Abstract
PURPOSE
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), delaying progression to castration-resistant disease is important not only for overall survival (OS) but also for patients' quality of life. Darolutamide plus androgen-deprivation therapy (ADT) with docetaxel improved OS versus ADT and docetaxel in patients with mHSPC. The ARANOTE trial evaluated darolutamide and ADT without chemotherapy in patients with mHSPC.
METHODS
In this global phase III trial, patients were randomly assigned 2:1 to receive darolutamide 600 mg twice daily or placebo, with concomitant ADT. The primary end point was radiological progression-free survival (rPFS).
RESULTS
From March 2021 to August 2022, 669 patients were randomly assigned (darolutamide n = 446; placebo n = 223). At the primary cutoff date (June 7, 2024), darolutamide plus ADT significantly improved rPFS, reducing the risk of radiological progression or death by 46% versus placebo plus ADT (hazard ratio [HR], 0.54 [95% CI, 0.41 to 0.71]; P < .0001), with consistent benefits across subgroups, including high- and low-volume disease. OS results were suggestive of benefit with darolutamide versus placebo (HR, 0.81 [95% CI, 0.59 to 1.12]), and clinical benefits were seen across all other secondary end points, including delayed time to metastatic castration-resistant prostate cancer (HR, 0.40 [95% CI, 0.32 to 0.51]) and time to pain progression (HR, 0.72 [95% CI, 0.54 to 0.96]). Adverse events were similar in the two groups. Notably, the incidence of fatigue was lower in patients receiving darolutamide (5.6%) versus those receiving placebo (8.1%), and fewer patients receiving darolutamide (6.1%) versus placebo (9.0%) discontinued treatment because of adverse events.
CONCLUSION
These results confirm the efficacy and tolerability of darolutamide plus ADT in patients with mHSPC, demonstrating clinically and statistically significant improvement in rPFS and a favorable safety profile consistent with prior phase III darolutamide trials.
Darolutamide + ADT improved rPFS versus ADT in ARANOTE, offering options with/without docetaxel for mHSPC.
INTRODUCTION
Androgen-deprivation therapy (ADT) has been the standard of care for patients with metastatic hormone-sensitive prostate cancer (mHSPC) for decades.1,2 However, despite initial response to ADT monotherapy, most patients develop metastatic castration-resistant prostate cancer (mCRPC) on average within 1 year,3-6 which is associated with poor prognosis and declining health-related quality of life.7 Several phase III trials have demonstrated improved overall survival (OS) and delayed progression to mCRPC when ADT is combined with an androgen receptor pathway inhibitor (abiraterone acetate, enzalutamide, or apalutamide), and the ARASENS and PEACE-1 trials have demonstrated survival benefits with the triplet combination of darolutamide or abiraterone, respectively, plus ADT and docetaxel.5,6,8-15 However, these doublet and triplet regimens are underutilized, and many patients with mHSPC continue to receive treatment with ADT alone because of concerns about drug accessibility, tolerability, safety, drug-drug interactions, and health care provider education.16-19 Thus, an unmet need remains for treatments that delay progression to mCRPC with recognized tolerability.
CONTEXT
Key Objective
We evaluated the efficacy and safety outcomes of patients with metastatic hormone-sensitive prostate cancer treated with darolutamide plus androgen-deprivation therapy (ADT) versus ADT alone.
Knowledge Generated
Darolutamide plus ADT significantly improved radiological progression-free survival, reducing the risk of radiological progression or death by 46% versus placebo plus ADT and demonstrated clear clinical benefit in secondary efficacy end points, including time to castration-resistant prostate cancer and time to pain progression. Incidences of adverse events were low and similar in the darolutamide and placebo groups, with a lower incidence of fatigue in the darolutamide group.
Relevance (A. Necchi)
The safety profile of darolutamide and diverse geographical representation of the study population distinguished the ARANOTE trial. Both these features should be addressed by future studies aimed at clarifying the optimal therapeutic approach of patients with metastatic hormone-sensitive prostate cancer.*
*Relevance section written by JCO Associate Editor Andrea Necchi, MD.
Darolutamide is a structurally distinct and highly potent androgen receptor inhibitor that has low blood-brain barrier penetration and limited potential for drug-drug interactions,20-23 which may be advantageous for the mHSPC population with age-related comorbidities and polypharmacy requirements.22,24,25 In the phase III ARASENS trial in patients with mHSPC, darolutamide plus ADT and docetaxel significantly reduced the risk of death by 32.5% and significantly delayed progression to mCRPC versus placebo plus ADT and docetaxel, regardless of disease volume and prognostic risk subgroups.15,26 Darolutamide plus ADT also demonstrated improved metastasis-free survival and OS versus ADT alone in patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) in the phase III ARAMIS trial.27 In both trials, incidences of adverse events and treatment discontinuations due to adverse events were similar between the darolutamide and placebo groups,15,27,28 and darolutamide demonstrated long-term tolerability.29,30 The positive clinical results of the ARAMIS and ARASENS trials provided strong rationale to proceed with ARANOTE to assess the efficacy and safety of darolutamide plus ADT without chemotherapy in patients with mHSPC, which, if positive, should increase therapeutic options for patients with mHSPC.
METHODS
Trial Design
This global, randomized, double-blind, placebo-controlled phase III trial was sponsored by Bayer and Orion Pharma. The trial was designed by the first author and Bayer and included 133 sites in 15 countries across Asia, Latin America, Europe, Australia, New Zealand, Canada, and South Africa. Institutional review boards or ethics committees at each site approved the study protocol, protocol amendments, and relevant documents. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guideline. Written informed consent was obtained from all patients before enrollment.
An independent data monitoring committee reviewed unblinded safety data throughout the study. Study data were collected by the investigators, analyzed by Bayer statisticians, and interpreted by the authors, including Bayer employees. Bayer funded medical writing and editorial assistance. All authors reviewed and approved the submitted manuscript. The authors assume responsibility for the completeness and accuracy of the data and for the fidelity of the trial to the protocol and statistical analysis plan, which are available in the Protocol (online only).
Patients and Interventions
Adults age 18 years and older were eligible if they had histologically or cytologically confirmed adenocarcinoma of the prostate and metastatic disease was centrally confirmed by conventional imaging. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2; had adequate bone marrow, liver, and renal function; and could have started ADT within 12 weeks of random assignment. Patients were excluded if they had regional lymph node metastases only, had a baseline superscan, or had received androgen receptor pathway inhibitors or chemotherapy for prostate cancer. Subgroups on the basis of disease volume were defined according to CHAARTED, with high-volume disease defined by the presence of visceral metastases and/or at least four bone lesions with at least one beyond the vertebral bodies and pelvis.6 Full eligibility criteria are provided in Appendix 1 (online only).
All patients started ADT of the investigator's choice (luteinizing hormone-releasing hormone agonist or antagonist or orchiectomy) within 12 weeks before initiating study treatment. Patients were randomly assigned in a 2:1 ratio to receive darolutamide 600 mg twice daily or matched placebo and stratified on the basis of the presence of visceral metastases and use of prior local therapy. All patients received study drug until radiological disease progression, unacceptable toxicity, initiation of new anticancer therapy, patient or physician decision, or study drug interruption of more than 28 consecutive days.
Study Assessments
During treatment and active follow-up, patients were evaluated at clinic visits every 12 weeks. The primary end point was radiological progression-free survival (rPFS) on the basis of central review of conventional imaging and using Response Evaluation Criteria in Solid Tumors v1.1 for soft-tissue metastases31 and Prostate Cancer Working Group 3 criteria for bone metastases.32 rPFS was defined as the time from random assignment to the first documentation of radiological progressive disease in soft tissue or bone or death due to any cause.
Secondary efficacy end points were OS, time to initiation of subsequent systemic anticancer therapy for prostate cancer, time to mCRPC, time to prostate-specific antigen (PSA) progression, rates of PSA <0.2 ng/mL in patients with baseline PSA ≥0.2 ng/mL, and time to pain progression. The secondary efficacy end points are defined in Appendix 1. Adverse events were assessed from the first dose of study drug until 30 days after the last treatment and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
Statistical Analysis
Sample size calculations were based on determining a difference in the primary end point (rPFS) between the darolutamide and placebo groups. Approximately 665 patients were required to observe 214 progression events, allowing for a 33% dropout rate for rPFS follow-up, and to provide the trial with 90% power using a two-sided alpha of .05 with a hazard ratio (HR) of 0.625 for rPFS. The full analysis set included all randomly assigned patients grouped according to their treatment at random assignment, irrespective of actual treatment. The safety analysis set included all randomly assigned patients who received at least one dose of study drug and are analyzed according to the treatment they received.
The full analysis set was analyzed for rPFS using a stratified log-rank test with the random assignment stratification factors (visceral metastases and prior local therapy). The Cox regression model was used to determine stratified HRs and 95% CIs for the treatment comparison, and Kaplan-Meier estimates present rPFS at various time points with 95% CIs for both groups. Subgroup analyses of rPFS and OS were performed to determine the effect of demographic and baseline characteristics using an unstratified Cox regression model. Sensitivity analyses of rPFS are summarized in Appendix 1.
Secondary efficacy end points were tested for statistical significance using a hierarchical gatekeeping procedure only if the primary end point was statistically significant (two-sided alpha of .05) using the same alpha in the following order: OS, time to initiation of subsequent systemic anticancer therapy, time to mCRPC, time to PSA progression, rates of PSA <0.2 ng/mL, and time to pain progression. Secondary time-to-event end points were analyzed in a similar manner as the primary end point, and rates of PSA <0.2 ng/mL were compared between treatment groups using a stratified Cochran-Mantel–Haenszel test. An interim analysis of OS was conducted at the time of the primary rPFS analysis; final analysis of OS will be performed when approximately 180 events have occurred. The stopping boundaries for these two survival analyses will be calculated with an O'Brien-Fleming alpha-spending function based on the actual number of survival events observed up to the primary data cutoff date and the expected OS at final analysis. Descriptive statistics summarize demographic and baseline characteristics, rates of PSA <0.2 ng/mL, and adverse events by treatment group. Statistical evaluations were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC).
