ABSTRACT
We isolated endophytic Fusarium strains from the healthy roots, stems, and leaves of Dendrobium moschatum to investigate their plant growth-promoting activities in vitro. Subsequently, Indole acetic acid (IAA) was quantified and the IaaM gene (responsible for IAA synthesis in fungi) was amplified and sequenced. Finally, a germination assay was performed with seeds of D. moschatum and a plant growth assay with protocorms of Dendrobium longicornu to test their plant growth-promoting activities. Five Fusarium isolates (CDS11, PDL1, PDL3, PDR6, PDR7) were identified in this study. The highest amount (60µgml−1) of indole acetic acid was recorded in the PDR7 extract, whereas it was not detected in PDR6 and CDS11. The fungal extracts of isolates PDR6 and PDR7 were highly effective for seed germination by approximately 80% and 90% (respectively) of the host plant. The fungal extract of PDR7 showed a high IAA content and promoted in vitro seed germination of the host (D. moschatum) and protocorm development of the non-host (D. longicornu). In contrast, IAA content in the fungal extract of PDR6 remained undetected but was effective in both seed germination and protocorm development. Our results demonstrated the potential beneficial application of endophytic Fusarium in orchid mass propagation.
KEYWORDS: Dendrobium, fungal extract, Fusarium, Indole acetic acid, orchid
Introduction
Orchids are the most diverse group of plants occurring in all continents, except Antarctica. According to a recent study, Nepal’s diverse ecosystems are home to approximately 501 species of orchids [1]. Due to overexploitation and forest loss, several species are in danger of extinction [1,2]. Orchidaceae has been reported as the second-largest plant family in Nepal [3,4]. Dendrobium is the largest genus in the orchid family in Nepal. Twenty-nine Dendrobium species have been reported in Nepal [5]. Dendrobium species have been extensively studied for their phytochemical, antioxidant, and free radical scavenging properties [6–9]. Dendrobium moschatum considered in this study is well-known for its musk-smelling fragrance and its ornamental and therapeutic qualities [10]. It is a common epiphytic orchid with an upright cylindrical stem, uneven bilobed leathery leaves and deep yellow colored flower that grows in the temperate regions of northeastern India, Nepal, and Bhutan [11].
Plant growth-promoting effects of endophytic microbes have been reported in various orchids [12–21]. Indole-3-acetic acid (IAA) is a naturally occurring plant hormone that is generally referred to as auxin. They play a crucial role in plant growth and development. It participates in cell signaling and coordination, cell elongation, cell division, and root initiation and elongation [22]. IAA is produced by two pathways: tryptophan-dependent and tryptophan-independent. The try-dependent pathway plays a critical role in embryonic development, seedling growth, flower and vascular tissue development, and other physiological processes. Several reports have demonstrated the ability of endophytic fungi to use the tryptophan dependent indole-3-acetamide (IAM pathway) for IAA production [23–25]. The first described orchid endophytes were Fusarium spp., which used the IAM pathway for IAA synthesis [26]. Non-pathogenic, endophytic Fusarium spp. have been consistently reported in orchid species as plant growth promoters, stimulators for seed germination, and immunomodulators for abiotic and biotic stress [4,27]. In this study, we aimed to 1) isolate and identify endophytic fungi from native Dendrobium moschatum, 2) quantify the IAA concentrations in fungal extracts, and 3) experimentally test the ability of the extracts to improve germination and plantlet development in the host (Dendrobium moschatum) and a non-host orchid (Dendrobium longicornu) in vitro.
Material and methods
Sample collection and isolation and identification of fungi
In the present study, we selected Dendrobium moschatum growing in a natural temperate habitat, Makwanpur District, central hills (Hetauda) of Nepal. Roots were excised from the wild species, wrapped with tissue paper, placed in zipper bags, and transferred to the laboratory. Healthy D. moschatum roots, stems, and leaves of Dendrobium moschatum were processed for surface sterilization [28]. Roots were gently washed in running water to remove dust particles and then treated with 3% sodium hypochlorite (NaOCl) for 30 s, followed by treatment with 75% ethanol for 1 min. After this step, it was rinsed with sterile distilled water and dried with a sterile paper towel in laminar wood under aseptic conditions. The outer epidermal layer was removed to prevent contamination. The fungi were isolated from sterile plant tissues. The Plant tissues (root, stem, and leaves) were placed in potato dextrose agar (PDA) or Czapek Dox Agar (CDA) medium and incubated for 7 days at 28°C. Fungi grown from plant tissues were isolated and cultured based on their growth patterns and morphotypes.
