Abstract
The vast majority of cases of gram-negative meningitis in neonates are caused by K1-encapsulated Escherichia coli. The role of the K1 capsule in the pathogenesis of E. coli meningitis was examined with an in vivo model of experimental hematogenous E. coli K1 meningitis and an in vitro model of the blood-brain barrier. Bacteremia was induced in neonatal rats with the E. coli K1 strain C5 (O18:K1) or its K1− derivative, C5ME. Subsequently, blood and cerebrospinal fluid (CSF) were obtained for culture. Viable bacteria were recovered from the CSF of animals infected with E. coli K1 strains only; none of the animals infected with K1− strains had positive CSF cultures. However, despite the fact that their cultures were sterile, the presence of O18 E. coli was demonstrated immunocytochemically in the brains of animals infected with K1− strains and was seen by staining of CSF samples. In vitro, brain microvascular endothelial cells (BMEC) were incubated with K1+ and K1− E. coli strains. The recovery of viable intracellular organisms of the K1+ strain was significantly higher than that for the K1− strain (P = 0.0005). The recovery of viable intracellular K1− E. coli bacteria was increased by cycloheximide treatment of BMEC (P = 0.0059) but was not affected by nitric oxide synthase inhibitors or oxygen radical scavengers. We conclude that the K1 capsule is not necessary for the invasion of bacteria into brain endothelial cells but is responsible for helping to maintain bacterial viability during invasion of the blood-brain barrier.
Meningitis remains a potentially devastating disease. In the neonatal period Escherichia coli is the most common gram-negative pathogen responsible for meningitis (9, 31). It is associated with a mortality rate as high as 40%, and more than half of the survivors have neurologic sequelae (9, 31). The poor outcome statistics, despite medical advances, including bactericidal antibiotics and improved intensive-care unit care, point to our incomplete knowledge of the pathogenesis and pathophysiology of neonatal E. coli meningitis. It is well documented that the majority of cases of neonatal E. coli meningitis are caused by K1-encapsulated bacteria (26). The reasons for this association are myriad and may include the neonatal immune system’s incomplete ability to localize and fight infection and the propensity of certain strains of E. coli to invade the central nervous system. In addition, studies of infants with meningitis and animal models of meningitis have shown that a high level of bacteremia is required for the development of E. coli meningitis (7, 15). Previous investigations have determined that the K1 capsule contributes to this high level of bacteremia by virtue of its serum resistance and antiphagocytic properties (15).
In an effort to better understand how systemically circulating E. coli bacteria cross the blood-brain barrier, we have used an in vivo model of neonatal rat meningitis (13, 15). This model shares several characteristics with human neonatal meningitis, most notably hematogenous infection of the meninges. In addition we have used an in vitro model of the blood-brain barrier, with bovine brain microvascular endothelial cell (BMEC) monolayers (11, 25, 29), to examine the process of invasion by K1+ and K1− E. coli. While the use of two different species of brain tissues may be questioned, in detailed experiments performed in our laboratory, the interactions between E. coli and brain endothelial cells were found to be similar, regardless of the cell’s species of derivation (25, 29). Given that bovine brain cells are more readily available, we opted to use these cells for our in vitro experiments. The present research uses these experimental models to examine, in part, the process by which bacteria gain access to the central nervous system and remain viable. We hypothesize that the K1 capsule is not necessary for the invasion of brain endothelial cells. It is, however, an important virulence factor, protecting E. coli from host defenses, and thus the bacterium is able to cross the blood-brain barrier alive, ultimately leading to meningitis.
MATERIALS AND METHODS
Bacterial strains.
The clinical isolate of K1-encapsulated E. coli, strain C5 (O18:K1), and its unencapsulated mutant C5ME have been characterized previously (15). Briefly, strain C5 was isolated from the cerebrospinal fluid (CSF) of a newborn infant with E. coli meningitis. Strain C5ME was obtained by selection for resistance to the K1-specific bacteriophages. Strain C5ME was examined for the loss of capsule production by the antiserum agar technique, testing for agglutination with an anti-K1 monoclonal antibody as well as lytic sensitivity to the K1-specific bacteriophages, as described previously (15). Extensive investigations have been undertaken to examine known virulence factors in the K1 mutant in order to ensure that these phenotypic characteristics remained intact. There were no phenotypic alterations in virulence factors such as outer membrane protein, S fimbriae, O18 lipopolysaccharide (LPS), and the invasion protein Ibe10 (11, 15, 24). The parent K1+ strain and the K1 mutant strain possess identical hemolysin, biochemical reactions, and patterns of binding to homologous LPS monoclonal antibody (15). In addition, these strains were found to have identical genotypes when they were examined by multilocus enzyme electrophoresis (15).
