Agency and the National Health Insurance Service for policy and academic research (KDCA-NHIS-2023-1-488).
Abbreviations
- COVID-19
Coronavirus disease 2019
- MV
Mechanical ventilation
- PS
Propensity score
- TPN
Total parenteral nutrition
Funding
This research was supported by the National Research Foundation of Korea grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (2022R1F1A1067609). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study protocol for the utilization of de-identified patient data was exempt from review by the Institutional Review Board of Chung-Ang University (1041078-20230306-HR-055).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sun-Young Jung, Email: jsyoung@cau.ac.kr.
Won-Young Kim, Email: wykim81@cau.ac.kr.
References
- 1.Wei JCC, Kuo P, Shih PC. Commenting on baricitinib versus tocilizumab in mechanically ventilated patients with COVID-19: a nationwide cohort study. Crit Care. 2024;28:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.You SH, Baek MS, Kim TW, Jung SY, Kim WY. Baricitinib versus tocilizumab in mechanically ventilated patients with COVID-19: a nationwide cohort study. Crit Care. 2024;28:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Insurance Sharing Service. https://nhiss.nhis.or.kr/en/z/a/001/lpza001m01en.do (2024). Accessed 23 Nov 2024.
- 4.U.S. Food and Drug Administration: Coronavirus (COVID-19) update: FDA authorizes drug combination for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19 (2020). Accessed 23 Nov 2024.
- 5.U.S. Food and Drug Administration: Coronavirus (COVID-19) update: FDA authorizes drug for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-treatment-covid-19 (2021). Accessed 23 Nov 2024.
- 6.Reintam Blaser A, Poeze M, Malbrain ML, Bjorck M, Oudemans-van Straaten HM, Starkopf J, et al. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med. 2013;39:899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheon S, Oh SH, Kim JT, Choi HG, Park H, Chung JE. Nutrition therapy by nutrition support team: a comparison of multi-chamber bag and customized parenteral nutrition in hospitalized patients. Nutrients. 2023;15:2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tetamo R, Fittipaldi C, Buono S, Umbrello M. Nutrition support for critically ill patients during the COVID-19 pandemic: the Italian SIAARTI survey. J Anesth Analg Crit Care. 2022;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.