DNA isolation of DNA the CTAB method. In this regard, 5 g of the plant part was harvested and ground in liquid nitrogen with a pre-chilled mortar and pestle. The entire content was then transferred to an oak ridge tube. CTAB extraction buffer (10 ml of CTAB extraction buffer was added to the powder and the tube was incubated for 1 h with occasional mixing by gently inverting the tubes. After 1 h, 10 ml of chloroform: isoamyl alcohol was added to the tube to separate DNA from other impurities. The upper layer was transferred to a new tube with the help of cut tips to this 0.1 ml of 3 M sodium acetate buffer (pH 5.2). Thereafter, 2 ml of ice-cold ethanol was added. The tube was spun at 12,000 rpm and the supernatant was removed, and the pellet was washed with 70% ethanol and again spun for 10 min at 10,000 rpm. The pellet was dried at room temperature, and 200 μL of TE buffer was added to it along with 10 μL of RNase -A and incubated for 30 min at 37°C. DNA aliquots were stored at 4°C.Polymerase chain reactions (PCR) were performed on a Mini-cycler TM (MJ Research, Reno, NV, USA). PCR Products were analyzed by gel electrophoresis on 1% agarose gels, stained with ethidium bromide, and visualized under UV light. After purification with mini-columns, the purified DNA was directly sequenced. The primers used were ITS1 and ITS4. The PCR was programmed as follows: initial denaturation at 94°C for 5 min. followed by 35 cycles at 94°C for 5 min and 57°C for 1 min and at 72°C for 1 min with a final extension step at 72°C for 10 min. The amplified fragments were separated on 1% agarose gel using 1 × TAE buffer at 80 V for 20 min. and was examined using a gel documentation system.
Phylogenetic analyses
The generated consensus sequences of ITS and LSU were subjected to BLASTn search against the GenBank database for identifying closely related taxa. Reference sequences of closely related taxa were selected based on NCBI nBLAST. The sequences were automatically aligned in the MAFFT version 7 (http://mafft.cbrc.jp/alignment/server/index.html) using default settings [29] and trimmed using trimAl (https://ngphylogeny.fr/tools/) [30]. Maximum likelihood (ML) and phylogenetic analyses were performed for combined gene datasets ITS was done on MEGA 11 tool [31] under the GTR+GAMMA substitution model and 1,000 bootstrap iterations.
IAA quantification and gene amplification
Czapek dox agar (CDA) medium (20 mL, pH 6.5) was used to cultivate the fungal isolates, either with or without 1 mg L-tryptophan supplement [32,33]. The inoculated broths were incubated in a shaker incubator set to 25°C and 120 rpm for ten days. Following incubation, they were centrifuged for 10 minutes at 4°C at 12,000 rpm. One milliliter of the supernatant and two milliliters of Salkowski reagent were combined, and the mixture was incubated in the dark for 30 min to measure the amount of IAA produced. The optical density was measured at 530 nm once a pink hue appeared using a UV-VIS spectrophotometer (ChromTech-CT 8200). The IAA concentration in the extract was measured using independently created standard IAA curves (10–100 µg ml−1). Three biological replicates were used for each experiment. Amplification of IaaM gene was performed as described with primers F’- AGT GAC CAG CCT GCT GAT TTC CCT CG and R’- AAG ATC GCA GCC ATT GAG TTG TGC [26]. The PCR amplifications were performed in Veriti thermal cycler (Applied Biosystems, Singapore), following conditions: initial denaturation at 94°C for 5 min. followed by 35 cycles at 94°C for 5 min and 57°C for 1 min and at 72°C for 1 min with a final extension step at 72°C for 10 min. The amplified fragments were separated on 1% agarose gel using 1 × TAE buffer at 80 V for 20 min. and examined using a gel documentation system [26].
Germination and protocorm development experiment
The seed germination assay was performed with the seeds of D. moschatum whereas protocorm development experiment was done for D. longicornu. The fungal extracts were prepared from CDS11, PDL1, PDL3, PDR6 and PDR7. For each of the five isolates, fungal extract solution was prepared from the supernatant of 10-day old isolates inoculated in Czapek broth medium supplemented with 1 mg tryptophan. The broth was centrifuged at 5000 rpm, and the supernatant was filtered through Whatman filter paper grade 1:11 μm (medium flow filter paper). MS medium supplemented with fungal extracts (250 µl per liter) was prepared. The medium was then autoclaved 121°C for 20 min. The surface-sterilized seed pod of Dendrobium moschatum was placed on a sterile Petri dish containing sterile filter paper to soak the surface moisture from its surface. The seed pod was then cut longitudinally into two halves using a sterile surgical blade. Microscopic seeds of orchids were scooped out with the help of a sterile spatula and inoculated on full-strength MS medium as a control and medium supplemented with extracts from the five Fusarium isolates. Approximately 15–20 seeds were plated in each culture tube, and 15 replicates were established to represent each of the six experimental treatments. Seeds were incubated at 25 ± 2°C under a 16/8 hrs photoperiod for ten weeks. Another experiment was performed using previously grown protocorms of the congeneric orchid D. longicornu. Aseptically grown initial-stage protocorms of D. longicornu were inoculated on full-strength MS medium as a control and medium supplemented with 2% fungal extract. We prepared six replicates for each of the six treatments. Each replicate vessel contained 15 protocorms, which were maintained at 25 ± 2°C under the range of 500–1000 lux illuminance for 16/8 h (light/dark) photoperiod using white fluorescent tubes (Philips, India) [34]. Germination was recorded at an interval of three weeks for ten weeks. Protocorm development was estimated by recording the number of roots and shoots at an interval of two weeks for seven weeks.