Animal model for E. coli bacteremia and meningitis.
E. coli bacteremia and meningitis, defined as a positive CSF culture, were induced in 5-day-old rats by a method described previously (13, 15). Briefly, outbred, specific-pathogen-free, pregnant Sprague-Dawley rats with timed conception were purchased from Charles River Breeding Laboratories (Wilmington, Mass.); the rats delivered in our vivarium 5 to 7 days after arrival. Each adult rat and her pups (average litter size, 10; range, 8 to 16) were housed in an opaque solid-polypropylene cage under a Small Animal Isolator (model 1894; Forma Scientific, Inc., Marietta, Ohio).
At the age of 5 days, all members of each litter were randomly divided into two groups to receive E. coli C5 (O18:K1; wild type) or C5ME (K1 mutant) subcutaneously. Pilot experiments were performed with each bacterial strain to determine the inoculum size that would induce a level of bacteremia (105 to 108 CFU/ml of blood) found to be necessary for hematogenous bacteria to enter the central nervous system (15). Inoculum sizes were 1 × 102 to 2.5 × 102 CFU for C5 and 5.6 × 106 to 1.4 × 107 CFU for C5ME. Eighteen hours after bacterial inoculation, blood and CSF (approximately 15- to 20-μl) specimens were obtained as described previously for quantitative cultures (13, 15). A 10-μl portion of each CSF specimen was used for quantitative cultures, and the remaining CSF (5 to 10 μl) from each animal was pooled (total volume, approximately 30 to 50 μl) for the detection of bacteria as described below. Immediately after blood and CSF specimens were obtained, the brains of selected animals were removed for examination of the presence of bacteria by immunocytochemistry (see below). Because the animals soon die after they reach the high degree of bacteremia necessary for the development of meningitis, this model does not permit us to investigate the effect of the duration of high-degree bacteremia on the development of meningitis.
Detection of bacteria in CSF.
Two methods were used to demonstrate morphologically the presence of bacteria in CSF. First, selected CSF specimens were pooled and concentrated by cytospin centrifugation, stained with acridine orange, and examined under a fluorescence microscope (Olympus BH2, equipped with a wide-band BPu 95 filter set).
Second, selected CSF specimens were pooled and centrifuged, and sediments were fixed with 2% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) for 1 h. The sediments were rinsed twice, for 5 min each time, with 0.1 M PBS, and then 1% gelatin (J.T. Parker Chemical Co., Philipsburg, N.J.) was added to the vial to form a pellet. The pellet was fixed with 1% OsO4 in 0.1 M PBS for 1 h, dehydrated in graded alcohols, and embedded in Epon 812. One-micron-thick sections were cut and stained with 1% Azure II, 1% methylene blue, and 0.5% basic fuchsin for light microscopy examination.
Immunocytochemical detection of bacteria in brains.
Brains from infected animals were embedded in OCT compound (Tissue Tek; Sakura Finetek) and cut by using a B1-H1 cryostat. Sections were fixed in acetone, preincubated with 1% acetic acid to block endogenous alkaline phosphatase activity, and then blocked with 5% heat-inactivated serum to avoid nonspecific binding of immunoglobulin (Ig) to neuronal tissues. The sections were then incubated with the primary antibody to O18 LPS (murine IgG monoclonal anti-O18 antibody) (14), followed by incubation with the secondary antibody (biotinylated sheep anti-mouse IgG). The sections were further incubated with alkaline phosphatase-conjugated streptavidin. Visualization of the antigen-antibody complex (red color) was done with the Alkaline Phosphatase Substrate Kit I (Vector Laboratories), and sections were counterstained with hematoxylin. Controls used for tissue specimens as well as for antibodies included uninfected brains and omission of the primary and/or secondary antibody.