RESULTS
Patients
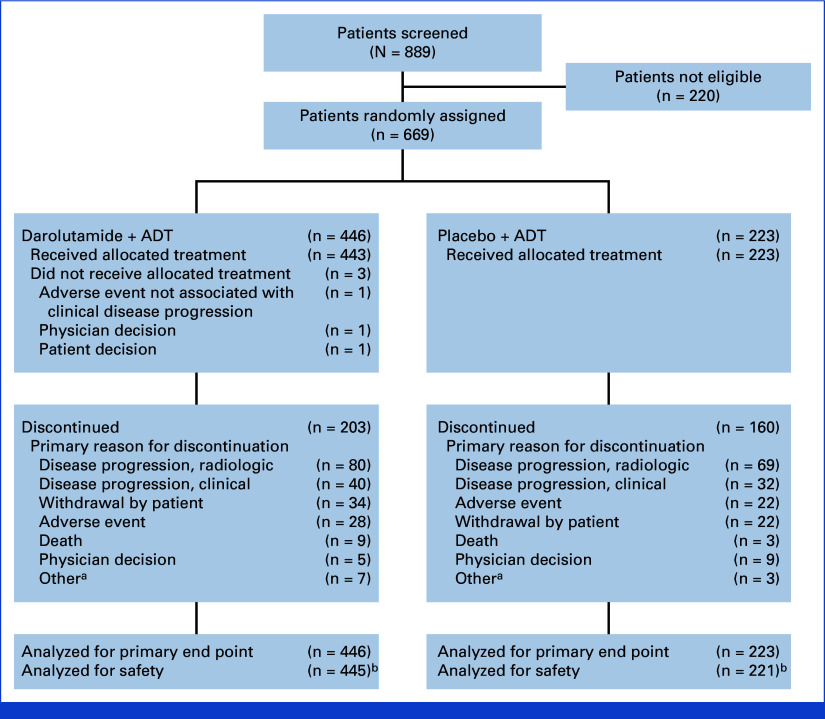
From March 2021 to August 2022, 669 patients were randomly assigned: 446 to darolutamide and 223 to placebo, both with ADT. The full analysis set included 669 patients (darolutamide, n = 446; placebo, n = 223) and the safety analysis set included 666 patients (darolutamide, n = 445; placebo, n = 221; Fig 1). Demographic and baseline characteristics of the patients were well balanced between the treatment groups with global representation (Table 1; Appendix Table A1). The median (range) age of patients was 70 (43-93) years, 31.2% of patients were Asian, and 9.7% were Black. Most patients had an ECOG performance status of 0 (49.8%) or 1 (47.2%), and 68.3% had a Gleason score of 8 or greater. The median PSA at baseline was 21.3 ng/mL (after ADT initiation within the previous 12 weeks). De novo metastatic disease was present in 72.5% of patients, and 12.0% of patients had visceral metastases by central review. At the data cutoff date for the primary analysis (June 7, 2024), the median treatment duration was 24.2 months in the darolutamide group compared with 17.3 months in the placebo group, with a greater proportion of patients in the darolutamide group (53.8%) still receiving study treatment than in the placebo group (28.3%). The median follow-up time was 25.3 months in the darolutamide group and 25.0 months in the placebo group.
FIG 1.
CONSORT flow diagram. aOther includes required study drug interruption longer than allowed per protocol, additional primary malignancy, noncompliance with study drug, loss to follow-up, and unspecified other reason. bTwo patients who were randomly assigned to the placebo group but received darolutamide are analyzed in the darolutamide group for the safety analysis set. ADT, androgen deprivation therapy.
TABLE 1.
Patient Demographic and Clinical Characteristics at Baseline (full analysis set)
| Characteristic | Darolutamide + ADT (n = 446) | Placebo + ADT (n = 223) |
|---|---|---|
| Median age (range), year | 70 (43-93) | 70 (45-91) |
| Age group, year, No. (%) | ||
| <65 | 118 (26.5) | 65 (29.1) |
| 65-74 | 193 (43.3) | 96 (43.0) |
| 75-84 | 117 (26.2) | 52 (23.3) |
| ≥85 | 18 (4.0) | 10 (4.5) |
| ECOG performance status, No. (%) | ||
| 0 | 235 (52.7) | 98 (43.9) |
| 1 | 199 (44.6) | 117 (52.5) |
| 2 | 12 (2.7) | 8 (3.6) |
| Race, No. (%) | ||
| White | 251 (56.3) | 125 (56.1) |
| Asian | 144 (32.3) | 65 (29.1) |
| Black | 41 (9.2) | 24 (10.8) |
| Other | 10 (2.2) | 9 (4.0) |
| Region, No. (%) | ||
| Asia | 141 (31.6) | 63 (28.3) |
| Latin American | 119 (26.7) | 72 (32.3) |
| Europe and rest of the world | 186 (41.7) | 88 (39.5) |
| Gleason score at initial diagnosis, No. (%) | ||
| <8 | 122 (27.4) | 67 (30.0) |
| ≥8 | 311 (69.7) | 146 (65.5) |
| Data missing | 13 (2.9) | 10 (4.5) |
| Metastasis stage at initial diagnosis,a No. (%) | ||
| De novo | 317 (71.1) | 168 (75.3) |
| Recurrent | 100 (22.4) | 45 (20.2) |
| Unknown | 29 (6.5) | 10 (4.5) |
| Extent of metastatic disease stage at screening, No. (%) | ||
| Nonregional lymph node metastases only | 17 (3.8) | 10 (4.5) |
| Bone metastases with or without lymph node metastases | 344 (77.1) | 171 (76.7) |
| Visceral metastases with or without lymph node metastases or with or without bone metastases | 85 (19.1) | 42 (18.8) |
| Disease volume,b No. (%) | ||
| High volume | 315 (70.6) | 157 (70.4) |
| Low volume | 131 (29.4) | 66 (29.6) |
| Median PSA level (range), ng/mLc | 21.4 (0.02-15,915) | 21.2 (0.02-8,533) |
| Median alkaline phosphatase level (range), U/L | 132.7 (34-4,286) | 147.0 (36-3,764) |
| Random assignment stratification factors | ||
| Visceral metastasesc | ||
| Present | 53 (11.9) | 27 (12.1) |
| Absent | 393 (88.1) | 196 (87.9) |
| Prior local therapy | ||
| Yes | 80 (17.9) | 40 (17.9) |
| No | 366 (82.1) | 183 (82.1) |
Abbreviations: ADT, androgen-deprivation therapy; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
Recurrent disease is defined as stage I to IVA, and de novo is defined as stage IVB at initial diagnosis.
Disease volume defined by CHAARTED criteria: presence of visceral metastases and/or at least four bone metastases with at least one beyond vertebral bodies and pelvis.6
Centrally assessed.
Primary End Point
The primary analysis of rPFS was performed after 222 patients had an rPFS event, with a smaller proportion of patients in the darolutamide group (128/446; 28.7%) compared with the placebo group (94/223; 42.2%). Darolutamide significantly improved rPFS, reducing the risk of radiological progression or death by 46% versus placebo (HR, 0.54 [95% CI, 0.41 to 0.71]; P < .0001; Fig 2A), with median rPFS not reached in the darolutamide group versus 25.0 months in the placebo group. The rPFS rates at 24 months were 70.3% in the darolutamide group and 52.1% in the placebo group. The benefit of darolutamide on rPFS was consistent across prespecified patient subgroups, including patients with high- and low-volume mHSPC (Fig 2B).
FIG 2.
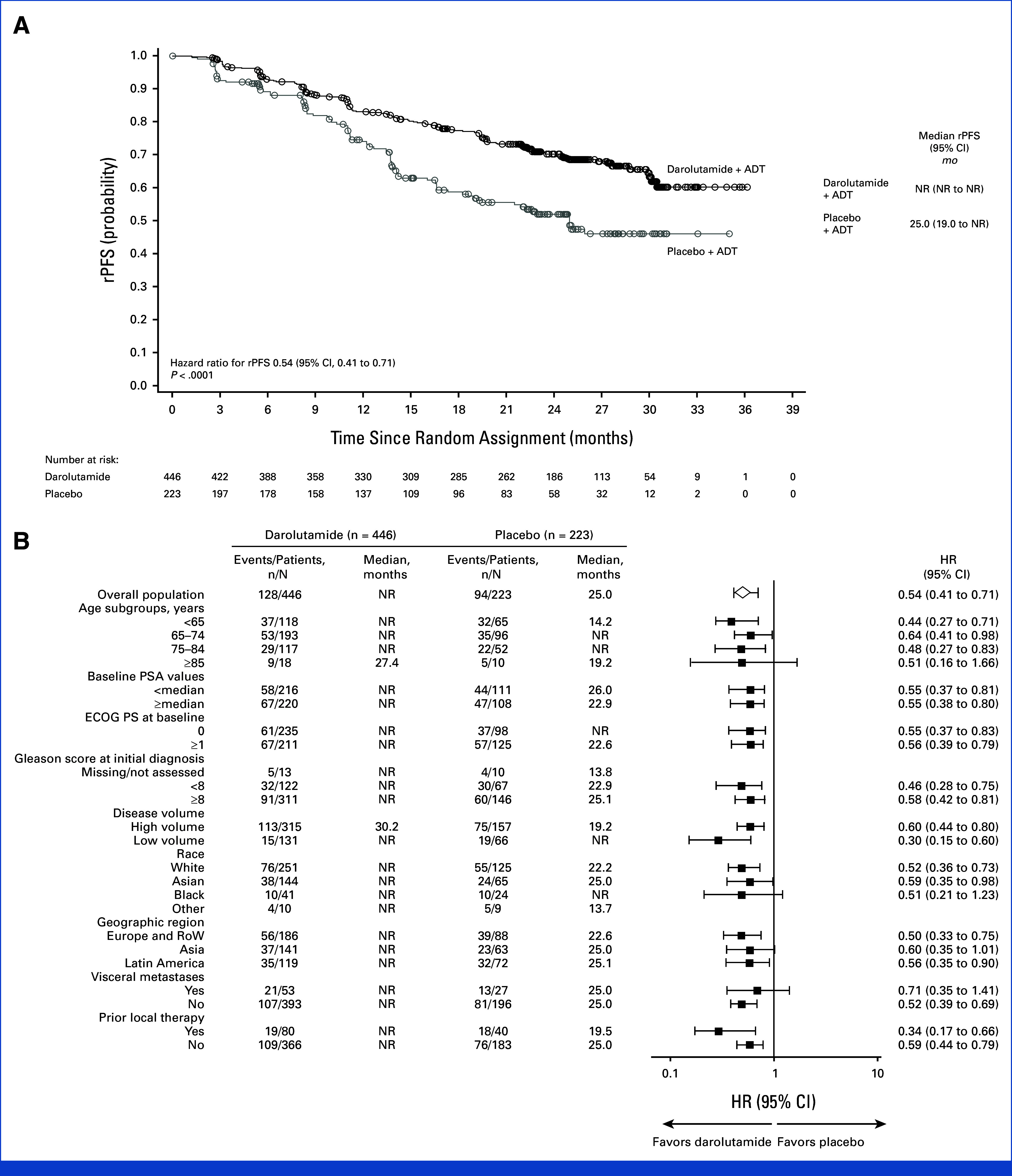
rPFS (full analysis set). (A) Kaplan-Meier estimates and (B) subgroup analyses. The HR and 95% CI were calculated using the Cox regression model stratified by the presence of visceral metastases and prior therapy. Subgroup analyses of rPFS provide HRs and 95% CIs obtained from univariate analysis using an unstratified Cox regression model. ADT, androgen-deprivation therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NR, not reached; PSA, prostate-specific antigen; RoW, rest of the world; rPFS, radiological progression-free survival.
