ABSTRACT
Pertussis resurgence has been documented even in countries with high pediatric vaccine coverage. The proportion of Bordetella pertussis isolates not expressing pertactin (PRN) has increased in several countries where acellular pertussis (aP) vaccines are used. We systematically reviewed published literature up to July 2023 on PRN-negative B. pertussis isolates in MEDLINE and Embase with no geographical limitations, complemented with a gray literature search. An increase in the proportion of PRN-negative isolates was observed in countries where aP vaccines were used, while such isolates seem to be absent in countries using whole-cell pertussis vaccination. We reviewed the data supporting aP vaccine-driven evolution of B. pertussis, explored the effects of PRN deficiency on the clinical presentation of pertussis, summarized the evidence for preserved aP vaccine effectiveness, and proposed actions to further improve assessment of the clinical significance of PRN deficiency and its potential impact on pertussis prevention.
KEYWORDS: Acellular pertussis vaccine, Bordetella pertussis, pertactin, pertussis (whooping cough), reemergence, surveillance, vaccine pressure, vaccine effectiveness, whole-cell pertussis vaccine
Plain Language Summary
The burden of pertussis (whooping cough), once a common cause of morbidity and mortality among children, was greatly reduced by the introduction of pediatric pertussis vaccination. Two types of pertussis vaccines exist: whole-cell pertussis (wP) vaccines, containing inactivated Bordetella pertussis bacteria, and acellular pertussis (aP) vaccines, containing purified antigens. Having lower reactogenicity, aP vaccines have replaced wP vaccines in many developed countries. In other parts of the world, wP vaccines are still used. Over time, the proportion of B. pertussis isolates not expressing pertactin (PRN, a surface antigen involved in virulence and contained in most aP vaccines) increased in countries using aP vaccines, but not in those using only wP vaccines. We performed a systematic literature review to collect data on the epidemiology of PRN-negative B. pertussis isolates identified worldwide. We combined these data with information on the type of pertussis vaccine used in each country and on the year aP vaccines were introduced, to assess if aP vaccine use was associated with a higher proportion of PRN-negative B. pertussis strains. We then selected three countries (Belgium, France, and Canada), for which we present data on pertussis diagnosis, surveillance, epidemiology, pertussis vaccine recommendations, and vaccine coverage, in connection with the PRN-negative B. pertussis data retrieved by our search. We found that the symptoms and severity of pertussis cases appear to be similar regardless of whether they were caused by PRN-negative or PRN-positive strains. There is no evidence of impaired protection by aP vaccines in countries where most circulating B. pertussis strains are PRN-negative.
Introduction
The first effective pertussis vaccine, developed in the 1930s,1 was obtained by culturing and inactivating Bordetella pertussis. This whole-cell pertussis (wP) vaccine represented a breakthrough in the prevention of pertussis: its widespread use as part of the diphtheria-tetanus-pertussis combination vaccines has led to a major reduction in pertussis incidence.2 While wP vaccines are effective and relatively inexpensive, producing consistent lots is difficult. wP vaccines are also quite reactogenic, frequently causing adverse reactions such as fever as well as redness or swelling at the site of injection, which tend to increase with age and the number of injections.3 In addition, the use of wP vaccines had decreased in many countries in the 1970s and 1980s based on suspicions that these vaccines may be linked to neurological damage in children,2,4–6 although this hypothesis was subsequently disproved.7–9
To address the concerns related to the adverse reactions observed following wP vaccination, acellular pertussis (aP) vaccines were developed3 and progressively introduced in national immunization programs. These aP vaccines contain 1–5 purified components of B. pertussis, all of them including pertussis toxin (PT), and, optionally, filamentous hemagglutinin (FHA, in 2-, 3-, and 5-component aP vaccines), pertactin (PRN, in 3- and 5-component aP vaccines), and/or fimbrial proteins (FIM2 and FIM3, in 5-component aP vaccines).10 Their introduction in many developed countries increased vaccine acceptance and further decreased the global burden of pertussis, by allowing introduction of boosters in older children and adolescents, in whom the use of wP vaccines is not recommended due to high reactogenicity.11,12 The aP vaccines were demonstrated to protect against severe and typical symptomatic pertussis disease. They do not, however, seem to prevent nasopharyngeal colonization and transmission of B. pertussis from asymptomatic or oligosymptomatic vaccinated individuals based on evidence from non-human primates13 and mathematical models14 (reviewed in15). Following their introduction, aP vaccines have often been used in parallel with wP vaccines for some time, before completely replacing them in most developed countries. There are differences in the antigens contained by the aP vaccines used in different countries, as well in the number of recommended doses and the ages at which they are administered.16 The World Health Organization recommends that countries currently using wP vaccines continue their use for primary vaccination series.12
Over the past decades, pertussis resurgences have been reported, even in countries with high pediatric vaccination coverage irrespective of the use of wP or aP vaccines.17,18 Multiple factors are thought to contribute to these resurgences.19 Increased disease awareness and improved diagnostics have resulted in a better detection of cases. Additionally, as aP vaccines seem less effective than wP vaccines at preventing infection and transmission of B. pertussis (as outlined above),13–15 a greater circulation of B. pertussis could occur in populations vaccinated with aP vaccines compared to wP vaccines, leading to more infections in susceptible individuals. Furthermore, the immunity induced by aP vaccines wanes more rapidly than the immunity induced by wP vaccines.19–21 Vaccine hesitancy could increase the risk of pertussis and further contribute to the pertussis resurgences as it leads to lower vaccine coverage.22 Another factor could be represented by the genetic changes exhibited by B. pertussis, which are the result of vaccine-induced selective pressure.15,19 In line with this hypothesis, several independent mutations have been observed in phylogenetically different B. pertussis strains circulating since pertussis vaccine introduction, some of which result in isolates not producing one or more antigenic proteins.11,15,23 One of the most impressive changes is represented by the increase in B. pertussis strains not expressing PRN.24 PRN is an autotransporter protein on the outer membrane of B. pertussis.25 Although it has been proposed to play a role in host cell adhesion26–28 and immune modulation,29 its exact role in pathogenesis is not completely understood.15 Antibodies against PRN play a major role in the protection against pertussis,15,30,31 and therefore several aP vaccines feature the PRN antigen in their composition.
Multiple mechanisms for PRN deficiency have been identified, including insertions, deletions, inversions, and point mutations affecting the genes encoding the PRN protein.23 Despite the proposed role of PRN as a virulence factor, PRN-negative strains showed higher fitness than PRN-positive strains in mice immunized with an aP vaccine in terms of their ability to colonize the respiratory tract.32 Another study showed that aP vaccines (containing PRN as antigen) generate bactericidal antibodies that are only directed against PRN, as opposed to natural infection, which induces bactericidal antibodies against PRN and other antigens.33 This suggests that in the context of widespread aP vaccine use, the cost of decreased virulence due to PRN loss might be compensated by a gain in fitness, potentially allowing increased transmission among vaccinated individuals, thus representing a selective advantage for PRN-negative strains.11,15,24 Although concerns have been raised regarding the potential decrease of pertussis vaccine effectiveness (VE) against disease caused by the increasing proportion of PRN-negative B. pertussis strains, no conclusive evidence confirming this hypothesis has been presented to date.11,15,34 Moreover, additional information on the severity and clinical presentation of pertussis cases caused by PRN-negative strains (in comparison with those caused by PRN-positive strains) is needed to define the clinical significance of PRN deficiency.
In this review, we aimed to i) provide an overview of the published data on the proportion of PRN-negative B. pertussis isolates, without imposing any geographical limitation; ii) assess the significance of a higher circulation of PRN-negative B. pertussis strains in three countries (Belgium, France, and Canada), by considering contextual epidemiological data, the pertussis surveillance system used, diagnostic practices, and vaccination policy; iii) propose actions that would further improve assessment of the clinical significance of PRN deficiency and its potential impact on pertussis prevention.
Materials & methods
Systematic literature review
For the first part of this review, a systematic literature search was performed on 21 July 2023 in MEDLINE (via the PubMed interface) and Embase. No limits regarding the year of publication, language, geographical scope, or publication type were applied.
This systematic search was complemented by a gray literature search, which included a Google search (performed on 14 February 2022 and on 2 August 2023) and screening of more than 24 websites (performed on 2 August 2023), including national websites/registries for six countries known to have relevant data (Australia, France, Germany, the Netherlands, the United Kingdom [UK], and the United States of America [USA]). Details regarding the search strategies employed can be found in Supplementary file 1.
Publications that included relevant data on the incidence or prevalence of PRN-negative B. pertussis strains, regardless of the age group from which the strains were isolated, were selected for analysis. Vaccine efficacy/effectiveness studies, studies presenting diagnostic tools/methods for genotyping, animal studies, and modeling studies without original data were excluded. After the title and abstract screening by two independent reviewers in duplicate, the full-text articles were screened for relevance by two reviewers (20% in duplicate). At this stage, studies that did not perform relevant methods or did not contain data relevant for the review objective (i.e., not identifying B. pertussis strains), studies that did not mention PRN-negative strains, narrative reviews, studies of insufficient methodological quality, studies that contained no relevant quantitative data, and studies not published in peer-reviewed journals were excluded. An effort was made to identify and eliminate duplicate data, i.e., similar results from identical samples originating from the same source and covering the same period. Data from the included full-text articles were then extracted using the systematic review software DistillerSR. The study selection, data extraction, and quality assessment processes, as well as the statistical methods used to analyze the extracted data are described in Supplementary file 1.
The percentage of PRN-negative B. pertussis strains and both numerator and denominator of this proportion were recorded. The denominator was the sample of confirmed B. pertussis isolates evaluated for PRN deficiency. Whenever possible (i.e., if data was available per isolate), data was extracted separately for each year covered by a particular study.
Contextual analysis of PRN deficiency proportion in selected countries
Three countries (Belgium, France, and Canada) were selected for performing an in-depth, contextual analysis of PRN-negative B. pertussis strain prevalence. These countries were chosen based on the availability of recent data regarding the national vaccination schedule (including recommendations for booster vaccination), the type(s) of vaccine administered, pertussis vaccine uptake, diagnostic practices, surveillance of pertussis disease and availability of B. pertussis strains (including data on PRN deficiency), and/or VE evaluations.
Results
Systematic literature review
Study characteristics
Overall, 47 records were included in this review (Figure 1). All 47 studies had a retrospective design and were specifically targeting B. pertussis isolates or pertussis-suspected cases.
Figure 1.
PRISMA flow diagram.
N, number of records; B. pertussis, Bordetella pertussis; PRN, pertactin
Samples were mainly acquired from two types of sources: either already-confirmed B. pertussis isolates from a national reference institution or from suspected/confirmed cases of (a) specific hospital(s). Isolates were obtained either from a regular country surveillance program or from a specific outbreak. Studies covered time periods spanning from 1 to 88 years; the sample size had a mean of 72, a median of 22, and ranged from 1 to 1,428 (Supplementary table S1). Most studies were of sufficient quality, as described in Supplementary file 2.
Epidemiological mapping of PRN-negative B. pertussis strains
The 47 studies were conducted in 33 countries across all continents; most were from Europe. The countries with most publications were the USA (11) and France (8), followed by Canada and Italy (5 publications each), China, Finland, Japan, the Netherlands, Sweden, and the UK (4 publications each), Australia, Denmark, Norway, and Iran (3 publications each), Argentina, Belgium, Poland, and Spain (2 publications each). For all other countries with available data, these were extracted from one publication for each country (Supplementary table S1).