In vitro invasion assays.
An in vitro model of the blood-brain barrier was developed with bovine BMECs. These cells were isolated and cultured as described previously (29) and were used in invasion experiments. Ten million bacteria in 500 μl of experimental media (Ham’s F-12, medium 199, 1× Earle salts [1:1], 5% heat inactivated fetal bovine serum [FBS], 1% sodium pyruvate, and 0.5% glutamine) (Irvine Scientific) were added to confluent BMEC monolayers at a multiplicity of infection of 100. The cells and bacteria were incubated for 1½ h at 37°C under 5% CO2 without shaking. The monolayers were then washed four times with M199 and reincubated with experimental medium containing gentamicin (100 μg/ml) for 1 h at 37°C to kill extracellular bacteria. In pilot experiments performed previously, there was no bacterial survival in the absence of BMECs if gentamicin was present. To determine the number of viable intracellular bacteria, the monolayers were washed five times with M199 and lysed with 100 μl of 0.1% Triton X-100 for 10 min. Four hundred microliters of M199 was then added to the wells for a final volume of 500 μl. Bacterial viability was not affected by this Triton X-100 treatment. CFUs were determined for each well by plating 50 μl undiluted and three serial 10-fold dilutions on blood agar plates. These experiments were conducted in triplicate and were repeated a minimum of three times.
Effects of eukaryotic inhibitors on E. coli invasion.
Cycloheximide (20 μg/ml), Nω-nitro-l-arginine (NNLA) (1 mM), Nγ-methyl-l-arginine (NMLA) (1 mM), superoxide dismutase (SOD) (100 μg/ml), or catalase (5,000 U/ml) (all from Sigma) was added to the wells in 400 μl of experimental medium 1 h before the bacteria. None of these inhibitors affected the viability or morphology of the BMEC monolayers. Bacteria were then added in 100-μl volumes to the wells, thereby decreasing the concentrations of inhibitors by 20%. Following this, the procedure was the same as that for the invasion assay described above. Experiments with cycloheximide were run in multiples of 3, 6, or 9 and repeated five times, while those done with other inhibitors were performed in replicates of 3 or 6 and repeated three times.
Statistics.
Nonparametric (Mann-Whitney), unpaired, two-tailed tests were used to examine the differences between experimental study groups.
RESULTS
Demonstration of live bacteria in the CSF from the animal model.
To examine whether the entry of E. coli K1 into the central nervous system requires the capsule, E. coli bacteremia and meningitis were induced in 5-day-old rats with a K1-encapsulated and a K1− E. coli strain. The isolation of E. coli from CSF was, as expected, observed in animals infected with the K1+ strain, who developed a high degree of bacteremia (e.g., >105 CFU/ml of blood). Overall, 14 of the 24 (58%) animals who were infected with strain C5 and had levels of bacteremia greater than 105 CFU/ml of blood were found to have positive CSF cultures. In contrast, none of the 20 animals infected with the K1− strain (C5ME) were found to have positive CSF cultures, despite the fact that all animals developed similarly high levels of bacteremia. However, we were not able to exclude the possibility that these CSF specimens might contain viable bacterial counts below the lower limit of detection, e.g., <10 CFU/total CSF, assuming that the total CSF of a 5-day-old rat is approximately 50 to 100 μl.
Detection of bacteria in CSF.
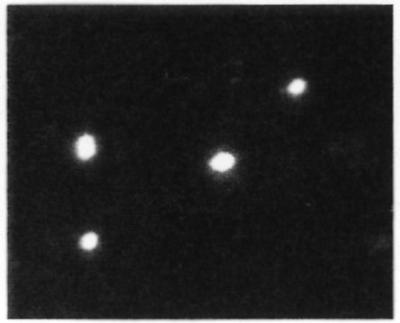
Due to the observation that animals infected with K1− bacteria did not develop meningitis, despite reaching high levels of bacteremia, we sought to determine if the K1 capsule was necessary for blood-brain barrier traversal or if it might function to facilitate bacterial survival instead. If the latter hypothesis is true, one would expect to find evidence of nonviable K1− E. coli in the CSF or brains of animals infected with this strain. The presence of bacteria was demonstrated in the CSF of these animals by two methods as described above. Figure 1 demonstrates the presence of bacteria with a morphology consistent with that of E. coli in acridine orange-stained cytospin specimens from animals infected with K1− E. coli whose CSF cultures were sterile. Similarly, methylene blue staining of semithin sections of pelleted and fixed sterile CSF specimens from animals infected with C5ME (K1−) revealed the presence of bacteria with a bacillus morphology consistent with that of E. coli (data not shown). These data suggest that the K1− strain is able to cross the blood-brain barrier and thus that sterile CSF cultures may not represent failure of the organism to invade the central nervous system. Instead, these organisms may be unable to survive the invasion process.