Secondary Efficacy End Points
At the primary analysis data cutoff date after 163 deaths (103/446 [23.1%] in the darolutamide group and 60/223 [26.9%] in the placebo group), results for OS were suggestive of a benefit with darolutamide versus placebo (HR, 0.81 [95% CI, 0.59 to 1.12]; Table 2) which was generally consistent across prespecified subgroups (Appendix Fig A1). The OS rates at 24 months were 79.8% in the darolutamide group and 75.5% in the placebo group. The benefit was observed despite a greater proportion of patients in the placebo group (42.5%) than in the darolutamide group (32.5%) receiving subsequent life-prolonging anticancer therapy, primarily docetaxel (Appendix Table A2). Clear benefits were seen across all other secondary end points. The time to mCRPC (HR, 0.40 [95% CI, 0.32 to 0.51]) and time to PSA progression were longer with darolutamide versus placebo (HR, 0.31 [95% CI, 0.23 to 0.41]; Fig 3), and a higher proportion of patients receiving darolutamide achieved PSA <0.2 ng/mL at any time during the treatment period (62.6%) versus those receiving placebo (18.5%). The time to initiation of subsequent systemic anticancer therapy (HR, 0.40 [95% CI, 0.29 to 0.56]) and time to pain progression, a key patient-relevant end point, were also delayed in the darolutamide group compared with the placebo group (HR, 0.72 [95% CI, 0.54 to 0.96]).
TABLE 2.
Secondary Time-To-Event End Points (full analysis set)
| End point | Darolutamide + ADT (n = 446) | Placebo + ADT (n = 223) | Hazard Ratioa (95% CI) | ||
|---|---|---|---|---|---|
| Median, months | Events, No. (%) | Median, months | Events, No. (%) | ||
| Overall survival | NR | 103 (23.1) | NR | 60 (26.9) | 0.81 (0.59 to 1.12) |
| Time to initiation of subsequent systemic anticancer therapy | NR | 68 (15.2) | NR | 74 (33.2) | 0.40 (0.29 to 0.56) |
| Time to metastatic castration-resistant prostate cancer | NR | 154 (34.5) | 13.8 | 143 (64.1) | 0.40 (0.32 to 0.51) |
| Time to PSA progression | NR | 93 (20.9) | 16.8 | 108 (48.4) | 0.31 (0.23 to 0.41) |
| Time to pain progression | NR | 124 (27.8) | 29.9 | 79 (35.4) | 0.72 (0.54 to 0.96) |
Abbreviations: ADT, androgen-deprivation therapy; NR, not reached; PSA, prostate-specific antigen.
Hazard ratio and 95% CI are based on Cox regression model, stratified by visceral disease (present v absent) and prior local therapy (yes v no).
FIG 3.
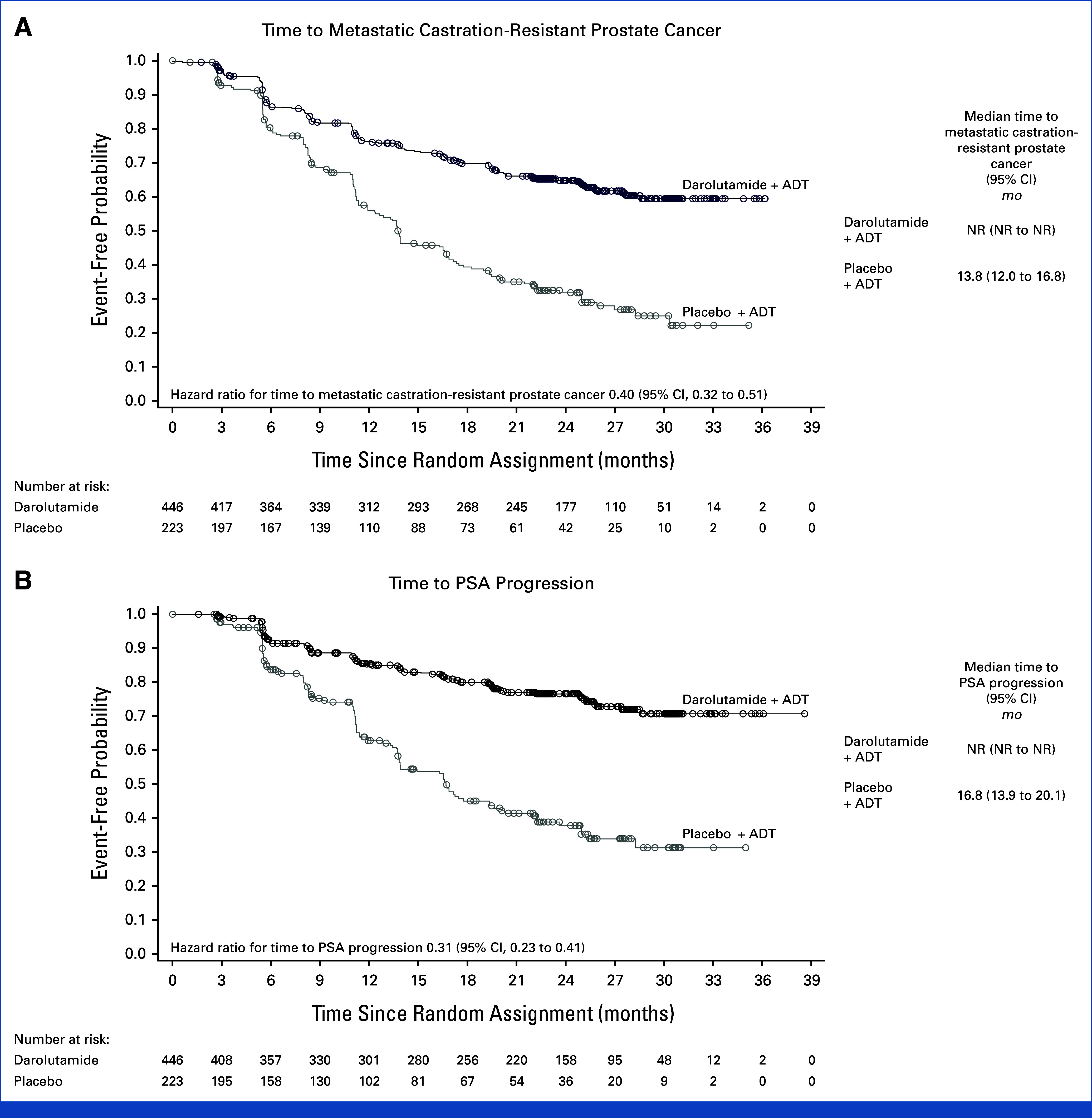
Additional secondary time-to-event end points (full analysis set). (A) Time to metastatic castration-resistant prostate cancer and (B) time to PSA progression. Hazard ratios and 95% CIs were calculated using the Cox regression model stratified by the presence of visceral metastases and prior therapy. ADT, androgen-deprivation therapy; NR, not reached; PSA, prostate-specific antigen.
The incidences of any-grade, grade 3, 4, or 5, and serious adverse events were similar in the darolutamide and placebo groups (Table 3). Most adverse events were grade 1 or 2 (darolutamide: 55.5%; placebo: 54.3%). Grade 3 or 4 adverse events occurred in 30.8% and 30.3% of patients receiving darolutamide and placebo, respectively. The frequency of death due to adverse events was low and similar in the two groups (21 of 445 patients in the darolutamide group [4.7%] and 12 of 221 patients in the placebo group [5.4%]), with no individual grade 5 adverse event occurring in more than two patients in either group (Appendix Table A3). Serious adverse events were reported in 23.6% of patients in the darolutamide group and 23.5% of patients in the placebo group. A smaller proportion of patients receiving darolutamide (6.1%) versus placebo (9.0%) discontinued treatment because of adverse events.
TABLE 3.
Treatment-Emergent Adverse Events (safety analysis set)
| Adverse Event, No. of Patients (%) | Darolutamide + ADT (n = 445a) | Placebo + ADT (n = 221a) | ||
|---|---|---|---|---|
| Any adverse event | 405 (91.0) | 199 (90.0) | ||
| Serious adverse event | 105 (23.6) | 52 (23.5) | ||
| Grade 3 or 4 adverse event | 137 (30.8) | 67 (30.3) | ||
| Grade 5 adverse event | 21 (4.7) | 12 (5.4) | ||
| Adverse event leading to permanent discontinuation of study drug | 27 (6.1) | 20 (9.0) | ||
| Most common adverse events, occurring in ≥5% of patients in either group | Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 |
|---|---|---|---|---|
| Anemia | 91 (20.4) | 14 (3.1) | 39 (17.6) | 8 (3.6) |
| Arthralgia | 55 (12.4) | 5 (1.1) | 25 (11.3) | 0 |
| Urinary tract infection | 52 (11.7) | 8 (1.8) | 17 (7.7) | 1 (0.5) |
| Back pain | 43 (9.7) | 5 (1.1) | 23 (10.4) | 2 (0.9) |
| Increased aspartate aminotransferase | 43 (9.7) | 10 (2.2) | 17 (7.7) | 1 (0.5) |
| Constipation | 42 (9.4) | 0 | 16 (7.2) | 0 |
| Hot flush | 41 (9.2) | 0 | 16 (7.2) | 0 |
| Increased alanine aminotransferase | 40 (9.0) | 9 (2.0) | 18 (8.1) | 1 (0.5) |
| Pain in extremity | 38 (8.5) | 1 (0.2) | 20 (9.0) | 4 (1.8) |
| Hypertension | 38 (8.5) | 19 (4.3) | 19 (8.6) | 8 (3.6) |
| Bone pain | 33 (7.4) | 9 (2.0) | 27 (12.2) | 3 (1.4) |
| Increased weight | 33 (7.4) | 4 (0.9) | 17 (7.7) | 1 (0.5) |
| COVID-19 | 32 (7.2) | 1 (0.2) | 15 (6.8) | 2 (0.9) |
| Increased alkaline phosphatase | 30 (6.7) | 0 | 13 (5.9) | 3 (1.4) |
| Insomnia | 28 (6.3) | 0 | 6 (2.7) | 1 (0.5) |
| Hyperglycemia | 27 (6.1) | 1 (0.2) | 8 (3.6) | 0 |
| Fatigue | 25 (5.6) | 0 | 18 (8.1) | 1 (0.5) |
| Increased creatinine | 21 (4.7) | 2 (0.4) | 15 (6.8) | 0 |
| Headache | 18 (4.0) | 0 | 14 (6.3) | 2 (0.9) |
Abbreviations: ADT, androgen-deprivation therapy; COVID-19, coronavirus disease 2019.
Two patients who were randomly assigned to the placebo group but received darolutamide are analyzed in the darolutamide group for the safety analysis set.
