Abstract
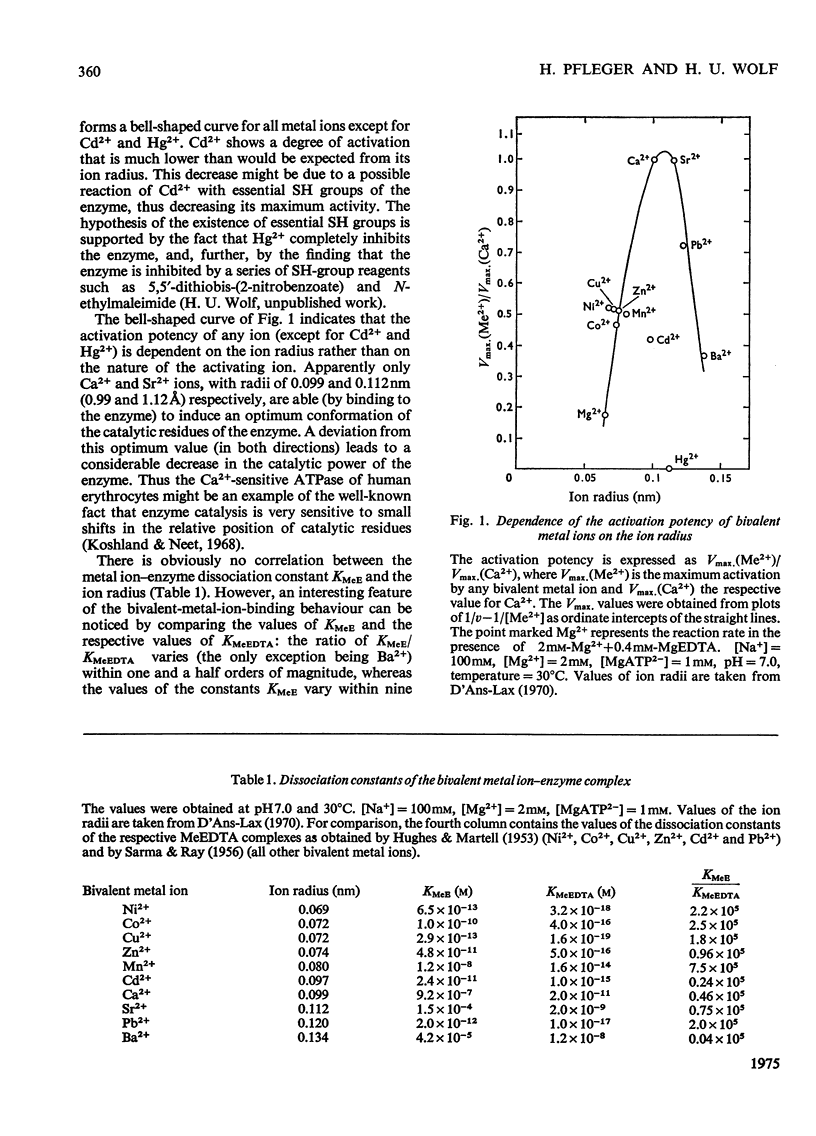
The Ca2+-sensitive ATPase (adenosine triphosphatase) of human erythrocyte membranes is activated, not only by Ca2+ ions, but also by a series of other bivalent metal ions including Sr2+, Ba2+, Mn2+, Ni2+, Co2+, Cd2+, Cu2+, Zn2+ and Pb2+. The degree of activation is dependent on the radius of the ion rather than on its nature, in contrast with the dissociation constant of the enzyme--metal ion complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond G. H., Green J. W. Effects of monovalent cations on the (Mg 2+ + Ca 2+ )-dependent ATPase of the red cell membrane. Biochim Biophys Acta. 1971 Aug 13;241(2):393–398. doi: 10.1016/0005-2736(71)90038-1. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Petitclerc C., Lazdunski M. Structure-function relationships for some metalloalkaline phosphatases of E. coli. Eur J Biochem. 1969 Apr;8(4):510–517. doi: 10.1111/j.1432-1033.1969.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Olson E. J., Cazort R. J. Active calcium and strontium transport in human erythrocyte ghosts. J Gen Physiol. 1969 Mar;53(3):311–322. doi: 10.1085/jgp.53.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray W. J., Jr Role of bivalent cations in the phosphoglucomutase system. I. Characterization of enzyme-metal complexes. J Biol Chem. 1969 Jul 25;244(14):3740–3747. [PubMed] [Google Scholar]
- Rifkin R. J. In vitro inhibition of Na+-K+ and Mg2+ ATPases by mono, di and trivalent cations. Proc Soc Exp Biol Med. 1965 Dec;120(3):802–804. doi: 10.3181/00379727-120-30658. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Enzyme action: views derived from metalloenzyme studies. Chem Br. 1968 Sep;4(9):397–402. [PubMed] [Google Scholar]
- Voth D. Uber das Verhalten von Adenosintriphosphatasen (ATPasen) in verschiedenen Rattenhirnfraktionen unter besonderer Berücksichtigung des Einflusses mono- und bivalenter Kationen. Brain Res. 1967 Feb;4(1):60–80. doi: 10.1016/0006-8993(67)90149-7. [DOI] [PubMed] [Google Scholar]
- Wolf H. U. Studies on a Ca 2+ -dependent ATPase of human erythrocyte membranes. Effects of Ca 2+ and H + . Biochim Biophys Acta. 1972 May 9;266(2):361–375. doi: 10.1016/0005-2736(72)90094-6. [DOI] [PubMed] [Google Scholar]


