Abstract
Salmonellae are gastrointestinal pathogens of man and animals. However, strains that are host-specific avian pathogens are often avirulent in mammals, and those which are nonspecific are commensal in poultry. The objective of this study was to determine whether host specificity was exhibited by bacterial abilities to invade epithelial cells or resist leukocyte killing. In this study, leukocytes isolated from humans and chickens were used to kill Salmonella in vitro. Both Salmonella pullorum, an avian-specific serotype, and Salmonella typhimurium, a broad-host-range serotype, were sensitive to killing by polymorphonuclear leukocytes isolated from both species. Both serotypes replicated in cells of the MQ-NCSU avian-macrophage cell line. In contrast, S. pullorum was noninvasive for cultured epithelial Henle 407, chick kidney, chick ovary, and budgerigar abdominal tumor cells. In the bird challenge, however, S. typhimurium rapidly caused inflammation of the intestinal mucosa, but S. pullorum preferentially targeted the bursa of Fabricius prior to eliciting intestinal inflammation. Salmonella serotypes which cause typhoid fever in mice have been shown to target the gut-associated lymphoid tissue. Observations from this study show that S. pullorum initiated a route of infection in chicks comparable to the route it takes in cases of enteric fever.
Salmonella is a facultative, intracellular pathogen capable of infecting a variety of hosts, resulting in several manifestations of disease, including enteric fever, bacteremia, and gastroenteritis (20). Following oral ingestion, Salmonella penetrates the mucosal epithelium of the small intestine, interacting with columnar epithelial cells and microfold cells overlaying the Peyer’s patches (12). Interaction between Salmonella and the epithelium triggers the chemotaxis of phagocytic cells to the infected site (40). This cellular response involves both neutrophils and macrophages migrating to the lumenal surface where they begin eradicating the bacterial pathogen (34). Penetration of microfold cells results in the presentation of Salmonella to macrophages residing in the lymphoid follicles (26). Salmonella has been shown to survive and replicate within macrophages from many hosts, including mice and chickens (1–3, 10, 11, 16, 42). Previous studies have demonstrated that macrophages play a role in the dissemination of Salmonella to organs of the reticuloendothelial system, such as mesenteric lymph nodes, liver, and spleen (12). Survival within macrophages is essential for the full expression of Salmonella virulence in mice (16).
While the pathogenesis of salmonellosis is well defined in the rabbit and mouse mammalian models, there is a surprisingly limited amount of literature describing Salmonella pathogenesis in an avian model. Light and electron microscopic examinations of intestine taken from chickens experimentally infected with various Salmonella species demonstrate similar cellular responses to these organisms, including the influx of heterophils and macrophages to the lumenal surface of the intestine (6, 46). Heterophils are considered to be the avian counterpart to mammalian neutrophils in their action as tissue phagocytes and their importance in host defense against bacterial infections (9, 37, 45). The capacity of heterophils and avian macrophages to kill Salmonella has been demonstrated through bactericidal assays performed in vitro (42). In addition, studies of salmonellosis in experimentally infected birds have obtained data that localize the presence of Salmonella to the intestine, liver, and spleen (46). This indicates that the pathogenesis of avian salmonellosis involves a dissemination of the organism that is similar to what has been established in the mammalian models.
The above-mentioned studies have reported observations and data from the experimental infection of birds by broad-host-range Salmonella serotypes. Salmonella typhimurium is a broad-range pathogen whose pathogenicity depends on the species of the host infected. For example, while S. typhimurium colonization in humans commonly produces gastroenteritis, this same organism causes lethal enteric fever in mice (12, 33). The colonization of S. typhimurium in chickens may elicit gastroenteritis in young birds; however, adult birds can serve as lifetime hosts for this organism without showing signs of infection (6). In contrast, Salmonella pullorum is a host-specific avian pathogen whose colonization in chickens results in a septic disease that kills young birds (41). S. pullorum is not often associated with disease in any other species (44). Recent studies performed in vitro have demonstrated that transepithelial signaling is crucial for those Salmonella species known to elicit gastroenteritis in humans (30). Serotypes that were non-human pathogens did not exhibit this signaling, indicating that virulence mechanisms which contribute to host specificity are expressed during the initial steps of colonization (31). Thus, the intensity and outcome of the disease produced by host-specific Salmonella serotypes depend largely on how these organisms interaction with the intestinal mucosa. The objective of this study was to compare the pathogenesis of disease in birds experimentally infected with either S. typhimurium or S. pullorum.
MATERIALS AND METHODS
Bacteria.
S. typhimurium SR-11 has been previously described (29). The host-specific avian pathogen S. pullorum χ3423 was a gift from Roy Curtiss III (Washington University, St. Louis, Mo.). Cultures were statically grown overnight at 37°C in Luria broth (LB). Confirmation that these isolates contained the invA gene and the virulence plasmid was obtained through PCR with spvC- and invA-specific primers. The 21-mer primers for Salmonella spvC and invA gene probes were designed by using published sequences (GenBank accession no. M64295 and M90846, respectively) and Oligo software (National Biosciences, Plymouth, Minn.). The spvC primer sequences were 5′-CGGAAATACCATCTACAAATA-3′ and 5′-CCCAAACCCATACTTACTCTG-3′ and were predicted to yield a 669-bp product. The invA primer sequences were 5′-TTGTTACGGCTATTTTGACCA-3′ and 5′-CTGACTGCTACCTTGCTGATG-3′ and were predicted to yield a 521-bp product. Primers were prepared by the University of Georgia molecular genetics instrumentation laboratory with the ABI Model 394 DNA synthesizer. Template DNA was isolated from S. typhimurium and S. pullorum by boiling loopfuls of bacteria for 20 min in water. PCR was conducted by using a PTC-100 model thermocycler (MJ Research, Inc., Watertown, Maine) with denaturation at 93°C for 1 min, primer annealing at 42°C for 1 min, and primer extension at 72°C for 2 min, for a total of 30 cycles. The products were examined by agarose gel electrophoresis for the presence of DNA fragments of the appropriate size. PCR performed with primers in the absence of genomic DNA served as the negative control.