The only adverse events exceeding an incidence of 10% in the darolutamide group were anemia, arthralgia, and urinary tract infection (Table 3). Incidences of adverse events commonly associated with other androgen receptor pathway inhibitors were only 2% greater with darolutamide than with placebo for coronary artery disorders, cardiac arrhythmias, and vasodilatation and flushing and <2% greater with darolutamide than placebo for bone fracture, mental-impairment disorder, rash, falls, and decreased weight (Table 4). No difference between treatment groups was observed for heart failure, and higher incidences of fatigue, cerebral ischemia, and diabetes mellitus and hyperglycemia were reported in the placebo group versus the darolutamide group. Patients with a history of seizures were not excluded from the study, and no seizures were reported.
TABLE 4.
Treatment-Emergent Adverse Events of Special Interest (safety analysis set)
| Adverse Events Commonly Associated with Androgen Receptor Pathway Inhibitors | Darolutamide + ADT (n = 445a) | Placebo + ADT (n = 221a) | ||
|---|---|---|---|---|
| No. of Patients (%) | EAIRb/100 PY | No. of Patients (%) | EAIRb/100 PY | |
| Hypertensionc | 42 (9.4) | 5.5 | 21 (9.5) | 6.7 |
| Vasodilatation and flushing | 41 (9.2) | 5.6 | 16 (7.2) | 5.0 |
| Diabetes mellitus and hyperglycemia | 40 (9.0) | 5.3 | 21 (9.5) | 6.7 |
| Cardiac arrhythmiasc | 39 (8.8) | 5.1 | 15 (6.8) | 4.7 |
| Fatigue | 25 (5.6) | 3.2 | 18 (8.1) | 5.7 |
| Rashd | 19 (4.3) | 2.4 | 8 (3.6) | 2.4 |
| Bone fracturee | 18 (4.0) | 2.3 | 5 (2.3) | 1.5 |
| Coronary artery disordersc | 16 (3.6) | 2.0 | 3 (1.4) | 0.9 |
| Decreased weight | 14 (3.1) | 1.8 | 6 (2.7) | 1.8 |
| Mental-impairment disorderc | 7 (1.6) | 0.9 | 1 (0.5) | 0.3 |
| Falls, including accident | 6 (1.3) | 0.8 | 2 (0.9) | 0.6 |
| Heart failurec | 4 (0.9) | 0.5 | 2 (0.9) | 0.6 |
| Depressed-mood disorderc | 2 (0.4) | 0.2 | 2 (0.9) | 0.6 |
| Cerebral ischemia | 1 (0.2) | 0.1 | 3 (1.4) | 0.9 |
Abbreviations: ADT, androgen-deprivation therapy; EAIR, exposure-adjusted incidence rate; MedDRA, Medical Dictionary for Regulatory Activities; PY, patient years.
Two patients who were randomly assigned to the placebo group but received darolutamide are analyzed in the darolutamide group for the safety analysis set.
EAIR of treatment-emergent adverse events is defined as the number of patients with a given event divided by the total darolutamide/placebo treatment duration of all patients in years. The rate is expressed in 100 PY.
This category is a MedDRA High-Level Group Term.
This category combines the following MedDRA terms: rash, maculopapular rash, papular rash, pustular rash, and dermatitis.
Excluding pathologic fractures. This category combines the following MedDRA terms: any fractures and dislocations, limb fractures and dislocations, pelvic fractures and dislocations, spinal fractures and dislocations, and thoracic cage fractures and dislocations.
DISCUSSION
Despite substantial evidence supporting the benefits of combination regimens for patients with mHSPC, real-world prescribing patterns continue to show high use of ADT alone, including data after approval of androgen receptor pathway inhibitors (through September 2023).16,18,33-35 Recent studies in the United States and Germany have shown that, even in 2023, around 30% of patients with mHSPC still receive ADT alone.35,36
Darolutamide significantly improved OS in phase III trials involving patients with nmCRPC (in combination with ADT) and mHSPC (with ADT and docetaxel), with a consistent, favorable safety profile.15,28 To provide an effective and well-tolerated treatment alternative to ADT monotherapy for patients with mHSPC, the ARANOTE trial was designed to evaluate darolutamide with ADT versus ADT alone. In this global phase III trial, darolutamide plus ADT demonstrated a statistically significant and clinically meaningful improvement in rPFS versus ADT alone and a favorable HR for OS. Clear clinical benefits were observed in other secondary end points including deep and durable PSA responses and increased time to pain progression, suggesting a positive impact on patients' quality of life. Thus, the ARANOTE and ARASENS trials demonstrate efficacy benefits with darolutamide plus ADT with and without docetaxel for patients with mHSPC. The efficacy of darolutamide in ARANOTE, notably the OS results, are consistent with findings from the ARCHES trial of enzalutamide plus ADT, which reported an identical HR for OS (0.81; 95% CI, 0.53 to 1.25) at the time of the primary analysis.13 The results of the TITAN trial of apalutamide are different because the trial had coprimary end points of rPFS and OS.10 Additionally, docetaxel was the primary choice of post-trial treatment received in ARANOTE and in the TITAN and ARCHES trials,10,13 which is not a surprising choice because switching the mechanism of action in mCRPC is recommended over back-to-back treatment with androgen receptor inhibitors.2
ARANOTE confirmed the favorable safety and tolerability profile of darolutamide seen in ARAMIS and ARASENS,15,27,28 with most adverse events being grade 1 or 2 and no differences observed between treatment groups in the frequency of any-grade, grade 3, 4, or 5, or serious adverse events. Adverse events commonly associated with androgen receptor pathway inhibitors, which can be burdensome to patients, occurred at low and mostly similar incidences in the darolutamide and placebo groups. Indeed, ARANOTE is the first study of an androgen receptor pathway inhibitor showing a lower rate of fatigue in the treatment group (5.6%) compared with the placebo group (8.1%), representing a 30% lower incidence of fatigue with darolutamide versus placebo. Enzalutamide and apalutamide are associated with higher rates of fatigue (24.1% and 19.7% in the ARCHES and TITAN trials, respectively).10,13 Moreover, discontinuation rates because of adverse events were lower in the darolutamide group (6.1%) than in the placebo group (9.0%), supporting a favorable tolerability across this widely diverse patient population. These findings are consistent with studies of darolutamide plus ADT in the nmCRPC setting, namely the phase III ARAMIS trial27,28 and a large real-world cohort study, in which the rate of discontinuation was significantly reduced with darolutamide compared with enzalutamide and apalutamide.37 The safety and tolerability results from ARANOTE are especially relevant because use of ADT alone continues worldwide because of concerns about adverse events associated with hormonal therapies.16,34
Use of placebo plus ADT as the comparator may be considered a limitation of the ARANOTE trial, as ADT monotherapy is no longer considered a standard of care for patients with mHSPC. However, the evidence of continued use of ADT monotherapy indicates that the findings are relevant to physicians making treatment decisions for patients with mHSPC. The 2:1 random assignment resulted in a smaller control group population that may limit subgroup analyses. ARANOTE was designed to show a benefit in rPFS and was not powered for OS as a secondary end point. The strengths of the trial include the highly diversified population, broadly representing patients with prostate cancer, including elderly patients (median age of patients was 70 years, with the eldest patient being 93 years at baseline), Asian (31%) and Black (10%) populations, and patients treated in a range of health care settings (Asia, 30%; Latin America, 29%; Europe and the rest of the world, 41%). Furthermore, subgroup analyses of rPFS showed a significant benefit in patients with low-volume mHSPC, demonstrating the importance of an effective combination therapy with a favorable safety profile for this often undertreated population.
In conclusion, the results of this second pivotal trial of darolutamide in patients with mHSPC add to the body of evidence from ARASENS, providing the option to select treatment in mHSPC with and without docetaxel to meet patients' individual needs and preferences. The results of ARANOTE confirm the proven efficacy and well-established favorable safety profile of darolutamide, including a low discontinuation rate due to adverse events. Darolutamide plus ADT in patients with mHSPC demonstrated a clinically significant delay in radiographic progression with minimal treatment burden and may further expand therapeutic options for these patients.
ACKNOWLEDGMENT
The authors thank the patients and their families and all of the investigators involved in the ARANOTE trial (Appendix 1). Statistical analyses were provided by Mindy Mo, Charles Duan, and Anna Liu of Bayer. Writing and editorial support in the development of this manuscript was provided by Michelle McDermott, PharmD, and Sara Black, ISMPP CMPP, of Luna, OPEN Health Communications, and funded by Bayer HealthCare, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
APPENDIX 1. ARANOTE Study Investigators
Australia: Vinod Ganju; Howard Gurney; Laurence Krieger; Vineet Kwatra; Sanjeev Sewak; Amanda Stevanovic; Andrew Weickhardt; Brazil: Alan Azambuja; Flavio Mavignier Carcano; Marcio Valerio Costa; Felipe Cruz; Juliana de Menezes; Charles Andree Joseph de Padua; Adriano Augusto de Paula; Carlos Eugenio Santiago Escovar; Fabio Leite Couto Fernandez; Otavio Gampel; Andrea Juliana P. de Santana Gomes; Murilo Luz; Gisele Marinho dos Santos; Augusto Cesar de Andrade Mota; Lucas Nogueira; Daniel D'Almeida Preto; Alexandre Sant’Anna; Katsuki Aruma Tiscoski; Canada: Jonathan Giddens; Godfrey Jansz; Julian Kim; Paul Quellette; Fred Saad; George Vrabec; Chile: Alejandro Acevedo Gaete; Christian Caglevic Medina; Javier Dominguez Cruzat; Marcelo Garrido Salvo; Pedro Octavio Pastor Arroyo; Anibal Salazar Huerta; Pamela Salman Boghikian; Yasna Daniela Valenzuela Velasquez; Ariel Zwenger; China: Cheng Fu; Hongqian Guo; Weiqing Han; Haowen Jiang; Junhui Jiang; Shusuan Jiang; Lei Li; Tongzu Liu; Zhenhua Liu; Lulin Ma; Jun Qi; Mingxing Qiu; Guowei Shi; Ye Tian; Ben Wan; Chun-Xi Wang; Dongwen Wang; Shaogang Wang; Xiaolin Wang; Shaozhong Wei; Jitao Wu; Jun Xiao; Keji Xie; Liping Xie; Nianzeng Xing; Boxin Xue; Zejun Yan; Yong Yang; Zhixian Yu; Dahong Zhang; Song Zheng; Fangjian Zhou; India: Suresh Advani; Pawan Agarwal; Niraj Bhatt; Dubashi Biswajit; Ghanashyam Biswas; Shailesh A. Bondarde; Chandan Das; SarojKumar Das Majumdar; Sujoy Gupta; Kunhi Parambath Haresh; Francis James; Pamela Jeyaraj; Amit Joshi; Suman Kalyan; Bhalchandra Kashyapi; Ashish Kaushal; Raghunath Krishnappa; Ravimohan Mavuduru; Rajanish Nagarkar; Harsha Panchal; Gourav Parkash; Ashwin Philips; Ginil Kumar Pooleri; Vikram Prabha; Krishna Kumar Rathnam; Naveen Ravel; Sudhir Rawal; Boya Rakesh Reddy; Manasi Shah; Praveena Voonna; Latvia: Andrejs Aleksandrovs; Maris Jakubovskis; Alvis Laukmanis; Vilnis Lietuvietis; Mareks Vejins; Egils Vjaters; Lithuania: Mindaugas Jievaltas; Albertas Ulys; Raimundas Venckus; Arunas Zelvys; New Zealand: Kevin Bax; Peter Gilling; Michael Holmes; Alvin Tan; Peru: Carlos Manuel Morante Deza; Alberto Juan Pazos Franco; Jorge Fernando Salas Sanchez; Alejandro Figueroa Torrejon; Russian Federation: Timur Andabekov; Vagif Atduev; Yana Chapko; Natalya Fadeeva; Alexander Filippov; Rustem Gafanov; Oleg Gladkov; Boris Kasparov; Denis Kholtobin; Evgeny Kopyltsov; Alexander Lykov; Marina Nechaeva; Alexey Plekhanov; Sufia Safina; Andrey Semenov; Mikhail Shkolnik; Pavel Skopin; Roman Smirnov; Ekaterina Solovyeva; Alexander Sultanbaev; Mikhail Zavyalov; Alexandr Zyryanov; South Africa: Khabane Chabane; Corlia Coetzee; Conrad Jacobs; Thamsanqa Madlala; Jorn Malan; Sophie Mathijs; Spain: Carlos Llorente Abarca; Daniel Ernesto Castellano Gauna; Jose Luis Alvarez-Ossorio Fernandez; Enrique Gallardo Diaz; Pablo Borrega Garcia; Bernardo Herrera Imbroda; Rafael Antonio Medina Lopez; Josep Maria Gaya Sopena; Taiwan: Hsiao-Jen Chung; Shu-Pin Huang; Yuh-Shya Tsai; Pai-Fu Wang; Shian-Shiang Wang; Ukraine: Igor Bondarenko; Yurii Golovko; Petro Ivashchenko; Viktor Paramonov.