Among the studied countries, PRN-negative B. pertussis strains were identified in Canada, the USA, Argentina, Brazil, all 16 European countries, China, Israel, Japan, Australia, and New Zealand. Overall, the proportion of PRN-negative strains increased in most countries where assessments were performed over several consecutive time periods. In some countries, such as the USA or New Zealand, PRN-negative strains have risen to dominance, representing > 80% of identified strains.23,35–40 In countries that primarily or exclusively use wP vaccines, no or few PRN-negative B. pertussis strains have been reported, as observed in Tunisia,41 Argentina,42,43 and Brazil.44
Vaccine types
Both wP and aP vaccines were used in the studied countries. Most countries included in this review have introduced the aP vaccine between 1997 and 2007 for primary and/or booster vaccination, often completely replacing wP vaccines with aP vaccines in the immunization schedules; 10 of the studied countries still use wP vaccines in combination with aP vaccines, and four countries never introduced aP vaccines in their national immunization programs (Iran, Tunisia, Kenya, and Senegal) (Figure 2). Most of the studies included in this review did not report the type of the vaccine used in the population from which the sample originated from, but rather the vaccine introduction year, an overall strategy of the country and/or the national vaccination schedule (for primary and booster vaccination). Comprehensive information on these parameters is presented in Supplementary table S2.
Figure 2.
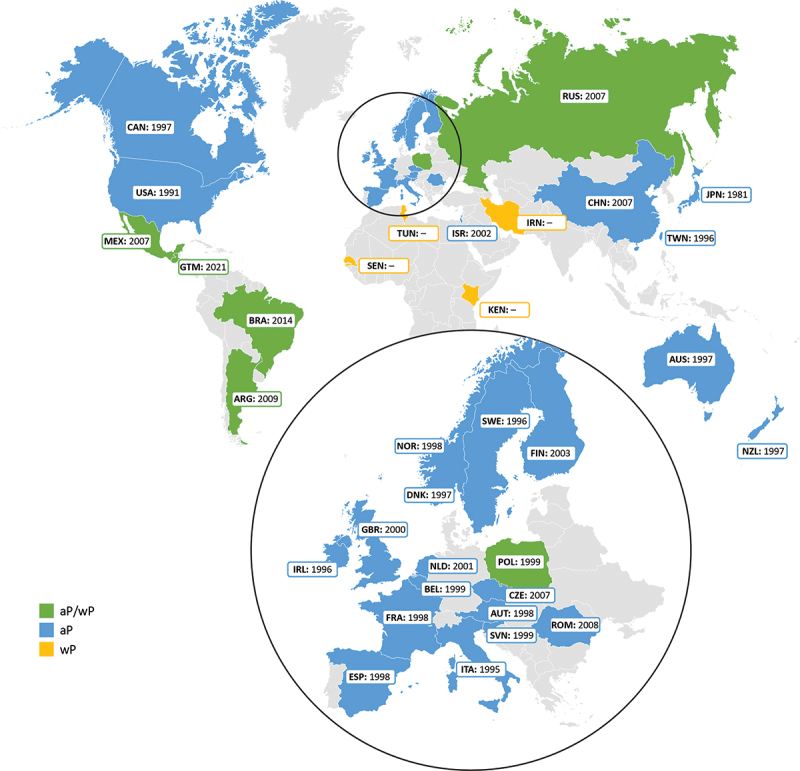
Current use of whole-cell pertussis (wP) and acellular pertussis (aP) vaccines and year of aP vaccine introduction (primary and/or booster) for countries included in the systematic literature review.
In countries using only wP vaccines, there might be aP vaccines available on the private market; however, the proportion of this private market may differ greatly from one country to another.
The major types of pertussis vaccines currently used worldwide for primary immunization are presented for each continent in Figure 3.45–58 In North America, both Canada and the USA currently use only aP vaccines that include the PRN antigen. In several regions of the world, wP and aP vaccines are both used.59 In Argentina, a wP vaccine is generally used for primary vaccination, while the aP vaccine is used in the private sector and for the recommended boosters in adolescents, healthcare workers in contact with infants <12 months of age, household contacts of low-birthweight infants, and pregnant women47 (Supplementary table S2). In Brazil, aP vaccines, which include the PRN antigen,44 are used for infants in special groups and booster doses for adults in certain categories (Supplementary table S2). Currently, all European countries exclusively use aP vaccines, except for Poland, where both wP and aP vaccines are used (Supplementary table S2). Of the countries using aP vaccines, only Denmark has solely been using a vaccine that does not contain the PRN antigen during the study period.60 Other countries have been using a mix of PRN-containing and non-PRN-containing aP vaccines, or only vaccines that contain PRN.60
Figure 3.
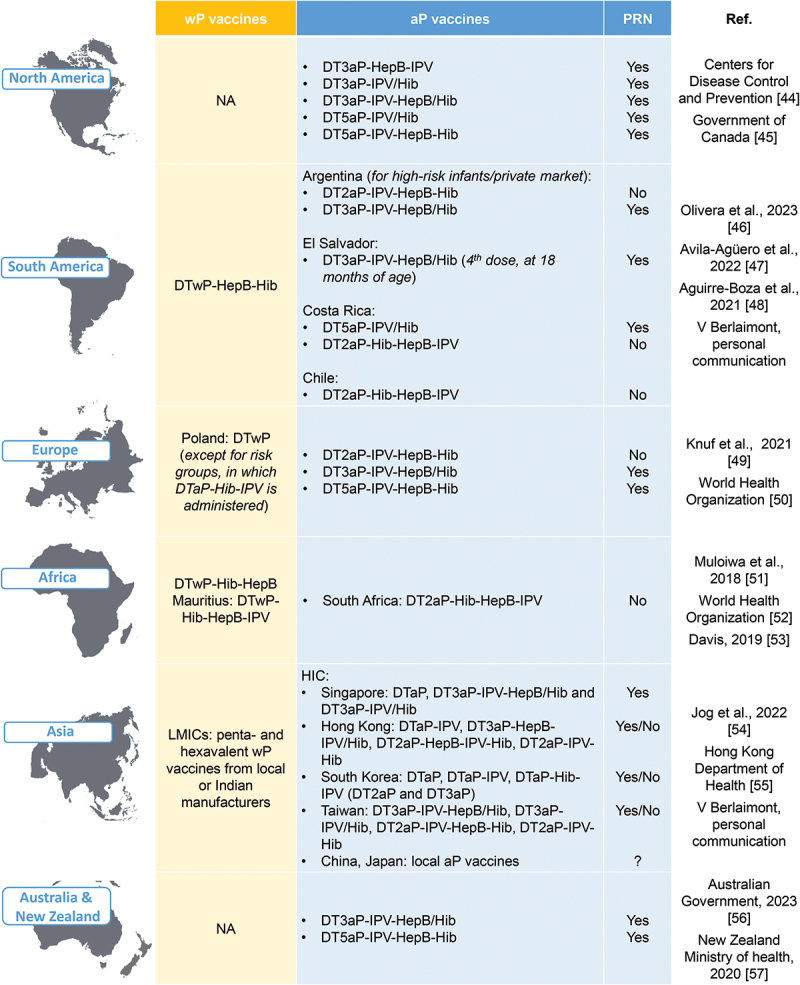
Major pertussis vaccine types for primary immunization currently in use worldwide.
aP, acellular pertussis (the number before aP indicates the number of pertussis antigens included in the vaccine); D, diphtheria toxoid; HepB, hepatitis B; Hib, Haemophilus influenzae type b; HIC, high-income country; IPV, inactivated poliovirus; LMIC, low- and middle-income country; NA, not applicable; T, tetanus toxoid; wP, whole-cell pertussis
Emergence of PRN-negative strains and the use of aP vaccines
Although a formal comparison between countries is methodologically challenging, almost all countries showed a consistent increase in PRN-negative strains approximately a decade after the introduction of aP vaccines in their immunization schedules. The proportion of PRN-negative isolates over time according to the sample size and ordered by the year of aP vaccine introduction is shown for the USA, Italy, Sweden, Australia, France, the Netherlands, and Finland isolates in Figure 4. While in countries like the USA, Italy, Sweden, or Australia, the percentage of PRN-negative B. pertussis isolates has increased following aP vaccine introduction, this pattern was not consistent across countries. This might be due to differences in vaccination schedule, vaccine coverage, and the marked heterogeneity of the aP vaccine types used with regards to PRN content. In most countries analyzed, PRN-containing aP vaccines have been used for primary vaccination and boosting alternatively or in parallel with non-PRN-containing aP vaccines. However, publicly available data for each vaccine type are not sufficiently granular to allow delimitating periods of predominant or exclusive PRN-containing aP vaccine use from periods when immunization with non-PRN-containing aP vaccines was more common. Therefore, no definitive conclusion regarding the effect of PRN-containing aP vaccines on the proportion of PRN-negative B. pertussis isolates can be drawn based on the available data.
Figure 4.
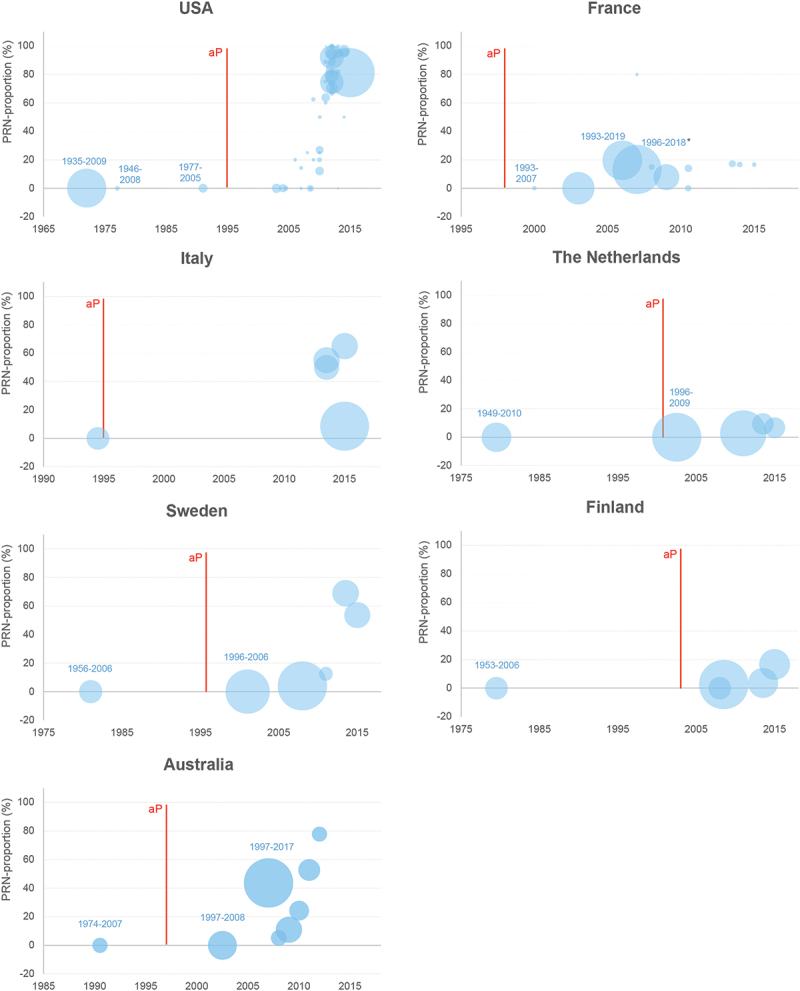
Proportion of PRN-negative strains over time in selected countries that use aP vaccines (regardless of PRN content).
aP, acellular pertussis; PRN, pertactin
*During the aP vaccine-only period, the proportion of PRN-negative B. pertussis strains increased from 5.6% in 2007 to 48.4% in 2018.10
Each bubble corresponds to a result in a study as presented in Supplementary table S1 (when a study is reporting results per year or several shorter periods, multiple results/bubbles will be presented for one study). Bubble size represents the sample size of the corresponding period (the denominator in the proportion), but it is not comparable across the charts for the different countries. For studies that report data over a time period, for the purpose of the plot, the midterm year (i.e., the average year between start and end years) was used. For all studies with a period longer than 10 years, the label is added (start year-end year). The red line represents the year of the first introduction of the aP vaccine for a given country.