Data analysis
IBM SPSS 20 was used to analyze the data generated from the IAA quantification experiment and the germination and protocorm growth experiments. IAA concentrations (with or without tryptophan) in the five isolates were compared using one-way Analysis of Variance (ANOVA) with the alpha error level set at p ≤ .01 (Tukey’s HSD test). Next, Analysis of Variance (ANOVA) was used to compare the germination percentages in D. moschatum and the root and shoot numbers in D. longicornu in response to the six experimental treatments. The responses were considered statistically different at p ≤ .05. (Tukey HSD test).
Results
Isolation and identification of plant growth-promoting fungi from Dendrobium moschatum
The fungal isolates PDR6 and PDR7 were obtained from the roots, PDL1 and PDL3 from the leaves, and CDS11 from the stems. All five isolates belonged to the Fusarium genus and were identified using internal transcribed spacer sequence (ITS) analysis. The phylogenetic analysis of ITS sequences data of 61 strains of Fusarium species of which Fusarium zealandicum CBS 11.93 and Fusarium venezuelense NRRL 2239 were outgroup as displayed in Figure 1. Our isolates CDS11, PDR6 and PDL3 showed clustered with Fusarium verticillioides, whereas PDL1 clustered with Fusarium oxysporum. The number of fungal isolates, their identities, and their ITS GenBank accession numbers are listed in Table 1.
Figure 1.
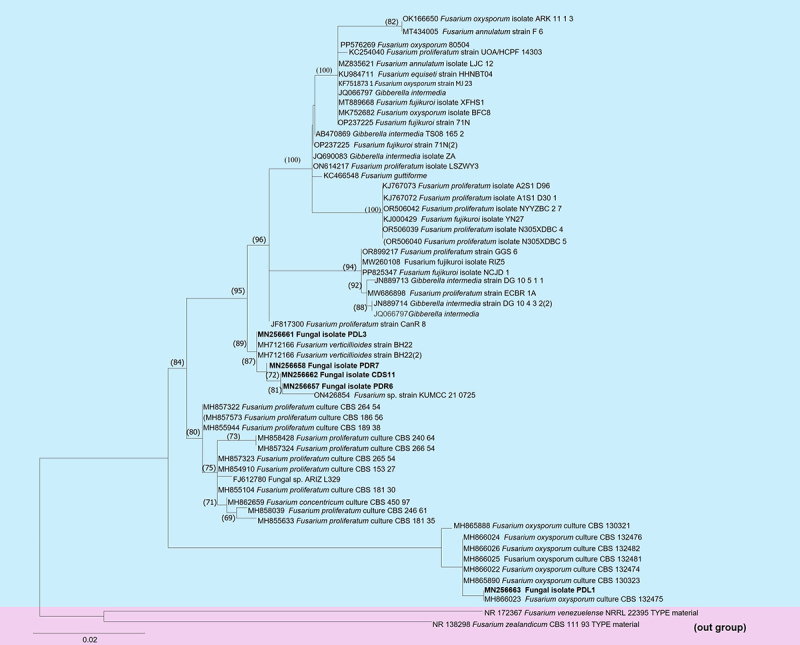
Phylogram generated from maximum likelihood analysis based ITS sequences of the Fusarium species. Maximum likelihood bootstrap support values ≥ 69% are shown in the nodes. Newly generated sequences of the isolates CD11, PDL1, PDL3, PDR6 and PDR7 are indicated in bold. The tree was rooted with Fusarium zealandicum CBS 11.93 and Fusarium venezuelense NRRL 2239.
Table 1.
Molecular identification of endophytic fungi isolated from healthy tissues of Dendrobium moschatum.
| Isolation medium and source tissue | Fungal taxonomy | Isolates | Query Coverage | Identity match (%) | GenBank Accession no. (ITS) |
|---|---|---|---|---|---|
| CDS11* | Fusarium sp. | 2 | 100% | 100% | MN256662 |
| PDL1** | Fusarium sp. | 2 | 100% | 100% | MN256663 |
| PDL3** | Fusarium sp. | 3 | 99% | 99.82% | MN256661 |
| PDR6*** | Fusarium sp. | 2 | 99% | 99.64% | MN256657 |
| PDR7*** | Fusarium sp. | 1 | 99% | 99.64% | MN256658 |
*CDS ‘CD’ represents CDA medium, ‘S’ Stem.