Criteria for Patient Selection
Inclusion Criteria
Participants are eligible to be included in the study only if all of the following criteria apply:
Written informed consent obtained.
Men age ≥18 years.
Histologically or cytologically confirmed adenocarcinoma of the prostate.
Metastatic disease documented by conventional imaging, either by a positive 99mTc-phosphonate bone scan or, for soft tissue or visceral metastases, by contrast-enhanced abdominal/pelvic/chest computed tomography (CT) or magnetic resonance imaging (MRI) assessed by central review. Participants with a baseline superscan (bone scan showing a diffuse, intense, skeletal uptake of the tracer with absent renal and background activity) are considered ineligible. Metastatic disease is defined as either malignant lesions on bone scan or measurable lymph nodes above the aortic bifurcation or soft tissue or visceral lesions according to RECIST v1.1. Lymph nodes are measurable if the short axis diameter is ≥15 mm, and soft tissue or visceral lesions are measurable if the long axis diameter is ≥10 mm. Regional lymph node metastases only (N1, below the aortic bifurcation) will not be considered as metastases eligible for the study. Only participants with nonregional lymph node metastases (M1a) and/or bone metastases (M1b) and/or other sites of metastases with or without bone disease (M1c) will be eligible.
Started androgen deprivation therapy (ADT; luteinizing hormone-releasing hormone [LHRH] agonist or antagonist or orchiectomy) with or without first-generation antiandrogen therapy, but not earlier than 12 weeks before random assignment. For participants receiving LHRH agonists, treatment in combination with a first-generation antiandrogen for at least 14 days before random assignment is recommended.
First-generation antiandrogen therapy must be discontinued at least 1 day before study treatment start.
Eastern Cooperative Oncology Group performance status of 0, 1, or 2.
Blood counts at screening: hemoglobin ≥9.0 g/dL, absolute neutrophil count ≥1.5 × 109/L, platelet count ≥100 × 109/L (participant must not receive any growth factor within 4 weeks or a blood transfusion within 7 days of the hematology laboratory sample obtained at screening).
Screening values of serum alanine aminotransferase and aspartate aminotransferase ≤1.5 × the upper limit of normal (ULN), total bilirubin ≤1.5 × ULN, and creatinine ≤2.0 × ULN (for Canada: screening values of serum alanine aminotransferase and aspartate aminotransferase ≤1.5 × ULN and total bilirubin ≤1.5 × ULN).
Sexually active men must agree to use condoms as an effective barrier method and refrain from sperm donation and/or their female partners of reproductive potential to use a method of effective birth control, during the treatment with study drug and for 4 weeks after the last dose of the study drug.
For Canada: at screening, estimated glomerular filtration rate ≥30 mL/min/1.73 m2 (calculated by the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula).
Exclusion Criteria
Participants are excluded from the study if any of the following criteria apply:
Pathological finding consistent with small cell, ductal, or neuroendocrine carcinoma of the prostate.
Known brain or leptomeningeal metastases. Brain CT or MRI should be performed only in case of symptoms.
-
Prior treatment with the following:
a. An LHRH agonist or antagonist started more than 12 weeks before random assignment, except neoadjuvant or adjuvant therapy, or both, for a maximum of 24 months and completed at least 12 months before random assignment
b. Second-generation androgen receptor inhibitors such as enzalutamide, darolutamide, apalutamide, or other investigational androgen receptor inhibitors
c. Cytochrome P450 (CYP) 17 enzyme inhibitor such as abiraterone acetate or oral ketoconazole as anticancer treatment for prostate cancer
d. Chemotherapy including docetaxel or immunotherapy for prostate cancer
e. A systemic corticosteroid with a dose greater than the equivalent 10 mg of prednisone/day within 28 days before random assignment
f. Radiopharmaceuticals
g. Any other anticancer treatment for prostate cancer, excluding local therapies and ADT
Treatment with radiotherapy (external beam radiation therapy or brachytherapy) within 2 weeks before random assignment.
Known hypersensitivity to any of the study drugs, study drug classes, or excipients in the formulation of the study drugs.
Contraindication to iodinated CT and gadolinium chelate MRI intravenous contrast agent(s).
Any prior malignancy (other than adequately treated basal cell or squamous cell skin cancer, superficial bladder cancer, or any other cancer in situ currently in complete remission) within 5 years before random assignment.
Active viral hepatitis (defined as hepatitis B surface antigen reactive or detectable [qualitative] hepatitis B virus DNA or as hepatitis C virus RNA [qualitative] is detected), known human immunodeficiency virus infection with detectable viral load, or chronic liver disease with a need for treatment. No testing for hepatitis B or C is required unless mandated by local authority. No human immunodeficiency virus testing is required unless mandated by local authority.
Any of the following within 6 months before random assignment: stroke, myocardial infarction, severe or unstable angina pectoris, coronary or peripheral artery bypass graft, congestive heart failure (New York Heart Association Class III or IV).
Uncontrolled hypertension as indicated by a resting systolic blood pressure ≥160 mm Hg or diastolic blood pressure ≥100 mm Hg despite medical management.
A gastrointestinal disorder or procedure that is expected to interfere substantially with absorption of the study drug.
Previous (within 28 days before the start of the study drug or five half-lives of the investigational treatment of the previous study, whichever is longer) or concomitant participation in another clinical study with investigational medicinal product(s).
Any other serious or unstable illness, or medical, social, or psychological condition, that could jeopardize the safety of the participant and/or their compliance with study procedures or may interfere with the participant's participation in the study or evaluation of the study results.
Inability to swallow oral medications.
Definitions of Secondary Efficacy End Points
Overall survival is defined as the time from the date of random assignment to the date of death from any cause.
Time to initiation of subsequent systemic anticancer therapy is defined as the time from the date of random assignment to the date of initiation of first subsequent systemic anticancer therapy for prostate cancer.
-
Time to castration-resistant prostate cancer is defined as the time from the date of random assignment to the date of occurrence of the following events, whatever comes first:
Prostate-specific antigen (PSA) progression with serum testosterone at castrate level <0.50 ng/mL, which is defined as a ≥25% increase above the nadir (lowest at or after baseline) and an increase in absolute value of ≥2 ng/mL above the nadir and at least 12 weeks from the random assignment date, which is confirmed by a second value three or more weeks later. All PSA values between the initial assessment meeting the PSA progression criteria and confirmation assessment must be ≥2 ng/mL and ≥25% increase above the nadir; serum testosterone at castrate level <0.50 ng/mL is requested at initial assessment.
Radiological progression by malignant soft tissue lesions, which is determined by central review based on RECIST v1.1.
Radiological progression by bone lesions, which is determined according to Prostate Cancer Working Group 3 criteria based on whole-body 99mTc-methylene diphosphonate bone scans assessed by central review.
Occurrence of a symptomatic skeletal event.
-
Time to PSA progression is defined as the time from the date of random assignment to the date of first PSA progression.
PSA progression with serum testosterone at castrate level <0.50 ng/mL, which is defined as a ≥25% increase above the nadir (lowest at or after baseline) and an increase in absolute value of ≥2 ng/mL above the nadir and at least 12 weeks from the random assignment date, which is confirmed by a second value three or more weeks later. All PSA values between the initial assessment meeting the PSA progression criteria and confirmation assessment must be ≥2 ng/mL and ≥25% increase above the nadir; serum testosterone at castrate level <0.50 ng/mL is requested at initial assessment.
-
PSA undetectable rate is defined as the proportion of participants with detectable PSA values of ≥0.2 ng/mL at baseline that become undetectable (<0.2 ng/mL) during the period between random assignment and 30 days after last dose of study drug or start of new anticancer therapy, whichever occurs first, based on the participants who had detectable PSA value at baseline. The analysis of PSA undetectable rate will be based on central PSA assessments.
PSA undetectable rate will be evaluated up to 3, 6, and 9 months after random assignment.
-
Time to pain progression is defined as the time from the date of random assignment to the date of first pain progression. Pain progression will be assessed by Question three of the Brief Pain Inventory—Short Form (BPI-SF) questionnaire related to the worst pain in the last 24 hours (worst pain subscale [WPS]) taken as an average for postbaseline score, or initiation of short or long-acting opioids for malignant disease for ≥7 consecutive days after random assignment. Initiation or change in the use of other nonopioid analgesics is not used in the assessment of pain progression.
WPS score is taken as an average score of Q3 of the BPI-SF questionnaires answered within 7 days before each reporting time point. If there are more than seven daily questionnaires answered at a reporting time point, the latest seven questionnaires to the reporting time point will be used to assess pain progression.
-
Pain progression definitions:
For asymptomatic participants with WPS score of zero at baseline, pain progression is defined as an increase of two or more points in the WPS score from the nadir (ie, zero) observed at two consecutive evaluations at least 4 weeks apart, or initiation of short- or long-acting opioids for malignant disease for at least seven consecutive days after random assignment.