Japan was the first country to introduce aP vaccination in 1981, using both vaccines with and without PRN. Since the late 1990s, the proportion of PRN-negative B. pertussis has increased gradually, reaching a maximum of 41–100% (depending on the study) between 2005 and 2010.61,62 However, a subsequent decrease in PRN-negative isolates has been recorded, with only 8% of the isolates collected between 2014 and 2016 exhibiting this characteristic; this trend emerged a few years after PRN-containing vaccines were replaced with vaccines that did not contain PRN.61 Among European countries using aP vaccines, the proportion of PRN-negative B. pertussis isolates is lowest in Denmark: only four PRN-negative strains were detected since 2012 (4/27, 14.8%) (Supplementary table S1). These strains could have been isolated from cases potentially originating from neighboring countries, where the frequency of PRN-negative isolates is high.60 Denmark is the only European country where a monocomponent (PT only) aP vaccine was used until 2019.60,63
In countries where aP vaccines have not been introduced in the national immunization program (Iran, Senegal, Kenya, and Tunisia), no PRN-negative stains have been identified (Supplementary table S1).
Contextual analysis of PRN deficiency proportion in selected countries
Belgium
Pertussis is a notifiable disease in all three Belgian regions (Brussels, Flanders, and Wallonia). Until 2019, it was mandatory to report all possible, probable, and confirmed pertussis cases. In 2020, compulsory notification underwent major adaptations in Wallonia and Brussels. In Wallonia, only confirmed cases in children <3 years of age are required to be reported, while in Brussels, only confirmed cases (regardless of age) are to be declared. In Flanders, no changes to the notification rules have been made.64 In Belgium, pertussis is diagnosed by polymerase chain reaction (PCR) and serology;64,65 as of April 2019, PCR diagnosis is only reimbursed for suspected pediatric cases, under certain conditions (Table 1).
Table 1.
Recommended/reimbursed pertussis diagnosis methods in Belgium, France, and Canada.
| Country | Ref. | Diagnosis of pertussis |
|---|---|---|
| Belgium | 64,65 | Reimbursed tests: serology and PCR (under certain conditions, since 1 April 2019):
OR
|
| France | 66 | Reimbursed tests: PCR and culture (aspiration or nasopharyngeal swab) Serology is not recommended (reimbursement has ceased in 2011) |
| Canada | 67 | Laboratory confirmation of pertussis cases is based on culture or PCR |
PCR, polymerase chain reaction.
Pertussis surveillance relies on three different sources, with information being obtained from notified cases and a laboratory network (Table 2). Data from all sources combined showed an increase in the number of pertussis cases between 2011 and 2014. From 2015 to 2017, this trend was less clear, with variations observed according to region and the source of the data (Figure 5A, Supplementary table S3). In 2018, the number of pertussis cases reported by all sources decreased. This drop continued to be observed in 2019 in all data sources, except for mandatory reporting data in the Brussels region, where cases increased. In 2020, cases again dropped sharply, likely due to the non-pharmaceutical interventions adopted during the coronavirus disease 19 (COVID-19) pandemic.
Table 2.
Pertussis surveillance in Belgium, France, and Canada.
| Country | Ref. | Pertussis surveillance |
|---|---|---|
| Belgium | 64 | Sources:
Case classification:
|
| France | 66,68 | Sources:
Case classification:
|
| Canada | 67,69 | Sources:
Case classification:
|
IgG, immunoglobulin G; PCR, polymerase chain reaction; PT, pertussis toxin; RENACOQ, Réseau national de la coqueluche (National pertussis network).
Figure 5.
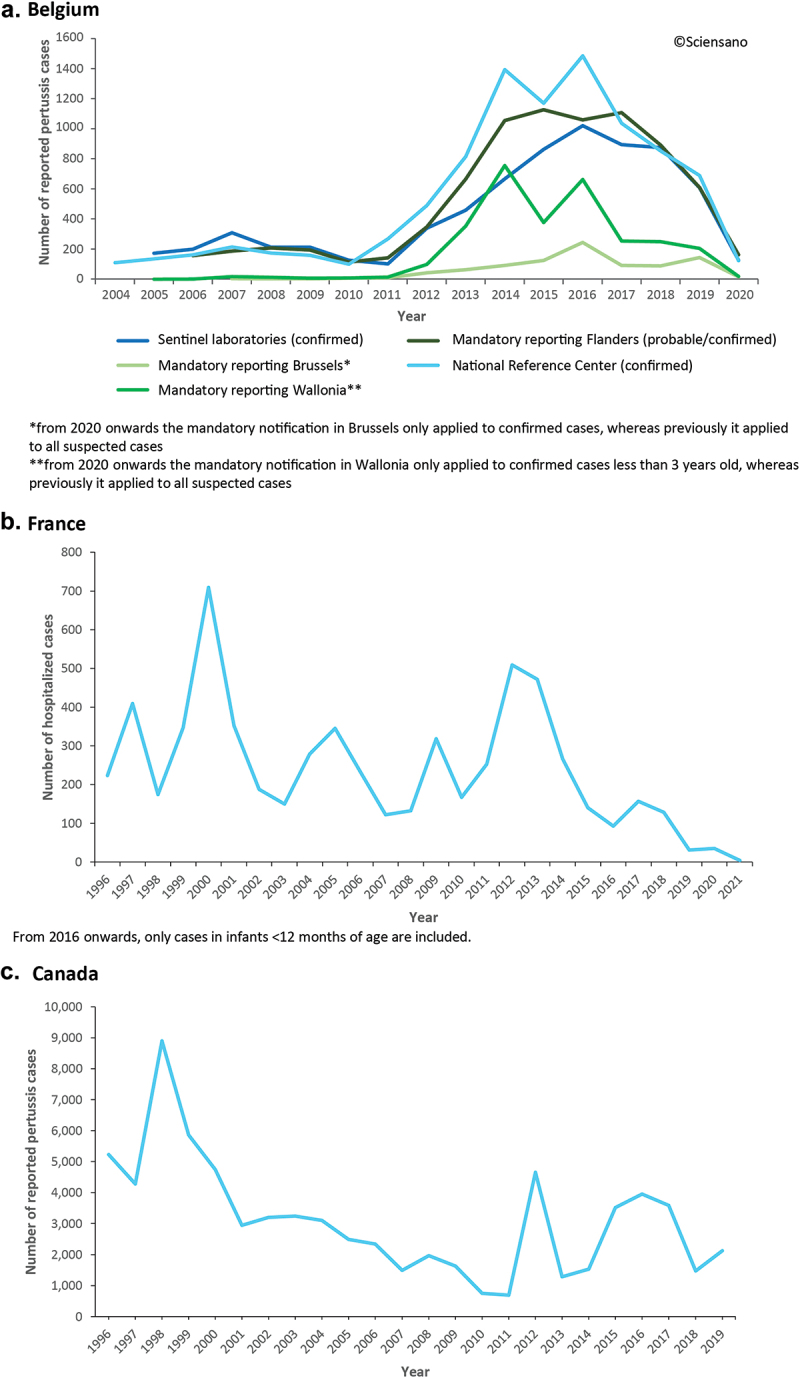
Trends in pertussis epidemiology in Belgium (a), France (b), and Canada (c).
a. Number of reported pertussis cases in Belgium between 2004 and 2020, by source;64 b. Number of hospitalized cases of pertussis in children under 17 years of age in France between 1996 and 2021, data reported by the RENACOQ network;70 c. Number of reported pertussis cases in Canada between 1996 and 2019.71
Currently available data on the proportion of PRN deficiency among strains isolated in Belgium comes from two published studies. Barkoff et al. (2019) found 10.5% (4/38) PRN-negative strains among B. pertussis isolates collected as part of the EUpertstrain IV study (2012 to 2015),60 while Lefrancq et al. (2021) reported 20% (6/30) PRN-negative strains among B. pertussis isolates collected between 2014 and 2016.72
Childhood immunization against pertussis starts with a 3 + 1 primary vaccination series (at 2, 3, 4, and 15 months, using a hexavalent vaccine against diphtheria, tetanus, pertussis, Haemophilus influenzae type b, hepatitis B, and poliomyelitis [DTaP-Hib-HepB-IPV]). Until 2014, all hexavalent vaccine formulations used in the national immunization program contained PRN; a hexavalent formulation that does not include PRN has replaced the PRN-containing aP vaccines in Flanders since July 2014, and in the entire country from September 2015.73 Starting from 2023, in Brussels and Wallonia a PRN-containing hexavalent vaccination is provided again as part of the national immunization schedule for primary vaccination; the hexavalent formulation not containing PRN is available in pharmacies for purchase.74 Following primary vaccination, two booster doses are administered in all three regions. The first booster, scheduled at 5–6 years of age is represented by a PRN-containing vaccine, DTaP-IPV, which is provided free of charge;74,75 a DTaP-IPV vaccine not containing PRN is also available for purchase in pharmacies.74,75 The second booster, recommended at 14–16 years of age, consists of a PRN-containing vaccine, the adult/adolescent formulation of tetanus toxoid, reduced diphtheria toxoid, and aP vaccine [Tdap]); some differences exist among the three regions regarding the age at which the pediatric booster doses are administered (Supplementary table S2). For adults, Tdap vaccination is recommended during each pregnancy and as part of the cocooning strategy for protecting newborns. In addition, decennial booster doses are also recommended (but not reimbursed) for adults (Table 3).16
Table 3.
Pertussis vaccination recommendations and pertussis vaccine type(s) used in Belgium, France, and Canada.
| Country | Ref. | Vaccination recommendations |
|---|---|---|
| Belgium | 16,60,73–77 | Current schedule:
Changes in the vaccination schedule:
|
| France | 16,60,68,78–83 | Current schedule:
Changes in the vaccination schedule:
|
| Canada | 16,46,69,84,85 | Current schedule:
Changes in the vaccination schedule:
|
aP, acellular pertussis; D, diphtheria toxoid; HepB, hepatitis B; Hib, Haemophilus influenzae type b; IPV, inactivated poliovirus; PRN, pertactin; T, tetanus toxoid; Td, tetanus toxoid and reduced diphtheria toxoid; Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis; wP, whole-cell pertussis.
Based on regional vaccination coverage studies, carried out between 2012 and 2021, pertussis vaccination coverage is ≥94% among children aged 18–24 months and varies between 31% and 85% in pregnant women (Table 4). We found no publicly available data regarding pertussis vaccine coverage in the general adult population.
Table 4.
Pertussis vaccination coverage in Belgium, France and Canada.
| Country | Ref. | Pertussis vaccination coverage |
|---|---|---|
| Belgium | 86 |
|
| France | 68,87,88 |
|
| Canada | 69,84 | Estimates from the 2019 Canadian Childhood National Immunization Coverage Survey:
Estimates of the 2018–2019 Seasonal Influenza Vaccination Coverage Survey:
Estimates of the Survey on Vaccination during Pregnancy 2021:
|
D, diphtheria toxoid; NIP, National Immunization Program; P, pertussis toxoid; T, tetanus toxoid; Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
France
In France, pertussis is not a notifiable disease since 1986; however, outbreaks have to be declared.66,68,70,78 Accepted (and reimbursed) diagnostic methods include PCR and culture from aspiration samples or nasopharyngeal swabs (Table 1).