**PDL represents the PDA medium, ‘L’leaf section.
***PDR represents ‘PD’ PDA medium, ‘R’ root.
Indole acetic acid quantification and gene amplificaiton
Indole acetic acid in the culture medium was quantified using Salkowski reagent. The absorbance of indole acetic acid (IAA) was measured at 530 nm, and the culture extracts contained varying amounts of IAA. The maximum concentration of indole acetic acid (60µgml-1) was found in broths supplemented with tryptophan for endophyte PDR7, whereas endophyte PDL1 had the lowest IAA content (24µgml-1). However, as illustrated in Figure 2, IAA was either undetectable or insignificant in the remaining fungal broth, both with and without tryptophan. The DNA extracts of all isolated endophytic fungi were subjected to IaaM gene amplification. In this regard, Fusarium sp. specific primers for the IaaM gene were used to amplify the IaaM gene present in the endophytic fungi. The partial amplified DNA fragment was approximately 400 bp. The amplified PCR products were purified, sequenced, and deposited in the NCBI database under GenBank accession numbers (Table 2).
Figure 2.
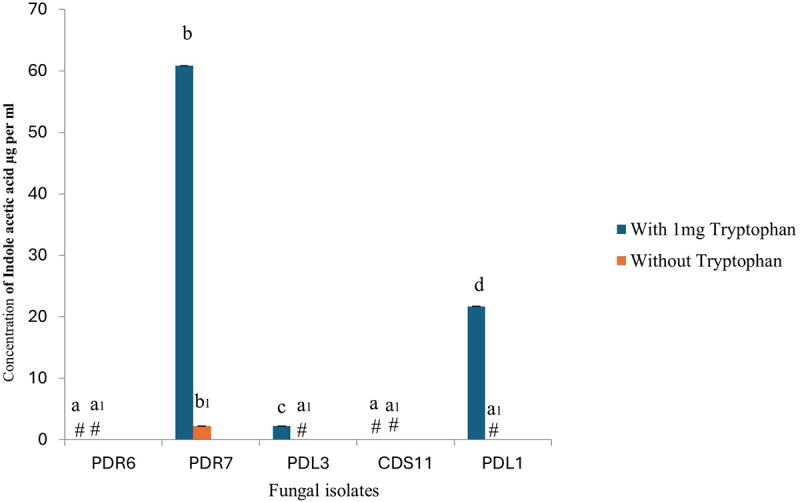
Auxin concentration in fungal extracts with and without added tryptophan. The experiment was repeated three times. Extract from PDR7 had the highest concentration of indole acetic acid (IAA). The bar represents mean ± SE (n = 3). Values with different letters are significantly different at p ≤ .05 (Tukey test). ‘#’ indicates the absence of IAA.
Table 2.
Five endophytic fungi and their IaaM GenBank accession number.
| Isolates | Taxon | GenBank Accession no. (IaaM gene) |
|---|---|---|
| CDS11* | Fusarium sp. | MK281637 |
| PDL1** | Fusarium sp. | MK281638 |
| PDL3** | Fusarium sp. | PQ067510 |
| PDR6*** | Fusarium sp. | PQ014737 |
| PDR7*** | Fusarium sp. | PQ067510 |
*CDS ‘CD’ represents CDA medium, ‘S’ Stem.
**PDL represents the PDA medium, ‘L’leaf section.
***PDR represents ‘PD’ PDA medium, ‘R’ root.
Germination and protocorm development experiment
The effect of the fungal extract on seed germination percentage was measured and compared with that of the control. The seed germination assay was kept for ten weeks until the seeds turned green (Figure 3). The significance level of the seed germination assay was set at p < .05. The fungal extracts of isolates PDR6 and PDR7 were highly effective at approximately 80 and 90% (respectively) for seed germination. The plant growth assay was performed with protocorms of D. longicornu and extracts prepared from the fungal isolates CDS11, PDR6, PDR7, PDL3, and PDL1. The protocorms were allowed to grow the MS media containing 2% of fungal extract for seven weeks to observe the growth of the protocorms (Figure 4). The protocorms treated with fungal extracts of PDR6 and PDR7 in this experiment showed enhanced growth in terms of length and number of roots and shoots. Both PDR6 and PDR7 showed a five-fold increase in root length and a four-fold increase in shoot length compared to the control. Comparatively, PDR6 and PDR7 both had two- and three-fold increases in the number of roots, and both showed a five-fold increase in the number of shoots. Whereas CDS11 showed the least growth (Figure 5). The significance level of the protocorms development assay was set at p < .05.
Figure 3.
