Abstract
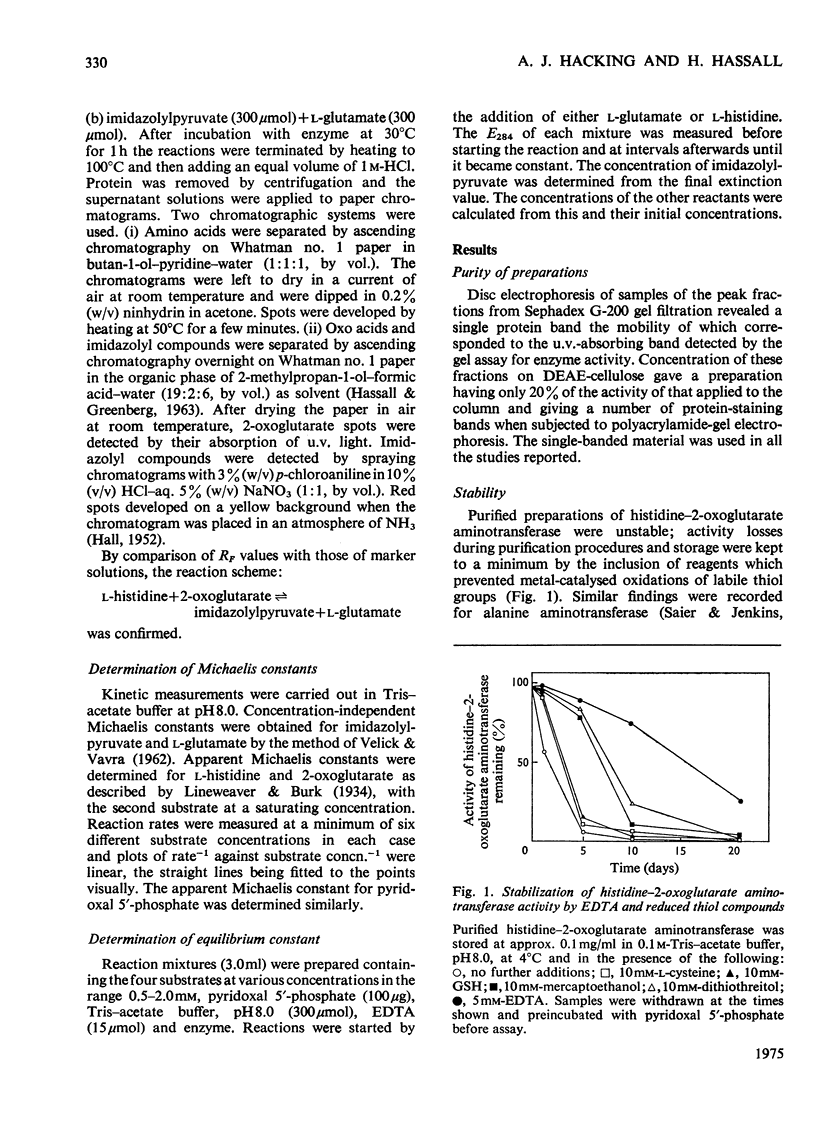
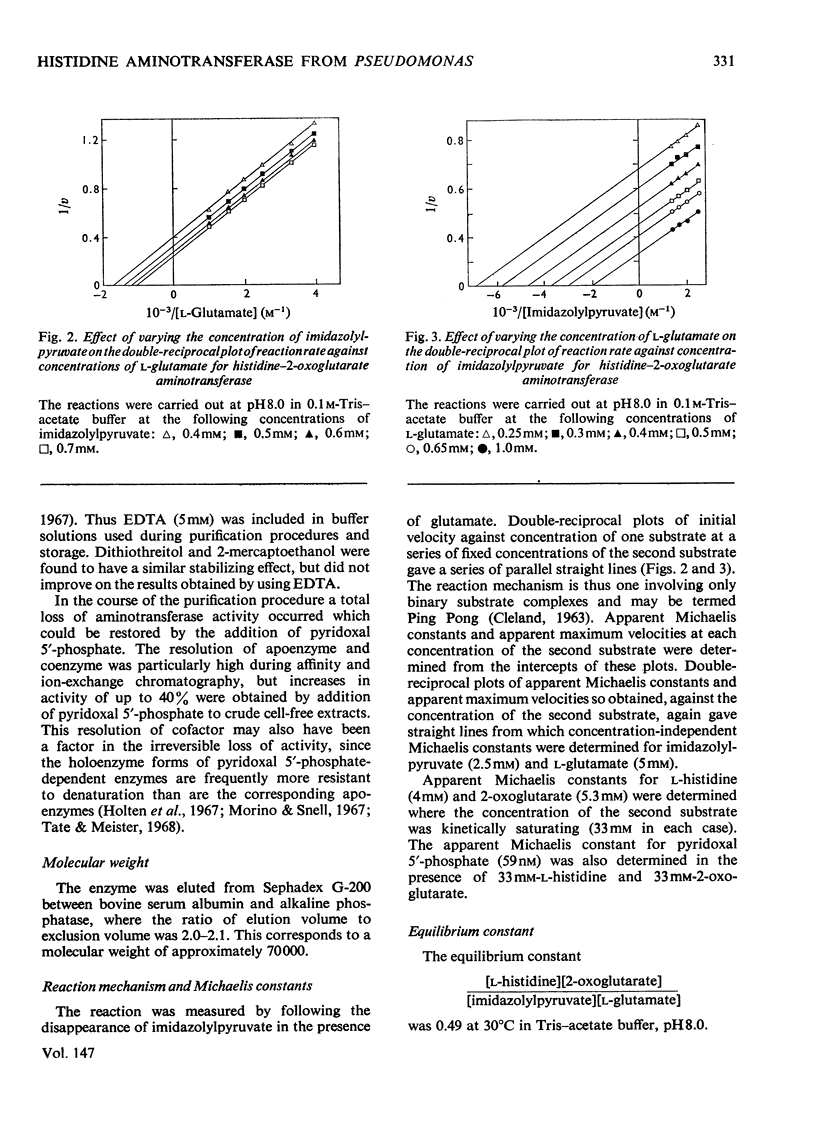
1. Inducible L-histidine--2-oxoglutarate aminotransferase was purified some 170-fold from extracts of Pseudomonas testosteroni. 2. The preparation showed only one major component after electrophoresis on polyacrylamide gels, though additional minor bands were observed when samples concentrated on a DEAE-cellulose column were used. 3. The molecular weight of the enzyme was found to be approx. 70000 by chromatography on Sephadex G-200. 4. The purification scheme produced enzyme that was inactive in the absence of pyridoxal 5'-phosphate. 5. The equilibrium constant for the reaction L-histidine+2-oxoglutarate equilibrium imidazolylpyruvate+L-glutamate was 0.49. 6. The reaction mechanism was Ping Pong. 7. The enzyme was shown to have only low activity towards aromatic amino acids and was highly specific for 2-oxoglutarate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Levin A. P. Histidine-2-oxoglutarate aminotransferase activity in Salmonella typhimurium. Biochem J. 1969 Sep;114(3):662–664. doi: 10.1042/bj1140662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet A., Hoffmeyer J., Roche J., Hedegaard J. Sur la réversibilité de la dégradation de la L-histidine en acide imidazolelactique chex différents microorganismes. C R Seances Soc Biol Fil. 1968;162(5):1054–1058. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Coote J. G., Hassall H. The control of the enzymes degrading histidine and related imidazolyl derivates in Pseudomonas testosteroni. Biochem J. 1973 Mar;132(3):423–433. doi: 10.1042/bj1320423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G., Hassall H. The degradation of L-histidine, imidazolyl-L-lactate and imidazolylpropionate by Pseudomonas testosteroni. Biochem J. 1973 Mar;132(3):409–422. doi: 10.1042/bj1320409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Emes