Pertussis surveillance is performed by combining information from several sources (Table 2). These include two nationwide outpatient laboratories (Cerba and Biomnis, which carry out >90% of the ambulatory testing for pertussis in mainland France), a nationwide network of >40 hospitals (Réseau national de la coqueluche [RENACOQ], covering ~30% of hospitalized pediatric pertussis cases), and a French pediatric ambulatory surveillance system (the Sentinelles network, including ~ 60 pediatricians working in private practices).10,68,70,89 As of 2016, only pertussis cases diagnosed in infants <12 months of age are reported by RENACOQ.70 Within the RENACOQ, data on pertussis culture and PCR results, as well as on the clinical and epidemiological characteristics of pertussis cases, are collected in hospitalized infants <6 months of age.68 Clinical isolates collected within the RENACOQ network and at other French hospitals are referred to the National Reference Center (Centre National de Référence de la coqueluche et autres Bordetelles);10,68 most of these isolates are collected from children, but some originate from their close contacts, i.e., older siblings, parents, or grandparents.10
The most recent epidemiological peak of pertussis cases was recorded in 2017 (Figure 5B); over the following years, the number of pertussis cases detected in infants <12 months of age has decreased, with historically low levels attained in 2021, in great part due to measures adopted during the COVID-19 pandemic (Supplementary table S3).
In the published literature, PRN deficiency of B. pertussis strains was assessed in multiple studies (Supplementary table S1), including a large study in which 2,280 isolates collected continuously between 1996 and 2018 were characterized.10 Of the 1,428 tested isolates, 188 (13.2%) were found to be PRN-negative: six of these were collected between 2004 and 2006, corresponding to the later part of the period when wP and aP vaccines were used in parallel (1996–2006), while 182 were collected after aP vaccines were exclusively used for both primary immunization and boosting (2007 to 2018). During this aP vaccine-only period, the proportion of PRN-negative B. pertussis isolates increased from 5.6% in 2007 to 48.4% in 2018.10 However, this documented increase in PRN deficiency proportion over the recent years does not seem to be temporally associated with a surge of pertussis cases in infants based on RENACOQ data.70
The current childhood immunization schedule in France comprises three doses of a hexavalent vaccine (DTaP-Hib-HepB-IPV) administered at 2, 4, and 11 months, one dose of DTaP-IPV at 6 years, and one dose of Tdap-IPV at 11–13 years.16 Vaccination with aP vaccines started in 1996; currently, nine aP-containing vaccines are used, of which three do not include PRN.60,90 Six are used for primary immunization, four for the pre-school booster, and two for the adult/maternal booster.90 For primary vaccination of infants, only one hexavalent vaccine (which contained PRN) was available between 2005 and 2015/2016.79,80 Among the three hexavalent vaccines currently on the market in France, one does not contain PRN. Since 2018, vaccination of children against pertussis is compulsory from the age of 2 months.68 In adults, aP vaccination is recommended at 25 years of age (unless a pertussis-containing vaccine has been administered in the past 5 years), and during each pregnancy (Table 3, Supplementary table S2).
Vaccination coverage tends to be high in infants and toddlers, reaching 98.8% in children 24 months of age in 2019; data available for older age groups indicates lower coverage (61.2% at 11–13 years of age, 36.0% at 29 years of age, and 21.7% at 69 years of age in 2017; Table 4).
Canada
Pertussis is a notifiable disease in Canada;67 only confirmed cases (defined as cases with laboratory confirmation or an epidemiological link to a confirmed case, and specific symptomatology) are to be reported (Table 1).
Pertussis surveillance in Canada includes a passive component, based on the confirmed cases reported by public health professionals through the Canadian Notifiable Disease Surveillance System. In addition, active surveillance data is also collected through the Immunization Monitoring Program, ACTive (IMPACT), a hospital-based pediatric surveillance system supported by the Public Health Agency of Canada (Table 2). At each hospital enrolled in IMPACT, a designated nurse monitor actively searches for children hospitalized with pertussis, under the supervision of a volunteer pediatrician/infectious diseases specialist.91
In a study that assessed pertussis epidemiology from 2005 to 2019 (after the recommendation for the aP vaccine adolescent booster was made), an average annual incidence rate per 100,000 population of 6.4 (ranging from 2.0 in 2011 to 13.4 in 2012) was reported.69 During this period, epidemiological peaks in pertussis cases were observed in 2012 and from 2015 to 2017 (Figure 5C). Most of the disease burden was seen in infants <1 year of age (Supplementary table S3).
Several studies reported data on PRN-negative B. pertussis strains isolated in Canada (Supplementary table S1). Tsang et al. (2019) identified PRN deficiency in 34.6% (143/413) of B. pertussis isolates cultured at the Public Health Ontario Laboratory.92 Over time, the proportion of PRN-negative B. pertussis isolates increased from 16.7% in 2011 to 70.8% in 2016; however, among isolates from 2017, only 46.2% were PRN-negative. This decrease in PRN-negative isolates might be linked to a decrease in the circulation of sequence type 2 of B. pertussis.92
The current childhood immunization program against pertussis varies across the different Canadian provinces and territories (Supplementary table S2). According to the Canadian Immunization Guide, a DTaP-IPV-Hib-containing vaccine should be used for primary vaccination, followed by a booster dose at 4–6 years of age (using DTaP-IPV or Tdap-IPV), and another booster dose at 14–16 years of age (using Tdap); the latter booster dose was introduced between 1999 and 2004.46,69 Pertussis vaccination is also recommended for adults and during pregnancy (Table 3).69 All aP vaccines currently used in Canada contain the PRN antigen.46
Based on 2018–2019 estimates, pertussis vaccine coverage in children is ~80%, and even lower in adults (Table 4), although the pertussis vaccine coverage during pregnancy has increased between 2019 and 2021.69,84
Discussion
In the first part of this review, we provided a comprehensive insight into the proportion of PRN deficiency in B. pertussis isolates, using a large body of systematically collected evidence. PRN deficiency arises as a result of mutations in the promoter or coding regions of the prn gene. However, it is important to note here that selection pressure is not exerted exclusively on the PRN antigen, but also on others. These include PT, the main factor responsible for overt symptomatology (through mutations in the PT A gene [ptxA], and the PT promoter region [ptxP]), and FIM2/3 (through mutations in the fim2 and fim3 genes). The fitness of genotypes with different combinations of mutations or alleles varies: in the aP vaccine era, PRN-negative genotypes appear to demonstrate a higher fitness than PRN-positive genotypes, but only when PRN deficiency is combined with a ptxP3 allele.72 The seemingly conserved pathogenicity of PRN-negative B. pertussis strains could contribute to the high number of isolates with this characteristic being identified in comparison to strains exhibiting other mutations. In comparison, PT-negative B. pertussis strains have been associated with a milder or even asymptomatic clinical course, meaning that such strains are less likely to be isolated in the context of the current surveillance systems.11,15
A study among infants <6 months old conducted in France93 and a study among different age groups conducted in the USA24 found no differences between the clinical presentation of pertussis cases in patients infected with PRN-negative strains and those infected with PRN-positive strains, except for a higher frequency of apnea in the latter category.24 This is in line with findings of other studies, in which PRN-negative strains were shown to cause similar clinical signs and symptoms as PRN-positive strains.38,94,95 Moreover, a recently published study from France among 361 symptomatic infants <6 months of age showed that PRN-positive strains collected between 2008 and 2019 were associated with an increased risk of fulminant pertussis.96
In the same study conducted in the USA, individuals vaccinated with PRN-containing aP vaccines appeared to have greater susceptibility to PRN-negative strains compared to PRN-positive strains.24 By contrast, the EUpertstrain IV study found no significant difference in the proportion of PRN-negative isolates between vaccinated and unvaccinated patients from the studied countries, except for the UK, where (in line with the study conducted in the USA)24 these isolates were found more in vaccinated patients.60
Clinical data is often not captured in studies evaluating PRN-negative B. pertussis prevalence, thus limiting the ability of formulating a comprehensive evaluation of the clinical significance of such strains. As a general practice, isolates are primarily obtained from infants and children, with a predominant focus on the hospitalized population subset; therefore, the representativeness of the characterized strains remains unknown.
We aimed to illustrate that the proportion of PRN-negative B. pertussis isolates exhibited a steady increase over the recent years in countries using aP vaccines; in most cases, these were PRN-containing aP vaccines, albeit they were often used in parallel with aP vaccines which do not include the PRN antigen. Although a correlation between the widespread use of (mostly PRN-containing) aP vaccines and an increase in the proportion of PRN-negative B. pertussis strains has been shown previously10,15,43,97 and appears to also be supported by the results of our systematic literature review, a causal relationship between these two findings cannot be automatically implied. Nevertheless, as very few PRN-negative B. pertussis isolates have been identified before the use of aP vaccines,10,97,98 evidence for the increase in PRN-negative strains as a result of vaccine pressure is strong.11,15,23 Moreover, the appearance and increase of PRN-negative strains does not seem to occur in countries that use aP vaccines without PRN, as suggested by the examples from Japan61 and Denmark.60
In some countries, >80% of B. pertussis isolates are PRN-negative.24,35–38,40,72 In contrast, no PRN-negative strains were identified in the countries included in this review in which only wP vaccines are being used.41,43,99,100 However, these results should be interpreted in the light of the overall low number of isolates and the limitations in surveillance, as well as strain isolation and their molecular characterization in developing countries. Interest in PRN deficiency (along with other mutations in circulating B. pertussis strains) has been increasing in the scientific community, as manifested by the growing number and scale of the studies exploring data related to B. pertussis strains exhibiting various mutations.
The PRN antigen is not specific to B. pertussis, as it can also be found in B. parapertussis and B. bronchiseptica.15 The emergence of PRN-negative B. parapertussis isolates has also been recorded in France from 2007 onwards.11,97,101,102 The absence of notable differences observed in the pathogenicity of PRN-negative and PRN-positive B. parapertussis strains (similar to what is seen in B. pertussis) supports the hypothesis that loss of PRN does not result in a significant change in virulence.101 Furthermore, the simultaneous increase in the proportion of PRN-negative B. pertussis and B. parapertussis strains following the introduction of aP vaccines, including those that contain PRN, could be interpreted as evidence supporting a potential impact (and subsequent selection pressure) of PRN-containing aP vaccines on B. parapertussis, although studies assessing the effectiveness of aP vaccines (including those that contain PRN) against B. parapertussis are scarce.11,103 To date, this increase in PRN-negative B. parapertussis strain proportion has only been reported in France; in Iran, where wP vaccines are used, none of the seven B. parapertussis isolates collected between 2012 and 2015 were PRN-negative.104
In the second part of the review, we gathered in-depth information on pertussis surveillance, epidemiology, and prevention in Belgium, France, and Canada; this allowed interpretation of the findings related to the proportion of PRN-negative B. pertussis isolates in a broader context. Pertussis is a notifiable disease in Belgium and Canada, but not in France.64,67,105 In all three countries, pertussis surveillance generally relies on different data sources, such as hospital surveillance networks or laboratory networks. Another common aspect is represented by the strong focus on pediatric cases across all three countries analyzed.64,68–70,91 In the two federal countries assessed (Belgium and Canada), regional differences have been identified in terms of reporting requirements, vaccine types used, or ages at which the various vaccine doses are recommended. For example, in Belgium, all possible, probable, and confirmed pertussis cases must be reported in Flanders, while only laboratory-confirmed cases are to be reported in Wallonia (in children under 3 years of age) and Brussels (regardless of age).64 Nevertheless, PCR (the more frequently used laboratory confirmation method), is only reimbursed in unvaccinated/partially vaccinated children,64 meaning that breakthrough cases could be more easily missed in Wallonia and Brussels, while the probability of detecting them would be higher in Flanders.