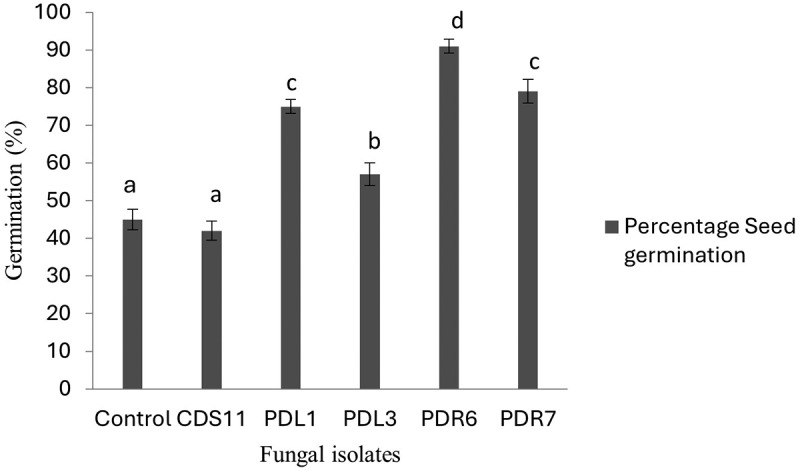
Germination response in seeds of Dendrobium moschatum. Supplementation with the extract of the isolated PDR6 yielded the highest percentage of seed germination as compared to other treatments. Bar represents mean ± SE (n= 15). p≤0.05.
Figure 4.
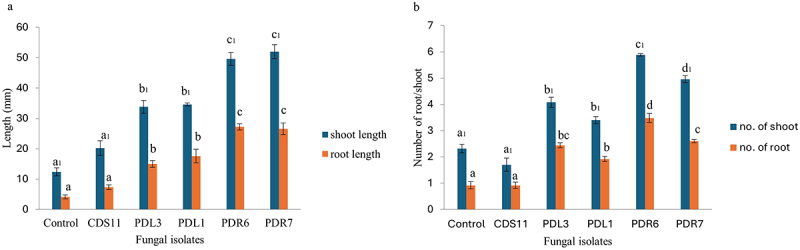
The protocorm development assay showing the effect of fungal extract in protocorm development of Dendrobium longicornu. The growth pattern treated with 2% fungal extract of PDR6 and PDR7 is higher in terms of mean of root and shoot length (a) as well as mean of roots and shoots number (b). Bar represents mean ± SE (n= 15). The data is significant at the level of p≤.05.
Figure 5.
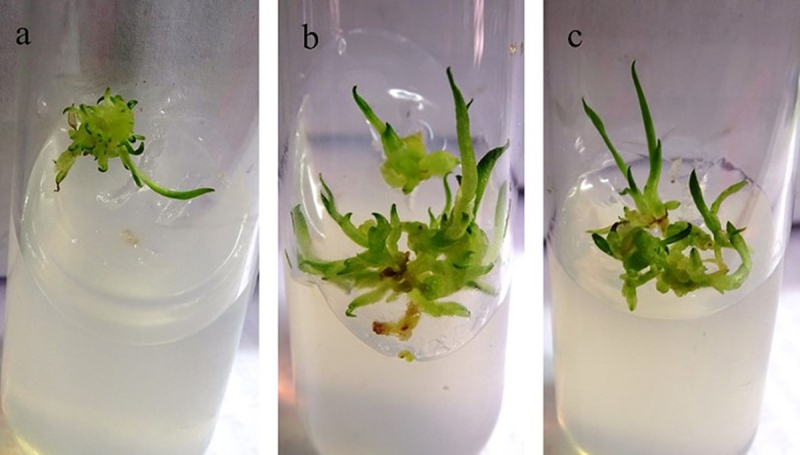
In-vitro plant growth assay of Dendrobium longicornu grown (a) on Murashige & Skoog (MS) media (control), or in MS media supplemented with fungal extract: (b) PDR6, (c) PDR7.
Discussion
Fusarium is an important group of non-mycorrhizal orchid fungi that have been reported as endophytes or pathogens based on the type of host tissue (healthy or diseased) [27,28]. They have been reported to originate from the roots, stems, and leaves of several orchids and are known to stimulate seed germination and plant growth [35–40]. Fusarium sp. was the first non-Rhizoctonia-like fungus reported to show a symbiotic association with orchid species [41]. Moreover, it is an orchid endophyte known to produce active metabolites in the host orchid [42–45] Crous et al 2019. Recent studies have shown that Fusarium is a common endophyte isolated from Dendrobium species [46]. A previous study indicated that Fusarium which may be a pathogenic or orchid-associated endophyte, may have a distinctive interaction with Dendrobium spp [47]. Dendrobium nobile and D. moschatum were infected with Fusarium proliferatum NRRL and F. fujikuroi IMI58289. F. proliferatum ET1 did not show any fungal infection. In addition, the plants remained healthy and asymptomatic which indicates the endophytic and beneficial nature of Fusarium species isolated from healthy plant material. Previous studies have shown that Fusarium sp. extracts isolated from D. longicornuu and Vanda cristata significantly promote the growth of in vitro-grown protocorms of Cymbidium and D. longicornu [14,15]. However, we cannot deny the pathogenicity of Fusarium species in orchids species. There are several reports regarding leaf-blight disease in orchids [48]. Fusarium and any other endophytes may become pathogenic depending on the plant’s age, immunity, abiotic stress or nutrient deficient condition [49,50].