Leukocyte isolation.
We used 20 specific-pathogen-free White Leghorn chickens, 5 to 8 weeks in age, as avian blood donors. The birds were placed in poultry house floor pens on a 16 h of light/8 h of dark cycle and were provided water and growth ration (The University of Georgia, Athens) ad libitum.
Avian heterophils were obtained from blood by using a modification described by Brooks et al. of a procedure previously described by Glick et al. (8, 19). Neutrophils were isolated from venous blood samples taken from healthy male and female human volunteers as previously described (15). Whole blood was collected in EDTA-containing Vacutainer tubes (Becton Dickinson, Rutherford, N.J.) from the median cubital vein and then subjected to a discontinuous Ficoll-Hypaque density gradient. Contaminating erythrocytes were lysed with phosphate-buffered deionized water, and the remaining cells were washed three times in magnesium-free Hanks balanced salt solution (HBSS) (Sigma Chemical Co., St. Louis, Mo.) supplemented with 1% fetal bovine serum (FBS) and suspended to a concentration of 3 × 106 cells/ml in HBSS.
Adherence and invasion assay.
Adherence and invasion assays were performed as previously described (17). The budgerigar abdominal tumor cells (BAT) were a gift from Phil Lukert (University of Georgia, Athens). Both the human Henle 407 and avian BAT cell lines were grown in Eagle’s minimal essential medium (EMEM) (Sigma Chemical Co.) supplemented with 5% each of FBS and chicken serum. Chick ovary and chick kidney cells were isolated as previously described and cultured in a 50:50 mixture of MEM and Ham’s F-12 medium (Sigma Chemical Co.) supplemented with 5% chicken and 2.5% horse sera (27). Monolayers were subjected to a bacterial inoculation at a multiplicity of infection of 100 organisms/epithelial cell. Plates were then centrifuged at 50 × g for 5 min at room temperature to facilitate contact of bacterium with the monolayers. Cells were then incubated at 37°C for either 1 h for adherence or 2 h for invasion. Monolayers were washed with EMEM three times to remove nonadherent bacteria. Noninvasive bacteria were killed with a polymyxin B-gentamicin overlay (100 μg/ml of each in EMEM for 1 h at 37°C). Monolayers were then lysed with 0.1% sodium deoxycholate in LB, and the bacteria were titered on LB agar.
Bactericidal assay.
The abilities of neutrophils and heterophils to kill Salmonella were evaluated by a modified colorimetric bactericidal assay (35). Isolates were opsonized with heat-inactivated chicken serum in HBSS for 20 min and then adjusted to 5 × 107 organisms/ml. Briefly, 106 opsonized bacteria in 50 μl of HBSS were added to 3 × 105 phagocytes in 100 μl of HBSS in quadruplicate wells in 96-well tissue culture plates. In order to enhance bacterium-phagocyte interaction, plates were centrifuged at 100 × g for 10 min at room temperature and then incubated at 37°C for 1 h. Suspensions were washed three times with HBSS and then lysed with 80 μl of distilled, deionized water before 150 μl of LB was added to the wells and the plates were incubated for 3 h at 37°C. At the end of this incubation, 10-μl aliquots of a 5-mg/ml solution of 3[4,5-dimethylthiazol-2-yl]-2,5-methyltetrazolium bromide (Sigma Chemical Co.) were added to all wells, and the plates were incubated for 10 min at 37°C. The optical density (OD) for formazan development was read at 570 nm in an automated microplate reader. ODs obtained from wells containing bacteria alone were fitted to a standard curve, and the calculated bacterial concentration in each set of test wells was based on that standard. Percent killing was calculated by using the following formula: {[OD of wells containing bacteria only − (OD of wells containing phagocytes and bacteria − OD of wells containing phagocytes only)]/OD of wells containing bacteria only} × 100. The data were analyzed using a one- or two-factor analysis of variance (48).
Phagocytosis and intracellular-survival assay.
A chicken-derived mononuclear cell line, MQ-NCSU, was used for the intracellular survival assay (38). The macrophage cell line was incubated with lipopolysaccharide for 24 h (10 μg of LPS/ml) (Sigma Chemical Co.) to elicit phagocytic ability (38). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma Chemical Co.) supplemented with 5% each of heat-inactivated FBS and chicken serum (Sigma Chemical Co.), 1 μM sodium pyruvate, and 10 μM 2-mercaptoethanol. A homogenate of 3 × 106 cells was resuspended in a 1-ml suspension of DMEM in a 24-well tissue culture plate (Nunc, Naperville, Ill.), subjected to an inoculum of 1 × 106 bacteria, centrifuged at 100 × g for 10 min at room temperature to enhance bacterium-phagocyte interaction, and incubated at 37°C for 2.5 h. The monolayers were then subjected to a gentamicin overlay (100 μg/ml) for 1 h to kill extracellular bacteria. For bacterial replication experiments, the medium was changed to DMEM containing 10 μg of gentamicin per ml. Macrophages were washed and lysed with distilled water, and surviving bacteria were titered by standard methods on MacConkey agar plates. These experiments were repeated eight times. Macrophage viability was confirmed by exclusion with 0.2% trypan blue dye. Visible confirmation of replication was obtained by Wright’s staining after 2.5 h of incubation.