All three countries are currently using aP vaccines that contain two (i.e., without PRN), three and/or five pertussis antigens.16,46,60,76 In France, six of the nine pertussis-containing vaccines used include the PRN antigen;90 the proportion in which these vaccines were used has evolved across time following their successive introduction, with an aP vaccine that does not contain PRN being currently used to vaccinate ~70% of infants. The uptake of pertussis-containing vaccines is monitored in young children in all three countries; vaccination coverage among 2-year-old children was high in Belgium and France (≥93%), and somewhat lower in Canada (<80%).69,86–88 However, vaccine coverage data in adolescents and adults is scarce.
Coverage data regarding adult pertussis vaccination administered as part of the cocooning strategy or during pregnancy were available for two of the three countries included in the in-depth analysis. A recently published study used vaccination coverage rates among parents of newborns to evaluate the implementation of the cocooning strategy introduced 12 years earlier in France; in 2016–2017, 46–47% of both mothers and fathers of newborns were up to date on their pertussis vaccination.88 In Belgium, regional Tdap vaccination uptake in pregnant women varied greatly, ranging from 31% in Brussels to 85% in Flanders.86 For Canada, only data on pertussis booster vaccination coverage in the general population were available, showing a coverage rate of 33–65%.69,84 These data suggest that there is room for improvement when pertussis vaccine uptake in adults is concerned.
Consistent with the overall findings of the systematic literature review, PRN-negative B. pertussis isolates were observed in all three countries evaluated in detail. Even though in some years the number of collected samples was small, the proportion of PRN-negative isolates followed an overall increasing trend, although yearly variations were also observed.92,106 PRN-negative strains appear to be more prevalent in Canada, with recent estimates of up to 70%,92 compared to less than 48% in France.10,60,72,106 Differences in vaccine types used, as well as in vaccine coverage among the different age groups could explain some of the variability in the proportion of PRN-negative isolates observed in the three countries. However, these findings should be interpreted with caution, considering that the representativeness of the characterized isolates is unknown, and the significance of the apparent differences in proportion of PRN-negative B. pertussis strains has not been established. In most cases, limited information was available on the demographic profile of the patients and the clinical characteristics of the cases from which the B. pertussis isolates were obtained. When examining this increasing trend in the context of pertussis epidemiology, we found that the recent increase in PRN-negative strain proportion among isolates from France did not seem to coincide with an overall increase of pertussis cases in infants.10,70 More recently (1 January 2024 to 31 May 2024), pertussis cases in France peaked, with 66 out of 67 isolates being PRN-positive. This is in contrast with the 228 isolates collected between 2016 and 2020, of which 51.3% were PRN-negative. The resurgence of PRN-positive strains may have been facilitated by the use of an aP vaccine that does not contain PRN, thereby enabling PRN-positive strains to spread more efficiently. Similar increases were seen in Czechia, Denmark, and Spain.107
As mentioned previously, the clinical significance of PRN-negative B. pertussis strains is not yet defined; nevertheless, these findings suggest that increases in PRN deficiency are not associated with increases in the number of pertussis cases. Furthermore, an equally reassuring finding is that the efficacy of aP vaccines, including those that contain PRN, appears to be preserved in the first years following childhood vaccination even in settings with a high proportion of PRN-negative isolates, although a decline in VE is observed 4–8 years after the last dose.108,109 To date, limited data on effectiveness of aP vaccines against PRN-negative B. pertussis strains are available, and concerns have been expressed that PRN-negative strains might have a higher potential to escape immunity induced by aP vaccines.11,15 Studies evaluating the pertussis VE often use the case–control methodology or the screening method. They are generally conducted over several years and very rarely provide information on the molecular evolution of the strains during the studied period.
In contrast, during an outbreak, the high incidence makes it possible to assess VE over a shorter period, often in a restricted geographical area (school, region). We found only one example of a VE evaluation conducted in the USA following a major pertussis outbreak in 2012, which also reported information on the characteristics of the isolated strains.34 Overall, the effectiveness of a five-dose primary vaccination schedule against pertussis disease in children 4–10 years of age was 84%, even though more than 90% of the B. pertussis isolates collected at the time of the study were PRN-negative. A VE case–control study conducted between 2009 and 2015 in Ontario, Canada, estimated that pertussis vaccination was ~ 90% effective during the first years after primary vaccination in children up to 8–9 years of age, ~50% in 12–13-year-olds, and ~80% after the adolescent booster in those 14–16 and 16–22 years of age.108 While this study did not report data resulting from strain characterization, such data is available for Ontario for approximately the same period, showing a steady increase in the proportion of PRN-negative isolates over time, from 16.7% in 2011 to 61.3% in 2015.92 In 2017, a year during which the most recent epidemiological peak of pertussis was recorded in Ontario, the proportion of PRN-negative isolates declined.92 This could indicate a greater transmission potential of B. pertussis strains expressing PRN and suggests that aP vaccines continue to be protective despite a high proportion of PRN-negative strains.
In the absence of studies directly evaluating VE on PRN-negative versus PRN-positive strains, circumstantial evidence can be inferred from other sources. For example, surveillance data collected in France, corroborated with data on PRN-negative strain circulation, do not suggest a decreased VE against these isolates, as the steady increase in the proportion of PRN-negative strains isolated was not reflected in a corresponding increase of pertussis cases.70,106 In contrast, a recent increase in the number of pertussis cases in 2024 coincided with an increase in the proportion of PRN-positive strains.107 Taken together, this suggests that the PRN-negative strains that are currently circulating may not be able to escape immunity induced by aP vaccines.
Future studies should assess vaccine coverage and dominant strains as well as the clinical significance of PRN deficiency and its potential impact on pertussis disease control. Guidelines on laboratory diagnosis and molecular surveillance of B. pertussis, issued by the European Centre for Disease Control and Prevention in 2022, mention that, although the use of culture has been decreasing from year to year, it is still highly recommended as the isolates can be used for molecular surveillance of the changes in bacterial populations.110 Monitoring of the circulating isolate variants is important for evaluation of VE and for future vaccine development.
To fulfill this requirement, a public health surveillance system needs to incorporate a strong laboratory component to collect and characterize representative isolates. Ideally, isolates (B. pertussis and B. parapertussis) should be collected from patients of different ages (i.e., from both children and adults), various degrees of illness severity (i.e., from both hospitalized and outpatient cases), and a broad geographical distribution of samples within a given country should also be ensured. This is challenging, as the routine use of culture for diagnosis of pertussis has declined since the introduction of PCR methods.111 Moreover, it is often not possible to isolate B. pertussis from cultures from adolescents and adults because they tend to seek medical advice only after several weeks of coughing.110 In these patients, diagnosis by serology (based on anti-PT immunoglobulin G) remains the most commonly used method. Ideally, information to be collected should include patient demographics (age, geographical area) and vaccination status (including the type of vaccine received, and the administration dates), clinical characteristics of the illness episode, and information on whether the case belongs to an outbreak. Furthermore, sequencing of collected isolates should be performed whenever possible.
Pertussis vaccine uptake studies beyond early childhood are important to accurately estimate the coverage in the entire population. Obtaining a broad coverage in all age groups through regular booster vaccination is important to prevent pertussis resurgence, especially in the context of increasing prevalence of mutations observed in B. pertussis strains.
Future effectiveness studies should include collection of contextual information on PRN deficiency during the study period and within the covered geographical area. An effectiveness study design in which strains would be characterized (e.g., case–control study) could also be considered.
Of note, steps are already being taken to better characterize the landscape of pertussis in the context of bacterial evolution under selection pressure. To study genetic changes in B. pertussis populations in Europe, panels of B. pertussis isolates have been serially collected during four periods, starting in 1999, in a total of 11 European countries.60 The ongoing EUpertstrain V project aims to continue monitoring the proportion of PRN-negative isolates and to collect information on the vaccination status and clinical severity associated to pertussis cases across several countries. This study, as well as similar ones that will potentially be conducted in other parts of the world, could provide additional insights to help decipher the mechanisms, clinical relevance, and required actions in the face of vaccine-driven selection pressure exerted on B. pertussis strains.
The systematic literature review presented here has limitations. The main limitations of the data on PRN-negative B. pertussis isolates relate to the heterogeneity between the included studies, variable and sometimes very long study periods, different vaccination strategies, overlapping samples, and very limited data for countries and/or periods when wP vaccines were primarily used. Furthermore, the information available on the PRN-negative strains and the population in which they were isolated is often limited. Genetic characterization of the isolates is rarely performed, thus data on the genomic events that cause PRN deficiency is scarce. Data on the type, number, and timing of pertussis vaccines received is not usually recorded for individuals from whom B. pertussis strains were isolated, hampering the identification of breakthrough pertussis cases. On a population level, the proportion of the various types of pertussis vaccines administered is not readily available, further limiting the possibility of correlating the extent of PRN-containing aP vaccine use and the proportion of PRN-negative B. pertussis isolates. Finally, finding an association between the increase of PRN-negative strains following introduction of aP vaccines is not irrefutable proof of cause and effect. For this, additional studies are needed, especially in countries where more types of pertussis vaccine (wP and aP, aP vaccines with and without a PRN component) are used concurrently, to analyze isolates from individuals with breakthrough infection following vaccination with the different vaccine types.
In conclusion, the proportion of PRN-negative B. pertussis strains has been increasing in most countries that use aP vaccines. Although currently available data do not suggest a decreased protection against pertussis disease conferred by aP vaccines in this context of increasing PRN deficiency, further studies are needed to provide stronger evidence and more data regarding VE and the duration of protection. Ideally, these studies should link the clinical and epidemiological characteristics, as well as the vaccination status of the cases with the molecular characterization of the strains. Enhanced disease surveillance and effectiveness estimation can only be achieved by incorporating a strong laboratory component in pertussis surveillance, including selection of representative B. pertussis (and B. parapertussis) isolates for strain characterization. In the context of waning immunity, and the increase in PRN-negative isolates (or other mutations driven by vaccine pressure), regular aP vaccine booster doses across life may be needed to limit pertussis resurgence.
Highlights
Pertussis childhood immunization programs using combination vaccines containing either whole-cell (wP) or acellular pertussis (aP) components remain crucial in reducing the burden of disease worldwide; however, pertussis resurgences have been documented, even in countries with high pediatric vaccine coverage.
Bordetella pertussis strains not expressing pertactin (PRN) have been increasingly identified in countries where aP vaccines are used but remain low or absent in countries with predominant wP vaccination, suggesting selection pressure of aP vaccines, in particular those that contain PRN as an antigen, on bacterial strain evolution.
In-depth analysis of contextual data regarding pertussis surveillance in three selected countries (Belgium, France, and Canada) revealed a strong focus on pediatric pertussis cases, although variations in surveillance systems among countries or regions within the same country were noted; data on pertussis cases in adults were not systematically collected. As PRN-containing and non-PRN-containing aP vaccines have been used alternatively or concomitantly in most countries, and the vaccination status of the patients from whom PRN-negative strains have been isolated is rarely reported, no definitive conclusion regarding the effect of PRN-containing aP vaccines on the proportion of PRN-negative B. pertussis isolates can be drawn based on the available data.