FIG. 1.
Acridine orange staining of cytospin specimens of pooled CSF derived from animals infected with C5ME (O18+ K1−) revealed the presence of bacilli despite the fact that their CSF cultures were sterile. Magnification, ×400.
Immunocytochemical detection of bacteria in the brain.
Corroborating evidence that K1− organisms could be found in the brains of animals infected with the K1 mutant, despite their having sterile CSF cultures, comes from immunocytochemical studies of brain sections from K1−-infected animals. The presence of O18 E. coli was demonstrated in the brain sections of two animals infected with E. coli K1 and two animals infected with its K1− derivative. Figure 2 shows representative brain cortex slices from three different experimental conditions: infection with E. coli K1 C5 (panel A), omission of primary antibody (panel B), and infection with C5ME (K1−) (panel C). The brain of an animal with a positive CSF culture infected with strain C5 revealed red precipitates, indicating E. coli stained by an O18 LPS antibody in the brain cortex (Fig. 2A). Controls (uninfected brains or brain sections for which the primary anti-O18 antibody was omitted) did not show any red precipitates (Fig. 2B), supporting the specificity of O18 E. coli interaction with the anti-O18 antibody. Similarly, E. coli bacteria, stained by O18 LPS antibody, were demonstrated in the brain of an animal infected with strain C5ME (O18+ K1−) despite the fact that its CSF cultures were sterile (Fig. 2C). This methodology does not permit the identification of the cellular location of these bacteria. Nevertheless, these results suggest that animals infected with K1− E. coli had bacteria present in their brains, despite having sterile CSF cultures.
FIG. 2.
Immunocytochemical detection of O18 E. coli (red dots) in brain sections. (A) The animal was infected with strain C5 (O18+ K1+) and had a positive CSF culture. (B) The primary O18 antibody was omitted. (C) The animal was infected with strain C5ME (O18+ K1−) and had a sterile CSF culture. Magnification in all panels, ×400.
Invasion assay and effects of eukaryotic inhibitors.
Our hypothesis, as supported by the previous data, is that the K1 capsule is not required for the invasion of E. coli into BMECs but does serve to protect the bacteria from being killed during the invasion process. To better understand the role of the K1 capsule in the invasion of BMECs by E. coli, we performed the following in vitro tissue culture invasion assays.
An in vitro model of the blood-brain barrier was developed with BMECs as described above. Experiments with the K1+ and K1− E. coli strains revealed a fourfold increase in the intracellular recovery of viable C5 (K1+) organisms over that of viable C5ME (K1−) organisms (P = 0.0005) after invasion of BMEC monolayers (Fig. 3). This correlates with our in vivo finding that viable bacteria were recovered only from CSF specimens of animals infected with E. coli K1.
FIG. 3.
Intracellular recovery of viable C5 (O18+ K1+) and C5ME (O18+ K1−) organisms from BMECs that were left untreated (solid bars) or pretreated with cycloheximide (20 μg/ml) (hatched bars). Error bars, standard errors of the means.