In order to confirm phagocytosis, a double-fluorescence staining technique was used to discriminate between intra- and extracellular bacteria (14). Salmonella was labeled with rabbit anti-serotype B (S. typhimurium) or anti-serotype D (S. pullorum) antiserum as the primary antibody and goat anti-rabbit fluorescein isothiocyanate-conjugated antiserum as the secondary antibody. Epifluorescence microscopy was used to examine the preparations.
Animal challenge.
Forty day-of-hatch, specific-pathogen-free White Leghorn chicks were used for the animal challenge. The birds were housed in biosafety level 3 Horsfal units at the Southeastern Poultry Research Laboratory (U.S. Department of Agriculture, Athens, Ga.) and provided with water and growth ration ad libitum. Bacteria (106 CFU) from a static overnight culture grown in LB were used to inoculate chicks per os prior to food and water access. Three chicks from each challenge group were euthanatized by cervical disarticulation each day for 4 days postchallenge. Large and small intestine en bloc, cecum, bursa, liver, and spleen were removed aseptically from control and challenged birds and examined for gross pathology. Samples taken for bacteriological culture were placed in sterile phosphate-buffered saline. Organs from two birds per time point from each group were homogenized in a stomacher (Tekmar, Cincinnati, Ohio) for 30 s, serially diluted, and titered on brilliant green agar (Difco, Detroit, Mich.). Samples testing negative for bacteria were enriched in nutrient broth for 24 h at 37°C and plated on brilliant green agar. Organs from one chick per time point from each group were placed in 10% buffered formalin, embedded in paraffin, and cut for hematoxylin and eosin staining and immunohistochemistry. S. typhimurium was labeled with rabbit anti-serotype B and S. pullorum was labeled with anti-serotype D antiserum as the primary antibody for immunohistochemical staining. The peroxidase method was used according to the manufacturer’s protocols to visualize the reaction (Vectastain Elite ABC Kit; Vector, Burlingame, Calif.).
RESULTS
Adherence and invasion.
Results are shown in Table 1. Approximately 4.5% of inoculated S. typhimurium bacteria were adherent to either Henle 407 or BAT cells, and about half of the attached cells invaded the epithelial cells. Less than 1% of the S. pullorum inoculum was adherent, and only 4% of the attached cells invaded epithelia. In addition, S. pullorum was poorly invasive on chick ovary and chick kidney cells with only 0.5 and 0.02% of inoculum recovered from those respective monolayers (data not shown).
TABLE 1.
Percent adherence and invasion of salmonellae on cultured human and avian epithelial cellsa
| Isolate | % Adherence
|
% Invasion
|
||
|---|---|---|---|---|
| Human | Avian | Human | Avian | |
| S. typhimurium χ3181 | 4.23 ± 0.077 | 4.85 ± 0.22 | 1.3 ± 0.25 | 2.65 ± 0.44 |
| S. pullorum χ3423 | 0.42 ± 0.022 | 0.88 ± 0.2 | 0.019 ± 0.006 | 0.03 ± 0.003 |
| Escherichia coli HB101 | 2.35 ± 0.08 | 2.74 ± 0.38 | 0.002 ± 0.000007 | 0.002 ± 0.00000002 |
Percent adherence and invasion were calculated as the percent of inoculum detected after four washes at a multiplicity of infection of 100 bacteria per epithelial cell. The human cells were Henle 407 and the avian cells were the budgerigar abdominal tumor cell line. Results and standard errors were calculated from two experiments with duplicate wells.
Killing by leukocytes.
The ability of polymorphonuclear leukocytes (PMNs) to kill the isolates is represented in Table 2. Differential staining after density gradient centrifugation confirmed that each leukocyte preparation resulted in greater than 90% purity. Human PMNs were significantly more efficient than heterophils at killing Salmonella (P < 0.05). S. pullorum was significantly more sensitive to PMN killing than S. typhimurium, suggesting that the host specificity of this isolate is not dependent on resistance to PMN killing.
TABLE 2.
Percent killing of salmonellae by PMNs isolated from chickens (heterophils) and humans (neutrophils)
| Isolate | % Killing by PMNs froma:
|
|
|---|---|---|
| Chicken | Human | |
| S. typhimurium χ3181 | 51.57 ± 5.23 | 73.2 ± 5.99 |
| S. pullorum χ3423 | 77.29 ± 5.03 | 93 ± 1.9 |
Results were determined by using a colorimetric assay of bacterial viability in at least three trials with duplicate wells. Killing was determined by the difference in bacterial viability prior to and after a 2-h incubation with the leukocytes.
Intracellular survival and replication.