Surveillance data do not show an increase of pertussis pediatric cases during the period in which the prevalence of PRN-negative strains increases, suggesting no critical impairment of the vaccine effectiveness against PRN-negative strains; although aP vaccines are very effective, immunity conferred by aP vaccines is waning significantly after 5–10 years following childhood vaccination, regardless of PRN expression.
To accurately assess changes in the circulating B. pertussis strains as well as the impact of these changes, surveillance systems should include steps for adequate characterization of the isolates; to do so, nasopharyngeal specimens from patients with cough illnesses should not only be tested by polymerase chain reaction (PCR), but growth of B. pertussis in culture should also be encouraged. In addition, sampling should ideally target all age groups instead of predominantly focusing on the pediatric population. This is challenging, as the routine use of culture as a method for diagnosing pertussis has declined since the introduction of PCR and as it is often not possible to isolate B. pertussis from cultures in older children and adults because they tend to seek medical advice only after several weeks of coughing.
Supplementary Material
Acknowledgments
The authors would like to thank Dasha Shamarina and Fabrice Godfroid for their valuable contribution to this manuscript. The authors would like to acknowledge Leonie de Munter, Ana Goios and Sanskruti Gaikwad from P95 for help with the SLR work. The authors would like to thank the Akkodis Belgium platform for writing and editorial assistance (by Timea Kiss), manuscript coordination, and design support, on behalf of GSK.
Biography
Valérie Berlaimont is holder of a PhD in Pharmaceutical Sciences and has more than 20 years experience in local, regional (APAC) and global Medical Affairs, successively at Sanofi, Boehringer-Ingelheim and GSK.
Funding Statement
This study and related publication were sponsored by GSK. Authors did not receive an honorarium related to this scientific publication.
Disclosure statement
UH declares to have received consulting fees for the Global Pertussis Initiative (Sanofi-Pasteur), the Central and Eastern Europe Pertussis Awareness Group (Sanofi-Pasteur), and a pertussis consultation (GSK), and product-independent lecture fees from GSK, InfectoPharm, Merck, Moderna, Pfizer, Roche, Sanofi Genzyme, and Sanofi-Pasteur. UH participated on the Data Monitoring Committees of a poliomyelitis vaccine (Takeda), a phase II study of an adjuvanted pandemic influenza vaccine (GSK/Watermark), the norovirus bivalent VLP vaccine program (Takeda/HilleVax), and the cell culture influenza vaccine (Seqirus/IQVIA). UH is a member of the Meta Data Safety Monitoring Board for CEPI (Coalition for Epidemic Preparedness Innovations) and the Varicella Advisory Board, Switzerland (Merck). UH has been a member of STIKO (Ständige Impfkommission, the National Immunization Technical Advisory Group of Germany; 2001–March 2024), the president of the Paediatric Infectious Diseases Group, Switzerland, and the chair of the Committee for Infectious Diseases and Vaccines, Bündnis Kinder- und Jugendgesundheit Germany (all without financial support). HM declares to have received financial support for attending EUPert-LabNet meetings from the EUpertstrain organization. JE and IP are employees of P95/Pallas, a company that received funding from GSK for performing the systematic literature review. Their company, P95, has held contracts with GSK, AstraZeneca, Pfizer, Sanofi, and Seqirus. Pallas, part of P95, has collaborated with GSK, Orchard, BioMarin, Daiichi, Bavarian Nordic, Bayer, and Sanofi. IP is an unpaid executive board member of the NGO UPK (United against COVID) in Serbia. AG, ET, MD, and VB are/were employed by GSK and hold financial equities in GSK.
Author contributions statement
All authors made substantial contributions to the conception and design, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2435134
References
- 1.Kendrick P, Eldering G.. A study in active immunization against pertussis. Am J Hyg. 1939;29 Sec B(3):133–19. doi: 10.1093/oxfordjournals.aje.a118485. [DOI] [Google Scholar]
- 2.Guiso N, Meade BD, Wirsing von König CH. Pertussis vaccines: the first hundred years. Vaccine. 2020;38(5):1271–1276. doi: 10.1016/j.vaccine.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Pertussis [accessed 2024 Oct 17]. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/pertussis.
- 4.Ström J. Is universal vaccination against pertussis always justified? Br Med J. 1960;2(5207):1184–1186. doi: 10.1136/bmj.2.5207.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulenkampff M, Schwartzman JS, Wilson J. Neurological complications of pertussis inoculation. Arch Dis Child. 1974;49(1):46–49. doi: 10.1136/adc.49.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrengut W. Über konvulsive Reaktionen nach Pertussis-Schutzimpfung [Convulsive reactions after pertussis immunization]. Dtsch Med Wschr. 1974;99(45):2273–2279. doi: 10.1055/s-0028-1108124. [DOI] [PubMed] [Google Scholar]
- 7.Gale JL, Thapa PB, Wassilak SGF, Bobo JK, Mendelman PM, Foy HM. Risk of serious acute neurological illness after immunization with diphtheria-tetanus-pertussis vaccine: a population-based case-control study. JAMA. 1994;271(1):37–41. doi: 10.1001/jama.1994.03510250053034. [DOI] [PubMed] [Google Scholar]
- 8.Berkovic SF, Harkin L, McMahon JM, Pelekanos JT, Zuberi SM, Wirrell EC, Gill DS, Iona X, Mulley JC, Scheffer IE. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006;5(6):488–492. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- 9.Ray P, Hayward J, Michelson D, Lewis E, Schwalbe J, Black S, Shinefield H, Marcy M, Huff K, Ward J, et al. Encephalopathy after whole-cell pertussis or measles vaccination: lack of evidence for a causal association in a retrospective case-control study. Pediatr Infect Dis J. 2006;25(9):768–773. doi: 10.1097/01.inf.0000234067.84848.e1. [DOI] [PubMed] [Google Scholar]
- 10.Bouchez V, Guillot S, Landier A, Armatys N, Matczak S, Toubiana J, Brisse S. Evolution of Bordetella pertussis over a 23-year period in France, 1996 to 2018. Euro Surveill. 2021;26(37):2001213. doi: 10.2807/1560-7917.ES.2021.26.37.2001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiso N, Soubeyrand B, Macina D. Can vaccines control bacterial virulence and pathogenicity? Bordetella pertussis: the advantage of fitness over virulence. Evol Med Public Health. 2022;10(1):363–370. doi: 10.1093/emph/eoac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Pertussis vaccines: WHO position paper, August 2015—recommendations. Vaccine. 2016;34(12):1423–1425. doi: 10.1016/j.vaccine.2015.10.136. [DOI] [PubMed] [Google Scholar]
- 13.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. 2014;111(2):787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015;13(1):146. doi: 10.1186/s12916-015-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Caulfield A, Dewan KK, Harvill ET. Pertactin-deficient Bordetella pertussis, vaccine-driven evolution, and reemergence of pertussis. Emerg Infect Dis. 2021;27(6):1561–1566. doi: 10.3201/eid2706.203850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Immunization data. Vaccination schedule for pertussis. [accessed 2024 Jul 15]. https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-pertussis?ISO_3_CODE=&TARGETPOP_GENERAL=.
- 17.Mooi FR. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect Genet Evol. 2010;10(1):36–49. doi: 10.1016/j.meegid.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Heininger U, André P, Chlibek R, Kristufkova Z, Kutsar K, Mangarov A, Mészner Z, Nitsch-Osuch A, Petrović V, Prymula R, et al. Comparative epidemiologic characteristics of pertussis in 10 central and eastern European countries, 2000-2013. PLOS ONE. 2016;11(6):e0155949. doi: 10.1371/journal.pone.0155949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diavatopoulos DA, Mills KHG, Kester KE, Kampmann B, Silerova M, Heininger U, van Dongen JJM, van der Most RG, Huijnen MA, Siena E, et al. PERISCOPE: road towards effective control of pertussis. Lancet Infect Dis. 2019;19(5):e179–e186. doi: 10.1016/S1473-3099(18)30646-7. [DOI] [PubMed] [Google Scholar]
- 20.Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation – two sides of the same coin. Epidemiol Infect. 2014;142(4):685–694. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alghounaim M, Alsaffar Z, Alfraij A, Bin-Hasan S, Hussain E. Whole-cell and acellular pertussis vaccine: reflections on efficacy. Med Princ Pract. 2022;31(4):313–321. doi: 10.1159/000525468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Shi N, Wang Q, Yang L, Cui T, Jin H. The association between vaccine hesitancy and pertussis: a systematic review and meta-analysis. Ital J Pediatr. 2023;49(1):81. doi: 10.1186/s13052-023-01495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mir-Cros A, Moreno-Mingorance A, Martín-Gómez MT, Abad R, Bloise I, Campins M, González-Praetorius A, Gutiérrez MN, Martín-González H, Muñoz-Almagro C, et al. Pertactin-deficient Bordetella pertussis with unusual mechanism of pertactin disruption, Spain, 1986–2018. Emerg Infect Dis. 2022;28(5):967–976. doi: 10.3201/eid2805.211958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, et al. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis. 2015;60(2):223–227. doi: 10.1093/cid/ciu788. [DOI] [PubMed] [Google Scholar]
- 25.Brennan MJ, Li ZM, Cowell JL, Bisher ME, Steven AC, Novotny P, Manclark CR. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley P, McDermott G, Charles IG, Fairweather NF, Isaacs NW. Crystallographic characterization of pertactin, a membrane-associated protein from Bordetella pertussis. J Mol Biol. 1994;235(2):772–773. doi: 10.1006/jmbi.1994.1029. [DOI] [PubMed] [Google Scholar]
- 27.Everest P, Li J, Douce G, Charles I, De Azavedo J, Chatfield S, Dougan G, Roberts M. Role of the Bordetella pertussis P.69/pertactin protein and the P.69/pertactin RGD motif in the adherence to and invasion of mammalian cells. Microbiology (Reading). 1996;142(Pt 11):3261–3268. doi: 10.1099/13500872-142-11-3261. [DOI] [PubMed] [Google Scholar]
- 28.Leininger E, Roberts M, Kenimer JG, Charles IG, Fairweather N, Novotny P, Brennan MJ. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88(2):345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hovingh ES, Mariman R, Solans L, Hijdra D, Hamstra H-J, Jongerius I, van Gent M, Mooi F, Locht C, Pinelli E. Bordetella pertussis pertactin knock-out strains reveal immunomodulatory properties of this virulence factor. Emerg Microbes Infect. 2018;7(1):1–13. doi: 10.1038/s41426-018-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16(20):1901–1906. doi: 10.1016/S0264-410X(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 31.Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16(20):1907–1916. doi: 10.1016/S0264-410X(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 32.Safarchi A, Octavia S, Luu LD, Tay CY, Sintchenko V, Wood N, Marshall H, McIntyre P, Lan R. Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine. 2015;33(46):6277–6281. doi: 10.1016/j.vaccine.2015.09.064. [DOI] [PubMed] [Google Scholar]
- 33.Lesne E, Cavell BE, Freire-Martin I, Persaud R, Alexander F, Taylor S, Matheson M, van Els CACM, Gorringe A. Acellular pertussis vaccines induce anti-pertactin bactericidal antibodies which drives the emergence of pertactin-negative strains. Front Microbiol. 2020;11:2108. doi: 10.3389/fmicb.2020.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breakwell L, Kelso P, Finley C, Schoenfeld S, Goode B, Misegades LK, Martin SW, Acosta AM. Pertussis vaccine effectiveness in the setting of pertactin-deficient pertussis. Pediatrics. 2016;137(5):e20153973. doi: 10.1542/peds.2015-3973. [DOI] [PubMed] [Google Scholar]
- 35.Weigand MR, Peng Y, Loparev V, Batra D, Bowden KE, Burroughs M, Cassiday PK, Davis JK, Johnson T, Juieng P, et al. The history of Bordetella pertussis genome evolution includes structural rearrangement. J Bacteriol. 2017;199(8):e00806–16. doi: 10.1128/JB.00806-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weigand MR, Pawloski LC, Peng Y, Ju H, Burroughs M, Cassiday PK, Davis JK, DuVall M, Johnson T, Juieng P, et al. Screening and genomic characterization of filamentous hemagglutinin-deficient Bordetella pertussis. Infect Immun. 2018;86(4):e00869–17. doi: 10.1128/IAI.00869-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weigand MR, Williams MM, Peng Y, Kania D, Pawloski LC, Tondella ML. Genomic survey of Bordetella pertussis diversity, United States, 2000–2013. Emerg Infect Dis. 2019;25(4):780–783. doi: 10.3201/eid2504.180812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vodzak J, Queenan AM, Souder E, Evangelista AT, Long SS. Clinical manifestations and molecular characterization of pertactin-deficient and pertactin-producing Bordetella pertussis in children, Philadelphia 2007–2014. Clin Infect Dis. 2017;64(1):60–66. doi: 10.1093/cid/ciw632. [DOI] [PubMed] [Google Scholar]
- 39.Theofiles AG, Cunningham SA, Chia N, Jeraldo PR, Quest DJ, Mandrekar JN, Patel R. Pertussis outbreak, southeastern Minnesota, 2012. Mayo Clin Proc. 2014;89(10):1378–1388. doi: 10.1016/j.mayocp.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ring N, Davies H, Morgan J, Sundaresan M, Tiong A, Preston A, Bagby S. Comparative genomics of Bordetella pertussis isolates from New Zealand, a country with an uncommonly high incidence of whooping cough. Microb Genom. 2022;8(1):000756. doi: 10.1099/mgen.0.000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben Fraj I, Kechrid A, Guillot S, Bouchez V, Brisse S, Guiso N, Smaoui H. Pertussis epidemiology in Tunisian infants and children and characterization of Bordetella pertussis isolates: results of a 9-year surveillance study, 2007 to 2016. J Med Microbiol. 2019;68(2):241–247. doi: 10.1099/jmm.0.000892. [DOI] [PubMed] [Google Scholar]
- 42.Carriquiriborde F, Regidor V, Aispuro PM, Magali G, Bartel E, Bottero D, Hozbor D. Rare detection of Bordetella pertussis pertactin-deficient strains in Argentina. Emerg Infect Dis. 2019;25(11):2048–2054. doi: 10.3201/eid2511.190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, Cassiday PK, Chiang C-S, Dalby T, Fry NK, et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio. 2014;5(2):e01074. doi: 10.1128/mBio.01074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leite D, Camargo CH, Kashino SS, Polatto R, Martins LM, Pereira JC, Pawloski L, Tondella ML, Oliveira RSD, Vaz de Lima LRDA. Prevalence and characterization of pertactin deficient Bordetella pertussis strains in Brazil, a whole-cell vaccine country. Vaccine X. 2021;8:100103. doi: 10.1016/j.jvacx.2021.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention . About diphtheria, tetanus, and pertussis vaccines. 2022. [accessed 2023 Aug 22]. https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/about-vaccine.html.