For symptomatic participants with WPS score more than zero at baseline, pain progression is defined as an increase of two or more points in the WPS score from the nadir observed at two consecutive evaluations at least 4 weeks apart and a WPS score of at least five, or initiation of short- or long-acting opioids for malignant disease for at least seven consecutive days after random assignment.
Sensitivity Analyses of the Primary End Point
The following sensitivity analyses will be performed for the primary end point, radiological progression-free survival (rPFS):
Analysis with all deaths from any cause at any time before the data cutoff date considered as an event, unless radiological progressive disease is documented
Analysis with rPFS on the basis of investigator radiological assessment
Analysis without stratification using an unstratified log-rank test and unstratified Cox model
Analysis considering the additional primary malignancy (except basal cell carcinoma) diagnosed before progression or death, which was censored at the date of last adequate tumor assessment before or at diagnosis of additional primary malignancy
Analysis without considering the censoring rule of radiological progression or death occurring later than (24 + 1) weeks of last adequate scan
-
Analysis considering the impact of radiological progressive disease by central review documented between the scheduled scans as per the protocol (every 12 weeks); the following will be implemented:
For the tumor assessment within the scheduled visit time interval (every 12 ± 1 weeks from random assignment), the actual tumor assessment date will be used for rPFS
For the tumor assessment outside the scheduled visit time interval (every 12 ± 1 weeks from random assignment), tumor assessment date of radiological progressive disease will be moved forward to the date of the next scheduled visit; the tumor assessment date of nonradiological progressive disease will be moved backward to the closest prior scheduled visit
rPFS is the time from random assignment to radiological progressive disease or death or withdrawal of informed consent or data cutoff, whichever comes first
This sensitivity analysis will not consider the censoring rules of radiological progression or death occurring later than 24 + 1 weeks of the last adequate scan, and subsequent systemic anticancer therapy starting before or without radiological progression or death
TABLE A1.
Participants by Country
| Region and Country | No. of Patients |
|---|---|
| Asia | 204 |
| China | 90 |
| India | 93 |
| Taiwan | 21 |
| Latin America | 191 |
| Brazil | 148 |
| Chile | 29 |
| Peru | 14 |
| Europe and rest of the world | 274 |
| Latvia | 57 |
| Lithuania | 34 |
| Russia | 83 |
| Spain | 14 |
| Ukraine | 32 |
| Australia | 28 |
| Canada | 2 |
| New Zealand | 12 |
| South Africa | 12 |
FIG A1.
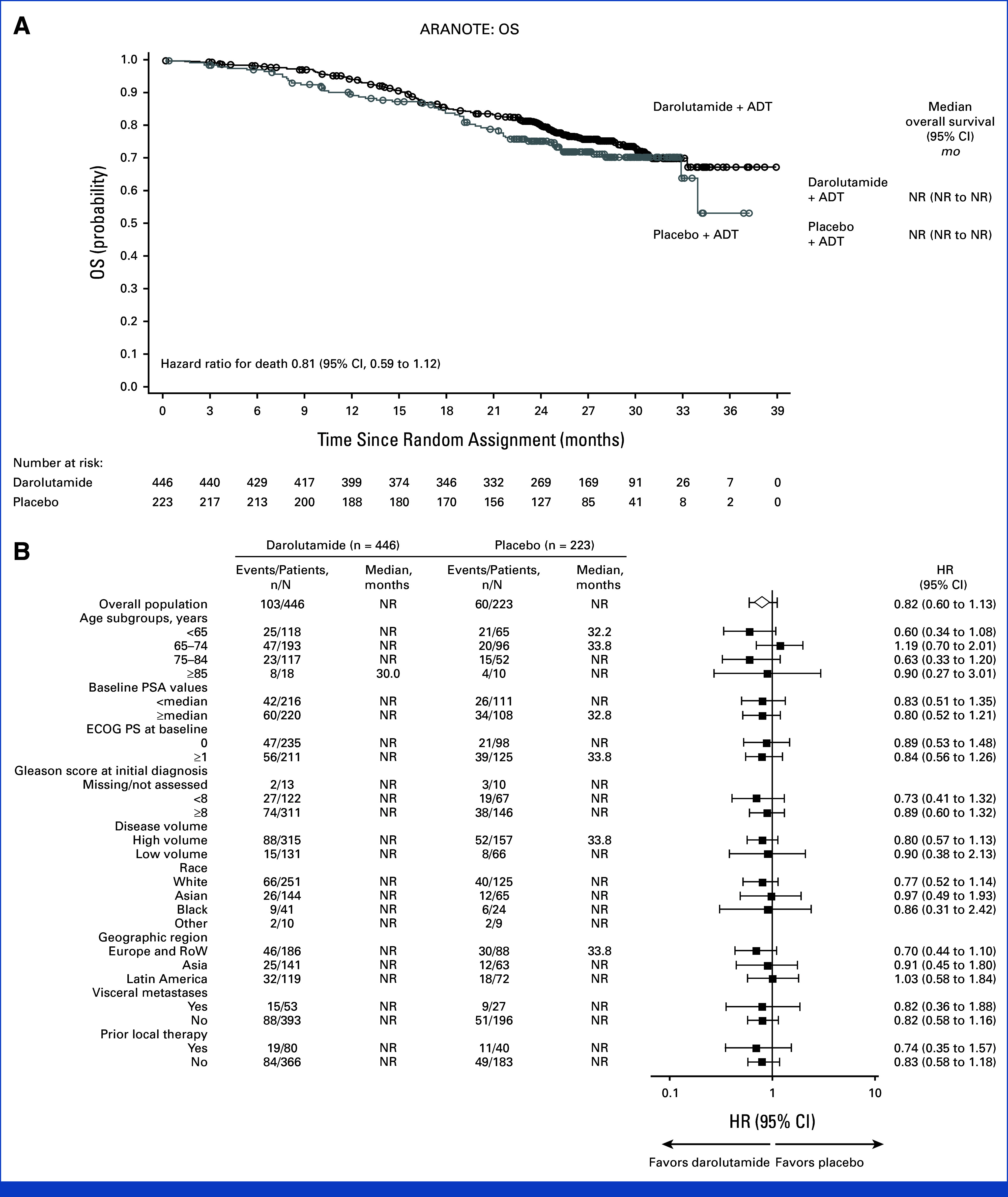
OS (full analysis set). (A) Kaplan–Meier estimates and (B) subgroup analyses. The HR and 95% CI were calculated using the Cox regression model stratified by the presence of visceral metastases and prior therapy. Subgroup analyses of OS provide HRs and 95% CIs obtained from univariate analysis using an unstratified Cox regression model. ADT, androgen-deprivation therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NR, not reached; OS, overall survival; PSA prostate-specific antigen; RoW, rest of the world.
TABLE A2.
Subsequent Life-Prolonging Anticancer Therapy
| No. (%) of Patientsa | Darolutamide (n = 446) | Placebo (n = 223) |
|---|---|---|
| Discontinued study treatment | 203 (45.5) | 160 (71.7) |
| Received subsequent life-prolonging anticancer therapyb | 66/203 (32.5) | 68/160 (42.5) |
| Docetaxel | 46/203 (22.7) | 46/160 (28.8) |
| Abiraterone acetate | 26/203 (12.8) | 21/160 (13.1) |
| Enzalutamide | 6/203 (3.0) | 12/160 (7.5) |
| Apalutamide | 3/203 (1.5) | 0 |
| Cabazitaxel | 2/203 (1.0) | 1/160 (0.6) |
| Radium-223 | 2/203 (1.0) | 0 |
| Olaparib | 1/203 (0.5) | 0 |
NOTE. Subsequent life-prolonging therapies for prostate cancer are defined as abiraterone acetate, apalutamide, enzalutamide, docetaxel, cabazitaxel, radium-223, sipuleucel-T, lutetium-177–PSMA-617, rucaparib, and olaparib.
Patients could receive more than one subsequent life-prolonging anticancer therapy.
Four patients who started life-prolonging therapy before study treatment discontinuation are included.
TABLE A3.
Treatment-Emergent Grade 5 Adverse Events by MedDRA Preferred Term (safety analysis set)
| Grade 5 Adverse Event, No. (%) of Patientsa | Darolutamide (n = 445) | Placebo (n = 221) |
|---|---|---|
| Any | 21 (4.7) | 12 (5.4) |
| Death (not otherwise specified) | 2 (0.4) | 2 (0.9) |
| Craniocerebral injury | 2 (0.4) | 0 |
| Myocardial infarction | 2 (0.4) | 0 |
| Septic shock | 2 (0.4) | 0 |
| Sepsis | 1 (0.2) | 1 (0.5) |
| Acinetobacter sepsis | 1 (0.2) | 0 |
| COVID-19 pneumonia | 1 (0.2) | 0 |
| COVID-19 test positive | 1 (0.2) | 0 |
| Disease progression | 1 (0.2) | 0 |
| Dyspnea | 1 (0.2) | 0 |
| Hyponatremia | 1 (0.2) | 0 |
| Multiple organ dysfunction syndrome | 1 (0.2) | 0 |
| Oncologic complication | 1 (0.2) | 0 |
| Pneumonia, viral | 1 (0.2) | 0 |
| Prostate cancer, metastatic | 1 (0.2) | 0 |
| Pulmonary edema | 1 (0.2) | 0 |
| Pulmonary sepsis | 1 (0.2) | 0 |
| Sudden death | 1 (0.2) | 0 |
| Urinary tract infection | 1 (0.2) | 0 |
| Urosepsis | 1 (0.2) | 0 |
| Acute coronary syndrome | 0 | 1 (0.5) |
| Acute myocardial infarction | 0 | 1 (0.5) |
| Cardiac arrest | 0 | 1 (0.5) |
| Cerebral infarction | 0 | 1 (0.5) |
| Gastrointestinal hemorrhage | 0 | 1 (0.5) |
| Intestinal ischemia | 0 | 1 (0.5) |
| Ischemic stroke | 0 | 1 (0.5) |
| Pulmonary congestion | 0 | 1 (0.5) |
| Pulmonary embolism | 0 | 1 (0.5) |
| Renal failure | 0 | 1 (0.5) |
| Respiratory failure | 0 | 1 (0.5) |
NOTE. MedDRA denotes Medical Dictionary for Regulatory Activities.
Patients may have had more than one grade 5 co-occurring adverse events.