Epifluorescent microscopy confirmed that the bacteria had been phagocytosed by the MQ-NCSU macrophages after 2.5 h of incubation. Surviving organisms detected after the 1-h gentamicin kill step reflect the number of bacteria internalized by the avian cell line macrophages. Table 3 shows data obtained after phagocytosis and the extracellular kill. An increase in the number of detected bacteria at 5 h postphagocytosis indicated bacterial replication had occurred by both isolates. No decrease in phagocyte viability was detected by trypan blue exclusion (data not shown). In some experiments, an average of 30 bacteria per macrophage were cultured after 5 h of incubation. Visual confirmation of replication was obtained by Wright’s staining of MQ-NCSU macrophages experimentally infected with S. typhimurium and S. pullorum. At 1 h postphagocytosis, small numbers of both S. typhimurium and S. pullorum were seen within the vacuoles. By 7 h post-gentamicin treatment, most vacuoles contained large clusters of bacteria (data not shown).
TABLE 3.
Percent survival of salmonellae in MQ-NCSU cells after phagocytosisa
| Isolate | % Survival aftera:
|
||||
|---|---|---|---|---|---|
| 1 h | 2.5 h | 5 h | 7.5 h | 10 h | |
| S. typhimurium χ3181 | 18.82 ± 3.16 | 14.42 ± 3.99 | 43.72 ± 17.06 | 58.65 ± 33.98 | 52.26 ± 25.64 |
| S. pullorum χ3423 | 5.68 ± 1.06 | 13.84 ± 10.69 | 24.52 ± 19.73 | 2.22 ± 0.58 | 3.86 ± 1.74 |
Percent survival was calculated as the percent of inoculum cultured after phagocytosis and gentamicin killing of extracellular bacteria. An increase in percent indicates bacterial replication; a decrease indicates killing of the bacteria.
Live animal challenge studies.
Salmonella was not cultured from control birds at any time during the course of the experiment. Immunohistochemical staining was negative for Salmonella in the control birds throughout the study, and tissues were observed to be histologically normal. Organized lymphoid tissue was not observed in the intestines of the control birds.
The isolates demonstrated different intestinal colonization dynamics in this study. Both S. typhimurium and S. pullorum were isolated from the cecum of challenged birds 1 day after inoculation at approximately 108 CFU. While this level was maintained in the S. typhimurium-challenged birds throughout the experiment, the number of S. pullorum cells decreased to levels only detected by enrichment on the 2nd day postchallenge. Both isolates were cultured from small and large intestinal tissues 1 day postchallenge at approximately 106 CFU. However, while S. typhimurium maintained this level throughout the experiment, S. pullorum decreased by 4 log units on the 2nd day postchallenge.
Immunohistochemical staining identified numerous S. typhimurium cells associated with the superficial surface of the mucosal epithelium, the lamina propria, and the lumenal contents of the cecum, 1 day after challenge. Figure 1 shows the microscopic events 2 days after S. typhimurium challenge. The presence of S. typhimurium was accompanied by marked heterophilic and mononuclear infiltration into the lamina propria, and heterophils were migrating between mucosal epithelial cells into the intestinal lumen (Fig. 1A). Multiple crypt abscesses were also present. On day 3 postchallenge, the luminal contents of the cecum and the ileum were filled with heterophils in the S. typhimurium-challenged bird; however, few heterophils were seen in the lamina propria or epithelium. The mucosal villi of the cecum were markedly flattened. On both days, large numbers of S. typhimurium bacteria were confirmed by immunohistochemical staining in areas of inflammation in the cecum (Fig. 1B), with moderate numbers in the small intestine. On day 4, the inflammatory infiltrate was primarily mononuclear and present in the cecum and small intestine in the S. typhimurium-infected bird. The cecal mucosal epithelium was attenuated, abscesses were present in the cecal crypts, and individual cell necrosis was distributed throughout the lamina propria. Intestinal luminal contents contained numerous necrotic heterophils and cellular debris. Large numbers of S. typhimurium bacteria were seen within areas of inflammation of the cecum as well as within the intestinal lumen. Clusters of bacteria were seen within vacuoles in intestinal epithelial cells and within mononuclear cells.
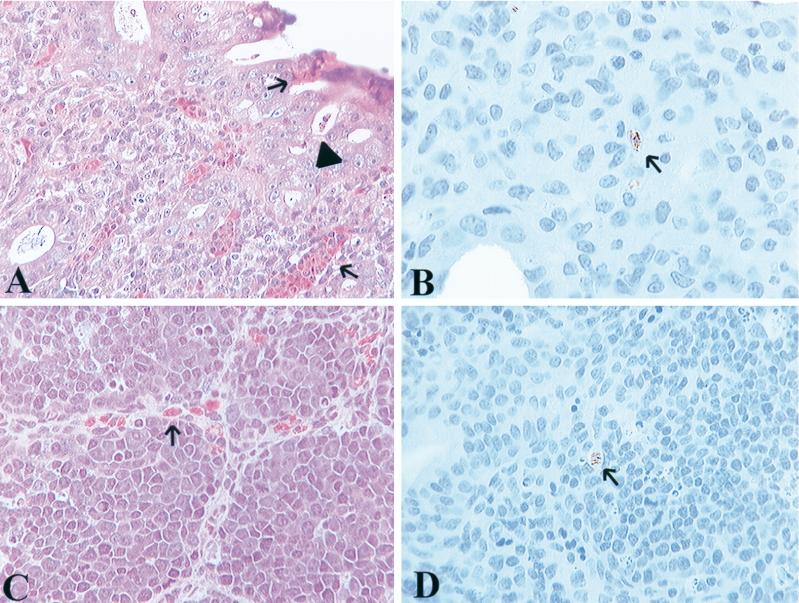
FIG. 1.