We then endeavored to define the mechanism of E. coli killing by a putative substance produced de novo by BMECs. To inhibit eukaryotic protein synthesis, BMECs were preincubated with cycloheximide (20 μg/ml) for 1 h prior to the addition of the bacteria. When BMEC monolayers were preincubated with cycloheximide prior to the invasion experiment, there was a statistically significant 2.5-fold increase (P = 0.0059) in the intracellular recovery of K1− E. coli (C5ME) organisms but not in that of K1+ E. coli (C5) organisms (Fig. 3). These findings suggest that a newly synthesized endothelial-cell product might be responsible for the decreased recovery of the K1− strain. Nitric oxide (NO) and oxygen intermediates have been shown to have antimicrobial properties (2, 8, 20). NO and/or nitric oxide synthase (NOS) has been isolated from a variety of different types of endothelial cells (12, 23), including cerebrovascular endothelium (5, 21). Superoxide and hydrogen peroxide have also been detected in cultured endothelial cells (4, 27). To see if NO and/or oxygen radicals produced by BMECs might be involved in the killing of K1− organisms, one of the NOS inhibitors NNLA (1 mM), NMLA (1 mM), SOD (100 μg/ml), and catalase (5,000 U/ml) (all from Sigma) was added to wells 1 h before the bacteria. None of these inhibitors affected the viability or morphology of the BMEC monolayers. Bacteria were then added in 100-μl volumes to the wells. As shown in Fig. 4, the addition of either of two NOS inhibitors (NNLA and NMLA), SOD–catalase, or a combination of SOD–catalase with NNLA or NMLA did not affect the intracellular recovery of K1− E. coli; the level of bacterial killing was similar to that in the standard invasion assay. Therefore, these agents could not duplicate the survival benefit seen with cycloheximide.
FIG. 4.
Effects of inhibitors on the intracellular recovery of viable C5ME (O18+ K1−) organisms from BMECs. CH, cycloheximide; cat, catalase; LNA, NNLA; LMA, NMLA; All#1, SOD–catalase and NNLA; All#2, SOD–catalase and NMLA. Error bars, standard errors of the means.
DISCUSSION
In the study of the pathogenesis of E. coli meningitis, we employed both in vitro and in vivo models of the blood-brain barrier, using BMEC monolayers and experimental hematogenous meningitis in neonatal rats. Our present findings revealed that both K1+ and K1− E. coli bacteria can invade the central nervous system and BMECs; however, significantly fewer K1− bacteria remain alive after invasion. The CSF clinical isolate of wild-type O18:K1+ E. coli, strain C5, and its K1− derivative, C5ME, have been shown to be identical in terms of several phenotypic properties examined (e.g., outer membrane protein, O18 LPS, S fimbriae, and invasion proteins) except for the presence of the capsule (11, 15, 24). Therefore, the differences between the recovery of live K1+ and K1− E. coli bacteria can likely be ascribed to the capsule. Because the genetic basis of the loss of the K1 capsule for the K1 phage-derived C5ME is undetermined, work to construct a genetically defined K1 mutant strain is in progress. This strain will contribute to further understanding of the role of the K1 capsule in central nervous system invasion by E. coli K1. Despite this limitation, we believe that the present study supports our hypothesis that the capsule is a crucial component of the bacterium’s armamentarium, allowing it to cross the blood-brain barrier and remain viable.
Prior in vivo studies of E. coli K1 meningitis have shown that the K1 capsule is a critical factor in the development of meningitis by virtue of its serum resistance and antiphagocytic properties (15). This is again demonstrated in this study by the differences in inoculum size required for the K1+ and K1− strains to reach high levels of bacteremia in neonatal rats. An inoculum approximately 104- to 105-fold greater was required for the K1− strain to achieve a high degree of bacteremia (e.g., >105 CFU/ml of blood) compared to the parent K1+ strain. The sterile CSF cultures from animals infected with K1− strains were previously interpreted to mean that the K1 capsule was necessary for the bacterial crossing of the blood-brain barrier (15). Several lines of evidence presented in this paper now suggest that the capsule is not necessary for invading BMECs but is responsible for maintaining the viability of the bacteria inside the BMECs. This is supported by the in vivo observation that while both K1+ and K1− E. coli bacteria are found in brains by immunocytochemical assays and in CSF by acridine orange and methylene blue staining, CSF cultures did not reveal any viable K1− E. coli organisms. These bacteria were therefore able to enter the central nervous system but were presumably killed in the process. We cannot completely exclude the possibility that sterile CSF cultures from the animals infected with K1− E. coli might represent viable counts below the limit of detection, e.g., <102 CFU/ml of CSF. We also recognize that the use of pooled specimens may not be the optimal experimental design; however, the volume of CSF that can be removed from a rat pup is small (15 to 20 μl) and necessitates the pooling of specimens. Therefore, to support these data, several different techniques (e.g., cytospins and fixed sections of pelleted CSF, and O18 LPS monoclonal antibody staining of brain sections) were used to demonstrate the presence of O18 bacteria in the central nervous systems of these animals, despite their having sterile CSF cultures. The concept that bacteria can be demonstrated in the central nervous systems of K1− E. coli-infected animals, without the evidence of viable bacteria, is a novel observation and potentially important for our understanding of the pathogenesis of E. coli meningitis.