Fred Saad
Honoraria: Astellas Pharma, Janssen Oncology, Sanofi, Bayer, AstraZeneca, AbbVie, Myovant Sciences, Pfizer, BMS, Novartis, Advanced Accelerator Applications, Merck, Knight Therapeutics, Tolmar
Consulting or Advisory Role: Astellas Pharma, Janssen Oncology, Sanofi, AstraZeneca/MedImmune, Bayer, Pfizer, Myovant Sciences, AbbVie, Novartis, Advanced Accelerator Applications, Knight Therapeutics, Tolmar
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Janssen Oncology (Inst), Sanofi (Inst), AstraZeneca (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Advanced Accelerator Applications (Inst), Merck (Inst)
Egils Vjaters
Consulting or Advisory Role: Janssen, Bayer, Ipsen
Neal Shore
Employment: GenesisCare
Leadership: Photocure, Alessa Therapeutics
Stock and Other Ownership Interests: Alessa Therapeutics, Photocure
Consulting or Advisory Role: Bayer, Janssen Scientific Affairs, Dendreon, Tolmar, Ferring, Medivation/Astellas, Amgen, Pfizer, AstraZeneca, Astellas Pharma, AbbVie, Merck, Bristol Myers Squibb/Sanofi, Exact Imaging, FerGene, InVitae, MDxHealth, Myriad Genetics, Propella Therapeutics, Genzyme, Sanofi, CG Oncology, Genesis Cancer Care, Urogen pharma, Speciality Networks, PeerView, Clarity Pharmaceuticals, Lantheus Medical Imaging, Lilly, Photocure, Telix Pharmaceuticals, AIkido Pharma, Arquer Diagnostics, Asieris Pharmaceuticals, Minomic, Novartis, PlatformQ Health, Promaxo, Protara Therapeutics, Fize Medical, Accord Research, Antev, Aura Biosciences, Bioprotect, Sumitomo Pharma Oncology
Research Funding: AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Boston Scientific, Clovis Oncology, Dendreon, Exact Imaging, Ferring, Foundation Medicine, InVitae, Janssen, MDxHealth, Merck, Myovant Sciences, Myriad Genetics, Nymox, Pfizer, Sanofi, Sesen Bio, Tolmar, CG Oncology, DisperSol, FORMA Therapeutics, Guardant Health, Jiangsu Yahong Meditech, Novartis, Pacific Edge, POINT Biopharma, Propella Therapeutics, SeaGen, MT Group, Theralase, Veru, Zenflow, Advantagene, Aragon Pharmaceuticals, Endocyte, Exelixis, FKD Therapies, Genentech, Istari Oncology, Medivation, OncoCellMDx, ORIC Pharmaceuticals, Palette Life Sciences, Plexxikon, RhoVac, Steba Biotech, Urogen pharma, Urotronic, US Biotest, Vaxiion
Expert Testimony: Ferring
David Olmos
Honoraria: Janssen, Bayer
Consulting or Advisory Role: Janssen, AstraZeneca, Bayer, MSD Oncology, Pfizer
Research Funding: Pfizer (Inst), Johnson & Johnson/Janssen (Inst)
Travel, Accommodations, Expenses: Bayer, Janssen, AstraZeneca Spain
Andrea Juliana P. de Santana Gomes
Honoraria: Astellas Pharma, Bayer, Janssen Oncology, AstraZeneca, Adium Pharma
Consulting or Advisory Role: Janssen Oncology, Bayer
Speakers' Bureau: Janssen Oncology, Astellas Pharma, Bayer, AstraZeneca
Research Funding: Janssen Oncology, MSD Oncology, Bayer, Roche, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology
Augusto Cesar de Andrade Mota
Consulting or Advisory Role: Pfizer, Janssen Oncology, Merck, Bayer
Travel, Accommodations, Expenses: Bayer
Pamela Salman
Consulting or Advisory Role: Roche/Genentech, Novartis, Lilly, Merck Serono
Speakers' Bureau: Roche/Genentech, Novartis, Lilly
Mindaugas Jievaltas
Research Funding: Janssen (Inst)
Travel, Accommodations, Expenses: IPSEN, Janssen, Recordati
Maris Jakubovskis
Employment: Riga East University Hospital, JSC Health Center Association
Leadership: Ltd Urologs
Consulting or Advisory Role: Joint stock company “Olainfarm” Registration Nr.LV40003007246
Liina Nevalaita
Employment: Orion
Patents, Royalties, Other Intellectual Property: Nevalaita L and Saarma M. United States patent “Splice variants of GDNF and uses thereof”. Patent No.: US 9,579,362 B2. Date of patent: Feb. 28, 2017. Nevalaita L and Saarma M. European patent “Splice variants of GDNF and uses thereof”. Patent No.: EP 2 551 281 B1. Validated in France, UK, Germany, Sweden and the Netherlands
Isabella Testa
Employment: Bayer
Leadership: Bayer
Research Funding: Bayer
Marie Aude Le Berre
Employment: Bayer
Iris Kuss
Employment: Bayer Germany
Stock and Other Ownership Interests: Bayer
No other potential conflicts of interest were reported.
PRIORI PRESENTATION
Presented in part at the European Society of Medical Oncology Congress Barcelona, Spain, September 13-17, 2024.
SUPPORT
Supported by Bayer AG and Orion Pharma.
CLINICAL TRIAL INFORMATION
Contributor Information
Collaborators: Vinod Ganju, Howard Gurney, Laurence Krieger, Vineet Kwatra, Sanjeev Sewak, Amanda Stevanovic, Andrew Weickhardt, Alan Azambuja, Flavio Mavignier Carcano, Marcio Valerio Costa, Felipe Cruz, Juliana de Menezes, Charles Andree Joseph de Padua, Adriano Augusto de Paula, Carlos Eugenio Santiago Escovar, Fabio Leite Couto Fernandez, Otavio Gampel, Andrea Juliana P. de Santana Gomes, Murilo Luz, Gisele Marinho dos Santos, Augusto Cesar de Andrade Mota, Lucas Nogueira, Daniel D'Almeida Preto, Alexandre Sant'Anna, Katsuki Aruma Tiscoski, Jonathan Giddens, Godfrey Jansz, Julian Kim, Paul Quellette, Fred Saad, George Vrabec, Alejandro Acevedo Gaete, Christian Caglevic Medina, Javier Dominguez Cruzat, Marcelo Garrido Salvo, Pedro Octavio Pastor Arroyo, Anibal Salazar Huerta, Pamela Salman Boghikian, Yasna Daniela Valenzuela Velasquez, Ariel Zwenger, Cheng Fu, Hongqian Guo, Weiqing Han, Haowen Jiang, Junhui Jiang, Shusuan Jiang, Lei Li, Tongzu Liu, Zhenhua Liu, Lulin Ma, Jun Qi, Mingxing Qiu, Guowei Shi, Ye Tian, Ben Wan, Chun-Xi Wang, Dongwen Wang, Shaogang Wang, Xiaolin Wang, Shaozhong Wei, Jitao Wu, Jun Xiao, Keji Xie, Liping Xie, Nianzeng Xing, Boxin Xue, Zejun Yan, Yong Yang, Zhixian Yu, Dahong Zhang, Song Zheng, Fangjian Zhou, Suresh Advani, Pawan Agarwal, Niraj Bhatt, Dubashi Biswajit, Ghanashyam Biswas, Shailesh A. Bondarde, Chandan Das, SarojKumar Das Majumdar, Sujoy Gupta, Kunhi Parambath Haresh, Francis James, Pamela Jeyaraj, Amit Joshi, Suman Kalyan, Bhalchandra Kashyapi, Ashish Kaushal, Raghunath Krishnappa, Ravimohan Mavuduru, Rajanish Nagarkar, Harsha Panchal, Gourav Parkash, Ashwin Philips, Ginil Kumar Pooleri, Vikram Prabha, Krishna Kumar Rathnam, Naveen Ravel, Sudhir Rawal, Boya Rakesh Reddy, Manasi Shah, Praveena Voonna, Andrejs Aleksandrovs, Maris Jakubovskis, Alvis Laukmanis, Vilnis Lietuvietis, Mareks Vejins, Egils Vjaters, Mindaugas Jievaltas, Albertas Ulys, Raimundas Venckus, Arunas Zelvys, Kevin Bax, Peter Gilling, Michael Holmes, Alvin Tan, Carlos Manuel Morante Deza, Alberto Juan Pazos Franco, Jorge Fernando Salas Sanchez, Alejandro Figueroa Torrejon, Timur Andabekov, Vagif Atduev, Yana Chapko, Natalya Fadeeva, Alexander Filippov, Rustem Gafanov, Oleg Gladkov, Boris Kasparov, Denis Kholtobin, Evgeny Kopyltsov, Alexander Lykov, Marina Nechaeva, Alexey Plekhanov, Sufia Safina, Andrey Semenov, Mikhail Shkolnik, Pavel Skopin, Roman Smirnov, Ekaterina Solovyeva, Alexander Sultanbaev, Mikhail Zavyalov, Alexandr Zyryanov, Khabane Chabane, Corlia Coetzee, Conrad Jacobs, Thamsanqa Madlala, Jorn Malan, Sophie Mathijs, Carlos Llorente Abarca, Daniel Ernesto Castellano Gauna, Jose Luis Alvarez-Ossorio Fernandez, Enrique Gallardo Diaz, Pablo Borrega Garcia, Bernardo Herrera Imbroda, Rafael Antonio Medina Lopez, Josep Maria Gaya Sopena, Hsiao-Jen Chung, Shu-Pin Huang, Yuh-Shya Tsai, Pai-Fu Wang, Shian-Shiang Wang, Igor Bondarenko, Yurii Golovko, Petro Ivashchenko, and Viktor Paramonov
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO-24-01798.