Histopathologic changes and immunohistochemical staining from S. typhimurium-challenged chicks, 2 days postchallenge. (A) Section taken from cecum shows marked infiltration of heterophils into the lamina propria and migration through the mucosal epithelium (arrows). Multiple crypt abscesses are identified by arrowheads. (B) Immunohistochemical staining of S. typhimurium in cecum from the same bird as in A. Bacteria are identified within a vacuole by brown staining (arrow). (C) Bursa contains mild infiltrates of heterophils (arrow) in the connective tissue between follicles, but not within the bursal follicles. (D) Immunohistochemical staining of S. typhimurium (arrow) within a vacuole in bursal follicles from the same bird as in C.
In contrast, no significant intestinal lesions were observed in the S. pullorum-challenged bird at 1 day postchallenge. Immunohistochemistry revealed that S. pullorum was present only within the lumen of the cecum and was rarely associated with the mucosal epithelium. On day 2 postchallenge, S. pullorum cells were rarely seen within vacuoles of the cecal epithelium, and only mild heterophilic infiltration was apparent (Fig. 2). By day 3, crypt abscesses and a marked heterophilic and mononuclear infiltration in the lamina propria were present in the cecum of the S. pullorum-challenged bird. Heterophils were seen between mucosal epithelial cells and in the intestinal lumen. The epithelium and lamina propria of the ileum were diffusely infiltrated by heterophils. Immunohistochemistry revealed S. pullorum within the contents of the cecal lumen and within vacuoles in the epithelial mucosa. The major inflammatory component of the cecum in the S. pullorum-challenged bird on day 4 was mononuclear, with very few heterophils except those present in the multifocal areas of crypt abscesses. The cecal lumen was filled with heterophils and cellular debris; immunohistochemistry showed bacteria primarily in the lumen of the cecum.
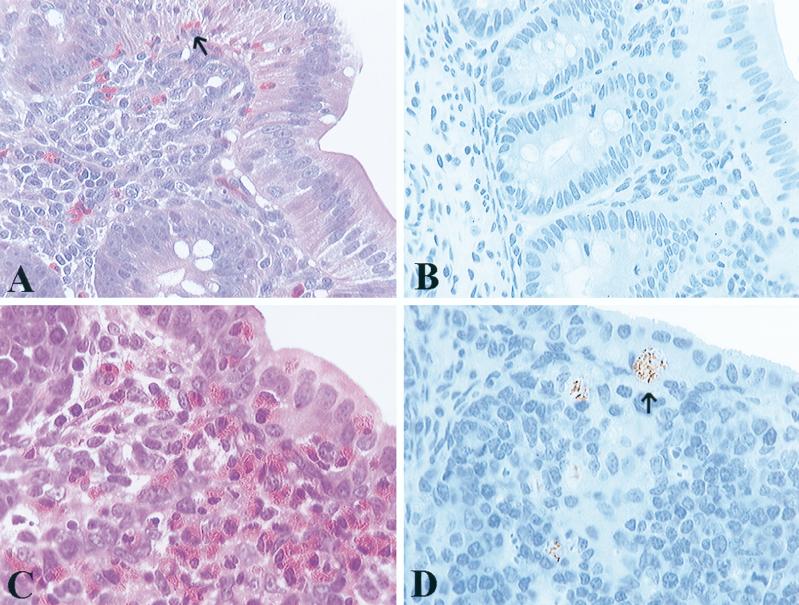
FIG. 2.
Histopathologic changes and immunohistochemical staining from S. pullorum-challenged chicks, 2 days postchallenge. (A) Section taken from cecum shows infiltration of heterophils into the lamina propria and migration into the mucosal epithelium (arrow). (B) Immunohistochemical staining of S. pullorum in cecum from the same bird as in A. Bacteria were found very infrequently and are not present in this section. (C) Bursa contains marked infiltrates of heterophils that have disrupted normal architecture. (D) Immunohistochemical staining of S. pullorum (arrow) within multiple vacuoles in bursal follicles from the same bird as in C.
The isolates were similar in their invasion of the liver; the number of each isolate peaked in the liver by 3 days postchallenge (Table 4). Bacteria were also demonstrated within areas of inflammation in the liver by using immunohistochemistry. In contrast, the isolates displayed different dynamics in invasion of the bursa. While both isolates were cultured from the bursa on all days, numbers of S. typhimurium bacteria were decreasing by day 4 postchallenge, while numbers of S. pullorum bacteria were increasing. The culture data were corroborated by microscopic pathology and immunohistochemistry. Surprisingly, the pathology associated with S. pullorum invasion of the bursa occurred earlier and was more severe than in the S. typhimurium lesions. On day 2 postchallenge, S. typhimurium elicited a mild heterophilic infiltration around the follicles of the bursa (Fig. 1C); immunohistochemistry confirmed the presence of bacteria in these areas (Fig. 1D). S. pullorum, in contrast, induced marked bursal inflammation (Fig. 2C). Bursal follicles were depleted of lymphocytes and infiltrated with heterophils and macrophages. Immunohistochemistry revealed that S. pullorum cells were present within the areas of bursal inflammation and within vacuoles of mononuclear cells in this organ (Fig. 2D); however, bacteria were not found in the bursal lumen. On day 4, the bursal architecture was effaced by a marked infiltration of heterophils and mononuclear cells with accompanying necrosis and marked lymphoid depletion. Large numbers of both organisms were present within areas of inflammation in the bursa. The pathology of the S. pullorum lesions was more severe, with bursal follicles exhibiting lymphoid lysis and necrosis.