- 46.Government of Canada . Pertussis (whooping cough) vaccines: Canadian immunization guide. 2023. [accessed 2023 June 9]. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-15-pertussis-vaccine.html.
- 47.Olivera I, Pérez CG, Lazarov L, Lopez E, Oddo C, Dibarboure H. Cost minimization analysis of a hexavalent vaccine in Argentina. BMC Health Serv Res. 2023;23(1):1067. doi: 10.1186/s12913-023-10038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avila-Agüero ML, Camacho-Badilla K, Ulloa-Gutierrez R, Espinal-Tejada C, Morice-Trejos A, Cherry JD. Epidemiology of pertussis in Costa Rica and the impact of vaccination: a 58-year experience (1961–2018). Vaccine. 2022;40(2):223–228. doi: 10.1016/j.vaccine.2021.11.078. [DOI] [PubMed] [Google Scholar]
- 49.Aguirre-Boza F, San Martín PP, Valenzuela BM. How were dtp-related adverse events reduced after the introduction of an acellular pertussis vaccine in Chile? Hum Vaccines Immunother. 2021;17(11):4225–4234. doi: 10.1080/21645515.2021.1965424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knuf M, Haas H, Garcia-Corbeira P, Turriani E, Mukherjee P, Janssens W, Berlaimont V. Hexavalent vaccines: what can we learn from head-to-head studies? Vaccine. 2021;39(41):6025–6036. doi: 10.1016/j.vaccine.2021.08.086. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization . Vaccination schedule for pertussis for Poland. [cited 2024 Jul 15]. https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-pertussis?ISO_3_CODE=POL&TARGETPOP_GENERAL=.
- 52.Muloiwa R, Wolter N, Mupere E, Tan T, Chitkara AJ, Forsyth KD, von König CHW, Hussey G. Pertussis in Africa: findings and recommendations of the global pertussis initiative (GPI). Vaccine. 2018;36(18):2385–2393. doi: 10.1016/j.vaccine.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization . Vaccination schedule for pertussis for Mauritius. [cited 2023 Sep 14]. https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-pertussis?ISO_3_CODE=MUS&TARGETPOP_GENERAL=.
- 54.Davis S. The what, why and when of childhood vaccination in South Africa – 2019. Prof Nurs Today. 2019;23(2):27–29. [Google Scholar]
- 55.Jog P, Memon IA, Thisyakorn U, Hozbor D, Heininger U, von König CHW, Tan T. Pertussis in Asia: recent country-specific data and recommendations. Vaccine. 2022;40(8):1170–1179. doi: 10.1016/j.vaccine.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Hong Kong Department of Health . Updated schedule of the hong kong childhood immunisation programme (HKCIP) recommended by the scientific committee on vaccine preventable diseases of the centre for health protection. Department of health. 2020. [cited 2023 Sep 14]. https://www.chp.gov.hk/files/pdf/updated_schedule_of_hkcip_recommended_by_scvpd_eng.pdf.
- 57.Australian Government . National immunisation program (NIP) changes from 1 July 2023. 2023. [cited 2023 Sep 14]. https://www.health.gov.au/news/national-immunisation-program-nip-changes-from-1-july-2023.
- 58.New Zealand Ministry of Health. Immunisation Handbook . Chapter 16: pertussis (whooping cough). 2024. [cited 2024 Oct 17]. https://www.tewhatuora.govt.nz/for-health-professionals/clinical-guidance/immunisation-handbook/16-pertussis-whooping-cough.
- 59.Chitkara AJ, Pujadas Ferrer M, Forsyth K, Guiso N, Heininger U, Hozbor DF, Muloiwa R, Tan TQ, Thisyakorn U, Wirsing von König CH. Pertussis vaccination in mixed markets: recommendations from the global pertussis initiative. Int J Infect Dis. 2020;96:482–488. doi: 10.1016/j.ijid.2020.04.081. [DOI] [PubMed] [Google Scholar]
- 60.Barkoff AM, Mertsola J, Pierard D, Dalby T, Hoegh SV, Guillot S, Stefanelli P, van Gent M, Berbers G, Vestrheim D, et al. Pertactin-deficient Bordetella pertussis isolates: evidence of increased circulation in Europe, 1998 to 2015. Euro Surveill. 2019;24(7):1700832. doi: 10.2807/1560-7917.ES.2019.24.7.1700832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiramatsu Y, Miyaji Y, Otsuka N, Arakawa Y, Shibayama K, Kamachi K. Significant decrease in pertactin-deficient Bordetella pertussis isolates, Japan. Emerg Infect Dis. 2017;23(4):699–701. doi: 10.3201/eid2304.161575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zomer A, Otsuka N, Hiramatsu Y, Kamachi K, Nishimura N, Ozaki T, Poolman J, Geurtsen J. Bordetella pertussis population dynamics and phylogeny in Japan after adoption of acellular pertussis vaccines. Microb Genom. 2018;4(5):e000180. doi: 10.1099/mgen.0.000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalby T. Clarifying pertussis in Denmark. Lancet Infect Dis. 2024;24(2):e77. doi: 10.1016/S1473-3099(23)00757-0. [DOI] [PubMed] [Google Scholar]
- 64.Litzroth A, Desombere I, Martini H, Piérard D. Surveillance épidémiologique de la coqueluche Bordetella pertussis - 2020 [Epidemiologic surveillance of pertussis - 2020]. 2020. [cited 2023 June 9]. https://www.sciensano.be/en/biblio/coqueluche-epidemiologie-rapport-annuel-2020. [Google Scholar]
- 65.Sciensano . IgG quantification (ELISA) against pertussis toxin (whooping cough) (sera). [cited 2023 Oct 19]. https://www.sciensano.be/en/analysis-request/igg-quantification-elisa-against-pertussis-toxin-whooping-cough-sera.
- 66.Institut Pasteur . Coqueluche [Pertussis]. 2021. [cited 2023 June 9]. https://www.pasteur.fr/fr/centre-medical/fiches-maladies/coqueluche.
- 67.Public Health Agency of Canada . Case definitions for communicable diseases under national surveillance. 2009. [cited 2023 June 9]. https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/publicat/ccdr-rmtc/09pdf/35s2-eng.pdf.
- 68.Debin M, Launay T, Rossignol L, Ait El Belghiti F, Brisse S, Guillot S, Guiso N, Levy-Bruhl D, Merdrignac L, Toubiana J, et al. Pertussis surveillance results from a French general practitioner network, France, 2017 to 2020. Euro Surveill. 2022;27(17):2100515. doi: 10.2807/1560-7917.ES.2022.27.17.2100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhagat D, Saboui M, Huang G, Reyes Domingo F, Squires SG, Salvadori MI, Li YA. Pertussis epidemiology in Canada, 2005–2019. Can Commun Dis Rep. 2023;49(1):21–28. doi: 10.14745/ccdr.v49i01a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santé Publique France . Coqueluche en France: données 2020-2021 [Pertussis in France - 2020-2021 data]. 2022. [cited 2023 June 9]. https://www.santepubliquefrance.fr/les-actualites/2022/coqueluche-en-france-donnees-2020-2021#.
- 71.Government of Canada . Vaccine preventable disease: surveillance report to December 31, 2019. 2022. [cited 2023 Aug 23]. https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-preventable-disease-surveillance-report-2019.html#b18. [Google Scholar]
- 72.Lefrancq N, Bouchez V, Fernandes N, Barkoff A-M, Bosch T, Dalby T, Åkerlund T, Darenberg J, Fabianova K, Vestrheim DF, et al. Global spatial dynamics and vaccine-induced fitness changes of Bordetella pertussis. Sci Transl Med. 2022;14(642):eabn3253. doi: 10.1126/scitranslmed.abn3253. [DOI] [PubMed] [Google Scholar]
- 73.Boisnard F, Caracci F, Badzhinerov N, Dhont P, Isnardy B, Kolenc M, Krogh K, Parisi S, Puchilita M, Ralfkiaer L. Use of hexyon/hexacima/hexaxim in preterm infants in Europe. Eur J Public Health. 2020;30(Supplement_5):ckaa166.1427. doi: 10.1093/eurpub/ckaa166.1427. [DOI] [Google Scholar]
- 74.Vaccination-info.be . Coqueluche [Pertussis]. 2023. [cited 2023 Oct 19]. https://www.vaccination-info.be/maladie/coqueluche/.