AUTHOR CONTRIBUTIONS
Conception and design: Fred Saad, Neal Shore, David Olmos, Nianzeng Xing, Albertas Ulys, Weiqing Han, Liina Nevalaita, Isabella Testa, Marie-Aude Le Berre, Iris Kuss, Kunhi Parambath Haresh
Administrative support: Neal Shore
Provision of study materials or patients: Fred Saad, Egils Vjaters, David Olmos, Nianzeng Xing, Andrea Juliana Pereira de Santanta Gomese, Neal Shore, Augusto Cesar de Andrade Mota, Pamela Salman, Mindaugas Jievaltas, Albertas Ulys, Maris Jakubovskis, Evgeny Kopyltsov, Weiqing Han, Kunhi Parambath Haresh
Collection and assembly of data: Fred Saad, Egils Vjaters, Neal Shore, David Olmos, Nianzeng Xing, Andrea Juliana Pereira de Santana Gomes, Augusto Cesar de Andrade Mota, Pamela Salman, Mindaugas Jievaltas, Albertas Ulys, Maris Jakubovskis, Evgeny Kopyltsov, Weiqing Han, Isabella Testa, Iris Kuss, Kunhi Parambath Haresh
Data analysis and interpretation: Fred Saad, Egils Vjaters, Neal Shore, David Olmos, Pamela Salman, Mindaugas Jievaltas, Albertas Ulys, Evgeny Kopyltsov, Weiqing Han, Isabella Testa, Marie-Aude Le Berre, Iris Kuss, Kunhi Parambath Haresh
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Darolutamide in Combination With Androgen-Deprivation Therapy in Patients With Metastatic Hormone-Sensitive Prostate Cancer From the Phase III ARANOTE Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Fred Saad
Honoraria: Astellas Pharma, Janssen Oncology, Sanofi, Bayer, AstraZeneca, AbbVie, Myovant Sciences, Pfizer, BMS, Novartis, Advanced Accelerator Applications, Merck, Knight Therapeutics, Tolmar
Consulting or Advisory Role: Astellas Pharma, Janssen Oncology, Sanofi, AstraZeneca/MedImmune, Bayer, Pfizer, Myovant Sciences, AbbVie, Novartis, Advanced Accelerator Applications, Knight Therapeutics, Tolmar
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Janssen Oncology (Inst), Sanofi (Inst), AstraZeneca (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Advanced Accelerator Applications (Inst), Merck (Inst)
Egils Vjaters
Consulting or Advisory Role: Janssen, Bayer, Ipsen
Neal Shore
Employment: GenesisCare
Leadership: Photocure, Alessa Therapeutics
Stock and Other Ownership Interests: Alessa Therapeutics, Photocure
Consulting or Advisory Role: Bayer, Janssen Scientific Affairs, Dendreon, Tolmar, Ferring, Medivation/Astellas, Amgen, Pfizer, AstraZeneca, Astellas Pharma, AbbVie, Merck, Bristol Myers Squibb/Sanofi, Exact Imaging, FerGene, InVitae, MDxHealth, Myriad Genetics, Propella Therapeutics, Genzyme, Sanofi, CG Oncology, Genesis Cancer Care, Urogen pharma, Speciality Networks, PeerView, Clarity Pharmaceuticals, Lantheus Medical Imaging, Lilly, Photocure, Telix Pharmaceuticals, AIkido Pharma, Arquer Diagnostics, Asieris Pharmaceuticals, Minomic, Novartis, PlatformQ Health, Promaxo, Protara Therapeutics, Fize Medical, Accord Research, Antev, Aura Biosciences, Bioprotect, Sumitomo Pharma Oncology
Research Funding: AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Boston Scientific, Clovis Oncology, Dendreon, Exact Imaging, Ferring, Foundation Medicine, InVitae, Janssen, MDxHealth, Merck, Myovant Sciences, Myriad Genetics, Nymox, Pfizer, Sanofi, Sesen Bio, Tolmar, CG Oncology, DisperSol, FORMA Therapeutics, Guardant Health, Jiangsu Yahong Meditech, Novartis, Pacific Edge, POINT Biopharma, Propella Therapeutics, SeaGen, MT Group, Theralase, Veru, Zenflow, Advantagene, Aragon Pharmaceuticals, Endocyte, Exelixis, FKD Therapies, Genentech, Istari Oncology, Medivation, OncoCellMDx, ORIC Pharmaceuticals, Palette Life Sciences, Plexxikon, RhoVac, Steba Biotech, Urogen pharma, Urotronic, US Biotest, Vaxiion
Expert Testimony: Ferring
David Olmos
Honoraria: Janssen, Bayer
Consulting or Advisory Role: Janssen, AstraZeneca, Bayer, MSD Oncology, Pfizer
Research Funding: Pfizer (Inst), Johnson & Johnson/Janssen (Inst)
Travel, Accommodations, Expenses: Bayer, Janssen, AstraZeneca Spain
Andrea Juliana P. de Santana Gomes
Honoraria: Astellas Pharma, Bayer, Janssen Oncology, AstraZeneca, Adium Pharma
Consulting or Advisory Role: Janssen Oncology, Bayer
Speakers' Bureau: Janssen Oncology, Astellas Pharma, Bayer, AstraZeneca
Research Funding: Janssen Oncology, MSD Oncology, Bayer, Roche, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology
Augusto Cesar de Andrade Mota
Consulting or Advisory Role: Pfizer, Janssen Oncology, Merck, Bayer
Travel, Accommodations, Expenses: Bayer
Pamela Salman
Consulting or Advisory Role: Roche/Genentech, Novartis, Lilly, Merck Serono
Speakers' Bureau: Roche/Genentech, Novartis, Lilly
Mindaugas Jievaltas
Research Funding: Janssen (Inst)
Travel, Accommodations, Expenses: IPSEN, Janssen, Recordati
Maris Jakubovskis
Employment: Riga East University Hospital, JSC Health Center Association
Leadership: Ltd Urologs
Consulting or Advisory Role: Joint stock company “Olainfarm” Registration Nr.LV40003007246
Liina Nevalaita
Employment: Orion
Patents, Royalties, Other Intellectual Property: Nevalaita L and Saarma M. United States patent “Splice variants of GDNF and uses thereof”. Patent No.: US 9,579,362 B2. Date of patent: Feb. 28, 2017. Nevalaita L and Saarma M. European patent “Splice variants of GDNF and uses thereof”. Patent No.: EP 2 551 281 B1. Validated in France, UK, Germany, Sweden and the Netherlands
Isabella Testa
Employment: Bayer
Leadership: Bayer
Research Funding: Bayer
Marie Aude Le Berre
Employment: Bayer
Iris Kuss
Employment: Bayer Germany
Stock and Other Ownership Interests: Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1. Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9(suppl 1):S3–S8. [PMC free article] [PubMed] [Google Scholar]
- 2.Cornford P, Tilki D, van den Bergh RCN, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Arnhem, the Netherlands: EAU Guidelines Office; 2024. [Google Scholar]
- 3. Fizazi K, Chi KN. Abiraterone in metastatic prostate cancer. N Engl J Med. 2017;377:1697–1698. doi: 10.1056/NEJMc1711029. [DOI] [PubMed] [Google Scholar]
- 4. Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 5. James ND, Spears MR, Sydes MR. Abiraterone in metastatic prostate cancer. N Engl J Med. 2017;377:1696–1697. doi: 10.1056/NEJMc1711029. [DOI] [PubMed] [Google Scholar]
- 6. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boye M, Ribbans A, Leith A, et al. Real-world health-related quality of life and caregiver need in patients with metastatic hormone-sensitive and metastatic castration-resistant prostate cancer. J Clin Oncol. 2022;40 (6_suppl; abstr 54) [Google Scholar]
- 8. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 9. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 11. Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: Final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39:2294–2303. doi: 10.1200/JCO.20.03488. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40:1616–1622. doi: 10.1200/JCO.22.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399:1695–1707. doi: 10.1016/S0140-6736(22)00367-1. [DOI] [PubMed] [Google Scholar]
- 15. Smith MR, Hussain M, Saad F, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386:1132–1142. doi: 10.1056/NEJMoa2119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leith A, Ribbands A, Kim J, et al. Impact of next-generation hormonal agents on treatment patterns among patients with metastatic hormone-sensitive prostate cancer: A real-world study from the United States, five European countries and Japan. BMC Urol. 2022;22:33. doi: 10.1186/s12894-022-00979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freedland SJ, Sandin R, Sah J, et al. Treatment patterns and survival in metastatic castration-sensitive prostate cancer in the US Veterans Health Administration. Cancer Med. 2021;10:8570–8580. doi: 10.1002/cam4.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raval AD, Chen S, Littleton N, et al. Underutilization of androgen deprivation therapy (ADT) intensification for the treatment of men with metastatic hormone-sensitive prostate cancer (mHSPC): A systematic review of real-world database studies. J Clin Oncol. 2024;42 (4_suppl; abstr 66) [Google Scholar]
- 19. Ryan CJ, Ke X, Lafeuille MH, et al. Management of patients with metastatic castration-sensitive prostate cancer in the real-world setting in the United States. J Urol. 2021;206:1420–1429. doi: 10.1097/JU.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi: 10.1038/srep12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zurth C, Sandmann S, Trummel D, et al. Blood-brain barrier penetration of [14C]darolutamide compared with [14C]enzalutamide in rats using whole body autoradiography. J Clin Oncol. 2018;36 (6_suppl; abstr 345) [Google Scholar]
- 22. Zurth C, Koskinen M, Fricke R, et al. Drug-drug interaction potential of darolutamide: In vitro and clinical studies. Eur J Drug Metab Pharmacokinet. 2019;44:747–759. doi: 10.1007/s13318-019-00577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams SCR, Mazibuko N, O'Daly O, et al. Comparison of cerebral blood flow in regions relevant to cognition after enzalutamide, darolutamide, and placebo in healthy volunteers: A randomized crossover trial. Target Oncol. 2023;18:403–413. doi: 10.1007/s11523-023-00959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shore N, Zurth C, Fricke R, et al. Evaluation of clinically relevant drug-drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: Results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol. 2019;14:527–539. doi: 10.1007/s11523-019-00674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shore N, Garcia-Horton V, Terasawa E, et al. Safety differences across androgen receptor inhibitors in nonmetastatic castration-resistant prostate cancer. Future Oncol. 2023;19:385–395. doi: 10.2217/fon-2022-1123. [DOI] [PubMed] [Google Scholar]
- 26. Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023;41:3595–3607. doi: 10.1200/JCO.23.00041. [DOI] [PubMed] [Google Scholar]
- 27. Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383:1040–1049. doi: 10.1056/NEJMoa2001342. [DOI] [PubMed] [Google Scholar]
- 28. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 29. Shore ND, Gratzke C, Feyerabend S, et al. Extended safety and tolerability of darolutamide for nonmetastatic castration-resistant prostate cancer and adverse event time course in ARAMIS. Oncologist. 2024;29:581–588. doi: 10.1093/oncolo/oyae019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shore ND, Luz M, Ulys A, et al. Long-term safety and tolerability of darolutamide and duration of treatment in patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) from the ARAMIS Rollover Study. J Clin Oncol. 2023;41 (6_suppl; abstr 147) [Google Scholar]
- 31. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dodkins J, Nossiter J, Cook A, et al. Does research from clinical trials in metastatic hormone-sensitive prostate cancer treatment translate into access to treatments for patients in the “real world”? A systematic review. Eur Urol Oncol. 2024;7:14–24. doi: 10.1016/j.euo.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 34. Freedland SJ, Klaassen ZWA, Agarwal N, et al. Reasons for oncologist and urologist treatment choice in metastatic castration sensitive prostate cancer (mCSPC): A physician survey linked to patient chart reviews in the United States. J Clin Oncol. 2022;40 (16_suppl; abstr 5065) [Google Scholar]
- 35. Raval AD, Lunacsek O, Kom MJ, et al. Real-world intensification beyond androgen deprivation therapy (ADT) in metastatic hormone sensitive prostate cancer (mHSPC) in the United States 2017-2023: An administrative claims database study. J Clin Oncol. 2024;42 (16_suppl; abstr e17082) [Google Scholar]
- 36.Wenzel M, Banek S, Chun FKH, et al. Contemporary treatment standards and trends of systemic therapy in metastatic hormone-sensitive prostate cancer—Implementing study data in clinical practice [article in German] Urologie. doi: 10.1007/s00120-024-02410-7. [epub ahead of print on May 13, 2024] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. George DJ, Morgans AK, Constantinovici N, et al. Androgen receptor inhibitors in patients with nonmetastatic castration-resistant prostate cancer. JAMA Netw Open. 2024;7:e2429783. doi: 10.1001/jamanetworkopen.2024.29783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO-24-01798.