TABLE 4.
Tracking viable salmonellae in vivo after oral challenge of 1-day-old chicks over a 4-day perioda
| Isolate | Salmonellae detected in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Intestine (days postchallenge)
|
Cecum (days postchallenge)
|
|||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| S. typhimurium χ3181 | 6.51 ± 0.36 | 6.77 ± 0.23 | 6.73 ± 0.27 | 6.96 ± 0.73 | 8.7 ± 0.53 | 9.12 ± 0.34 | 8.5 ± 0.1 | 8.69 ± 0.85 |
| S. pullorum χ3423 | 6.38 ± 0.84 | 2.51 ± 1.52 | 4.75 ± 1.15 | 3.7 ± 3.7 | 8.7 ± 0.6 | 1 ± 0 | 3.83 ± 3.83 | 4.18 ± 4.18 |
| Salmonellae detected in: | ||||||||
| Liver (days postchallenge) | Bursa (days postchallenge) | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 2.45 ± 0.45 | 4.53 ± 0.36 | 4.53 ± 0.53 | 3.13 ± 0.84 | 5.83 ± 0.17 | 4.74 ± 0.38 | 6.12 ± 0.58 | 3.8 ± 1.5 | |
| 1 ± 0 | 3.82 ± 0.18 | 4.31 ± 0.47 | 3.96 ± 0.73 | 4.09 ± 0.43 | 3.3 ± 2.3 | 3.37 ± 2.37 | 6.56 ± 0.14 | |
Two chicks were sacrificed daily, and the numbers of salmonellae were determined in selected organs. Results reflect the means (log10) of CFU detected per organ.
DISCUSSION
In this study, the broad-host-range pathogen S. typhimurium and the host-specific avian pathogen S. pullorum were shown to interact similarly with avian leukocytes. Both mammalian and avian PMNs were competent killers, although macrophages were not as efficient as PMNs at killing Salmonella. Furthermore, both isolates were also capable of replicating within macrophages in vitro in comparable numbers. The interactions of PMNs or macrophages with Salmonella have been studied in great detail by using mammalian models (1–4, 10, 39, 47), but only a few studies have evaluated the ability of avian leukocytes to kill Salmonella (42). Histological reports on experimental broad-host-range Salmonella infection in mammalian and avian models confirm that intestinal colonization by Salmonella initiates an inflammatory response characterized by infiltration of the infected site by PMNs (7, 18, 32, 37, 44). Studies establishing the importance of PMNs in host resistance have demonstrated that experimentally infected neutropenic animals do not exhibit gastroenteritis; however, these animals die from septic disease following systemic spread of the organism (18, 24). In addition, transepithelial signaling to PMNs occurs for those serotypes known to produce gastroenteritis in humans (30); however, non-human pathogens did not exhibit this signaling in human-derived cells, which suggests that the intensity and outcome of disease depend largely on the pathogen-host interactions involved in colonization (31). Thus, PMNs apparently play a crucial role in eradicating lumenal pathogens, thereby preventing their dissemination.
PMN migration is typically followed by an infiltration of macrophages (21). Although macrophages are important in clearing bacteria, there is evidence that these phagocytes, capable of surviving intracellularly, may play a key role in pathogenesis by serving as hosts for those organisms. The capacity for Salmonella species to intracellularly replicate within murine-derived macrophages has been previously demonstrated by several techniques (2, 10). For serotypes such as S. typhimurium, known to produce enteric fever in mice, survival and replication in macrophages are essential for virulence, and macrophages serve as a vehicle of dissemination (12, 16). Several studies suggest that the site of intestinal invasion may contribute to the host specificity of Salmonella serotypes (25, 36, 43). Barrow et al. have reported that, for Salmonella serotypes that exhibit host specificity for chickens and mice, specificity is the result of the ability to survive within organs of the reticuloendothelial system rather than the ability to penetrate intestinal epithelium (5). However, the gut-associated lymphoid tissue of birds is poorly organized; cecal tonsils and Peyer’s patches do not develop in young birds until 2 weeks of age (23, 44). In our study, even though S. pullorum was rarely found associated with epithelial cells, it was found within macrophages in the intestinal mucosa, bursal follicles, and liver on the 2nd day postchallenge, suggesting that these organisms were taken up and disseminated by macrophages. While S. typhimurium was primarily localized to intestinal tissues, the route of pathogenesis for S. pullorum involved rapid dissemination to the bursa, a lymphoid organ unique to birds.
Invasion assays performed in vitro demonstrated that S. typhimurium was capable of invading both human and avian cells, while S. pullorum was poorly invasive on these and other avian species-derived cells. Histopathology and immunohistochemistry confirmed that S. typhimurium was located within vacuoles in the intestinal epithelium and accompanied by an infiltrate of heterophils on the 1st day postchallenge. In contrast, while S. pullorum was seen in the luminal contents of the cecum and large intestine, close association with the mucosa was not observed until the 2nd day postchallenge, and inflammation was not observed until the 3rd day postchallenge. Nonetheless, bursal follicles were depleted and contained S. pullorum by this time.