A. V., Hassall H. The degradation of L-histidine in the rat. The formation of imidazolylpyruvate, imidazolyl-lactate and imidazolylpropionate. Biochem J. 1973 Nov;136(3):649–658. doi: 10.1042/bj1360649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL D. A. Histidine alpha-deaminase and the production of urocanic acid in the mammal. Biochem J. 1952 Jul;51(4):499–504. doi: 10.1042/bj0510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSALL H., GREENBERG D. M. Studies on the enzymic decomposition of urocanic acid. V. The formation of 4-oxoglutaramic acid, a nonenzymic oxidation product of 4(5)-imidazolone-5(4)-propionic acid. J Biol Chem. 1963 Apr;238:1423–1431. [PubMed] [Google Scholar]
- Hassall H., Soutar A. K. Amino acid sequence of a peptide containing the active cysteine residue of histidine ammonia-lyase. Biochem J. 1974 Mar;137(3):559–566. doi: 10.1042/bj1370559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. I., Granner D. K., Tomkins G. M. Tyrosine aminotransferase. Purificaton and characterization. J Biol Chem. 1967 Sep 25;242(18):3998–4006. [PubMed] [Google Scholar]
- Holten D., Wicks W. D., Kenney F. T. Studies on the role of vitamin B6 derivatives in regulating tyrosine alpha-ketoglutarate transaminase activity in vitro and in vivo. J Biol Chem. 1967 Mar 10;242(5):1053–1059. [PubMed] [Google Scholar]
- JENKINS W. T., YPHANTIS D. A., SIZER I. W. Glutamic aspartic transaminase. I. Assay, purification, and general properties. J Biol Chem. 1959 Jan;234(1):51–57. [PubMed] [Google Scholar]
- Karni-Katsadimas I., Dimitropoulos C., Evangelopoulos A. E. Substrate induced changes in the reactivity of the sulfhydryl groups of aspartate transaminase. Eur J Biochem. 1969 Mar;8(1):50–54. doi: 10.1111/j.1432-1033.1969.tb00493.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin R. G., Goldberger R. F. Imidazolylacetolphosphate:L-glutamate aminotransferase. Purification and physical properties. J Biol Chem. 1967 Mar 25;242(6):1168–1174. [PubMed] [Google Scholar]