Corroborating the in vivo finding are our tissue culture invasion data, which show that fourfold-fewer K1− bacteria can be recovered from BMECs in invasion assays. In addition, our in vitro data showed that by inhibition of BMEC protein synthesis with cycloheximide, the K1− strains were protected from killing. Our interpretation of these data is that brain endothelial cells may produce a substance that is bactericidal to E. coli strains without a capsule. The nature of this eukaryotic substance is unknown. Nitric oxide has been found to possess antimicrobial properties (8, 20) and is produced by endothelial cells via NOS (12, 23). NO has been shown, in vitro and in animal models, to be active against a wide variety of pathogens, including, but not limited to, the following organisms: bacteria (Mycobacterium spp., E. coli, Salmonella typhimurium [19, 22, 30]), viruses (herpes simplex virus type 1 and Japanese encephalitis virus [6, 18]), fungi (Cryptococcus neoformans [1]), and parasites (Leishmaniae major [17, 28]). We examined the possibility that NO or oxygen intermediates might be the agent of E. coli killing in our in vitro BMEC invasion assays. Using two different NOS inhibitors, which are analogues of the NOS substrate l-arginine, at concentrations found to inhibit NO production in endothelial cells (10), we were unable to reproduce the survival advantage to K1− E. coli seen with cycloheximide. This suggests that neither NO nor peroxynitrites, which are formed when NO reacts with oxygen radicals (3, 32), are responsible for the bacterial killing in this system. We also examined the possible effects of superoxides and other oxygen radicals, which are known antimicrobial products of professional phagocytes (2) and are produced by endothelial cells (4, 27), in the invasion process of meningitic E. coli K1 and its capsule-negative derivative. Experiments using SOD and catalase, which are scavengers of oxygen radicals, were performed. In contrast to the results of experiments with cycloheximide-treated BMECs, there was no increase in levels of viable intracellular K1− E. coli bacteria in these experiments. Because SOD and catalase are large proteins, it is possible that they do not freely enter endothelial cells, the putative site of the bacterial killing. There is some evidence that certain cells, such as hepatocytes, do actively take up SOD (16), but this has not been investigated in BMECs. Further experiments are needed to correlate BMEC oxygen radical production with bacterial killing and its inhibition with an increase in the survival of unencapsulated bacteria.
In summary, using an in vivo neonatal rat model of hematogenous meningitis and an in vitro model of the blood-brain barrier, we showed that both K1+ and K1− E. coli strains were able to penetrate BMECs and enter the central nervous system. However, only infections caused by K1+ strains resulted in positive CSF cultures in our animal model, and in vitro experiments yielded a significantly higher recovery of viable K1+ intracellular organisms compared to that of K1− strains. This strongly suggests that the K1 capsule has, in addition to its well-recognized serum resistance and antiphagocytic properties, a novel role in the transversal of E. coli K1 across the blood-brain barrier. It serves to protect K1-encapsulated E. coli strains from killing during invasion of the central nervous system, thus helping to explain the predominance of K1-encapsulated bacteria in neonatal E. coli meningitis.
ACKNOWLEDGMENTS
This work is supported in part by United States Public Health Service grant R01-NS-26310 and by a Pediatric Infectious Disease Society Fellowship Award sponsored by Lilly Research Laboratories.