- 75.Departement Zorg . Basisvaccinatieschema [Basic vaccination schedule]. [cited 2023 Oct 19]. https://www.zorg-en-gezondheid.be/per-domein/infectieziekten-en-vaccinaties/vaccinaties/basisvaccinatieschema.
- 76.Conseil Supérieur de la Santé . CSS 9606 - Calendrier vaccinal de base - 2021 [CSS 9606 - basic vaccination schedule - 2021]. 2021. [cited 2023 June 9]. https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/20210603_fiche_9606_calendrier_vaccinal_css_2021_0.pdf.
- 77.Hoge Gezondheidsraad . Vaccinatie tegen kinkhoest [Vaccination against pertussis]. 2013. [cited 2023 Aug 23]. https://www.zorg-en-gezondheid.be/sites/default/files/2023-03/Vaccinatie%20tegen%20kinkhoest%20%28april%202014%29.pdf.
- 78.Ministère de la Santé et de la Prévention . Coqueluche. 2022. [cited 2023 Oct 19]. https://sante.gouv.fr/soins-et-maladies/maladies/maladies-infectieuses/coqueluche.
- 79.European Medicines Agency . Public statement on hexavac (diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis, hepatitis B [recombinant] and Haemophilus influenzae type B conjugate vaccine, adjuvanted). Withdrawal of marketing authorisation in the European Union. 2012. [cited 2023 Sep 14]. https://www.ema.europa.eu/en/documents/public-statement/public-statement-hexavac-withdrawal-marketing-authorisation-european-union_en.pdf.
- 80.Haut Conseil de la Santé Publique . Utilisation du vaccin hexavalent Hexyon® pour la vaccination des nourrissons [Use of the Hexyon® hexavalent vaccine for vaccination of infants]. 2015. [cited 2023 Sep 14]. https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspr20150220_vaccnourrissonsplacehexyon.pdf.
- 81.Haute Autorité de Santé . Utilisation du vaccin hexavalent VAXELIS pour la vaccination des nourrissons [Use of the VAXELIS hexavalent vaccine for immunization of infants]. 2017. [cited 2023 Oct 19]. https://www.ordre-sages-femmes.fr/wp-content/uploads/2017/12/recommandation_vaccinale_utilisation_du_vaccin_hexavalent_vaxelis.pdf.
- 82.Ministère de la Santé et de la Prévention . Calendrier des vaccinations et recommandations vaccinales 2024 [Vaccination schedule and recommendations 2024]. 2024. [cited 2024 Oct 17]. https://sante.gouv.fr/IMG/pdf/calendrier_vaccinal_oct24.pdf.
- 83.Gaudelus J, Vié le Sage F, Dufour V, Lert F, Texier N, Pouriel M, Tehard B, Bréart G. Public health impact of infanrix hexa™ (DTPa-HBV-IPV/Hib) reimbursement: a study programme in France. Part 1: evolution of hepatitis B vaccine coverage rates in infants aged less than 27 months, in the general population – the PopCorn study. Revue d’Épidémiologie et de Santé Publique. 2016;64(1):23–32. doi: 10.1016/j.respe.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 84.Government of Canada . Results of the survey on vaccination during pregnancy 2021. 2022. [cited 2023 Oct 19]. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/survey-vaccination-during-pregnancy-2021.html.
- 85.Bettinger JA, Halperin SA, De Serres G, Scheifele DW, Tam T. The effect of changing from whole-cell to acellular pertussis vaccine on the epidemiology of hospitalized children with pertussis in Canada. Pediatr Infect Dis J. 2007;26(1):31–35. doi: 10.1097/01.inf.0000247055.81541.04. [DOI] [PubMed] [Google Scholar]
- 86.Grammens T, Cornelissen L. Couverture vaccinale [Vaccine coverage]. 2021. [cited 2023 June 9]. https://www.sciensano.be/sites/default/files/vaccine_coverage_2020-21_fr_final.pdf.
- 87.Santé Publique France . Données de couverture vaccinale diphtérie-tétanos, poliomyélite, coqueluche par groupe d’âge [Diphtheria-tetanus, poliomyelitis, pertussis vaccine coverage data by age group]. 2023. [cited 2023 June 9]. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-diphterie-tetanos-poliomyelite-coqueluche-par-groupe-d-age.
- 88.Marchal C, Belhassen M, Guiso N, Jacoud F, Cohen R, Le Pannerer M, Verdier R. Cocooning strategy: pertussis vaccination coverage rate of parents with a new-born in 2016 and 2017 in France. Front Pediatr. 2022;10:988674. doi: 10.3389/fped.2022.988674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guillot S, Bouchez V, Toubiana J, Brisse S. Rapport annuel d’activité du Centre National de Référence de la coqueluche et autres bordetelloses – Années 2019-2020 [Annual activity report of the National Reference Center for pertussis and other diseases caused by Bordetellae - Years 2019-2020]. 2021. [cited 2023 Aug 22]. https://www.pasteur.fr/fr/file/42803/download.
- 90.Vaccination Info Service.fr . Le vaccin contre la coqueluche [The pertussis vaccine]. 2024. [cited 2023 Aug 22]. https://vaccination-info-service.fr/Les-maladies-et-leurs-vaccins/Coqueluche.
- 91.Canadian Paediatric Society . Surveillance. 2024. [cited 2024 Oct 17].https://cps.ca/en/impact.
- 92.Tsang RSW, Shuel M, Cronin K, Deng S, Whyte K, Marchand-Austin A, Ma J, Bolotin S, Crowcroft N, Schwartz K, et al. The evolving nature of Bordetella pertussis in Ontario, Canada, 2009–2017: strains with shifting genotypes and pertactin deficiency. Can J Microbiol. 2019;65(11):823–830. doi: 10.1139/cjm-2019-0128. [DOI] [PubMed] [Google Scholar]
- 93.Bodilis H, Guiso N. Virulence of pertactin-negative Bordetella pertussis isolates from infants, France. Emerg Infect Dis. 2013;19(3):471–474. doi: 10.3201/eid1903.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barkoff AM, Mertsola J, Guillot S, Guiso N, Berbers G, He Q. Appearance of Bordetella pertussis strains not expressing the vaccine antigen pertactin in Finland. Clin Vaccine Immunol. 2012;19(10):1703–1704. doi: 10.1128/CVI.00367-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clarke M, McIntyre PB, Blyth CC, Wood N, Octavia S, Sintchenko V, Giles L, Quinn H, Hill V, Hanly G, et al. The relationship between Bordetella pertussis genotype and clinical severity in Australian children with pertussis. J Infect. 2016;72(2):171–178. doi: 10.1016/j.jinf.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 96.Leroux P, Matczak S, Bouchez V, Volant S, Ouziel A, Launay E, Faye A, Rabier V, Sarlangue J, Jeziorski E, et al. Association between pertactin-producing Bordetella pertussis and fulminant pertussis in infants: a multicentre study in France, 2008–2019. Clin Microbiol Infect. 2024; doi: 10.1016/j.cmi.2024.09.009. [DOI] [PubMed] [Google Scholar]
- 97.Hegerle N, Paris AS, Brun D, Dore G, Njamkepo E, Guillot S, Guiso N. Evolution of French Bordetella pertussis and Bordetella parapertussis isolates: increase of Bordetellae not expressing pertactin. Clin Microbiol Infect. 2012;18(9):E340–E346. doi: 10.1111/j.1469-0691.2012.03925.x. [DOI] [PubMed] [Google Scholar]
- 98.Pawloski LC, Queenan AM, Cassiday PK, Lynch AS, Harrison MJ, Shang W, Williams MM, Bowden KE, Burgos-Rivera B, Qin X, et al. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin Vaccine Immunol. 2014;21(2):119–125. doi: 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Safarchi A, Octavia S, Nikbin VS, Lotfi MN, Zahraei SM, Tay CY, Lamichhane B, Shahcheraghi F, Lan R. Genomic epidemiology of Iranian Bordetella pertussis: 50 years after the implementation of whole cell vaccine. Emerg Microbes Infect. 2019;8(1):1416–1427. doi: 10.1080/22221751.2019.1665479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saedi S, Safarchi A, Moghadam FT, Heidarzadeh S, Nikbin VS, Shahcheraghi F. Fha deficient Bordetella pertussis isolates in Iran with 50 years whole cell pertussis vaccination. Iran J Public Health. 2021;50(7):1454–1462. doi: 10.18502/ijph.v50i7.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bouchez V, Brun D, Dore G, Njamkepo E, Guiso N. Bordetella parapertussis isolates not expressing pertactin circulating in France. Clin Microbiol Infect. 2011;17(5):675–682. doi: 10.1111/j.1469-0691.2010.03303.x. [DOI] [PubMed] [Google Scholar]
- 102.Toubiana J, Azarnoush S, Bouchez V, Landier A, Guillot S, Matczak S, Bonacorsi S, Brisse S. Bordetella parapertussis bacteremia: clinical expression and bacterial genomics. Open Forum Infect Dis. 2019;6(4):ofz122. doi: 10.1093/ofid/ofz122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liko J, Robison SG, Cieslak PR. Do pertussis vaccines protect against Bordetella parapertussis? Clin Infect Dis. 2017;64(12):1795–1797. doi: 10.1093/cid/cix221. [DOI] [PubMed] [Google Scholar]
- 104.Safarchi A, Saedi S, Tay CY, Lamichhane B, Nakhost Lotfi M, Shahcheraghi F. Genome characteristic of Bordetella parapertussis isolated from Iran. Curr Microbiol. 2022;79(10):314. doi: 10.1007/s00284-022-03009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santé Publique France . Coqueluche [Pertussis]. [cited 2023 June 9]. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/coqueluche/notre-action.
- 106.Bouchez V, Guglielmini J, Dazas M, Landier A, Toubiana J, Guillot S, Criscuolo A, Brisse S. Genomic sequencing of Bordetella pertussis for epidemiology and global surveillance of whooping cough. Emerg Infect Dis. 2018;24(6):988–994. doi: 10.3201/eid2406.171464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodrigues C, Bouchez V, Soares A, Trombert-Paolantoni S, Aït El Belghiti F, Cohen JF, Armatys N, Landier A, Blanchot T, Hervo M, et al. Resurgence of Bordetella pertussis, including one macrolide-resistant isolate, France, 2024. Euro Surveill. 2024;29(31):2400459. doi: 10.2807/1560-7917.ES.2024.29.31.2400459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crowcroft NS, Schwartz KL, Chen C, Johnson C, Li Y, Marchand-Austin A, Bolotin S, Jamieson FB, Drews SJ, Russell ML, et al. Pertussis vaccine effectiveness in a frequency matched population-based case-control Canadian immunization research network study in Ontario, Canada 2009–2015. Vaccine. 2019;37(19):2617–2623. doi: 10.1016/j.vaccine.2019.02.047. [DOI] [PubMed] [Google Scholar]
- 109.Schwartz KL, Kwong JC, Deeks SL, Campitelli MA, Jamieson FB, Marchand-Austin A, Stukel TA, Rosella L, Daneman N, Bolotin S, et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ. 2016;188(16):E399–E406. doi: 10.1503/cmaj.160193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.European Centre for Disease Control and Prevention . Laboratory diagnosis and molecular surveillance of Bordetella pertussis. 2022. [cited 2023 June 12]. https://www.ecdc.europa.eu/sites/default/files/documents/bordetella-pertussis-laboratory-diagnosis-molecular-surveillance.pdf.
- 111.van der Zee A, Schellekens JF, Mooi FR. Laboratory diagnosis of pertussis. Clin Microbiol Rev. 2015;28(4):1005–1026. doi: 10.1128/CMR.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