The results from our study indicate that infection with S. pullorum is not dependent upon rapid penetration of the intestinal epithelial cells. Rather, dissemination to the bursa occurs prior to the manifestation of enteritis. Hassan and Curtiss also observed transient inflammation of the bursa 2 days after 1-day-old chicks were orally challenged with S. typhimurium (22). Our histopathology of S. pullorum infection illustrated a targeting of lymphoid tissue, similar to the progression of disease caused by other host-specific salmonellae (12, 13, 28). Although the presence of organized lymphoid tissue was not observed in the cecum and intestine of these chicks, the bursa was a major site of infection, as confirmed by culture and immunohistochemical staining. Therefore, by preferentially invading lymphoid tissues instead of targeting intestinal epithelium, it is possible that S. pullorum exhibits tissue tropism for organized lymphoid tissue in chickens similar to the way S. typhimurium targets gut-associated lymphoid tissue in mice.
REFERENCES
- 1.Abshire K Z, Neidhardt F C. Growth rate paradox of Salmonella typhimurium within host macrophages. J Bacteriol. 1993;175:3744–3748. doi: 10.1128/jb.175.12.3744-3748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abshire K Z, Neidhardt F C. Analysis of proteins synthesized by Salmonella typhimurium during growth within a host macrophage. J Bacteriol. 1993;175:3734–3743. doi: 10.1128/jb.175.12.3734-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpuche-Aranda C M, Berthiaume E P, Mock B, Swanson J A, Miller S I. Spacious phagosome formation within mouse macrophages correlates with Salmonella serotype pathogenicity and host susceptibility. Infect Immun. 1995;63:4456–4462. doi: 10.1128/iai.63.11.4456-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron E J, Proctor R A. Inefficient in vitro killing of virulent or nonvirulent Salmonella typhimurium by murine polymorphonuclear neutrophils. Can J Microbiol. 1984;30:1264–1270. doi: 10.1139/m84-199. [DOI] [PubMed] [Google Scholar]
- 5.Barrow P A, Huggins M B, Lovell M A. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect Immun. 1994;62:4602–4610. doi: 10.1128/iai.62.10.4602-4610.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrow P A, Huggins M B, Lovell M A, Simpson J M. Observations on the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res Vet Sci. 1987;42:194–199. [PubMed] [Google Scholar]
- 7.Bayer R C, Gershman M, Bryan T A, Rittenburg J H. Degeneration of the mucosal surface of the small intestine of the chicken in Salmonella infection. Poult Sci. 1977;56:1041–1042. doi: 10.3382/ps.0561041. [DOI] [PubMed] [Google Scholar]
- 8.Brooks R L, Jr, Bounous D I, Andreasen C B. Functional comparison of avian heterophils with human and canine neutrophils. Comp Haematol Int. 1996;6:153–159. [Google Scholar]
- 9.Brune K, Leffell M S, Spitznagel J K. Microbicidal activity of peroxidaseless chicken heterophile leukocytes. Infect Immun. 1972;5:283–287. doi: 10.1128/iai.5.3.283-287.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrol M E, Jackett P S, Aber V R, Lowrie D B. Phagolysosome formation, cyclic adenosine 3′: 5′ monophosphate and the fate of Salmonella typhimurium within mouse peritoneal macrophages. J Gen Microbiol. 1979;110:421–429. doi: 10.1099/00221287-110-2-421. [DOI] [PubMed] [Google Scholar]
- 12.Carter P B, Collins F M. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter P B, Collins F M. Peyer’s patch responsiveness to Salmonella in mice. J Reticuloendothel Soc. 1974;17:38–46. [PubMed] [Google Scholar]
- 14.Detilleux P G, Deyoe B I, Cheville N F. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect Immun. 1990;58:2320–2328. doi: 10.1128/iai.58.7.2320-2328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.English D, Andersen B R. Single-step separation of red blood cells, granulocytes and mononuclear leukocytes on a discontinuous density gradient of Ficoll-Hypaque. J Immunol Methods. 1974;5:249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- 16.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of S. typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galan J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianella R A. Importance of the intestinal inflammatory reaction in Salmonella-mediated intestinal secretion. Infect Immun. 1979;23:140–145. doi: 10.1128/iai.23.1.140-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glick B, Madyastha P, Koger B, LaVia M F. The use of Ficoll-Hypaque double density gradients in the separation of avian granulocytes from other cell types for the purpose of cell flow cytometric analysis. Dev Comp Immunol. 1985;9:477–484. doi: 10.1016/0145-305x(85)90010-2. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg M B, Rubin R H. The spectrum of Salmonella infection. Infect Dis Clin N Am. 1988;2:571–598. [PubMed] [Google Scholar]
- 21.Golemboski K A, Bloom S E, Dietert R R. Dynamics of avian inflammatory response to crosslinked dextran. Inflammation. 1990;14:31–40. doi: 10.1007/BF00914027. [DOI] [PubMed] [Google Scholar]