REFERENCES
- 1.Alspaugh J A, Granger D L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991;59:2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babior B M. Oxygen-dependent microbial killing by phagocytes. N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 3.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Apparent hydroxyl radical production by peroxynitrite: implications of endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter W O, Narayanan P K, Robinson J P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994;55:253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- 5.Catalan R E, Martinez A M, Aragones M D, Hernandez F. Identification of nitric oxide synthases in isolated bovine brain vessels. Neurosci Res. 1996;25:195–199. doi: 10.1016/0168-0102(96)01038-3. [DOI] [PubMed] [Google Scholar]
- 6.Croen K D. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Investig. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietzman D E, Fischer G W, Schoenknecht F D. Neonatal Escherichia coli septicemia—bacterial counts in blood. J Pediatr. 1974;85:128–130. doi: 10.1016/s0022-3476(74)80308-2. [DOI] [PubMed] [Google Scholar]
- 8.Fang C F. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feigin R D. Bacterial meningitis in the newborn infant. Clin Perinatol. 1977;4:103–116. [PubMed] [Google Scholar]
- 10.Gross S S, Jaffe E A, Levi R, Kilbourn R G. Cytokine-activated endothelial cells express an isotype of nitric oxide-dependent synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank order of potency characteristic of activated macrophages. Biochem Biophys Res Commun. 1991;178:823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- 11.Huang S H, Wass C A, Fu Q, Prasadaroa N V, Stins M, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssens S P, Shimouchi A, Quertermous T, Bloch D B, Bloch K D. Cloning and expression of a cDNA encoding human endothelium derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992;267:14519–14522. [PubMed] [Google Scholar]
- 13.Kim K S. Comparison of cefotaxime, imipenem-cilastin, ampicillin-gentamicin, and ampicillin-chloramphenicol in the treatment of experimental Escherichia coli bacteremia and meningitis. Antimicrob Agents Chemother. 1985;28:433–436. doi: 10.1128/aac.28.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K S, Kang J H, Cross A S, Kaufman B, Zollinger W, Sadoff J. Functional activities of monoclonal antibodies to the O side chain of Escherichia coli lipopolysaccharides in vitro and in vivo. J Infect Dis. 1988;157:47–53. doi: 10.1093/infdis/157.1.47. [DOI] [PubMed] [Google Scholar]
- 15.Kim K S, Itabashi H, Gemski P, Sadoff J, Warren R L, Cross A S. The K1 capsule is the critical determinant in the development of E. coli meningitis in the rat. J Clin Investig. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyle M E, Nakae D, Sakaida I, Miccadei S, Farber J L. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988;263:3784–3789. [PubMed] [Google Scholar]
- 17.Liew F Y, Millott S, Parkinson C, Palmer R M J, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- 18.Lin Y L, Huang Y L, Ma S H, Yeh C T, Chiou S Y, Chen L K, Liao C L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncada S, Palmer R M J, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharm Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 21.Morin A M, Stanboli A. Nitric oxide synthase localization in cultured cerebrovascular endothelium during mitosis. Exp Cell Res. 1994;211:183–188. doi: 10.1006/excr.1994.1076. [DOI] [PubMed] [Google Scholar]
- 22.Pacelli R, Wink D A, Cook J A, Krishna M C, Degraff W, Friedman N, Tsokos M, Samuni A, Mitchell J B. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J Exp Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer R M J, Ferrige A G, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 24.Prasadarao N V, Wass C A, Hacker J, Jann K, Kim K S. Adhesion of S fimbriated Escherichia coli to brain glycolipids mediated by sfaA gene-encoded protein of S fimbriae. J Biol Chem. 1993;268:10356–10363. [PubMed] [Google Scholar]
- 25.Prasadarao N V, Wass C A, Kim K S. Endothelial cell GlcNAcβ1–4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins J B, McCracken G H, Gotschlich E C, Orskov F, Orskov I, Hanson L A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974;290:1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- 27.Rosen G M, Freeman B A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci USA. 1984;81:7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stins M F, Prasadarao N V, Ibric L, Wass C A, Luckett P, Kim K S. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvessel endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 30.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthase and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unhanand M, Mustafa M M, McCracken G H., Jr Gram-negative enteric bacillary meningitis: a 21-year experience. J Pediatr. 1993;122:15–21. doi: 10.1016/s0022-3476(05)83480-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Gunn C, Beckman J S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]