- Morino Y., Snell E. E. The subunit structure of tryptophanase. I. The effect of pyridoxal phosphate on the subunit structure and physical properties of tryptophanase. J Biol Chem. 1967 Dec 10;242(23):5591–5601. [PubMed] [Google Scholar]
- SCARDI V., SCOTTO P., IACCARINO M., SCARANO E. The binding of pyridoxal 5-phosphate to aspartate aminotransferase of pig heart. Biochem J. 1963 Jul;88:172–175. doi: 10.1042/bj0880172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOLTER H., BALDRIDGE R. C. MULTIPLE FORMS OF HISTIDINE-PYRUVATE TRANSAMINASE IN RAT LIVER. Biochim Biophys Acta. 1964 Aug 19;90:287–290. doi: 10.1016/0304-4165(64)90191-6. [DOI] [PubMed] [Google Scholar]
- SPOLTER P. D., BALDRIDGE R. C. The metabolism of histidine. V. On the assay of enzymes in rat liver. J Biol Chem. 1963 Jun;238:2071–2074. [PubMed] [Google Scholar]
- Saier M. H., Jr, Jenkins W. T. Alanine aminotransferase. I. Purification and properties. J Biol Chem. 1967 Jan 10;242(1):91–100. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Studies on the sulfhydryl groups of L-aspartate beta-decarboxylase. Biochemistry. 1968 Sep;7(9):3240–3247. doi: 10.1021/bi00849a029. [DOI] [PubMed] [Google Scholar]
- VELICK S. F., VAVRA J. A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem. 1962 Jul;237:2109–2122. [PubMed] [Google Scholar]
- Wickramasinghe R. H. Multiple histidine degrading enzymes in Proteus vulgaris. Experientia. 1970 Jan 15;26(1):37–38. doi: 10.1007/BF01900377. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe R. H. Repressible histidine transamination in Escherichia coli and its retro-inhibition. Enzymologia. 1969 Aug 29;37(2):91–96. [PubMed] [Google Scholar]
- Wickramasinghe R. H. Studies on the histidine transaminating enzyme of Escherichia coli. Enzymologia. 1969;36(3):161–171. [PubMed] [Google Scholar]