Overall, the present study showed that most of the isolates were able to produce indole acetic acid as well as enhance in vitro plant growth and development. IAA production may be correlated with the coupling effect of two genes, IaaM (encoding tryptophan monooxygenase) and IaaH (indole-3-acetamide hydrolase) of the IAM pathway. This pathway has been reported to be fully functional only in orchid endophytes, especially Fusarium sp [26,51]. The fungal extracts of these isolates promoted growth of in vitro grown seeds of the host, as well as protocorm development of non-host orchids.
Acknowledgments
The authors are thankful to the University Grants Commission, Nepal; Dr. Yogesh S. Shouche director of National Centre for Microbial Resource, National Center for Cell Science, India; Centre for Co-operation in Science & Technology among Developing Societies (CCSTDS), Department of Science and Technology, Government of India for their extended support.
Funding Statement
This work was supported by the University Grants Commission, Nepal (NP) [PhD-72/73-S&T-Y-04]. Centre for Co-operation in Science & Technology among Developing Societies (CCSTDS), Department of Science and Technology, Government of India.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contribution
SS performed the experiments and wrote the manuscript; BBT performed data recording; MRP reviewed and edited the manuscript; HS and AKK performed phylogenetic tree and data analysis; BNR & RS facilitated space and instrumentation for the molecular work; JS supervised the research work and edited the manuscript; BP conceived and designed the research and supervised the research work and manuscript editing. All authors have read and approved the final version of the manuscript for publication.
Data availability statement
The authors confirm that the data supporting the findings of this study are available in the article. The datasets analyzed in this study are accessible from the corresponding author upon reasonable request.
Declarations
The field trip for sample collection was done in July 2018, as per the official guidelines provided by the Central Department of Botany, Tribhuvan University, Nepal. Dr. Keshav Raj Rajbhandri, botanist of National Herbarium and Plant Laboratories, Kathmandu, identified the plant. A voucher specimen of Dendrobium longicornu, voucher number N05, was deposited in the Tribhuvan University Central Herbarium (TUCH).
References
- [1].Shrestha KK, Bhandari P, Bhattarai S.. Plants of Nepal (gymnosperms and angiosperms). Heritage Publishers & Distributors Pvt. Limited; 2022. [Google Scholar]
- [2].Rokaya MB, Raskoti BB, Timsina B, et al. An annotated checklist of the orchids of Nepal. Nord J Bot. 2013;31(5):511–9. doi: 10.1111/j.1756-1051.2013.01230.x [DOI] [Google Scholar]
- [3].Pant B. Medicinal orchids and their uses: tissue culture a potential alternative for conservation. Afr J Plant Sci. 2013;7(10):448–467. doi: 10.5897/AJPS2013.1031 [DOI] [Google Scholar]
- [4].Pant B, Shah S, Shrestha R, et al. An overview on orchid endophytes. In: Varma A, Prasad R, Tuteja N, editors. Mycorrhiza-Nutrient Uptake, Biocontrol, Ecorestoration. 2017. p. 503–524. doi: 10.1007/978-3-319-68867-1_26 [DOI] [Google Scholar]
- [5].Pradhan BM, Bajracharya DM. Anatomical study of Dendrobium (Orchidaceae) of Nepal. Ann. Plant Sci. 2020;9(7):3916–3948. [Google Scholar]
- [6].Hlosrichok A, Sumkhemthong S, Sritularak B, et al. A bibenzyl from Dendrobium ellipsophyllum induces apoptosis in human lung cancer cells. J Nat Med. 2018;72(3):615–625. doi: 10.1007/s11418-018-1186-x [DOI] [PubMed] [Google Scholar]
- [7].Paudel MR, Chand MB, Pant B, et al. Antioxidant and cytotoxic activities of Dendrobium moniliforme extracts and the detection of related compounds by GC-MS. BMC Complement Altern Med. 2018;18(1):134. doi: 10.1186/s12906-018-2197-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shrestha R, Sujit S, Bijaya P. Identification of endophytic fungi from roots of two Dendrobium species and evaluation of their antibacterial property. Afr J Microbiol Res. 2018;12(29):697–704. doi: 10.5897/AJMR2018.8924 [DOI] [Google Scholar]
- [9].Pant B, Joshi PR, Maharjan S, et al. Comparative cytotoxic activity of wild harvested stems and in vitro-raised protocorms of Dendrobium chryseum Rolfe in human cervical carcinoma and glioblastoma cell lines. Adv Pharmacol Pharm Sci. 2021;2021:1–8. doi: 10.1155/2021/8839728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].della Cuna Fs R, Boselli C, Papetti A, et al. Composition of volatile fraction from inflorescences and leaves of Dendrobium moschatum (Orchidaceae). Natural Product Communications. 2018;13(1):1934578X1801300127. doi: 10.1177/1934578X1801300127 [DOI] [Google Scholar]