- 22.Hassan J O, Curtiss R., III Virulent Salmonella typhimurium-induced lymphocyte depletion and immunosuppression in chickens. Infect Immun. 1994;62:2027–2036. doi: 10.1128/iai.62.5.2027-2036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeurissen S H M, Janse E M, Koch G, DeBoer G F. Postnatal development of mucosa-associated lymphoid tissues in chickens. Cell Tissue Res. 1989;258:119–124. doi: 10.1007/BF00223151. [DOI] [PubMed] [Google Scholar]
- 24.Kogut, M. H., G. I. Tellez, E. D. McGruder, B. M. Hargis, J. D. Williams, D. E. Corrier, and J. R. DeLoach. Heterophils are decisive components in the early responses of chickens to Salmonella enteritidis infections. Microb. Pathog. 16:141–151. [DOI] [PubMed]
- 25.Kohbata S, Yokoyama H, Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer’s patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol. 1986;30:1225–1237. doi: 10.1111/j.1348-0421.1986.tb03055.x. [DOI] [PubMed] [Google Scholar]
- 26.Kraehenbuhl J P, Neutra M R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Soc Rev. 1992;72:853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- 27.Lee M D, Curtiss III R, Peay T. The effect of bacterial surface structures on the pathogenesis of Salmonella typhimurium infection in chickens. Avian Dis. 1996;40:28–36. [PubMed] [Google Scholar]
- 28.Liebler E M, McPress C L, Landsverk T. Lymphocyte subpopulations in jejunal and ileal Peyer’s patches of calves with experimental Salmonella dublin infection. J Vet Med. 1994;41:113–125. doi: 10.1111/j.1439-0450.1994.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie G M, Fitzgerald H, Pike R. Interrelationships of antigenic structure, virulence and immunizing properties of smooth and rough cultures of Salmonella aertrycke. Trans Assoc Amer Physicians. 1935;50:242–248. [Google Scholar]
- 30.McCormick B A, Colgan S P, Archer C D, Miller S I, Madara J L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signaling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick B A, Miller S I, Carnes D, Madara J L. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGovern V J, Slavutin L J. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3:483–490. doi: 10.1097/00000478-197912000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Miller S I, Hohmann E L, Pegues D A. Salmonella (including Salmonella typhi) In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2013–2033. [Google Scholar]
- 34.Ozawa A, Goto J, Ito Y, Shibata H. Histopathological and biochemical responses of germfree and conventional mice with Salmonella infection. In: Heneghan J, editor. Germfree research: biological effect of gnotobiotic environments. New York, N.Y: Academic Press; 1973. pp. 325–330. [Google Scholar]
- 35.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985;82:131–140. doi: 10.1016/0022-1759(85)90232-7. [DOI] [PubMed] [Google Scholar]
- 36.Pospischil A, Wood R L, Anderson T D. Peroxidase-antiperoxidase and immunogold labeling of Salmonella typhimurium and Salmonella choleraesuis var kunzendorf in tissues of experimentally infected swine. Am J Vet Res. 1990;51:619–624. [PubMed] [Google Scholar]
- 37.Powell P C. Immune mechanisms in infections of poultry. Vet Immunol Immunopathol. 1987;15:87–113. doi: 10.1016/0165-2427(87)90107-3. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi M A, Miller L, Lillehoj H S, Ficken M D. Establishment and characterization of a chicken mononuclear cell line. Vet Immunol Immunopathol. 1990;26:237–250. doi: 10.1016/0165-2427(90)90094-9. [DOI] [PubMed] [Google Scholar]
- 39.Roof M B, Kramer T T. Porcine neutrophil function in the presence of virulent and avirulent Salmonella choleraesuis. Vet Immunol Immunopathol. 1989;23:365–376. doi: 10.1016/0165-2427(89)90148-7. [DOI] [PubMed] [Google Scholar]
- 40.Ruitenberg E G, Guinee P A, Kruyt B C, Berkvens J M. Salmonella pathogenesis in germ-free mice: a bacteriological and histological study. Br J Exp Pathol. 1971;52:192–196. [PMC free article] [PubMed] [Google Scholar]
- 41.Snoeyenbos G H. Pullorum disease. In: Calnek B W, Barnes H J, Beard C W, Reid W M, Yoder H W, editors. Diseases of poultry. Ames, Iowa: Iowa State University Press; 1991. pp. 73–86. [Google Scholar]
- 42.Stabler J G, McCormick T W, Powell K C, Kogut M H. Avian heterophils and monocytes: phagocytic and bactericidal activities against Salmonella enteritidis. Vet Microbiol. 1994;38:293–305. doi: 10.1016/0378-1135(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967;50:109–136. [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor J, McCoy H. Salmonella and Arizona infection. In: Riemann H, editor. Food-borne infections and intoxications. New York, N.Y: Academic Press; 1969. pp. 3–72. [Google Scholar]
- 45.Topp R C, Carlson H C. Studies on avian heterophils. Avian Dis. 1972;16:374–380. [PubMed] [Google Scholar]
- 46.Turnbull P C B, Snoeyenbos G H. Experimental salmonellosis in the chicken. Fate and host response in the alimentary canal, liver, and spleen. Avian Dis. 1973;18:153–177. [PubMed] [Google Scholar]
- 47.Wallis T S, Starkey W G, Haddon S J, Osborne M P, Candy D C. The nature and role of mucosal damage in relation to Salmonella typhimurium-induced fluid secretion in the rabbit ileum. Med Microbiol. 1986;22:39–49. doi: 10.1099/00222615-22-1-39. [DOI] [PubMed] [Google Scholar]
- 48.Zar J H. Biostatistical analysis. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1974. [Google Scholar]