- [11].Thapa Magar MS, Maharjan S, Pathak J, et al. DNA barcoding of dendrobium moschatum (banks) sw. Specimen from Makawanpur, Central Nepal. J Pl Reso. 2022;20(2):124–129. doi: 10.3126/bdpr.v20i2.57001 [DOI] [Google Scholar]
- [12].Pant B, Pradhan S, Paudel MR, et al. Various culture techniques for the mass propagation of medicinal orchids from Nepal. Acta Hortic. 2018;(1262):109–124. doi: 10.17660/ActaHortic.2019.1262.16 [DOI] [Google Scholar]
- [13].Shah S, Thapa BB, Chand K, et al. Piriformospora indica promotes the growth of the in-vitro-raised cymbidium aloifolium plantlet and their acclimatization. Plant Signal Behav. 2019;14(6):1596716. doi: 10.1080/15592324.2019.1596716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shah S, Shah B, Sharma R, et al. Colonization with non-mycorrhizal culturable endophytic fungi enhances orchid growth and indole acetic acid production. BMC Micrbiol. 2022;22(1):101. doi: 10.1186/s12866-022-02507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chand K, Shah S, Sharma J, et al. Isolation, characterization, and plant growth-promoting activities of endophytic fungi from a wild orchid Vanda cristata. Plant Signal Behav. 2020;15(5):1744294. doi: 10.1080/15592324.2020.1744294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bhantana P, Rana MS, Sun XC, et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis. 2021;84(1):19–37. doi: 10.1007/s13199-021-00756-6 [DOI] [Google Scholar]
- [17].Selosse MA, Petrolli R, Mujica MI, et al. The waiting room hypothesis revisited by orchids: were orchid mycorrhizal fungi recruited among root endophytes? Ann Bot. 2022;129(3):259–270. doi: 10.1093/aob/mcab134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rekadwad BN, González Grau JM, Shah S, et al. Microbiome perspective: multisectorial exploitations of chitinases. Bentham Science Publishers. 2022. doi: 10.2174/9789815040340122020016 [DOI] [Google Scholar]
- [19].Kashyap AK, Shah S, Pant KK, et al. Metabolomics and genomics for understanding stress biology of plant metabolites. In: Swamy MK, Kumar A, editors. Phytochemical genomics: plant metabolomics and medicinal plant genomics. Springer Nature Singapore; 2023. p. 629–649. [Google Scholar]
- [20].Kashyap AK, Dubey SK, Shah S, et al. A short review on genes regulating biosynthesis of Major secondary metabolites. Phytochem Gen Plant Metabol Med Plant Gen. 2023;1:501–519. [Google Scholar]
- [21].Thakur M, Khushboo SS, Kumari P, et al. Unlocking the secrets of rhizosphere microbes: a new dimension for agriculture. Symbiosis. 2024;92(3):305–322. doi: 10.1007/s13199-024-00980-w [DOI] [Google Scholar]
- [22].Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61(1):49–64. doi: 10.1146/annurev-arplant-042809-112308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling, FEMS microbiol. FEMS Microbiol Rev. 2007;31(4):425–448. doi: 10.1111/j.1574-6976.2007.00072.x [DOI] [PubMed] [Google Scholar]
- [24].Zhao Y. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol Plant. 2012;5(2):334–338. doi: 10.1093/mp/ssr104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fu SF, Wei JY, Chen HW, et al. Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Signal Behav. 2015;10(8):e1048052. doi: 10.1080/15592324.2015.1048052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tsavkelova E, Oeser B, Oren-Young L, et al. Identification and functional characterization of indole-3-acetamide-mediated IAA biosynthesis in plant-associated Fusarium species. Fungal Genet Biol. 2012;49(1):48–57. doi: 10.1016/j.fgb.2011.10.005 [DOI] [PubMed] [Google Scholar]
- [27].Bhatti SK, Thakur M. An overview on orchids and their interaction with endophytes. Bot Rev. 2022;88(4):485–504. doi: 10.1007/s12229-022-09275-5 [DOI] [Google Scholar]
- [28].Shah S, Chand K, Rekadwad B, et al. A prospectus of plant growth promoting endophytic bacterium from orchid (Vanda cristata). BMC Biotech. 2021;21(1):1–9. doi: 10.1186/s12896-021-00676-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Katoh K, Misawa K, Kuma K, et al. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi: 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Capella-Gutiérrez S, Silla-Martinez JM, Gabaldòn T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–1973. doi: 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tamura K, Stecher G, Kumar S, et al. MEGA 11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–3027. doi: 10.1093/molbev/msab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khan AL, Halo BA, Elyassi A, et al. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron J Biotechnol. 2016;21:58–64. doi: 10.1016/j.ejbt.2016.02.001 [DOI] [Google Scholar]
- [33].Shah S, Shrestha R, Maharjan S, et al. Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants. 2019;8(1):5. doi: 10.3390/plants8010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pradhan S, Tiruwa B, Subedee BR, et al. In vitro germination and propagation of a threatened medicinal orchid, cymbidium aloifolium (L.) sw. through artificial seed. Asian Pac J Trop Biomed. 2014;4(12):971–976. doi: 10.12980/APJTB.4.2014APJTB-2014-0369 [DOI] [Google Scholar]
- [35].Srivastava S, Kadooka C, Uchida JY. Fusarium species as pathogen on orchids. Microbiol Res. 2018;207:188–195. doi: 10.1016/j.micres.2017.12.002 [DOI] [PubMed] [Google Scholar]
- [36].Sarsaiya S, Shi J, Chen J. A comprehensive review on fungal endophytes and its dynamics on Orchidaceae plants: current research, challenges, and future possibilities. Bioengineered. 2019;10(1):316–334. doi: 10.1080/21655979.2019.1644854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peschke HC, Volz PA. Fusarium moniliforme sheld. Association with species of orchids. Phytologia. 1978;40:347–356. [Google Scholar]
- [38].Bayman P, Otero JT. Microbial endophytes of orchid roots. In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 153–177. [Google Scholar]
- [39].Sufaati S, Agustini V, Suharno S. Short communication: fusarium as endophyte of some terrestrial orchid from Papua, Indonesia. Biodiversitas J Biolog Divers. 2016;17(1). doi: 10.13057/biodiv/d170149 [DOI] [Google Scholar]
- [40].Maldonado GP, Yarzábal LA, Cevallos-Cevallos JM, et al. Root endophytic fungi promote in vitro seed germination in Pleurothallis coriacardia (Orchidaceae). Lankesteriana. 2020;20(1):107–119. doi: 10.15517/lank.v20i1.41472 [DOI] [Google Scholar]
- [41].Vujanovic V, St-Arnaud M, Barabé D, et al. Viability testing of orchid seed and the promotion of colouration and germination. Ann Bot. 2000;86(1):79–86. doi: 10.1006/anbo.2000.1162 [DOI] [Google Scholar]
- [42].Strobel GA. Rainforest endophytes and bioactive products. Critical reviews in biotechnology. Critical Rev Biotechnol. 2002;22(4):315–333. doi: 10.1080/07388550290789531 [DOI] [PubMed] [Google Scholar]
- [43].Yuan ZL, Chen YC, Yang Y. Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): estimation and characterization. World J Microbiol Biotechnol. 2009;25(2):295–303. doi: 10.1007/s11274-008-9893-1 [DOI] [Google Scholar]
- [44].Crous PW, Carnegie AJ, Wingfield MJ, et al. Fungal planet description sheets: 868–950. Persoonia. 2019;42:291–473. doi: 10.3767/persoonia.2019.42.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hou XQ, Guo SX. Interaction between a dark septate endophytic isolate from Dendrobium sp. and roots of D. nobile seedlings. J Integr Plant Biol. 2009;51(4):374–381. doi: 10.1111/j.1744-7909.2008.00777.x [DOI] [PubMed] [Google Scholar]
- [46].Hajong S, Kapoor R. An amalgam of pathogenic and beneficial endophytic fungi colonizing four Dendrobium species from Meghalaya, India. J Basic Microbiol. 2020;60(5):415–423. doi: 10.1002/jobm.201900631 [DOI] [PubMed] [Google Scholar]
- [47].Tsavkelova EA, Kolomeitseva GL. Fusarium–orchid interactions under greenhouse conditions. S Afr J Bot. 2022;146:889–896. doi: 10.1016/j.sajb.2022.03.038 [DOI] [Google Scholar]
- [48].Yang J, Ahmed W, Zhang J, et al. Identification of Fusarium oxysporum causing leaf blight on Dendrobium chrysotoxum in Yunnan Province, China. Life. 2024;14(3):285. doi: 10.3390/life14030285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Redkar A, Sabale M, Zuccaro A, et al. Determinants of endophytic and pathogenic lifestyle in root colonizing fungi. Curr Opin Plant Biol. 2022;67:102226. doi: 10.1016/j.pbi.2022.102226 [DOI] [PubMed] [Google Scholar]
- [50].Collinge DB, Jensen B, J JH. Fungal endophytes in plants and their relationship to plant disease. Curr Opin Microbiol. 2022;69:102177. doi: 10.1016/j.mib.2022.102177 [DOI] [PubMed] [Google Scholar]
- [51].Tang J, Li Y, Zhang L, et al. Biosynthetic pathways and functions of indole-3-acetic acid in microorganisms. Microorganisms. 2023;11(8):2077. doi: 10.3390/microorganisms11082077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available in the article. The datasets analyzed in this study are accessible from the corresponding author upon reasonable request.


